Abstract
Herpes simplex virus (HSV) is associated with a variety of ocular diseases, including epithelial and stromal keratitis. HSV can cause stromal opacification and is believed to be the leading cause of infectious blindness in the developed world. An improved understanding of the global burden of HSV keratitis, including the incidence of severe vision loss, could have a significant effect on prevention and treatment and place it in perspective among causes of corneal ulceration. We found that the global incidence of HSV keratitis is roughly 1.5 million, including 40,000 new cases of severe monocular visual impairment or blindness each year. We also discuss relevant epidemiologic issues regarding HSV epithelial and stromal disease.
Keywords: antiviral resistance, corneal latency, epidemiology, HSV keratitis, infectious blindness, surveillance, vision losss
Introduction
Herpes simplex virus (HSV) is a double-stranded DNA virus belonging to Alphaherpesvirinae, a subfamily of the Herpesviridae family. The three members of the subfamily are Herpes simplex virus type-1 (HSV-1), Herpes simplex virus type-2 (HSV-2) and varicella zoster virus (VZV). HSV-1 and HSV-2 in particular are highly related viruses, although HSV-1 has a much greater association with ocular pathology. Ocular HSV manifests as conjunctivitis, iridocyclitis, acute retinal necrosis and keratitis. HSV keratitis is believed to be an important cause of infectious blindness, mainly resulting from stromal opacification. An estimated 500,000 people in the United States have ocular HSV, and treatment of new and recurrent cases costs the country US$ 17.7 million annually.38,45 The global impact of ocular HSV is difficult to ascertain because of a lack of surveillance-based epidemiologic studies.
HSV keratitis is often cited as the leading cause of infectious blindness in developed nations, although it appears that the burden of vision loss has not been determined. Furthermore, the impact of the disease in developing nations is currently unknown, with limited access to treatment and immunosuppression perhaps contributing to a significantly higher visual morbidity. While the World Health Organization (WHO) has identified several diseases among its priority targets for the Vision 2020 program, corneal opacity has not been included as a prevention category (Table 1). Here we update the review by Dawson and Togni published in 1976 that first described HSV as the leading cause of infectious blindness in the developed world.16
TABLE 1.
World Health Organization Vision 2020 Program Priority Eye Diseases
| Cataract |
| Refractive error and low vision |
| Trachoma |
| Diabetic retinopathy |
| Onchocerciasis (river blindness) |
| Glaucoma |
| Childhood blindness |
The HSV-2 epidemic, as well as the decrease in early HSV-1 seropositivity in developed nations, both may have implications for HSV keratitis. The issue of corneal latency of HSV, although it requires further investigation, may influence eye banking and corneal transplantation. There have recently been reports of resistance to acyclovir, which has been an important form of antiviral therapy. Finally, the prospect for developing a vaccine for HSV has recently been discussed.
Pathophysiology
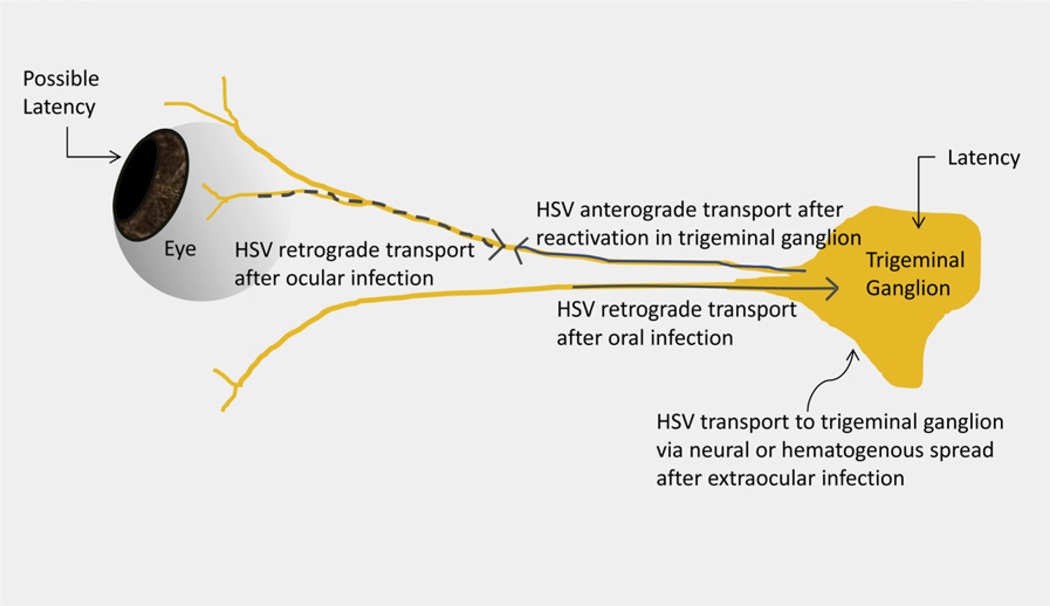
The main route of HSV spread is via direct contact, as the virus enters at the mucous membrane of the host.1 Ocular infection can occur as primary or recurrent episodes (Table 2). HSV epithelial keratitis begins as a superficial punctate lesion, progressing to a stellate erosion and, finally, a dendritic ulcer.12,55,56 The immune system is believed to be instrumental in clearing the corneal epithelium of HSV; the virus is able to travel via retrograde axonal transport along sensory nerves to the trigeminal ganglion, however, where it remains latent until reactivated (Fig. 1).6,8,10,17,21,41,42,47,50,66,102,103
TABLE 2.
Primary and Recurrent Ocular HSV
| Primary ocular HSV (no previous exposure) |
| Recurrent ocular HSV via reactivation after primary ocular infection |
| Recurrent ocular HSV via reactivation after primary extraocular infection |
| Recurrent ocular HSV via exposure to the same or different viral strain |
Fig. 1.
Schematic for HSV ocular infection, latency, and recurrence. HSV travels retrograde along the ophthalmic division of the fifth cranial nerve after ocular infection or via other routes after extraocular infection to develop latency in trigeminal ganglia. It also may develop latency locally in the cornea.
HSV stromal keratitis is thought to occur more commonly in recurrences. Much of the morbidity in stromal disease is thought to result from CD4+ T-cell destruction in the inflammatory response to the virus, in addition to direct viral effects.25,63,82 The severity of disease may increase with each subsequent episode, and inflammatory changes may be seen long after viral activity is no longer detected.33,85 Non-necrotizing stromal keratitis presents with localized corneal edema and is often self-limited, whereas necrotizing stromal keratitis has a rapid progression with stromal infiltrates and widespread inflammation. Both can lead to corneal neovascularization and scarring, with blindness as an end result.59
Corneal latency of HSV is a controversial topic.23 Several studies have contributed to supporting evidence for corneal latency, as well as to the possibility of long-term viral activity in corneal tissue, which could have similar implications (Table 3).52,58,68–70,73,76 These investigations point to several possibilities:
HSV can remain latent in the cornea with only some reactivation episodes leading to disease.
A low level of viral activity can persist within the cornea with some patients developing disease during increased viral activity or reactivation from trigeminal ganglia.
Viral DNA detected in the cornea is derived from defective genomes.
The virus can develop latency only in sensory ganglia with some individuals having a high rate of phenotypic reactivation.
TABLE 3.
Selected Studies in Corneal Latency and Persistence of HSV
| Author(s) | Summary | Conclusions |
|---|---|---|
| Remeijer et al69 | HSV-1 DNA load in corneas with HK correlated with age, recurrence-free interval, corneal neovascularization, disease severity, and graft rejection whereas qPCR in donor corneas was not predictive | HSV-1 qPCR has clinical value if performed on excised corneas of patients with HK, whereas screening donor corneas by qPCR may not |
| Polcicova et al68 | HSV-1 US9-mutant caused stromal keratitis in mice despite an impaired ability to travel anterograde along sensory nerves | HSV may not need to travel to and from trigeminal ganglia to cause stromal keratitis, supporting the idea that corneal latency may be possible |
| Robert et al73 | HSV in corneal tissue without clinical disease confirmed by PCR with infectivity demonstrated by culture | HSV DNA in corneal tissue can be transmitted during transplantation, consider excluding high risk eye bank tissue |
| Zheng102 | Corneas in rabbits latently infected with HSV-1 can transmit virus to naïve rabbits suggesting the possibility of corneal latency with increased transmissibility in LAT-positive viral strains | Consideration of ocular HSV history in donor is important along with close follow-up |
| Remeijer et al70 | HSV-1 transfer from donor to recipient confirmed by PCR led to blindness | Further studies are required to determine the nature of latency and localization of HSV in corneal tissue |
| Morris et al58 | Donor cornea culture media from 3 of 80 corneas were positive for HSV DNA by PCR, which did not result in ocular infectiona | Screening of donor culture medium for HSV could not be recommended |
qPCR = quantitative polymerase chain reaction; HK = herpetic keratitis.
