Abstract
Adult functional magnetic resonance imaging (fMRI) literature suggests that a left-right hemispheric dissociation may exist between verbal and spatial working memory (WM), respectively. However, investigation of this type has been obscured by incomparable verbal and spatial WM tasks and/or visual inspection at arbitrary thresholds as means to assess lateralization. Furthermore, it is unclear whether this hemispheric lateralization is present during adolescence, a time in which WM skills are improving, and whether there is a developmental association with laterality of brain functioning. This study used comparable verbal and spatial WM n-back tasks during fMRI and a bootstrap analysis approach to calculate lateralization indices (LI) across several thresholds to examine the potential of a left-right WM hemispheric dissociation in healthy adolescents. We found significant left hemispheric lateralization for verbal WM, most notably in the frontal and parietal lobes, as well as right hemisphere lateralization for spatial WM, seen in frontal and temporal cortices. Although no significant relationships were observed between LI and age or LI and performance, significant age-related patterns of brain activity were demonstrated during both verbal and spatial WM. Specifically, increased adolescent age was associated with less activity in the default mode brain network during verbal WM. In contrast, increased adolescent age was associated with greater activity in task-positive posterior parietal cortex during spatial working memory. Our findings highlight the importance of utilizing non-biased statistical methods and comparable tasks for determining patterns of functional lateralization. Our findings also suggest that, while a left-right hemispheric dissociation of verbal and spatial WM is apparent by early adolescence, age-related changes in functional activation during WM are also present.
Keywords: Adolescence, working memory, fMRI, brain, lateralization
1. Introduction
Working memory (WM) refers to the ability to actively maintain and manipulate information in the brain for a short period of time (Baddeley, 1986). It has been posited that the system sub-serving this ability is comprised of a central executive control system and two subordinate systems responsible for the manipulation, rehearsal, and retention of domain-specific information – the phonological loop and the visuospatial sketchpad (Baddeley, 1992). Research suggests that these subsidiary systems, specific to the processing of verbal and visuospatial information, are functionally distinct by the time children reach school-age (Alloway, Gathercole, & Pickering, 2006; Gathercole, Pickering, Ambridge, & Wearing, 2004; Pickering, Gathercole, & Peaker, 1998). However, it has been demonstrated that WM abilities continue to improve throughout adolescence. Specifically, WM accuracy increases, and reaction times decrease as a function of age (Kwon, Reiss, & Menon, 2002; Luciana, Conklin, Hooper, & Yarger, 2005; Luna, Garver, Urban, Lazar, & Sweeney, 2004; Zald & Iacono, 1998). In addition, WM span, for both verbal and spatial information, expands across adolescence (Conklin, Luciana, Hooper, & Yarger, 2007). These improvements are thought to be due to better executive and rehearsal strategies and improved processing speed that occurs with continued adolescent neuromaturation (Cowan, 1997); however, the link between improved functioning and neurodevelopment remains unclear.
The advent of functional magnetic resonance imaging (fMRI) has made essential contributions to the current understanding of the neuroanatomical substrates underlying human WM. FMRI studies of adults have consistently revealed activation of premotor, lateral prefrontal and posterior parietal cortices during adequate performance on WM tasks (for review, see Fletcher & Henson, 2001; Owen, 1997; Owen, McMillan, Laird, & Bullmore, 2005). While fewer studies have utilized fMRI in normally developing youth to explore the neural underpinnings of WM, the limited work has revealed several patterns. Studies have shown that while similar patterns of spatial WM-related brain activity appear to be present in both children and adults (Thomas et al., 1999), children tend to show more widespread patterns of activation than adults (Geier, Garver, Terwilliger, & Luna, 2009), and with increased age, children and adolescents appear to exhibit greater activation in bilateral prefrontal and parietal brain regions to spatial WM tasks (Klingberg, Forssberg, & Westerberg, 2002; Kwon et al., 2002; Schweinsburg, Nagel, & Tapert, 2005). Adults, on the other hand, appear to show more refined and lateralized patterns of activity than seen during earlier stages of development (Scherf, Sweeney, & Luna, 2006). During verbal WM, children also appear to activate similar brain regions to adults, but in a more widely distributed fashion (Casey et al., 1995). However, studies of verbal WM also suggest that even children may show left hemispheric lateralization in frontal brain regions (Casey et al., 1995) and adult-comparable lateralized activation of brain response, with greater activation to verbal WM tasks in the left frontal and temporal lobes, and greater activation to spatial WM in right frontal, parietal, and occipital cortices (Thomason et al., 2009). While the modality-specific WM literature is somewhat mixed, a number of non-WM studies of both visuospatial and language processing have also demonstrated increased lateralized patterns and shifted patterns of activation in adults, as compared to children (for review, see Stiles, Moses, Passarotti, Dick, & Buxton, 2003).
Taken together, these data suggest that WM-related brain activation patterns, while similar to those in adulthood, change with age. Developing youths appear to display more diffuse and/or bilateral activation, particularly for spatial WM, while adults exhibit more refined, and possibly more lateralized brain response. However, it is unclear when during development these neurobiological shifts occur. These differences in brain activation patterns with age may be a function of increasing functional specialization and efficiency within developing brain regions (Durston et al., 2006), and may reflect less segregation across brain systems in the immature brain (Fair et al., 2007). However, little research has examined the contributions of different brain regions to specific aspects or types of WM during development.
In the adult literature, there has been effort toward identifying the brain regions responsible for subserving WM for different types of stimuli. To that end, there is some evidence that verbal and spatial WM functions are subserved by different hemispheres in the brain. The idea for this material-specific dissociation originally grew out of the lesion literature suggesting that the left hemisphere was more specialized in processing verbal information, while the right hemisphere was more apt to process spatial stimuli (Ojemann & Dodrill, 1985; M. L. Smith & Milner, 1981). Since then, a number of studies have examined whether or not such a hemispheric dissociation exists for WM functions in the adult brain. Findings from these studies, although disparate, suggest that there may be a hemispheric dissociation for verbal and spatial WM (E. E. Smith & Jonides, 1997); however, this functional distinction may be restricted to the regions of prefrontal cortex (Fiez et al., 1996; Manoach et al., 2004; Walter et al., 2003). Notably, studies that have failed to demonstrate this dissociation (D’Esposito et al., 1998; Nystrom et al., 2000) may have utilized spatial stimuli that were more amenable to verbal encoding strategies than those who were able to show this hemispheric distinction or may not have had sufficient power to detect lateralization effects. In addition, most studies addressing this question have done so by contrasting neural activation patterns in one cognitive condition versus another, and lateralization is examined by means of visual inspection. This type of voxel-based examination is insufficient to adequately address issues of lateralization in a statistically driven manner (Jernigan, Gamst, Fennema-Notestine, & Ostergaard, 2003). Although examined in adult and child populations, to date, no study has examined this potential material specific dissociation in developing adolescents or whether more distinct or lateralized patterns of WM brain response emerge across this developmental period.
Therefore, the goals of the current study were to examine brain functioning during comparable verbal and spatial WM tasks in a group of healthy adolescents and use a threshold-independent technique to examine the possibility of hemispheric lateralization. Based on previous imaging and lesion literature, we hypothesized that in adolescents, verbal WM would be lateralized to the left hemisphere and spatial WM would show a right-lateralized pattern of activity in the brain, particularly in frontal lobe regions. Further, based on evidence of more efficient and specialized brain activity across adolescent development (Durston et al., 2006; Finn, Sheridan, Kam, Hinshaw, & D’Esposito, 2010), we also hypothesized that the laterality of verbal and spatial WM would become stronger as a function of adolescent age.
2. Materials and methods
2.1 Participants
Sixty-seven healthy adolescents, ages 10–16 years, underwent fMRI during a verbal and spatial WM task. All participants were recruited and underwent comprehensive structured interviews as part of an ongoing study focused on typical adolescent neurodevelopment. Briefly, following written consent and assent from all youth and their parents in accordance with Oregon Health & Science University’s International Review Board, separate structured telephone interviews were conducted with both the youth and one of their parents. Interviews consisted of the Diagnostic Interview Schedule for Children Predictive Scales (DISC-PS-4.32b) (Lucas et al., 2001), the Family History Assessment Module (FHAM) (Rice et al., 1995), the Brief Lifetime version of the Customary Drinking and Drug Use Record (S. A. Brown et al., 1998), and the Structured Clinical Interview (S.A. Brown, Myers, Mott, & Vik, 1994). Exclusionary criteria included the inability of a parent to provide family history information; lifetime history of a diagnosed DSM-IV psychiatric disorder; significant substance use (10 lifetime alcoholic drinks or 2 drinks/occasion, > 5 uses of marijuana, any other drug use, or > 4 cigarettes per day); neurological illness; significant head trauma (loss of consciousness > 2 minutes); serious medical problems; mental retardation or learning disability; prenatal exposure to drugs or alcohol; reported history of psychotic disorders in biological parents (i.e. bipolar 1 or schizophrenia); left-handedness (Edinburgh Handedness Inventory, (Oldfield, 1971)); irremovable metal; and pregnancy. Left-handedness was exclusionary for the current study due to demonstrated handedness influences on cerebral laterality (Lux et al., 2008)
Participant characteristics are provided in Table 1. As an estimate of intellectual functioning (IQ), all teens were administered the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). To further characterize the sample, information was gathered on socioeconomic status by administering the Hollingshead Index of Social Position (ISP) to parents as part of the structured telephone interview. The Hollingshead ISP determines socioeconomic status based on occupation and educational attainment of each parent (Hollingshead, 1975). Pubertal status was assessed using the self-report Pubertal Development Scale (PDS) (Petersen, Crockett, Richards, & Boxer, 1988).
Table 1.
Participant characteristics
| Mean (Standard Deviation) or %
|
|||
|---|---|---|---|
| All participants (n=67) | 90% accuracy for Verbal (n=56) | 90% accuracy for Spatial (n=45) | |
| Female | 47.8% | 46.4% | 48.9% |
| Years of age (range: 10.19 to 16.14) | 13.11 (1.78) | 13.32 (1.72) | 13.49 (1.68) |
| Caucasian | 76.1% | 76.8% | 80.0% |
| SESa | 28.85 (11.94) | 29.00 (11.79) | 28.98 (12.22) |
| Verbal IQb | 120.67 (13.25) | 121.29 (13.35) | 122.89 (13.29) |
| Performance IQb | 112.54 (11.41) | 113.95 (11.47) | 115.38 (10.90) |
| Overall IQb | 118.69 (12.14) | 119.88 (12.47) | 121.53 (12.15) |
| Pubertal Statusc | 2.40 (.80) | 2.49 (.76) | 2.53 (.69) |
Hollingshead Index of Social Position - higher scores indicate lower socioeconomic status; mean scores here are commensurate with upper-middle class status,
Wechsler Abbreviated Scale of Intelligence,
Pubertal Development Scale composite score
2.2 Imaging Protocol
Adolescents were scanned on a 3.0 Tesla Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) with a twelve channel head coil at OHSU’s Advanced Imaging Research Center (AIRC). Whole-brain, high-resolution structural anatomical images were acquired in the sagittal plane using a T1 weighted MPRAGE scanning sequence (TI = 900ms, Flip Angle = 10 degrees, TE = 3.58 ms, TR = 2300 ms, acquisition matrix = 256×240, FOV = 256 mm, slice thickness = 1.1 mm, 33 slices). Functional images were acquired in the axial plane oblique to the AC-PC, using a high-angular resolution T2-weighted echo planar Blood Oxygen Level Dependent (BOLD) sequence (TR = 2000 ms, TE = 30 ms, FOV= 240 mm, flip-angle = 90°, 33 slices no gap, slice thickness = 3.8 mm, 166 repetitions). Task stimuli were rear-projected and viewed through a mirror mounted on the head coil. Participants made responses with an MRI-compatible button box, using their index finger.
2.3 Spatial and verbal n-back WM fMRI task
The task used in this study is similar to that used by Nystrom and colleagues (Nystrom et al., 2000), in that it was a block-design spatial and verbal WM 2-back task that differentiated between the two WM conditions only by way of task instruction (Figure 1). During the spatial WM condition, stimuli were presented in various spatial locations on the screen, so as to minimize the likelihood of verbal encoding strategies. In order to increase the demands on phonological rehearsal for the verbal working WM condition (Conrad, 1964), this task utilized phonemically similar capitalized letters (e.g., D, G, P, etc.) during both the spatial and verbal WM conditions. During spatial WM, participants were instructed to respond by a button press each time a stimulus appeared in the same spatial location as the stimulus 2 prior, regardless of stimulus content. During verbal WM, participants were to press a button each time the same letter appeared as the one 2 prior, regardless of spatial location. Pilot testing of the task was preformed to ensure matched difficulty in the spatial and verbal WM conditions (Nagel, Ohannessian, & Cummins, 2007). To control for the attention processes and sensory and motor demands of the WM conditions, blocks of a control vigilance task were used. During these blocks, participants viewed gray and white dots presented in the same spatial locations as the letter stimuli during the WM blocks. Participants were asked to press the button each time a gray dot appeared. The blocked design task consisted of 2 runs of 6 blocks (3 verbal WM, 3 spatial WM) counterbalanced per run, and 16 trials per block for a total of 192 trials. Stimuli were presented on the screen for 500 ms, with an inter-trial stimulus interval of 1500 ms in which subjects looked at a crosshair. The entire task lasted 11 minutes, 4 seconds. Task presentation was performed using Presentation software (Schneider, Eschman, & Zuccolotto, 2002). Response logging was performed, and accuracy and reaction time data were collected for all blocks, with the latter only computed for correct trials. Following scanning, an exit questionnaire was administered to assess self-reported verbal, spatial, or no strategy use during the working memory tasks.
Figure 1.
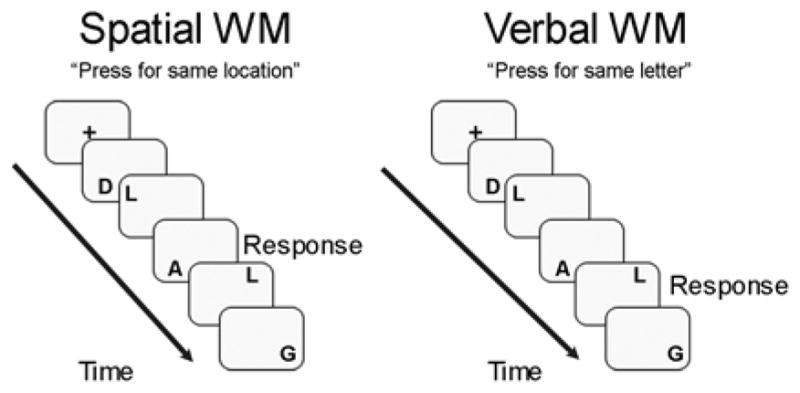
Verbal and spatial working memory 2-back paradigm.