Study based on organ culture eye-banking method.
The potential impact of these possibilities on eye banking and corneal transplantation is discussed in a later section.
HSV Seroprevalence
HSV seroprevalence is determined by blood testing that demonstrates antibodies to the virus indicating previous exposure. We include studies that present epidemiologic data from the National Health and Nutrition Examination Survey (NHANES). The NHANES data include HSV type-specific assays in people ages 14 to 49. The methods of inclusion for ocular HSV epidemiologic studies are summarized in Table 4. The NHANES data likely constitute the most complete information on HSV seroprevalence in the United States. The age limitations of the study, however, may limit the importance of the data. Some of the data on ocular HSV in this section are based on regional information that may be less predictive of national trends. The strengths and weaknesses of ocular HSV epidemiologic studies are summarized in Table 4.
TABLE 4.
Studies of HSV Keratitis Incidence
| Location and Dates | Method of Inclusion | Incidence Data | Trend | Strengths | Weaknesses |
|---|---|---|---|---|---|
| France; Sept–Dec 2002 | Dendritic or geographic ulcer (epithelial), stromal keratitis with identical previous events, and subjective description by investigators (low, medium, or high probability of herpetic origin) | New and recurrent epithelial keratitis in 22.0 per 100,000 person-years | None noted during study period (3 months) | Well-defined nationwide multicenter study with large number of randomized investigators reducing possibility of referral bias | Potential seasonal effect on keratitis incidence |
| New and recurrent stromal keratitis in 9.2 per 100,000 person-years | |||||
| Keratitis in 0.3 per 100,000 person-years that could not be classified | |||||
| New and recurrent epithelial and stromal keratitis cases that were “highly probable” in 25.8 per 100,00 person-years | |||||
| Rochester, MN (USA); 1950–1982 | Punctate keratitis, dendritic or geographic ulcer (epithelial), or disciform/necrotizing keratitis (stromal) | New and recurrent epithelial keratitis in 15.6 per 100,000 person-years | Statistically insignificant rise in incidence during study period, possibly related to changing HSV-1 seroprevalence | Well-defined long-term study data from single center that serves all of Rochester reducing possibility of referral bias | Data from single center serving mostly white patients |
| New and recurrent stromal keratitis in 2.6 per 100,000 person-years | |||||
| Funen, Denmark; 1976–1978 | Epithelial dendritic ulcer | Epithelial dendritic keratitis in 12 per 100,000 person-years | None noted during study period (24 months) | All cases in Funen that presented to an ophthalmologist were included (population 446,223) virtually eliminating referral bias | Only cases of epithelial dendritic keratitis included |
| Copenhagen, Denmark; (study dates not reported) | Epithelial dendritic ulcer | Epithelial dendritic keratitis in 5.9 per 100,000 person-years | None noted during study period (7 years) | Long-term study data from single center | Only cases of epithelial dendritic keratitis included, many cases seen in the community likely not included |
| Tunisia; Oct 1972– June 1973 | Dendritic or geographic ulcer (epithelial) | Epithelial keratitis in 1.4 per 100,000 person-years | None noted during study period (8 months) | Record-keeping included extraocular herpetic lesions and previous treatment | Only epithelial keratitis incidence determined, no efforts made to include all cases from l’Institute d’Ophthalmologie de Tunis, no other centers included |
| Rijeka, Croatia; (study dates not reported) | Keratitis | Keratitis in 4 per 100,000 person-years | None noted during study period (18 years) | Long-term study performed at single center | Methodology and results not well-described |
The seroprevalence of HSV-1 appears to be decreasing in the United States, whereas HSV-2 remains elevated compared to previous decades. The decrease in HSV-1 may be related to improved hygiene leading to delayed or reduced exposure to the virus. HSV-2 may be more common as the result of changes in sexual behavior.
Delayed exposure to HSV-1 may potentially cause more severe disease later in life that can include ocular involvement. HSV-2 may be transmitted during childbirth, leading to neonatal or delayed-onset ocular infection.29
There are several ways by which HSV can be detected in human beings. In one study of trigeminal ganglia harvested from cadavers, 89.1% were positive by polymerase chain reaction (PCR) for HSV-1 DNA, not correlated to sex or age.26 Kaufman et al investigated the asymptomatic shedding of HSV-1 DNA in saliva and tears in patients without ocular disease and found that 98% had at least one episode of HSV-1 shedding during the study period.32 The use of PCR will likely expand as correlations with ocular disease are determined.34,75,86,88 The recent epidemiologic studies of HSV-1 and HSV-2 prevalence have used type-specific serology, which utilizes glycoprotein assays to demonstrate past infection as well as an observable immune response, although it is less sensitive than PCR. Seropositivity to HSV-1 partially protects against HSV-2.53
HSV-1 SEROPREVALENCE AND THE EYE
Most people are seropositive for HSV-1, presumed to be the result of transmission by asymptomatic shedding from oral mucosa.90 The age-specific seroprevalence of HSV-1 in developed nations has been decreasing. A recent NHANES found that age-adjusted seroprevalence of HSV-1 in 1999–004 was 57.7%, representing a 6.9% relative decrease from 1988–1994 (95% confidence interval [CI], –11.6% to –2.3%; p = 0.006).97 Improved hygiene and living conditions are likely contributors. HSV-1 is also causing an increasing proportion of genital disease, especially in young people.40,49,74
There are implications of these findings for eye disease, as a delay in HSV-1 seropositivity may contribute to increased ocular HSV This is consistent with findings from two population-based Minnesota studies that point to a possible rise in ocular HSV incidence.45, A A recent large study of HSV keratitis in France was performed over a few months. The similarity of the initial case incidence from this study (13.2 per 100,000 person-years) to that from the second half of the more recent Minnesota study (10.4 per 100,000 person-years) suggests that their findings are close to the actual incidence of initial ocular HSV in developed nations.37
Uchio et al performed a retrospective study in Japan in which patients attending a single outpatient center over a 30-year period were divided into two temporal groups (1963 to 1979 and 1980 to 1992).84 In the first group initial keratitis occurred in 2.4% of patients at an average age of 7.06 years, whereas in the second group it occurred in 5.7% at an average age of 24.2 years. The rise in mean age of initial infection was attributed to decreasing seroprevalence. In the UK, reduced HSV-1 seropositivity during childhood was correlated with changes in ocular infection rates. Initial ocular HSV-1 in those under 5 years old decreased from 29% to 7% in the 1980s, whereas young adults showed an increase from 41% to 64%.39
HSV-2 SEROPREVALENCE AND THE EYE
The HSV-2 epidemic is of concern in part because of its potential to cause neonatal herpes.11,24,49,97 Through pooling of prevalence values by age and sex in a random-effect model, followed by the use of a constant-incidence model, the worldwide prevalence of HSV-2 among 15- to 49-year-olds is estimated to be 536 million (16%).48 NHANES data from 1999–2002 showed that 63% of pregnant women in the United States were seropositive for HSV-1, 22% for HSV-2, and 13% for both.96 The incidence of neonatal HSV was estimated to be 5.9 per 100,000 live births in a nationwide surveillance program in Canada and ranged from 5.8 to 11.5 per 100,000 live births in the United States.14 Various manifestations of ocular infection occur in an estimated 13–20% of neonates with HSV.54,61
An apparent rise in ocular HSV incidence suggested by the studies from Minnesota may partially be the result of delayed recurrences after neonatal HSV. Multiple case series suggest that HSV-2 is an important cause of acute retinal necrosis (ARN) in patients under 25 years of age.44 Because of the low incidence and severe nature of ARN, most reports come from tertiary centers. A population-based study in the UK using the British Ophthalmological Surveillance Unit reporting system estimated the incidence of ARN to be 1 in 1.6–2.0 million people per year.60 HSV-2 was the third most frequent cause, after VZV and HSV-1. Further studies are required to demonstrate whether HSV-2 epithelial and stromal keratitis are increasing.