2.4 Data Analysis
All demographic and task performance data were examined for normality and the presence of outliers using PASW Statistics 18 (PASW, Chicago, Illinois). All demographic variables were normally distributed, except for household income, for which both the mean and median are reported. Because all youth performed relatively well, task performance variables (accuracy and reaction time) were positively skewed. Thus, log transformations were applied to improve normality. Furthermore, given that laterality indices are inherently skewed, age, puberty, IQ, task strategy, and task performance were examined in relation to LI using nonparametric correlation analyses (Spearman’s rho).
Imaging data were processed and analyzed using Analysis of Functional NeuroImages (AFNI) (Cox, 1996). Using an iterated least squares algorithm, motion was corrected for in the time series data by registering each acquisition to a selected repetition (Cox & Jesmanowicz, 1999), and repetitions that showed greater than 2.5 mm in any of the 3 rotational or 3 displacement parameters were removed from subsequent analyses. Furthermore, an average root mean squared (RMS) value was calculated for within-run motion, across these 6 motion parameters for each subject. These values were log transformed to allow for a normal distribution, and used in subsequent age-related analyses as a covariate, as RMS values were found to be significantly associated with age (r = −.543, p <.001). Using a deconvolution process, time series data was correlated with a vector representing the task design, in light of the delay of the hemodynamic response, while covarying for motion and linear trends. The fit coefficients derived from fitting the time series data to the model represent the blood oxygen level dependent (BOLD) response, which was then contrasted between the verbal WM and vigilance, and spatial WM and vigilance, for each voxel of the brain. Functional data sets were resampled into 3mm3 voxels and were transformed into standard Talairach coordinates for anatomical localization and between-subject comparisons.
To examine whole-brain verbal and spatial WM activation, single-sample t-tests were performed on BOLD response for both task conditions (versus vigilance). In addition, follow-up multiple regression analyses were performed to examine the relationship between age and whole-brain BOLD response for each functional WM task, while covarying for RMS and task performance (accuracy and reaction time; covaried separately), as well as the relationship between pubertal status and WM task response, controlling for RMS, task performance, and age. To correct for Type I error when determining significant clusters of activation, a combined t-statistic magnitude and cluster volume thresholding technique was employed using AFNI’s AlphaSim program. To capture only the most significant task-related activation, for initial WM task-related t-tests, only clusters with a voxel threshold of p <.0001 exceeding 243 microliters, equal to 9 contiguous significant (α < .05) 3mm3 voxels were considered significant. For follow-up multiple regression analyses, multiple comparison correction followed more standard convention, and significance was determined for only voxels exceeding a threshold of p <.01 (voxel and clusterwise corrected), and part of a cluster greater to 648 microliters (24 voxels).
2.5 Lateralization Indices
For between verbal and vigilance and spatial and vigilance contrasts, individual as well as group t-maps were used to determine laterality of activation over the right and left hemisphere. Lateralization indices (LI) were calculated using a combined bootstrap/histogram analysis approach, previously reported (Wilke & Schmithorst, 2006). This approach is superior to other methods to examine lateralization, as it does not rely on visual inspection, or arbitrary thresholding. Briefly, using the LI-toolbox from SPM5, all voxel values from unthresholded individual and group t-maps, except those 5 mm left and right of the interhemispheric fissure (Wilke & Lidzba, 2007), were used to calculate a whole brain LI. Specifically, the common lateralization equation LI = (left-right)/(left+right) was used, resulting in a continuum of values between +1 and −1, with positive values representing left hemispheric lateralization, and negative values representing right hemispheric lateralization. A bootstrapping approach was simultaneously applied (Wilke & Schmithorst, 2006) to create threshold-dependent laterality curves as to avoid a fixed and arbitrary threshold which can obscure laterality findings (Wilke & Lidzba, 2007). Specifically, the interquartile range of 100 bootstrapped resamples was created. Using these interquartile ranges, 10,000 LI combinations were then computed. These three steps were repeated for twenty, equally spaced t-thresholds ranging from 0 to the maximum t-threshold for a given image. An overall LI was then derived by weighting each of the trimmed mean LI’s by the respective iterative t-threshold in which they were created and averaging them to create a weighted mean LI (LIw). Thus, this final LIw is resistant to outliers and allows for LI calculated at higher t-thresholds to have a larger impact in the overall average LI for an image (Wilke & Schmithorst, 2006). In order to also examine lobe specific lateralization, LIw were determined for frontal, parietal, and temporal lobes using the same methods as whole brain, except SPM pre-defined regional masks were applied to each t-map prior to calculating LIw using the bootstrap approach. Individual LIw for the frontal, parietal, and temporal lobes were then imported to SPSS in order to examine the relationship between verbal and spatial lateralization and age, while covarying for RMS, using Spearman’s rho nonparametric correlations. Based on previous literature (Everts et al., 2009; Wilke & Schmithorst, 2006) left hemispheric lateralization was determined by LIw values > 0.2, and right hemispheric lateralization as LI values < − 0.2.
3. Results
3.1 Behavioral performance
Task performance data were available for 66 participants, due to button box failure for one individual. Participants performed at 98.6 ± 1.9% accuracy on the vigilance condition, 94.2 ± 4.56% accuracy on the verbal WM condition, and 90.8 ± 7.8% accuracy on the spatial WM condition. Paired t-test results suggested that overall, youth performed more accurately during verbal compared to spatial WM (t = −4.98, p < .01), but were also slower on this task condition (t = 3.23, p < .01). Paired t-test of composite performance indices for verbal and spatial WM (summed z-scores of accuracy and reaction time) were not significant, providing support for a speed/accuracy trade-off on these two WM tasks (t = −.007, p = .99). Self-reported strategy use was largely as expected for the tasks, with 84% of youth reporting spatial strategy use during spatial WM (11% verbal; 5% none), and 78% of youth reporting a verbal strategy during verbal WM (12% spatial; 9% none). Across adolescent age and pubertal development, there were significant improvements in task performance. Specifically, age was correlated with faster performance (lower reaction times) and increased accuracy during both the verbal (reaction time: r = −.37, p < .01; accuracy: r = .29, p < .05) and spatial (reaction time: r = −.41, p < .01; accuracy: r = .30, p < .05) WM task conditions. Not surprisingly, pubertal stage, which was highly correlated with age (r = .79, p < .001), was also negatively correlated with reaction time and positively correlated with accuracy across the sample (verbal reaction time: r = −.31, p <.01; verbal accuracy: r = .26, p < .05); spatial reaction time: r = −.33, p < .01; spatial accuracy: r = .27, p < .05). In addition, as has been previously shown in the literature (Conway, Kane, & Engle, 2003), IQ was significantly related to WM task accuracy during both the verbal (r = .38, p < .01) and spatial (r = .44, p < .001) WM conditions, but not to task reaction time. While all participants had greater than 67% accuracy on both tasks, only a subset of the participants had ≥90% accuracy on both the verbal (n = 56) and spatial (n = 45) conditions. Because errors have been shown to impact brain response during fMRI, and often in a lateralized fashion (Dosenbach et al., 2006; Fair et al., 2009; Murphy & Garavan, 2004), the same imaging analyses were performed on this smaller subsample of subjects to ensure that the results were not confounded by error-related signal.