HSV Keratitis in Developed and Developing Nations
How cases of HSV keratitis were ascertained, as well as the definitions and methods to determine inclusion, are summarized in Table 4. Incidence rates of ocular HSV are based on regional information that may have limited external validity. Those studies that provided detailed definitions of inclusion criteria and used linked medical record systems or representative survey methods are likely to be more applicable to the general population.
The incidence of ocular HSV may be increasing in the developed world. This may be related to delayed exposure to HSV-1 or an increased prevalence of HSV-2. An increased rate of ocular HSV could mean increased morbidity as well as a greater economic burden of disease.
There are relatively few studies on the epidemiology of HSV keratitis in developed nations (Table 4). The landmark study of ocular herpes simplex epidemiology looked at cases in Rochester, Minnesota, from 1950 to 1982.46 The incidence of new epithelial keratitis was 5.6 per 100,000 person-years, with new and recurrent cases totaling 15.6 per 100,000 person-years. New stromal keratitis occurred at a rate of 0.6 per 100,000 person-years, with 2.6 per 100,000 person-years presenting with new and recurrent episodes. A statistically insignificant rise in ocular HSV incidence was noted. The Rochester study used a medical record linkage system that reduced the possibility of referral bias.
A nationwide, multicenter, prospective study in France was conducted from September to December 2002. The incidence of initial HSV keratitis episodes was 13.2 per 100,000 person-years, and initial and recurrent cases were estimated to occur in 31.5 per 100,000 person-years (95% CI, 25.5– 37.5).37 This included epithelial keratitis in 22.0 per 100,000 person-years and stromal keratitis in 9.2 per 100,000 person-years, with 0.3 per 100,000 that could not be classified. If only highly probable HSV lesions were included (e.g., dendritic or geographic epithelial ulceration, stromal disease with prior documented episodes), the incidence was 25.8 per 100,000 person-years (95% CI, 21.2–30.4). Incidence values were determined by multiplying the average incidence rate among participating physicians by the total number of French ophthalmologists, then dividing by the population of France. The 412 ophthalmologists participating in the study were considered representative of all French ophthalmologists based on several criteria.
The investigations performed in developing nations have limited external validity, and those that do provide incidence rates have substantial shortcomings (Table 4). In a hospital-based study of corneal ulceration in Tanzanian children, HSV keratitis was diagnosed in 35.5% from 1982 to 1984, and in 65.8% from 1986 to 1988.99 A higher rate in the latter group was correlated with malaria, which was also demonstrated in a study that included adults.100 Lewallen and Chirambo noted that HSV caused less than 10% of corneal ulcers in Malawai and did not correlate with measles or cerebral malaria infections in children; other diseases that may be hyperendemic were not mentioned, however.43 A study from a tertiary hospital in Nigeria reported that 49.5% of keratitis in children was attributed to HSV.3 HSV keratitis was second only to trauma as a cause of corneal perforation in a tertiary center in China.94 A number of studies from the developing world provide data on infectious keratitis, but exclude viral etiologies.
Because the data from France and the United States appeared to be the most generalizable and detailed, we made a rough estimation of incidence in developed nations (Table 5). This estimation is based upon several assumptions. For the data from France, we used the incidence of high probability cases in order to maintain a conservative projection and for applicability to visual prognosis studies. Similarly, the Rochester data was used to estimate incidence in the United States, although the trend indicates that the current incidence may be higher. The incidence in the United States was extrapolated to Canada, and the data for high probability cases from France was applied to the remaining developed nations (summarized in Table 5).
TABLE 5.
HSV Keratitis Incidence
| Location | Incidencea | Rationale | Author(s) | Populationb | Incidence in Population (per year) |
|---|---|---|---|---|---|
| France | 25.8 | Combined incidence of epithelial and stromal keratitis (high probability) | Labetoulle et al37 | 65,102,719 | 16,797 |
| USA | 18.2 | Combined incidence of epithelial and stromal keratitis | Liesegang et al45 | 313,232,044 | 57,008 |
| Developed nations | 23.3 | List of nations included: Norway, Australia, New Zealand, USA, Ireland, Liechtenstein, Netherlands, Canada, Sweden, Germany, Japan, South Korea,c Switzerland, France, Israel, Finland, Iceland, Belgium, Denmark, Spain, Hong Kong, Greece, Italy, Luxembourg, Austria, UK, Singapore, Czech Republic, Slovenia, Andorra, Slovakia, U.A.E., Malta, Estonia, Cyprus, Hungary, Brunei, Qatar, Bahrain, Portugal, Poland, Barbados Incidence from U.S. extrapolated to Canada Incidence from France extrapolated to remaining developed nations |
(extrapolated data) | 1,036,895,491 | 241,597 |
| Developing nations | Unknown | All nations not included in list of developed nations Incidence from developed nations extrapolated to developing nations as a minimum estimated |
(extrapolated data) | 5,891,302,762 | 1 to 1.5 million |
Developed nations were categorized as having “very high human development” by the United Nations Development Program. There is as such no agreed upon definition for developed versus developing nations.
Incidence values are given per 100,000 person-years.
Populations based on estimates from the U.S. Bureau of the Census and the Central Intelligence Agency World Factbook, updated 2011.
South Korea is officially the Republic of Korea.
Incidence rates were extrapolated from the French and U.S. studies to the population of the developing world. If our assumption is correct that the rates of HSV keratitis incidence are at least as high in the developing world as they are in the developed world, then this may provide us with a minimum estimate. Adding the estimates for developed and developing nations provides a combined annual global incidence of roughly 1.5 million.
The next issue was whether any studies could be used to estimate HSV keratitis incidence in developing nations. Unfortunately, the studies from the developing world were performed at single centers, with either incomplete data for the population served or a poorly described methodology (Table 5). It currently remains unknown whether HSV keratitis incidence is higher or lower in developing versus developed nations. Although some hospital-based studies of HSV suggest a high rate of infection, they do not provide a basis for determining incidence.
The data we have reviewed suggest an increasing rate of HSV keratitis in developed nations, yet the developing world appears to have a significant burden of risk factors. Viral recurrence, responsible for the majority of HSV keratitis incidence, may be associated with stress, ultraviolet radiation, corneal trauma, and immunosuppression.92 An increased rate of recurrence and resistance to treatment has been seen in patients with human immunodeficiency virus (HIV) / acquired immune deficiency syndrome.27,101 These factors, along with the severely limited access to treatment in the developing world, suggest that disease rates may be high.
Because these risks cannot be quantified, it is problematic to use studies from the developed world as a guide. Nonetheless, we may be able to obtain a basic understanding of the minimum annual incidence, particularly if our assumption that rates are higher in the developing world is correct. An extrapolation of data from France and the United States points to what we believe to be a minimum annual global incidence of roughly 1.5 million (summarized in Table 3). This is a gross estimate, and it remains unknown to what degree this may correlate with the actual global impact of HSV keratitis.
HSV: An Important Infectious Cause of Blindness
Studies that reported on the visual morbidity of ocular HSV used all patients diagnosed with the disease in a given period, often in a single center, that were followed longitudinally. There are several potential sources of error, including variable treatments and periods of follow-up. Studies with longer periods of follow-up are likely to be more accurate because they allow more time for recurrences.
In the developed world, although there may be an increasing incidence of ocular HSV, improved access to antiviral treatment may cause the overall visual burden of the disease to remain stable or decrease. This is less likely to occur in the developing world, where HSV and other causes of corneal ulceration have not been systematically addressed.
HSV is thought to be the leading cause of infectious blindness in developed nations.16 The burden of vision loss associated with the virus remains uncertain. Surveillance often focuses on etiologies of blindness that affect a large number in the population (e.g., age-related impairment) or are endemic in certain regions of the world (e.g., onchocerciasis). Unlike these causes, HSV is a ubiquitous infectious agent that causes blindness only rarely, making surveillance a difficult task. HSV may be the leading infectious indication for corneal transplant in developed nations, but this is affected by a number of factors, including disease incidence, availability of donor corneas, and overall access to health care.
One way to estimate vision loss in HSV would be to determine the proportion of HSV keratitis cases that lead to blindness in the affected eye and extrapolate this to annual incidence rates. A Moor-fields Eye Hospital study found that of 152 patients with epithelial keratitis, only 3% had a final visual acuity less than 20/200.93 Final visual acuity ranged from 20/60 to 20/200 in 24%, and 20/20 to 20/40 in 73%. Liesegang et al found a slightly lower incidence of vision impairment in ocular HSV (3 of 131 cases); impairment was defined as an acuity worse than 20/100, however, and the series included diseases other than keratitis.47 Final visual acuity was 20/40 or better in 78% of eyes. A study at the Aravind Eye Hospital in India found that at least 2% had visual acuity worse than 20/1200, and 62% improved to better than 20/40.31 Norn et al found nearly 6% of eyes were worse than 20/200, although some were treated with idoxuridine or steroids.64 In one series 20% of HSV uveitis cases led to severe vision loss; this did not include cases of uveitis without keratitis, which would be considered additive.55 Although blindness can occur via ocular HSV without corneal involvement (e.g., acute retinal necrosis), these cases are much rarer.
There were several factors that needed to be considered in projecting the rates of vision loss from HSV keratitis (Table 6). Based on these issues, and adjusting the Moorfields data for the effect of long-term antiviral treatment (which reduces the rate of recurrence), we estimate that at least 1.5% of clinically significant HSV keratitis leads to vision worse than 20/200, the WHO definition of severe visual impairment in developed nations (Table 6). This is based on the assumption that a reduction in recurrence rate leads to a proportional decrease in visual impairment. It is slightly lower than the rate of visual impairment in the Rochester study, where acyclovir was available for only a portion of the study period. In the developing world, including Africa and India, it may be 3% or higher as access to treatment is often severely limited, and other risk factors may play a role. We are unable to estimate the proportion of cases leading to monocular blindness (lower than 20/400). Longer study periods might reveal higher rates of vision loss as there would be more time for recurrences.
TABLE 6.
Summary of Issues for Visual Prognosis Data
| 1) The authors of the Moorfields study noted the data have limited implications because a selected population was used. It should be considered that the study overrepresented severe cases. | The study did not include patients with stromal keratitis, the percentage of dendritic versus geographic ulceration was similar to other studies, and a longer study period may have revealed a higher rate of visual morbidity. |
| 2) Long-term antiviral prophylaxis was found in the HEDS to reduce the recurrence rate by nearly 50%.81 This appears to limit the external validity of visual prognosis studies performed before the availability of oral acyclovir. | The rate of severe vision loss in the Moorfields study was approximately 3% with a slightly lower rate found in Rochester. In developing nations where access to treatment is often severely limited, this may still be predictive before other risk factors are taken into account. In developed nations we would expect to see up to nearly a 50% reduction in the recurrence rate of keratitis with a proportional improvement in the rate of vision loss. |
| 3) The Rochester study showed a slightly lower rate of vision loss than the Moorfields study. It may be a better source for visual morbidity in developed nations due to improved treatment used for part of the study period. | The limited efficacy of antivirals explains why, although rare, there were cases of vision loss in the Rochester study. Because acyclovir was only available for a portion of the study period, it may not reflect the current rate of vision loss. Adjusting the Moorfields data for the effect of treatment based on the HEDS results provides a slightly lower rate of vision loss than that found in Rochester and also describes the rate of severe visual impairment based on the WHO definition. |
| 4) The Aravind Eye Hospital study and Copenhagen study both showed substantially higher rates of vision loss. This may result from several factors that require consideration. | A higher rate of vision loss at the Aravind Eye Hospital could have resulted in part from ascertainment bias, although as the only eye center serving a large population, delayed presentation following lack of treatment is often the norm. This also may result from increased risk factors. Some patients in the Copenhagen study were exposed toidoxuridine or steroids, which likely contributed to vision loss. |
HEDS = Herpetic Eye Disease Study.
Using the available data on visual prognosis, therefore, our conservative estimate is that HSV keratitis is the cause of roughly 40,000 new cases of severe monocular visual impairment or blindness annually in the world (Table 7). This may not account for the effect of risk factors such as trauma and immunosuppression that may be contributing to higher rates of vision loss in the developing world. The HSV-2 epidemic may also be contributing in developing nations from its synergistic role in HIV spread. The current global prevalence of HSV-related blindness remains elusive.
TABLE 7.
HSV Keratitis Leading to Severe Vision Loss
| Keratitis Incidence per year) |
Rate of Severe Visual Impairment |
Incidence of Severe Visual Impairment (per year) |
|
|---|---|---|---|
| Developed nations | 241,597 | 0.015 (adjusted data) | 3,624 |
| Developing nations | 1 to 1.5 million | 0.03 (Moorfields data) | 30,000 to 45,000 |
| Total | ~;1.5 million | n/a | ~;40,000 |
The incidence of other infectious corneal diseases and their visual prognosis in developed nations are not well known, although trachoma, once the leading cause of blindness in the world, is rare in the developed world today. Other etiologies are more important: coagulase-negative Staphylococcus, Streptococcus pneumoniae, Pseudomonas aeruginosa, and Acanthamoeba. In a large population-based study of corneal ulcers in the UK, HSV was the most common cause of poor visual outcome, followed by Pseudomonas.28 As the burden of visual impairment due to traditional causes of infectious blindness in developing nations decreases, other causes may become increasingly recognized. The prevalence of trachoma across 57 endemic nations has decreased to 40.6 million and likely causes fewer than 5.9 million cases of severe visual impairment or blindness.51 This reduction is in part because of the Alliance for the Global Elimination of Blinding Trachoma by the year 2020, which implemented the SAFE (Surgery for entropion trichiasis, Antibiotics, Facial cleanliness, Environmental improvement) methodology. In 1995 onchocerciasis was estimated to cause severe visual impairment in 500,000 and blindness in an additional 270,000.B International programs for distribution of oral ivermectin in endemic areas will likely eliminate this disease by 2020. Leprosy has also declined as a cause of severe vision loss.91
Corneal Transplantation
The studies investigating the transmissibility of HSV by corneal transplantation have used different detection methods. Data on corneal transplant indications come from either hospital records or national databases. Highly specific assays that allow investigators to confirm the source of the virus are better for determining whether donor–host transmission has actually occurred, although they may have lower sensitivity. Studies on corneal transplant indication using national data are less likely to have referral bias than hospital-based studies.
It is unclear whether the transmission rate of HSV by corneal transplantation is changing. HSV as an indication for transplantation may be decreasing in tertiary centers in the developed world, possibly the result of improved treatment as well as transplants being performed in the community. National data in this area are limited. Much less is known about the developing world. A decrease in the number of transplants being performed secondary to HSV would correlate with decreased morbidity as well as a reduced the economic burden associated with the procedure.