3.2 Brain response during verbal WM and associations with age and pubertal status
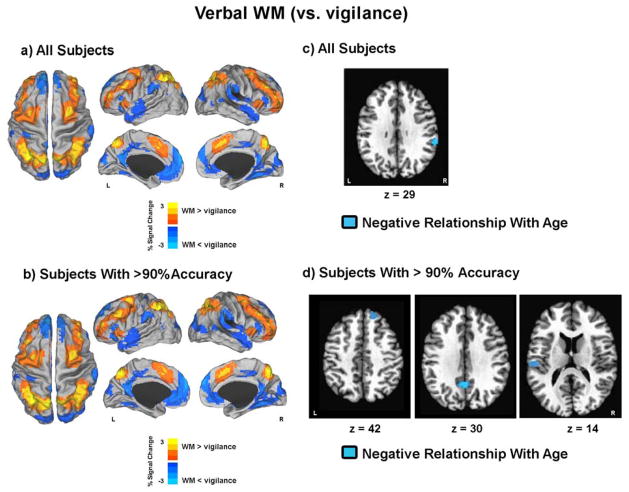
During the verbal WM condition (versus vigilance), adolescents demonstrated decreased BOLD activation bilaterally in the anterior and posterior cingulate, medial frontal gyrus, superior frontal gyrus, cuneus, parahippocampal gyrus, superior temporal gyrus, and precentral and postcentral gyri, as well as the middle frontal gyrus, inferior parietal lobe, middle temporal gyrus, and angular gyrus of the left hemisphere (Figure 2a, Table 2). Youth also displayed increased verbal WM-related BOLD response in bilateral cingulate, middle and superior frontal gyri, inferior and superior parietal lobe, precuneus, cerebellum, and the right insula and left precentral gyrus (Figure 2a, Table 2). Similar whole-brain patterns of activation were seen for verbal WM (versus vigilance) in youth who performed at >90% task accuracy on the verbal task condition (see Figure 2b, Table 2).
Figure 2. Verbal working memory (WM) brain response.
a and b) Results depict t-maps displayed on surface maps in Talairach space for a) all subjects and b) only for subjects with >90% accuracy. Red-Yellow reflects greater brain response for WM when compared to vigilance. Blue-Light Blue reflects less brain response for WM when compared to vigilance. c and d) Blue voxels reflect areas in which a negative relationship was seen between verbal WM percent signal change and age for c) all subjects and d) only for subjects with >90% accuracy. L = left; R = right.
Table 2.
Verbal WM BOLD Response
| Focal Point | Anatomic Region(s) Included | x | y | z | Volume (μl) | |
|---|---|---|---|---|---|---|
|
All Subjects
| ||||||
| VWM > DOTS | ||||||
| left medial frontal gyrus | bilateral cingulate, bilateral middle frontal gyrus, bilateral superior frontal gyrus, left precentral gyrus | −1.5 | 13.5 | 47.5 | 101385 | |
| left superior parietal lobe | bilateral inferior parietal lobe, bilateral precuneus, bilatral superior parietal lobe | −31.5 | −55.5 | 50.5 | 67527 | |
| left culmen (cerebellum) | right red nucleus, right mammillary body, bilateral culmen | −1.5 | −31.5 | −18.5 | 4509 | |
| right insula | right inferior frontal gyrus | 31.5 | 19.5 | 2.5 | 3591 | |
| left caudate | left thalamus, left caudate body | −13.5 | −4.5 | 20.5 | 1242 | |
| left declive (cerebellum) | bilateral pyramis, bilateral tuber of vermis | −4.5 | −73.5 | −21.5 | 1215 | |
| right culmen (cerebellum) | right cerebellar tonsil | 34.5 | −52.5 | −27.5 | 891 | |
| left tuber (cerebellum) | left culmen | −43.5 | −58.5 | −24.5 | 837 | |
|
| ||||||
| DOTS > VWM | ||||||
| left posterior cingulate | right superior temporal gyrus, bilateral cuneus, right postcentral gyrus, bilateral parahippocampal gyrus, left middle frontal gyrus | −1.5 | −49.5 | 17.5 | 198774 | |
| left vmpfc | medial frontal gyrus, bilateral anterior cingulate, left medial frontal and bilateral superior frontal gyrus | −1.5 | 49.5 | 2.5 | 64476 | |
| left inferior parietal lobe | left inferior parietal lobe, left precentral and postcentral gyrus | −58.5 | −31.5 | 26.5 | 20250 | |
| left middle temporal gyrus | left middle temporal gyrus, left superior temporal gyrus, left angular gyrus | −49.5 | −61.5 | 29.5 | 7101 | |
| right inferior frontal gyrus | 46.5 | 31.5 | −0.5 | 5022 | ||
| right precentral gyrus | right postcentral gyrus | 16.5 | −22.5 | 71.5 | 918 | |
|
| ||||||
|
Subjects with >90% Accuracy
| ||||||
|
VWM > DOTS
| ||||||
| left medial frontal gyrus | bilateral dLPFC, middle frontal gyrus, superior frontal gyrus, left inferior frontal gyrus, left claustrum, left anterior insula, left caudate, left thalamus, bilateral brainstem, red nuclei | −1.5 | 13.5 | 47.5 | 134109 | |
| right precuneus | bilateral precuneus, inferior and superior parietal lobule | 1.5 | −61.5 | 47.5 | 74763 | |
| right anterior insula | right BA 13 | 31.5 | 19.5 | 2.5 | 3834 | |
| left cerebellum | left lateral tuber and culmen | −43.5 | −61.5 | −24.5 | 1998 | |
| left declive of vermis | bilateral declive and tuber of vermis | −1.5 | −73.5 | −21.5 | 1647 | |
| right cerebellum | right lateral tuber and culmen | 34.5 | −52.5 | −27.5 | 999 | |
| right caudate | right cingulate gyrus | 16.5 | −1.5 | 23.5 | 972 | |
| left thalamus | left pulvinar | −13.5 | −25.5 | 17.5 | 918 | |
| right thalamus | 10.5 | −1.5 | 5.5 | 864 | ||
|
| ||||||
| DOTS > VWM | ||||||
| vmpfc | bilateral anterior and posterior cingulate, inferior frontal gyrus, insula, postcentral gyrus, inferior temporal gyrus, middle temporal gyrus, superior temporal gyrus, middle occipital gyrus, cuneus, bilateral parahippocampal gyrus | −1.5 | 58.5 | 14.5 | 366309 | |
| right medial frontal gyrus | right pre and postcentral gyrus, BA 3, BA 6 | 10.5 | −10.5 | 71.5 | 1971 | |
| right precentral gyrus | right BA 4 and 6 | 43.5 | −13.5 | 56.5 | 1242 | |
Multiple regression analyses run on the whole group examining the relationship between age and verbal WM activation, while covarying for motion (RMS) and task accuracy, showed no significant areas of age-related activation. Similar multivariate analyses examining this relationship, with RMS and reaction time covaried, showed one significant age-related cluster, with decreased activation in the right inferior parietal lobe with increased age (Figure 2c, Table 3). In the subset of youth with >90% verbal WM task accuracy (n = 56), no significant age-accuracy correlation remained. Multiple regression analyses examining the relationship between age and verbal WM BOLD response, while covarying for age-associated RMS and reaction time, showed three significant clusters of age-related BOLD response. These age-related clusters, where verbal WM-related activation decreased with increased adolescent age, included the right superior frontal, bilateral cingulate, and the left post-central gyri (BA 40) (Figure 2d, Table 3). No areas of verbal WM activity were uniquely related to pubertal status in either the entire or 90% accuracy restricted sample, after controlling for RMS, task accuracy or reaction time, and age.
Table 3.