Among patients undergoing corneal transplant by penetrating keratoplasty (PKP), those with a diagnosis of HSV keratitis remain at risk for recurrence and graft rejection despite antiviral prophylaxis.20 HSV-1 in donor corneal tissue can cause disease in the recipient, although this is rare. Remeijer et al reported the first confirmed case in which donor virus was demonstrated to cause blindness in the recipient.70 The ability of HSV to be transmitted by corneal transplant has been the subject of a number of investigations (Table 3). Whether these events result from true corneal latency or reactivated virus in donor tissue, there is concern over eye-bank screening related to HSV. The Eye Bank Association of America reviews all reported cases of disease transmission and sets standards for screening of donors and tissue. The role of a national reporting system for adverse events related to donor corneal tissue is to facilitate further improvement.80 At this time, screening of all donor corneas for HSV may not be indicated because of the rarity of reported transmission and the use of viral detection methods that may damage the graft itself.72
There are few nationwide studies on corneal transplant indications. In an investigation looking at risk factors for corneal regraft using the French national waiting list for PKP, 8,904 eyes underwent corneal transplant between 2000 and 2002, including 1,246 regrafts.83 HSV keratitis accounted for 952 (12.4%) of first-time PKPs and 203 (16.4%) repeat procedures. The multivariate relative risk for corneal regraft with primary HSV keratitis was 2.35 (95% CI, 1.67–3.31). That study did not provide treatment information. Several reports from tertiary centers have shown a decrease in rates of corneal transplant due to HSV, which can be contrasted with reports from developing nations (Table 8).2,9,18,95,98 The use of prophylactic antivirals as well as transplants performed outside of tertiary care facilities may explain this apparent decline in HSV as an indication in developed nations.
TABLE 8.
HSV as Indication for Corneal Transplant in Tertiary Centers
| Location | Author(s) | Summary |
|---|---|---|
| Ankara, Turkey | Yalniz-Akkaya et al98 | HSV associated with 10.9% of first-time PKPs and 9.4% of repeat procedures from 1995–2005 |
| Toronto, Canada | Dorrepaal et al18 | HSV associated with 3.9% of PKPs from 1996–2004 |
| Qingdao, China | Xie et al95 | HSV second leading indication for PKP accounting for 18% of cases from 1997–2002 |
| East Grinstead, UK | Al-Yousuf et al2 | HSV and HZV associated with 5.9% of primary PKPs and 21.2% of regrafts, with regraft being the leading indication, from 1990–1999 |
| San Francisco, California, USA | Branco et al9 | HSV associated with 6% of PKPs from 1972–1976 and 1% from 1997–2001 |
PKP = penetrating keratoplasty.
A study comparing indications for PKP at Queen Victoria Hospital in the UK to the national transplant database found that viral keratitis (including HSV and VZV) was the leading infectious indication for transplant (5.9% vs 4% nationwide).5 The leading indication overall was regraft, which another study at the same center attributed to viral keratitis 21.2% of the time.2 A higher rate of post-herpetic graft survival at 5 years (86%) compared to other studies looking at HSV was linked to the indefinite use of prophylactic antivirals, although this also included VZV. Patients were typically given acyclovir 400 mg four times a day tapered to 400 mg daily by 12 months postoperatively, whereas information on dosage and duration nationwide was not available. The relative disparity between HSV as an indication for PKP in France and the UK, two developed nations, indicates the multifactorial nature of transplantation rates.
Resistance to Acyclovir
Studies investigating the resistance of HSV to acyclovir have used variable methodologies, including laboratory testing and clinical determination of resistance. The use of different strains of HSV, as well as different patient populations, makes it difficult to assess the relevance of data to clinical rates of resistance. Studies that investigate resistance to acyclovir in a specific disease process (e.g., HSV keratitis) are likely to be more accurate.
It is unclear whether the rate of resistance to acyclovir is changing. If there has been an increase, it may likely be caused by the wide use of acyclovir in the treatment of both HSV-1 and HSV-2.C If there is an increase in resistance to acyclovir, this increases the importance of developing alternative treatments, particularly if cross-resistance to other antivirals is also demonstrated.
The wide use of acyclovir for the treatment of genital, orofacial, and other herpetic diseases, and the over-the-counter availability of the drug in certain countries, has raised concern over the development of resistance, particularly in immunosuppressed patients. Some studies have sought to determine the prevalence of either acyclovir-resistant strains or clinical resistance (Table 9).13,15,19,71,79, D The mechanism of resistance in the majority of cases appears to be a mutation or deletion of the thymidine kinase gene, which may be difficult to interpret due to gene polymorphisms.36 It appears that antiviral resistance remains low in immunocompetent individuals, likely because the immune system drives the virus into a latent state, whereas resistance is much higher in the immunocompromised.4 This should be considered as a cause of treatment failure so that alternative treatments can be used, although cross-resistance may also occur.57 Studies have used varying methodologies in different clinical scenarios; therefore there is no clear indication at this time that long-term prophylactic antivirals in the form of nucleoside analogues should be avoided in the management of ocular herpes.
TABLE 9.
HSV and Resistance to Acyclovir
| Author(s) | Method | Results |
|---|---|---|
| Duan et al19 | qPCR confirmed by plaque reduction assay | 6.4% resistance in immunocompetent patients with herpetic keratitis |
| Stránská et al79 | ELVIRA HSV screening assay | 0.27% resistance in immunocompetent and 7% resistance in immunocompromised patients |
| Danve-Szatanek et al15 | Chessboard technique | 0.3% resistance in immunocompetent and 3.6% resistance in immunocompromised patients, with highest resistance in bone marrow transplant (10.9%) |
| Reyes et al71 | Plaque reduction assay | 0.1% resistance in immunocompetent patients |
| Gnann et alD | Plaque reduction assay | 5.6% resistance in immunocompromised patients |
| Christophers et al13 | Plaque reduction assay | 0.1–0.7% resistance in immunocompetent and 6.3% resistance in immunocompromised patients |
ELVIRA = enzyme linked virus inhibitor reporter assay; qPCR = quantitative polymerase chain reaction.
Preventing HSV Keratitis
Studies investigating prevention of HSV with antibiotics have used variable methods, primarily in animal models. Even if successful prevention of HSV recurrence is demonstrated in animals, this requires confirmation in humans. Immune response variations between non-human and human study subjects are a significant limiting factor.
The development of a vaccine for HSV remains one of the greatest challenges to controlling its impact and spread. The majority of research has focused on HSV-2 because of its association with genital herpes and also because of its synergy with HIV.24 Although various vaccines have shown promise in animal models, only limited efficacy has been demonstrated in humans.30 The largest recent clinical trial for an HSV-2 vaccine is the Herpevac Trial, which tested a subunit glycoprotein vaccine that showed some benefit, although only in women seronegative for both HSV-1 and HSV-2.78 A high amount of genetic homogeneity between the two strains and changing seroprevalence suggest that an effective HSV vaccine should to some degree be protective against both.
Few studies have described a vaccine designed for the prevention of HSV keratitis. Some have investigated glycoprotein D (gD) vaccines, including one study that used a self-adjuvanting gD subunit vaccine to demonstrate prevention of stromal keratitis and lower viral titer in mice, which was correlated to higher CD4+ T-cell activity.7,62 This effect remains to be demonstrated in humans and also has raised concerns over exacerbation of stromal keratitis in those previously infected.65 Another study using a gD vaccine demonstrated differential effects on primary versus recurrent ocular infection based on formulation and delivery of the vaccine before or after primary exposure.35 It has also been proposed in mice that immunization with HSV-2 dl5-29, a replication-defective mutant, can prevent the development of ocular HSV.87 A limited study in humans using a heat-inactivated virus vaccine showed a decreased rate of HSV-1 recurrence leading to ocular disease.67 Other clinical trials are needed to determine if there is long-term benefit in humans.
Discussion and Conclusion
The estimates presented here for HSV keratitis incidence and for resulting visual loss are limited by several factors, many of which have been discussed. Additionally, the studies used to derive these estimates have variable methodologies and are based on assumptions and extrapolations that may serve as sources of error. In particular, some of the studies we used are population-based, and the data sets may not be representative of the populations to which they were applied. The season or time of year in short-duration studies may also have influenced incidence rates. The assumption that HSV keratitis incidence is at least as high in the developing world as what was observed in a developed nation between 30 and 60 years ago is suggested by some case series. Similarly, the assumption that visual morbidity is higher in developing nations—and at least as high as was seen in a developed nation prior to effective prophylaxis—relies on risk factor assessment and a limited number of studies, but is also suggested by trends in corneal transplantation. Finally, the visual morbidity in developing nations may have been underestimated.
The studies that were used from the developed world to determine the burden of HSV keratitis represent the best available in terms of methodology, population, and severity of disease. From the study by Labetoulle et al we used the incidence of “high probability” cases in order to arrive at a conservative estimation for incidence as well as to maintain congruity with the methodology used for diagnosis in the Moorfields study of visual prognosis. The effect of long-term prophylactic antivirals was taken from results from the Herpetic Eye Disease Study, the largest trial of its kind. A study from the Aravind Eye Hospital, although not used to project vision loss, did support the assumption that lack of treatment and increased risk factors may contribute to a worse visual prognosis in developing nations.
HSV, unlike many pathogens, has two strains that can infect and develop life-long latency. The rate of HSV-2 seropositivity in some regions approaches that of HSV-1.89 Most cases of ocular HSV have been attributed to HSV-1. Although the precise prevalence of vision loss from HSV keratitis remains unknown, our estimate of roughly 40,000 cases per year of severe visual impairment or blindness indicates that HSV is increasingly important relative to other infectious cause of blindness. Trachoma, once the leading cause of infectious blindness, is in marked decline as the result of improved hygiene and an effective international campaign. Onchocerciasis is projected to be eliminated by 2020.
An important consideration is that the incidence of other causes of corneal ulceration, which has been described as a silent epidemic, is not well known.91 We estimate that the annual incidence of corneal epithelial ulceration or stromal disease from HSV is roughly 1.5 million. The incidence of ulceration from all causes, therefore, is even higher than the 1.5 to 2 million estimated by Whitcher et al91 based on results from a study of presumed non-viral cases in the Madurai District in South India that were extrapolated to India and Africa.22,77
These findings also suggest a role for nationwide surveillance programs for ocular herpes. In the developed world, a changing HSV seroprevalence may be contributing to a rising incidence of HSV keratitis, partially counteracting the benefit of improved treatment. The burden of HSV keratitis in developing nations may be substantially higher than previously estimated. The role of HSV-2 in ocular infections also may become increasingly recognized with the use of more specific diagnostic tests. As primary exposure to HSV is not easily prevented, it is unlikely that its global impact on vision loss will be eliminated without a targeted monitoring approach. This also indicates the importance of developing improved treatments and eventually a vaccine to prevent HSV keratitis.
Method of Literature Search
A search of the PubMed database was conducted for several keywords, including ocular HSV (720 articles), HSV seroprevalence (397 articles), HSV keratitis (3,929 articles), penetrating keratoplasty indications (255 articles), and HSV antiviral resistance (488 articles). Those articles that provided information on the epidemiology of HSV keratitis were included, and further sources were derived from their bibliographies. For corneal latency, studies that were representative of its subtopics were chosen with a preference for those published from 1995 to present day.
Acknowledgments
The authors would like to thank Joel Sugar, MD (UIC) and Charlottle Joslin, OD, PhD (UIC) for critical review of this article.
Footnotes
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article. Publication of this article was supported in part by NIH grants AI057860, AI081869, Core Grant EY01792 and a Lew Wasserman Merit Award from Research to Prevent Blindness, Inc, New York, New York (Dr. Shukla). Asim Farooq is supported by a Research to Prevent Blindness Medical Student Eye Research Fellowship.
References
- 1.Akhtar J, Tiwari V, Oh M-J, et al. HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium. Invest Ophthalmol Vis Sci. 2008;49:4026–4035. doi: 10.1167/iovs.08-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Yousuf N, Mavrikakis I, Mavrikakis E, et al. Penetrating keratoplasty: indications over a ten year period. Br J Ophthalmol. 2004;88:998–1001. doi: 10.1136/bjo.2003.031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashaye A, Aimola A. Keratitis in children as seen in a tertiary hospital in Africa. J Natl Med Assoc. 2008;100:386–390. doi: 10.1016/s0027-9684(15)31270-0. [DOI] [PubMed] [Google Scholar]
- 4.Bacon TH, Levin MJ, Leary JJ, et al. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16:114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckingsdale P, Mavrikakis I, Al-Yousuf N, et al. Penetrating keratoplasty: outcomes from a corneal unit compared to national data. Br J Ophthalmol. 2006;90:728–731. doi: 10.1136/bjo.2005.086272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertke AS, Patel A, Krause PR. Herpes simplex virus latency-associated transcript sequence downstream of the promoter influences type-specific reactivation and viral neurotropism. J Virology. 2007;81:6605–6613. doi: 10.1128/JVI.02701-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettahi I, Nesburn AB, Yoon S, et al. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lip-opeptide vaccines. Invest Ophthalmol Vis Sci. 2007;48:4643–4653. doi: 10.1167/iovs.07-0356. [DOI] [PubMed] [Google Scholar]
- 8.Branco FJ, Fraser NW. Herpes simplex virus type 1 latency-associated transcript expression protects trigeminal ganglion neurons from apoptosis. J Virol. 2005;79:9019–9025. doi: 10.1128/JVI.79.14.9019-9025.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branco BC, Gaudio PA, Margolis TP. Epidemiology and molecular analysis of herpes simplex keratitis requiring primary penetrating keratoplasty. Br J Ophthalmol. 2004;88:1285–1288. doi: 10.1136/bjo.2003.040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton EA, Hong CS, Glorioso JC. The stable 2.0-kilobase intron of the herpes simplex virus type 1 latency-associated transcript does not function as an antisense repressor of ICP0 in nonneuronal cells. J Virology. 2003;77:3516–3530. doi: 10.1128/JVI.77.6.3516-3530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celum C, Levine R, Weaver M, et al. Genital herpes and human immunodeficiency virus: double trouble. Bull World Health Organ. 2003;82:447–453. [PMC free article] [PubMed] [Google Scholar]
- 12.Centifanto-Fitzgerald YM, Yamaguchi T, Kaufman HE, et al. Ocular disease pattern induced by herpes simplex virus is genetically determined by a specific region of viral DNA. J Exp Med. 1982;155:475–489. doi: 10.1084/jem.155.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christophers J, Clayton J, Craske J, et al. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob Agents Chemother. 1998:42868–42872. doi: 10.1128/aac.42.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376–1385. doi: 10.1056/NEJMra0807633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danve-Sztanek C, Aymard M, Thouvenot D, et al. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J Clin Microbiol. 2004;42:242–249. doi: 10.1128/JCM.42.1.242-249.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson CR, Togni B. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv Ophthalmol. 1976;21:121–135. doi: 10.1016/0039-6257(76)90090-4. [DOI] [PubMed] [Google Scholar]
- 17.Dixit R, Tiwari V, Shukla D. Herpes simplex virus type 1 induces filopodia in differentiated P19 neural cells to facilitate viral spread. Neurosci Lett. 2008;440:113–118. doi: 10.1016/j.neulet.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorrepaal SJ, Cao KY, Slomovic AR. Indications for penetrating keratoplasty in a tertiary referral centre in Canada, 1996–2004. Can J Ophthalmol. 2007;42:244–250. [PubMed] [Google Scholar]
- 19.Duan R, de Vries RD, Osterhaus AD, et al. Acyclovir-resistant HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198:659–663. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- 20.Garcia DD, Farjo Q, Musch DC, et al. Effect of prophylactic oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Cornea. 2007;26:930–934. doi: 10.1097/ICO.0b013e3180e79b77. [DOI] [PubMed] [Google Scholar]