Verbal WM BOLD Signal Relationships with Age, while controlling for motion (RMS) and RT
| Relationship | Focal Point | Anatomic Region(s) Included | x | y | z | Volume (μl) | |
|---|---|---|---|---|---|---|---|
|
All Subjects
| |||||||
| Negative | right inferior parietal lobule | right superior temporal gyrus and BA 40 | 59 | -35 | 30 | 918 | |
|
| |||||||
|
Subjects with >90% Accuracy
| |||||||
| Negative | right superior frontal gyrus | 16.5 | 40.5 | 44.5 | 1107 | ||
| Negative | left cingulate gyrus | bilateral cingulate gyrus | -1.5 | -52.5 | 29.5 | 1026 | |
| Negative | left postcentral gyrus | left BA 40 | -52.5 | -22.5 | 14.5 | 837 | |
BA = Brodmann’s Area
3.3 Brain response during spatial WM and associations with age and pubertal status
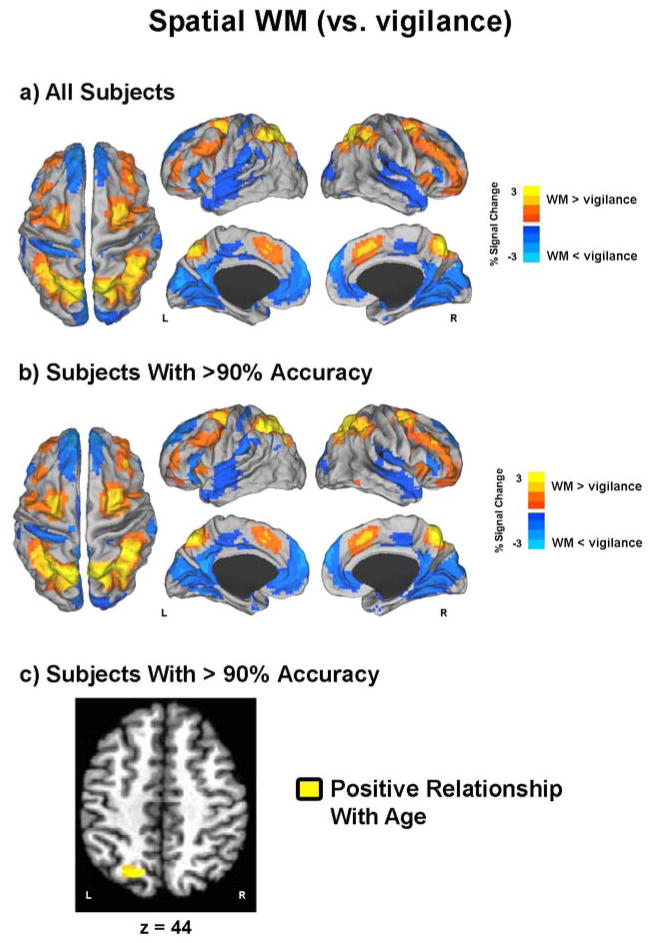
During spatial WM (versus vigilance), adolescents showed decreased BOLD activation bilaterally in anterior and posterior cingulate cortices, medial and superior frontal gyri, cuneus, the middle and superior temporal gyri, as well as in the right inferior frontal gyrus, precentral and postcentral gyri, and middle and superior temporal gyri (Figure 3a, Table 4). Increased spatial WM-related activation was demonstrated bilaterally in the insula, medial and superior frontal gyri, cingulate gyrus, inferior and superior parietal lobe, precuneus, and the left cerebellum (Figure 3a, Table 4). Similar findings were seen for the spatial WM condition (versus vigilance) in participants with >90% task accuracy on the spatial condition (Figure 3b, Table 4).
Figure 3. Spatial working memory (WM) brain response.
a and b) Results depict t-maps displayed on surface maps in Talairach space for a) all subjects and b) only for subjects with >90% accuracy. Red-Yellow reflects greater brain response for WM when compared to vigilance. Blue-Light Blue reflects less brain response for WM when compared to vigilance. c) Yellow voxels reflect areas in which a positive relationship was seen between spatial WM percent signal change and age for d) only for subjects with >90% accuracy. No age relationship was detected using all subjects. L = left; R = right.
Table 4.
Spatial WM BOLD Response
| Focal Point | Anatomic Region(s) Included | x | y | z | Volume (μl) | |
|---|---|---|---|---|---|---|
|
All Subjects
| ||||||
| SWM > DOTS | ||||||
| left precuneus | bilateral inferior parietal lobe, bilateral precuneus, bilateral superior parietal lobe | −10.5 | −67.5 | 50.5 | 79677 | |
| right middle frontal gyrus | bilateral superior frontal gyrus, right inferior frontal gyrus, bilateral medial frontal gyrus, left precentral gyrus, bilateral cingulate gyrus | 28.5 | 1.5 | 56.5 | 73872 | |
| left superior frontal gyrus | left middle frontal gyrus | −34.5 | 52.5 | 14.5 | 8370 | |
| right insula | right inferior frontal gyrus | 31.5 | 19.5 | 2.5 | 3213 | |
| left Tuber (cerebellum) | left cerebellar tonsil, left culmen | −40.5 | −61.5 | −27.5 | 2646 | |
| left insula | left claustrum | −31.5 | 16.5 | 5.5 | 1971 | |
|
| ||||||
| DOTS > SWM | ||||||
| ventral medial prefrontal cortex | anterior and posterior cingulate, anterior medial frontal gyrus, anterior superior frontal gyrus, bilateral cuneus, bilateral middle temporal and superior temporal gyrus, bilatreal parahippocampal gyrus, bilateral inferior parietal gyri, left angular gyrus | −1.5 | 49.5 | −0.5 | 433998 | |
| right inferior frontal gyrus | 49.5 | 34.5 | 5.5 | 5454 | ||
| right precentral gyrus | right postcentral gyrus | 37.5 | −22.5 | 59.5 | 1782 | |
| right superior temporal gyrus | right middle temporal gyrus | 58.5 | −52.5 | 11.5 | 567 | |
|
| ||||||
|
Subjects with >90% Accuracy
| ||||||
|
SWM > DOTS
| ||||||
| right superior parietal lobule | bilateral precuneus, inferior parietal lobule, superior parietal lobule | 13.5 | −64.5 | 53.5 | 84564 | |
| right middle frontal gyrus | right dLPFC, bilateral middle frontal gyrus, superior frontal gyrus, cingulate, medial frontal gyrus, and inferior frontal gyrus | 28.5 | 1.5 | 59.5 | 70173 | |
| left dLPFC | left middle and frontal gyri | −34.5 | 55.5 | 11.5 | 9774 | |
| left cerebellum | left culmen and cerebellar tonsils | −31.5 | −34.5 | −30.5 | 3807 | |
| right anterior insula | right BA 13 and claustrum | 31.5 | 19.5 | 2.5 | 2565 | |
| left anterior insula | left BA 13 and claustrum | −31.5 | 16.5 | 5.5 | 2133 | |
| bilateral brainstem | red nucleus | 1.5 | −7.5 | −12.5 | 1377 | |
| right inferior temporal gyrus | right BA 20 and 37 | 55.5 | −49.5 | −12.5 | 702 | |
| left cerebellum | medial left uvula, declive and pyramis of vermis, bilateral pyramis and tuber of vermis | −4.5 | −70.5 | −21.5 | 648 | |
|
| ||||||
|
DOTS > SWM
| ||||||
| ventral medial prefrontal cortex | anterior and posterior cingulate, anterior medial frontal gyrus, anterior superior frontal gyrus, bilateral cuneus, bilateral middle temporal and superior temporal gyrus, bilateral parahippocampal gyrus, bilateral inferior parietal gyri, left angular gyrus, left lateral cerebellum (declive) | −1.5 | 52.5 | 8.5 | 425655 | |
| right precentral gyrus | right BA 6 | 4.5 | −25.5 | 68.5 | 1647 | |
| right precentral gyrus | right BA 4 | 40.5 | −19.5 | 56.5 | 756 | |
| right cerebellum | right lateral uvula and declive | 25.5 | −76.5 | −21.5 | 648 | |
BA = Brodmann’s Area
Whole group multiple regression analyses examining the relationship between age and spatial WM activation, while covarying for RMS and task accuracy, revealed no significant age-related associations with spatial WM BOLD response. Comparable multivariate analyses examining this relationship controlling for RMS and reaction time (as opposed to accuracy) also showed no significant age-related clusters of spatial WM response. As was the case for verbal WM, among only youth with >90% accuracy on the spatial WM task (n=45), no significant age-accuracy correlation was present. Thus, multiple regression analyses examining the relationship between age and spatial WM-related BOLD response, covarying for age-associated RMS and reaction time, revealed one positive age-related relationship with increased brain response in the left superior parietal lobe/precuneus seen as a function of increased adolescent age (Figure 3c, Table 5). No areas of spatial WM activity were uniquely related to pubertal status in either the entire or 90% accuracy restricted sample, after controlling for RMS, task accuracy or reaction time, and age.