- 21.Ghiasi H, Cai S, Perng GC, et al. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br J Ophthalmol. 2000;84:408–412. doi: 10.1136/bjo.84.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzales CA, Srinivasan M, Whitcher JP, et al. Incidence of corneal ulceration in Madurai District, South India. Ophthalmic Epidemiol. 1996;3:159–166. doi: 10.3109/09286589609080122. [DOI] [PubMed] [Google Scholar]
- 23.Gordon YJ, Romanowski E, Araullo-Cruz T, McKnight JL. HSV-1 corneal latency. Invest Ophthalmol Vis Sci. 1991;32:663–665. [PubMed] [Google Scholar]
- 24.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370:2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 25.Halford WP, Balliet JW, Gebhardt BM. Re-evaluating natural resistance to herpes simplex virus type 1. J Virology. 2004;78:10086–10095. doi: 10.1128/JVI.78.18.10086-10095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill JM, Ball MJ, Neumann DM, et al. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virology. 2008;82:8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodge WG, Margolis TP. Herpes simplex virus keratitis among patients who are positive or negative for human immunodeficiency virus: an epidemiologic study. Ophthalmology. 1997;104:120–124. doi: 10.1016/s0161-6420(97)30351-0. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim YW, Boase DL, Cree IA. Epidemiologic characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth Corneal Ulcer Study. Br J Ophthalmol. 2009;93:1319–1324. doi: 10.1136/bjo.2008.151167. [DOI] [PubMed] [Google Scholar]
- 29.Inoda S, Wakakura M, Hirata J, et al. Stromal keratitis and anterior uveitis due to herpes simplex virus type-2 in a young child. Jpn J Ophthalmol. 2001;45:618–621. doi: 10.1016/s0021-5155(01)00409-9. [DOI] [PubMed] [Google Scholar]
- 30.Jones CA, Cunningham AL. Vaccination strategies to prevent genital herpes and neonatal herpes simplex virus (HSV) disease. Herpes. 2004;11:12–17. [PubMed] [Google Scholar]
- 31.Kabra A, Lalitha P, Mahadevan K, et al. Herpes simplex keratitis and visual impairment: a case series. Indian J Ophthalmol. 2006;54:23–27. doi: 10.4103/0301-4738.21610. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman HE, Azcuy AM, Varnell ED, et al. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46:241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25:355–380. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Kaye SB, Baker K, Bonshek R, et al. Human herpesviruses in the cornea. Br J Ophthalmol. 2000;84:563–571. doi: 10.1136/bjo.84.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keadle TL, Laycock KA, Miller JK, et al. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J Infect Dis. 1997;176:331–338. doi: 10.1086/514049. [DOI] [PubMed] [Google Scholar]
- 36.Kudo E, Shiota H, Naito T, et al. Polymorphisms of thymidine kinase gene in herpes simplex virus type 1: analysis of clinical isolates from herpetic keratitis patients and laboratory strains. J Med Virol. 1998;56:151–158. doi: 10.1002/(sici)1096-9071(199810)56:2<151::aid-jmv9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Labetoulle M, Auquier P, Conrad H, et al. Incidence of herpes simplex virus keratitis in France. Ophthalmology. 2005;112:888–895. doi: 10.1016/j.ophtha.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 38.Lairson DR, Begley CE, Reynolds TF, et al. Prevention of herpes simplex virus eye disease: a cost-effectiveness analysis. Arch Ophthalmol. 2003;121:108–112. doi: 10.1001/archopht.121.1.108. [DOI] [PubMed] [Google Scholar]
- 39.Lamey PJ, Hyland PL. Changing epidemiology of herpes simplex virus type 1 infections. Herpes. 1999;6:20–24. [Google Scholar]
- 40.Langenberg AG, Corey L, Ashley RL, et al. A prospective study of new infections with herpes simplex virus type 1 and 2 Chiron HSV Vaccine Study Group. N Engl J Med. 1999;341:1432–1438. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- 41.LaVail JH, Tauscher AN, Aghaian E, et al. Axonal transport and sorting of herpes simplex virus components in a mature mouse visual system. J Virology. 2003;77:6117–6126. doi: 10.1128/JVI.77.11.6117-6126.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leib DA, Coen DM, Bogard CL, et al. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virology. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewallen S, Chirambo MC. Herpetic corneal ulcers in Malawi. Br J Ophthalmol. 1993;77:827. doi: 10.1136/bjo.77.12.827-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Liesegang TJ, Melton LJ, Daly PJ, et al. Epidemiology of ocular herpes simplex: incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 46.Liesegang TJ. Epidemiology of ocular herpes simplex: natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1160–1165. doi: 10.1001/archopht.1989.01070020226030. [DOI] [PubMed] [Google Scholar]
- 47.Lilley CE, Carson CT, Muotri AR, et al. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc Natl Acad Sci. 2005;102:5844–5849. doi: 10.1073/pnas.0501916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus 2 infection. Bull World Health Organ. 2008;86:805–812. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malkin J. Epidemiology of genital herpes simplex virus infection in developed countries. Herpes. 2004;11(Suppl 1):2a–23a. [PubMed] [Google Scholar]
- 50.Margolis TP, LaVail JH, Setzer PY, et al. Selective spread of herpes simplex virus in the central nervous system after ocular inoculation. J Virol. 1989;63:4756–4761. doi: 10.1128/jvi.63.11.4756-4761.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mariotti SP, Pascolini D, Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol. 2009;93:563–538. doi: 10.1136/bjo.2008.148494. [DOI] [PubMed] [Google Scholar]
- 52.McGraw HM, Awasthi S, Wojcechowskyj JA, et al. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not US9. J Virol. 2009;83:8315–8326. doi: 10.1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mertz GJ, Benedetti J, Ashley R, et al. Risk factors for the sexual transmission of genital herpes. Ann Intern Med. 1992;116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 54.Mets MB, Chhabra MS. Eye manifestations of intrauterine infections and their impact on childhood blindness. Surv Ophthalmol. 2008;53:95–111. doi: 10.1016/j.survophthal.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Miserocchi E, Waheed NK, Dios E, et al. Visual outcome in herpes simplex virus and varicella zoster virus uveitis: a clinical evaluation and comparison. Ophthalmology. 2002;109:1532–1537. doi: 10.1016/s0161-6420(02)01113-2. [DOI] [PubMed] [Google Scholar]
- 56.Misson GP, Landini G, Murray PI. Size dependent variation in the fractal dimensions of herpes simplex epithelial keratitis. Curr Eye Res. 1993;12:957–961. doi: 10.3109/02713689309029221. [DOI] [PubMed] [Google Scholar]
- 57.Morfin F, Thouvenot D. Herpes simplex virus resistance to antiviral drugs. J Clin Virol. 2003;26:29–37. doi: 10.1016/s1386-6532(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 58.Morris DJ, Cleator GM, Klapper PE, et al. Detection of herpes simplex virus DNA in donor cornea culture medium by polymerase chain reaction. Br J Ophthalmol. 1996;80:654–657. doi: 10.1136/bjo.80.7.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mott KR, Bresee CJ, Allen SJ, et al. Level of herpes simplex virus type 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J Virology. 2009;83:2246–2254. doi: 10.1128/JVI.02234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muthiah MN, Michaelides M, Child CS, et al. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91:1452–1455. doi: 10.1136/bjo.2007.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nahmias AJ, Visintine AM, Caldwell DR, et al. Eye infections with herpes simplex virus in neonates. Surv Ophthalmol. 1976;21:100–105. doi: 10.1016/0039-6257(76)90086-2. [DOI] [PubMed] [Google Scholar]
- 62.Nesburn AB, Burke RL, Ghiasi H, et al. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Invest Ophthalmol Vis Sci. 1998;39:1163–1170. [PubMed] [Google Scholar]
- 63.Newell CK, Martin S, Sendele D, et al. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989;63:769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norn MS. Dendritic (herpetic) keratitis. I Incidence—seasonal variations—recurrence rate—visual impairment—therapy. Acta Ophthalmol (Copenh) 1970;48:91–107. doi: 10.1111/j.1755-3768.1970.tb06577.x. [DOI] [PubMed] [Google Scholar]
- 65.Pepose JS, Keadle TL, Morrison LA. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am J Ophthalmol. 2006;141:547–557. doi: 10.1016/j.ajo.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Perng G, Jones C, Ciacci-Zanella J, et al. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 67.Pivetti-Pezzi P, Accorinti M, Colabeli-Gisoldi RA, et al. Herpes simplex virus vaccine in recurrent herpetic ocular infection. Cornea. 1999;18:47–51. [PubMed] [Google Scholar]
- 68.Polcicova K, Biswas PS, Banerjee K, et al. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc Nat Acad Sci. 2005;102:11462–11467. doi: 10.1073/pnas.0503230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Remeijer L, Duan R, van Dun JM, et al. Prevalence and clinical consequences of herpes simplex virus type 1 DNA in human cornea tissues. J Infect Dis. 2009;200:11–19. doi: 10.1086/599329. [DOI] [PubMed] [Google Scholar]
- 70.Remeijer L, Maertzdorf J, Doomenbal P, et al. Herpes simplex virus 1 transmission through corneal transplantation. Lancet. 2001;357:442. doi: 10.1016/S0140-6736(00)04011-3. [DOI] [PubMed] [Google Scholar]
- 71.Reyes M, Shaik NS, Graber JM, et al. Acyclovir-resistant genital herpes among persons attending sexually transmitted disease and human immunodeficiency virus clinics. Arch Intern Med. 2003;163:76–80. doi: 10.1001/archinte.163.1.76. [DOI] [PubMed] [Google Scholar]
- 72.Robert P, Adenis J, Denis F, et al. Transmission of viruses through corneal transplantation. Clin Lab. 2005;51:419–423. [PubMed] [Google Scholar]
- 73.Robert P, Adenis J, Denis F, et al. Herpes simplex virus DNA in corneal transplants: prospective study of 38 recipients. J Med Virol. 2003;71:69–74. doi: 10.1002/jmv.10454. [DOI] [PubMed] [Google Scholar]
- 74.Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30:797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- 75.Scoular A. Using the evidence base on genital herpes: optimising the use of diagnostic tests and information provision. Sex Transm Infect. 2002;78:160–165. doi: 10.1136/sti.78.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimomura Y, Deai T, Fukuda M, et al. Corneal buttons obtained from patients with HSK harbor high copy numbers of the HSV genome. Cornea. 2007;26:190–193. doi: 10.1097/ICO.0b013e31802eaee6. [DOI] [PubMed] [Google Scholar]
- 77.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiologic diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stanberry LR. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes. 2004;11(Suppl 3):161a–169a. [PubMed] [Google Scholar]
- 79.Stránská R, Schuurman R, Nienhuis E, et al. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: prevalence and characterization. J Clin Virol. 2005;32:7–18. doi: 10.1016/j.jcv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Sugar J. Infectious disease risk factors of corneal donors: is there new cause for concern? Arch Ophthalmol. 2008;126:262. doi: 10.1001/archophthalmol.2007.52. [DOI] [PubMed] [Google Scholar]
- 81.The Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 82.Tiwari V, Shukla SY, Yue BY, et al. Herpes simplex virus type 2 entry into cultured human corneal fibroblasts is mediated by herpesvirus entry mediator. J Gen Virol. 2007;88:2106–2110. doi: 10.1099/vir.0.82830-0. [DOI] [PubMed] [Google Scholar]
- 83.Tuppin P, Poinard C, Loty B, et al. Risk factors for corneal regraft in patients on the French waiting list. Cornea. 2004;23:704–711. doi: 10.1097/01.ico.0000126438.10504.7c. [DOI] [PubMed] [Google Scholar]
- 84.Uchio E, Hatano H, Mitsui K, et al. A retrospective study of herpes simplex keratitis over the last 30 years. Jpn J Ophthalmol. 1994;38:196–201. [PubMed] [Google Scholar]
- 85.Valyi-Nagy T, Sheth V, Clement C, et al. Herpes simplex virus entry receptor nectin-1 is widely expressed in the murine eye. Curr Eye Res. 2004;29:303–309. doi: 10.1080/02713680490516756. [DOI] [PubMed] [Google Scholar]
- 86.van Gelderen BE, van der Lelij A, Treffer WF, van der Gaag R. Detection of herpes simplex virus type 1, 2 and varicella zoster virus DNA in recipient corneal buttons. Br J Ophthalmol. 2000;84:1238–1243. doi: 10.1136/bjo.84.11.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Lint AL, Torres-Lopez E, Knipe DM. Immunization with a replication-defective herpes simplex virus 2 mutant reduces herpes simplex virus 1 infection and prevents ocular disease. Virology. 2007;368:227–231. doi: 10.1016/j.virol.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wald A, Huang M, Carrell D, et al. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345–1351. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 89.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11:24A–35A. [PubMed] [Google Scholar]
- 90.Wheeler CE. The herpes simplex problem. J Am Acad Dermatol. 1988;18:163–168. doi: 10.1016/s0190-9622(88)70019-5. [DOI] [PubMed] [Google Scholar]
- 91.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 92.Wilhelmus KR. Epidemiology of ocular infections. In: Tasman W, Jaeger EA, editors. Duane’s Foundations of Clinical Ophthalmology. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. pp. 1–46. [Google Scholar]
- 93.Wilhelmus KR, Coster DJ, Donovan HC, et al. Prognostic indicators of herpetic keratitis: analysis of a five-year observation period after corneal ulceration. Arch Ophthalmol. 1981;99:1578–1582. doi: 10.1001/archopht.1981.03930020452009. [DOI] [PubMed] [Google Scholar]
- 94.Xie L, Zhai H, Dong X, et al. Primary diseases of corneal perforation in Shandong Province, China: a 10-year retrospective study. Am J Ophthalmol. 2008;145:662–666. doi: 10.1016/j.ajo.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 95.Xie L, Song Z, Zhao J, et al. Indications for penetrating keratoplasty in north China. Cornea. 2007;26:1070–1073. doi: 10.1097/ICO.0b013e318093de07. [DOI] [PubMed] [Google Scholar]
- 96.Xu F, Markowitz LE, Gottlieb SL, et al. Seroprevalence of herpes simplex virus types 1 and 2 in pregnant women in the United States. Am J Obstet Gynecol. 2007;196(43):e1–e6. doi: 10.1016/j.ajog.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 97.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 98.Yalniz-Akkaya Z, Nurozler AB, Yildiz EH, et al. Repeat penetrating keratoplasty: indications and prognosis, 1995–2005. Eur J Ophthalmol. 2009;19:362–368. doi: 10.1177/112067210901900306. [DOI] [PubMed] [Google Scholar]
- 99.Yorston D, Foster A. Corneal ulceration in Tanzanian children: relationship between malaria and herpes simplex keratitis. T Roy Soc Trop Med H. 1992;86:456–457. doi: 10.1016/0035-9203(92)90270-m. [DOI] [PubMed] [Google Scholar]
- 100.Yorston D, Foster A. Herpetic keratitis in Tanzania: association with malaria. Br J Ophthalmol. 1992;76:582–585. doi: 10.1136/bjo.76.10.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Young TL, Robin JB, Holland GN, et al. Herpes simplex keratitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1989;96:1476–1479. doi: 10.1016/s0161-6420(89)32706-0. [DOI] [PubMed] [Google Scholar]
- 102.Zheng X. Reactivation and donor-host transmission of herpes simplex virus after corneal transplantation. Cornea. 2002;21(suppl 7):S90–S93. doi: 10.1097/01.ico.0000263126.76392.cf. [DOI] [PubMed] [Google Scholar]
- 103.Zwaagstra JC, Ghiasi H, Nesburn AB, et al. Identification of a major regulatory sequence in the latency associated transcript (LAT) promoter of herpes simplex virus type 1 (HSV-1) Virology. 1991;182:287–297. doi: 10.1016/0042-6822(91)90672-x. [DOI] [PubMed] [Google Scholar]
Other Cited Material
- A.Baratz KH, Young RC, Hodge DO, et al. Incidence of herpes simplex eye disease in Olmsted County, Minnesota, 1976–2007. Invest Ophthalmol Vis Sci. 2009;50 doi: 10.1001/archophthalmol.2010.187. e-abstract5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B.World Health Organization. Onchocerciasis and its control. Report of a WHO expert committee on onchocerciasis. Geneva: Technical Report Series; 1995. No. 852. WHO. [PubMed] [Google Scholar]
- C.International Herpes Management Forum. The management of HSV-1 and ocular HSV diseases. Recommendations from the IHMF Management Strategies Workshop. IHMF. 2002. [Google Scholar]
- D.Gnann JW, Davis MG, Harden EA, et al. Task Force on HSV Resistance. Acyclovir-resistant HSV from HIV-infected individuals: population surveillance and in-vitro characterization of isolates (abstract); New Orleans LA. 38th Annual International Disease Society of America meeting.2000. [Google Scholar]