Table 5.
Spatial WM BOLD Signal Relationships with Age, while controlling for motion (RMS) and RT
| Relationship | Focal Point | Anatomic Region(s) Included | x | y | z | Volume (μl) | |
|---|---|---|---|---|---|---|---|
| All Subjects | |||||||
| None | |||||||
| Subjects with >90% Accuracy | |||||||
| Positive | left precuneus | left BA 7 | −19.5 | −64.5 | 44.5 | 837 | |
BA = Brodmann’s Area
3.4 Hemispheric laterality
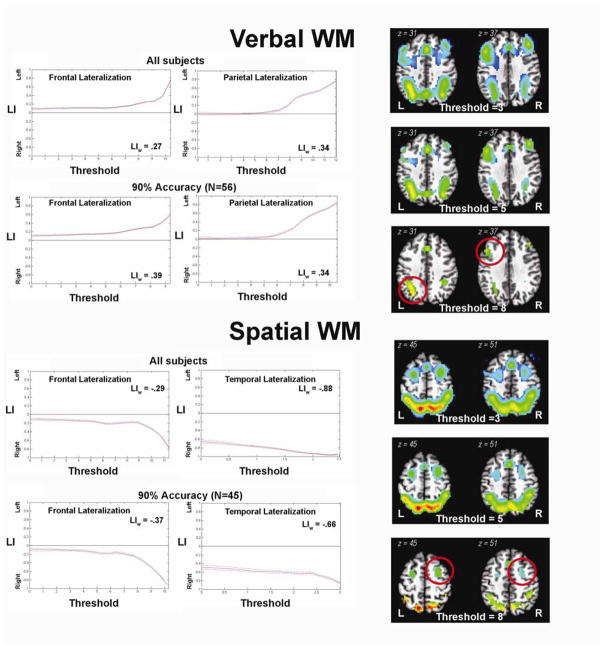
Lateralization of verbal and spatial WM was analyzed using a combined bootstrap/histogram analysis approach (Wilke and Schmithorst, 2006) (Figure 4). Independent of statistical threshold, whole brain lateralization analyses showed that verbal WM (versus vigilance) was left lateralized (LIw = 0.35). Restricted LI analyses to the frontal, parietal, and temporal lobes showed that verbal LI was robustly left lateralized in the frontal (LIw = 0.25; 90% accuracy LIw = 0.27) and parietal lobes (LIw = 0.34; 90% accuracy LIw = 0.34) (Figure 4), but not temporal lobes. In contrast, whole brain analyses showed that spatial WM (versus vigilance) was right lateralized (LIw = −0.18), independent of thresholding (Figure 4). Restricted LI analyses showed that LI was right lateralized in the frontal lobe (LIw = −0.29; 90% accuracy LIw = −0.37) and temporal lobes (LIw = −0.88; 90% accuracy LIw = −0.66), but not in the parietal lobes (Figure 4). These patterns of lateralization were slightly more robust in youth who performed greater than 90% task accuracy on the verbal condition (LIw = 0.39) and the spatial task accuracy (LIw = −0.26), again highlighting the potential confounding impact of error-related response in the brain (Dosenbach et al., 2006; Fair et al., 2009; Murphy & Garavan, 2004). Reported task strategy use, nor IQ, significantly related to laterality indices.
Figure 4. Working memory lateralization.
Bootstrap lateralization results for verbal and spatial WM fMRI brain activation for all subjects, as well as for subjects with >90% accuracy. Plots show stability of lateralization curves across multiple t-thresholds (bootstrap approach). Images reflect >90% accuracy t-images arbitrarily thresholded at t-values of 3, 5, and 8, to show the importance of using a bootstrap approach in determining lateralization. Of note, thresholds 3 and 5 show an “absence of lateralization”, but threshold 8 looks “lateralized” (red circles). Examining lateralization by creating threshold-dependent laterality curves allows for avoiding these fixed and arbitrary thresholds which can obscure laterality findings. Plots: y-axis = Lateralization Index (LI) values fall on a continuum of values between +1 (left hemispheric lateralization) and −1 (right hemispheric lateralization), zero line represents no lateralization; x-axis = t-thresholds used based on the maximum t-value for each image. LIw values reflect the weighted mean LI across the respective iterative t-thresholds. L = left; R = right.
3.5 Lateralization, age, and pubertal status
For verbal WM, lateralization in the frontal and parietal lobes did not significantly relate to age, after partialling out variance related to RMS (frontal LIw rho (67) = .12, p = .31; parietal LIw rho (67) = .08, p = .49). Among the >90% verbal WM accuracy subsample, similar results were observed (frontal LIw rho (56) = .12, p = .38; parietal LIw rho (56) = .09, p = .50). Similarly, spatial WM lateralization in the frontal and temporal lobes did not significantly relate to age, after controlling for RMS (frontal LIw rho (67) = −.10, p = .40, temporal LIw rho (67) = .03, p = .82), with comparable results in the >90% spatial WM accuracy subsample (frontal LIw rho (45) = −.11, p = .50, temporal LIw rho (45) = .09, p = .55). Similarly, regional LI also did not significantly relate to pubertal status (all p’s > .05).
3.6 Lateralization and performance
After controlling for age and RMS, lateralization in the frontal and parietal lobes did not significantly relate to verbal WM accuracy (frontal LIw rho (66) = −.10, p = .43; parietal LIw rho (66) = .08, p = .51) or reaction time (frontal LIw rho (66) = −.01, p = .93; parietal LIw rho (66) = .18, p = .16). These results were comparable when examining the >90% verbal WM accuracy sample (frontal LIw accuracy rho (56) = .02, p = .91; parietal LIw rho (56) =.19, p = .16); reaction time (frontal LIw rho (56) = .01, p = .96; parietal LIw rho (56) =.19, p = .17). Similarly, after controlling for age and RMS, spatial WM accuracy did not relate to lateralization in the frontal lobe (rho (66) = −.02, p = .89) or the temporal lobe (rho (66) = .17, p = .18). These results were also seen in the >90% verbal WM accuracy sample (frontal: rho (56) = −.19, p = .20; temporal: (rho (45) = .01, p = .96). In both the whole and performance-constrained groups, no significant relationships were seen between either frontal lobe (rho (66) = .13, p = .30; rho (56) = .02, p = .92) or temporal lobe (rho (66) = .12, p = .32; rho (45) = −.05, p = .76) lateralization and spatial WM reaction time.
4. Discussion
This is the first study, to our knowledge, to examine both verbal and spatial WM processes and associated brain activity in sample of healthy developing adolescents. As evidenced by utilizing comparable verbal and spatial WM paradigms during fMRI, working memory-related regions of increased brain activity were observed in premotor, lateral prefrontal, and posterior parietal cortices, and cerebellum, consistent with the existing literature documenting the neural substrates of WM across the lifespan. In addition, regions of the default-mode network (DMN) – a brain network more commonly active at rest and deactivated during task (Buckner & Vincent, 2007; Greicius, Krasnow, Reiss, & Menon, 2003; Raichle et al., 2001), including the posterior cingulate cortex, medial prefrontal cortex, and angular gyrus, showed reduced activation during the more demanding WM task conditions, compared to the easier vigilance control condition.
Using a threshold-independent measure of hemispheric lateralization, as hypothesized, we demonstrated that among our sample of youth, brain activity during verbal WM showed a more left-hemisphere lateralized pattern of BOLD response, particularly in the frontal and parietal lobes, while spatial WM invoked a pattern of more right-hemisphere lateralized activity, observed in both frontal and temporal lobe regions. Thus, in adolescence, there appear to be functionally lateralized patterns of brain activity, specific to working memory domain. This pattern is consistent with the adult literature showing this hemispheric distinction (E. E. Smith & Jonides, 1997), particularly within the frontal cortex (D’Esposito et al., 1998; Fiez et al., 1996; Manoach et al., 2004; Walter et al., 2003), and also with developmental literature showing lateralized profiles of brain activity distinguishing language-based versus visuospatial tasks, more broadly (Everts et al., 2009). Contrary to our hypotheses, as well as previous findings (Everts et al., 2009), the strength of lateralization in these regions did not vary as a function of adolescent age. Our lack of an observed age relationship with LI in this sample may suggest that, similar to behavioral work documenting the existence of separable verbal and spatial WM storage systems by early childhood (Alloway et al., 2006), associated neural substrates are in place prior to adolescence. Our findings are also consistent with previous work, albeit using a different working memory paradigm, demonstrating the presence of verbal and spatial WM hemispheric laterality by childhood (including children up to 12 years of age and overlapping in age with the current study) (Thomason et al., 2009). Notably, previous work has shown that greater neurodevelopmental differences can be captured by manipulating the difficulty of task demands (Jolles, Kleibeuker, Rombouts, & Crone, 2011; O’Hare, Lu, Houston, Bookheimer, & Sowell, 2008; Thomason et al., 2009). Thus, while future studies using parametrically varied verbal and spatial WM loads and manipulation in WM may better discern age-related changes in hemispheric laterality of brain activity subserving these functions, it is also possible that these lateralized brain response profiles are well in place prior to the adolescent years.
In contrast to developmentally stable hemispheric lateralization of verbal and spatial WM functions, we observed significant age-related improvements in both verbal and spatial WM behavior across adolescence. Despite no significant age relationship with strength of LI, we did observe unique age-related associations with BOLD signal during both verbal and spatial WM. Notably, age relationships with brain response were more detectable when using the higher performance-restricted sample, likely due to fewer error trials being included in the analyses and the reduction of a potential performance confound (Dosenbach et al., 2006; Fair et al., 2009; Murphy & Garavan, 2004). Specifically, during verbal WM, we showed several areas of reduced BOLD response as a function of increasing adolescent age. These age-relationships were observed only in areas of less activation during the verbal WM condition (compared to the vigilance contrast), including the right inferior parietal/superior temporal cortex (and temporoparietal junction), right superior frontal cortex, and bilateral cingulate/posterior cingulate, regions that overlap with the DMN (Buckner & Vincent, 2007; Greicius et al., 2003; Raichle et al., 2001). Given that age-relationships were predominantly seen in areas of decreased DMN activity during WM, this may further explain the absence of an age relationship with LI, as LI was calculated based on areas of increased activity during the WM conditions. Regions of superior temporal and inferior parietal cortex, namely the temporoparietal junction, have been implicated in the orienting of attention, particularly toward task relevant stimuli (Corbetta & Shulman, 2002). During working memory, however, it is possible that less attention allocated toward distractor stimuli may result in reduced activity in this region, representing a more developmentally mature response (Shulman, Astafiev, McAvoy, d’Avossa, & Corbetta, 2007; Todd, Fougnie, & Marois, 2005). Consistent with this, previous work examining deactivation of the temporoparietal junction, as well as other more traditional DMN regions during working memory suggests that suppression of activity in these regions is associated with better task performance (Anticevic, Repovs, Shulman, & Barch, 2010).
During spatial WM, unique age relationships with brain activity were also seen, with increased left hemisphere posterior parietal/precuneus activity with increasing age (seen only in the 90% accuracy-restricted sample). This increase in posterior parietal activation, particularly in better performance adolescents, with increasing age is not surprising. This finding has been documented in several developmental WM studies (Geier et al., 2009; Klingberg et al., 2002; Kwon et al., 2002), and likely reflects maturation of the posterior parietal executive attention network, necessary for accurate spatial WM task completion (Constantinidis, 2006; Ikkai & Curtis, 2011; Wager & Smith, 2003).
While the first of its kind, there are inherent limitations to the current study that should be considered. First, our use of a block design fMRI paradigm rendered us unable to distinguish between components of WM (e.g., maintenance, rehearsal, manipulation, etc.), each which have been shown to be associated with different neural substrates and show different developmental trajectories (Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006). Despite our inability to examine at this level of detail, the blocked design of this task minimized task switching, which may have confounded lateralization effects. In addition, we did not examine dorsal/ventral split in the prefrontal cortex using statistical methods, a distinction which has previously been seen to be associated with both domain and task-specific aspects of WM (Mohr, Goebel, & Linden, 2006). Thus, the contribution of ventral versus dorsal activation patterns to frontal lobe laterality findings must be considered in future efforts. It is also possible that differing hemispheric volumes (as well as lobe volumes) could have contributed to laterality findings. While it was beyond the scope of this paper to consider volumetric contributions to laterality calculations, this potential confound should be considered is future work. Further, the adolescents in the current study were medically and psychiatrically healthy and high functioning, which potentially limits generalizability of the findings; however, we believe the results here provide an initial framework from which to compare more heterogeneous, as well as clinical populations. Lastly, although we used a task that showed matched verbal and spatial WM performance in adults (Nagel et al., 2007) and our composite scores of accuracy and reaction time were comparable in the current sample, differences between verbal and spatial WM abilities may have clouded the true extent of laterality results. It is also the case that we could not directly compare the verbal and spatial WM conditions, as this contrast would exclude an appropriate control contrast condition, further confounding the interpretation of results.
Nonetheless, this study contributes to a growing body of developmental WM literature and demonstrates that the debated hemispheric laterality in the neural substrates of WM exists during adolescence. Although the adolescent brain is actively developing (for review, see Blakemore, 2012), the fact that these lateralized substrates appear in place prior to this developmental period may have implications for the continued maturation of working memory skills and performance. Future studies examining relationships between hemispheric white matter maturation and lateralization may provide insight into the extent of plasticity of these neural functions during the adolescent years. These findings also have relevance for the study of WM-related neural substrates in clinical populations, whereby one modality of working memory function or one hemisphere is differentially impacted (Rhodes, Riby, Fraser, & Campbell, 2011; Westmacott, Askalan, MacGregor, Anderson, & Deveber, 2010). Given the necessity of intact WM skills to numerous activities of daily living, additional developmental WM-related brain changes should be considered, including examination of laterality and WM functioning in clinical populations where it may be disrupted and the neurobiological mechanisms of WM-related intervention and remediation.
Highlights.
Adolescent working memory improves as a function of age and pubertal status.
Adolescents show lateralized brain activity during spatial and verbal working memory.
Adolescents show age-related changes in brain activation during working memory.
Adolescents do not show developmental change in brain activity lateralization.
Acknowledgments
Portions of this study were presented at the annual meeting of the Cognitive Neuroscience Society, March, 2009, San Francisco, California. This research was supported by Oregon Clinical and Translational Research Institute (UL1 RR024140), R01 AA017664 (Nagel), K08 NS52147 (Nagel), F31 AA019866 (Herting), and UNCF/MERCK Postdoctoral Science Research Fellowship (Fair), Ford Foundation Research Fellowship (Fair), and K99/R00 MH091238 (Fair). The authors express appreciation to Dr. Joan Stiles for her invaluable assistance in task conceptualization for this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alloway TP, Gathercole SE, Pickering SJ. Verbal and visuospatial short-term and working memory in children: are they separable? Child Development. 2006;77(6):1698–1716. doi: 10.1111/j.1467-8624.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49(3):2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working Memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. Imaging brain development: The adolescent brain. Neuroimage. 2012;61(2):397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Applied & Preventive Psychology. 1994;3:61–73. [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37(4):1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. NeuroImage. 1995;2(3):221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Developmental Neuropsychology. 2007;31(1):103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Conrad J. Acoustic confusion in immediate memory. British Journal of Psychology. 1964;55:75–84. doi: 10.1111/j.2044-8295.1964.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Constantinidis C. Posterior parietal mechanisms of visual attention. Reviews in Neuroscience. 2006;17(4):415–427. doi: 10.1515/revneuro.2006.17.4.415. [DOI] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences. 2003;7(12):547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cowan N. The development of working memory. In: Cowan N, editor. The development of memory in childhood. Hove, UK: Psychology Press; 1997. pp. 163–199. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42(6):1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103. Epub 2006 May 9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Research: Cognitive Brain Research. 1998;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Everts R, Lidzba K, Wilke M, Kiefer C, Mordasini M, Schroth G, et al. Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Human Brain Mapping. 2009;30(2):473–483. doi: 10.1002/hbm.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. The Journal of Neuroscience. 1996;16(2):808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AS, Sheridan MA, Kam CL, Hinshaw S, D’Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. The Journal Of Neuroscience. 2010;30(33):11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124(Pt 5):849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Developmental Psychology. 2004;40(2):177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. Journal of Neurophysiology. 2009;101(1):84–99. doi: 10.1152/jn.90562.2008. Epub 2008 Oct 2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status) NewHa ven, CT: Yale University; 1975. [Google Scholar]
- Ikkai A, Curtis CE. Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia. 2011;49(6):1428–1434. doi: 10.1016/j.neuropsychologia.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Fennema-Notestine C, Ostergaard AL. More “mapping” in brain mapping: statistical comparison of effects. Human Brain Mapping. 2003;19(2):90–95. doi: 10.1002/hbm.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles DD, Kleibeuker SW, Rombouts SA, Crone EA. Developmental differences in prefrontal activation during working memory maintenance and manipulation for different memory loads. Developmental Science. 2011;14(4):713–724. doi: 10.1111/j.1467-7687.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40(4):443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76(3):697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Lux S, Keller S, Mackay C, Ebers G, Marshall JC, Cherkas L, et al. Crossed cerebral lateralization for verbal and visuo-spatial function in a pair of handedness discordant monozygotic twins: MRI and fMRI brain imaging. J Anat. 2008;212(3):235–248. doi: 10.1111/j.1469-7580.2008.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, White NS, Lindgren KA, Heckers S, Coleman MJ, Dubal S, et al. Hemispheric specialization of the lateral prefrontal cortex for strategic processing during spatial and shape working memory. Neuroimage. 2004;21(3):894–903. doi: 10.1016/j.neuroimage.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Mohr HM, Goebel R, Linden DEJ. Content- and Task-Specific Dissociations of Frontal Activity during Maintenance and Manipulation in Visual Working Memory. The Journal Of Neuroscience. 2006;26(17):4465–4471. doi: 10.1523/JNEUROSCI.5232-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Garavan H. Artifactual fMRI group and condition differences driven by performance confounds. Neuroimage. 2004;21(1):219–228. doi: 10.1016/j.neuroimage.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Ohannessian A, Cummins K. Performance dissociation during verbal and spatial working memory tasks. Perceptual and Motor Skills. 2007;105(1):243–250. doi: 10.2466/pms.105.1.243-250. [DOI] [PubMed] [Google Scholar]
- Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, Cohen JD. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage. 2000;11(5 Pt 1):424–446. doi: 10.1006/nimg.2000.0572. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. Neuroimage. 2008;42(4):1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA, Dodrill CB. Verbal memory deficits after left temporal lobectomy for epilepsy. Mechanism and intraoperative prediction. Journal of Neurosurgery. 1985;62(1):101–107. doi: 10.3171/jns.1985.62.1.0101. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Owen AM. The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. The European journal of neuroscience. 1997;9(7):1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pickering SJ, Gathercole SE, Peaker SM. Verbal and visuospatial short-term memory in children: evidence for common and distinct mechanisms. Memory & cognition. 1998;26(6):1117–1130. doi: 10.3758/bf03201189. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SM, Riby DM, Fraser E, Campbell LE. The extent of working memory deficits associated with Williams syndrome: exploration of verbal and spatial domains and executively controlled processes. Brain Cogn. 2011;77(2):208–214. doi: 10.1016/j.bandc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism Clin Exp Res. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. Journal of Cognitive Neuroscience. 2006;18(7):1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide. Pittsburgh: Psychology Software Tools Inc; 2002. [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. FMRI reveals alteration of spatial working memory networks across adolescence. Journal of the International Neuropsychological Society. 2005;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, McAvoy MP, d’Avossa G, Corbetta M. Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cerebral Cortex. 2007;17(11):2625–2633. doi: 10.1093/cercor/bhl170. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory: a view from neuroimaging. Cognitive Psychology. 1997;33(1):5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19(6):781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Stiles J, Moses P, Passarotti A, Dick FK, Buxton R. Exploring developmental change in the neural bases of higher cognitive functions: the promise of functional magnetic resonance imaging. Developmental neuropsychology. 2003;24(2–3):641–668. doi: 10.1080/87565641.2003.9651914. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, et al. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10(3 Pt 1):327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Race E, Burrows B, Whitfield-Gabrieli S, Glover GH, Gabrieli JD. Development of spatial and verbal working memory capacity in the human brain. Journal of Cognitive Neuroscience. 2009;21(2):316–332. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JJ, Fougnie D, Marois R. Visual short-term memory load suppresses temporo-parietal junction activity and induces inattentional blindness. Psychological Science. 2005;16(12):965–972. doi: 10.1111/j.1467-9280.2005.01645.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, affective & behavioral neuroscience. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Walter H, Bretschneider V, Gron G, Zurowski B, Wunderlich AP, Tomczak R, et al. Evidence for quantitative domain dominance for verbal and spatial working memory in frontal and parietal cortex. Cortex. 2003;39(4–5):897–911. doi: 10.1016/s0010-9452(08)70869-4. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corp; 1999. [Google Scholar]
- Westmacott R, Askalan R, MacGregor D, Anderson P, Deveber G. Cognitive outcome following unilateral arterial ischaemic stroke in childhood: effects of age at stroke and lesion location. Dev Med Child Neurol. 2010;52(4):386–393. doi: 10.1111/j.1469-8749.2009.03403.x. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. Journal of Neuroscience Methods. 2007;163(1):128–136. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33(2):522–530. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Zald DH, Iacono WG. The development of spatial working memory abilities. Developmental Neuropsychology. 1998;14(4):563–578. [Google Scholar]