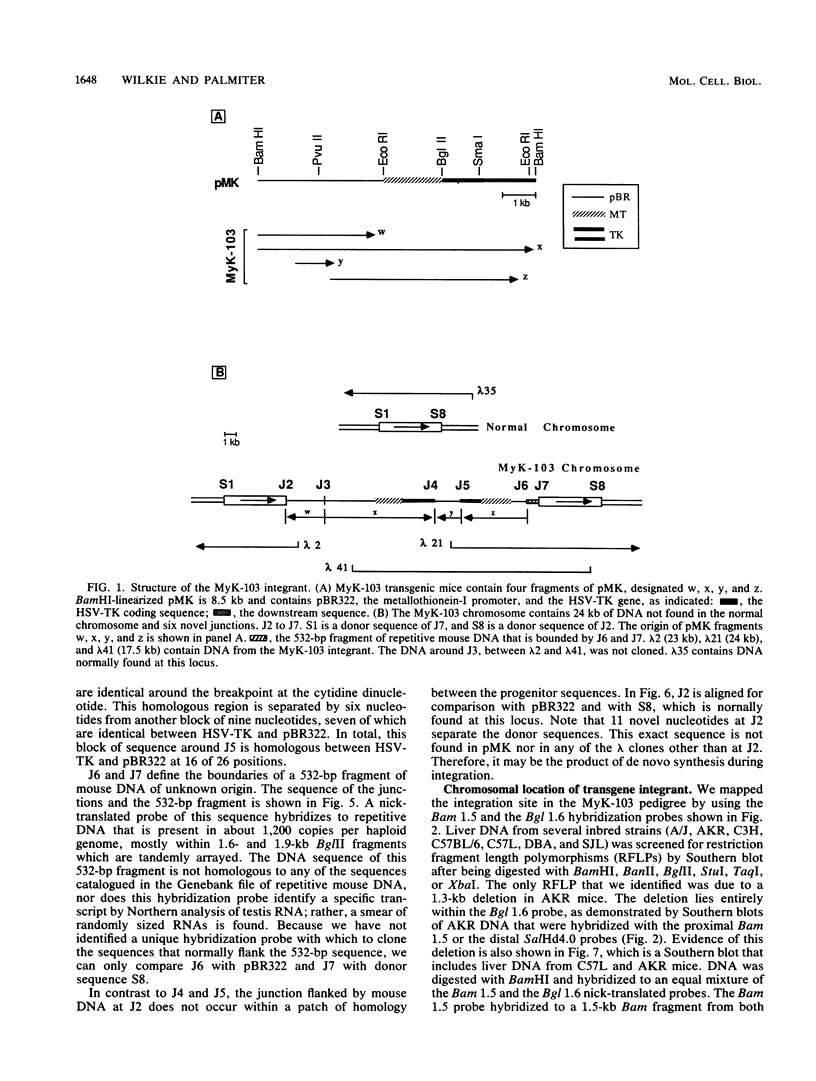
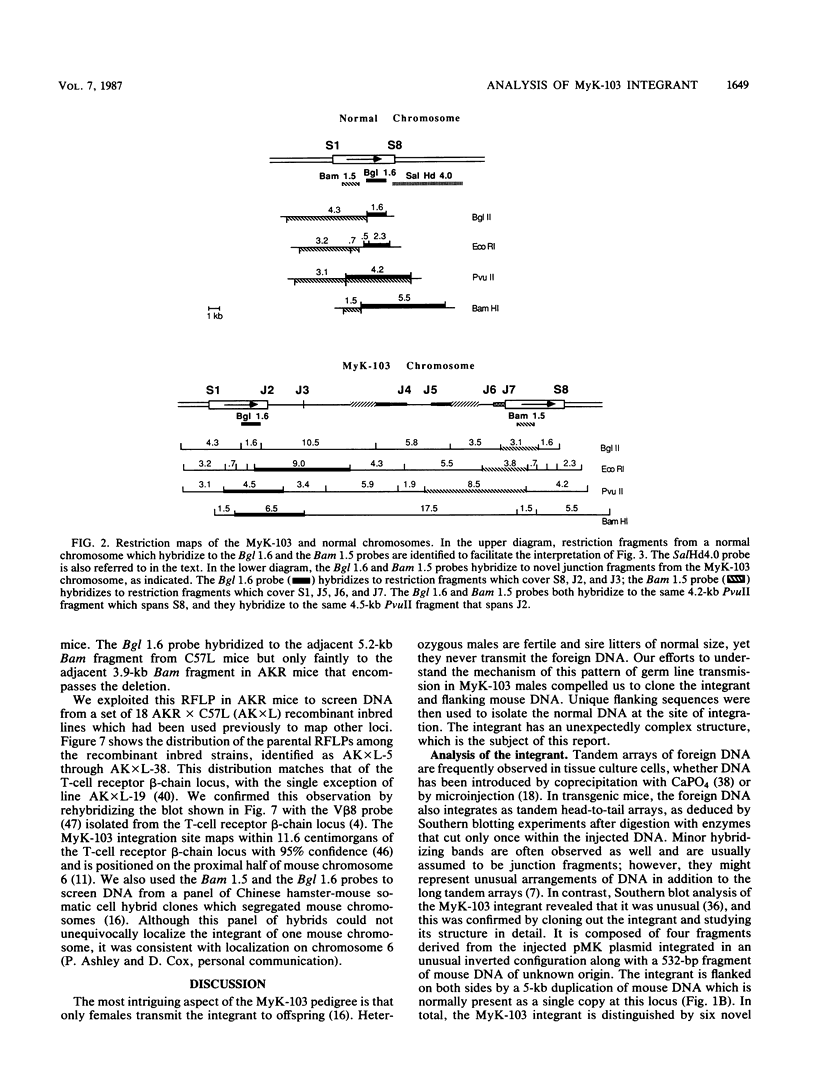
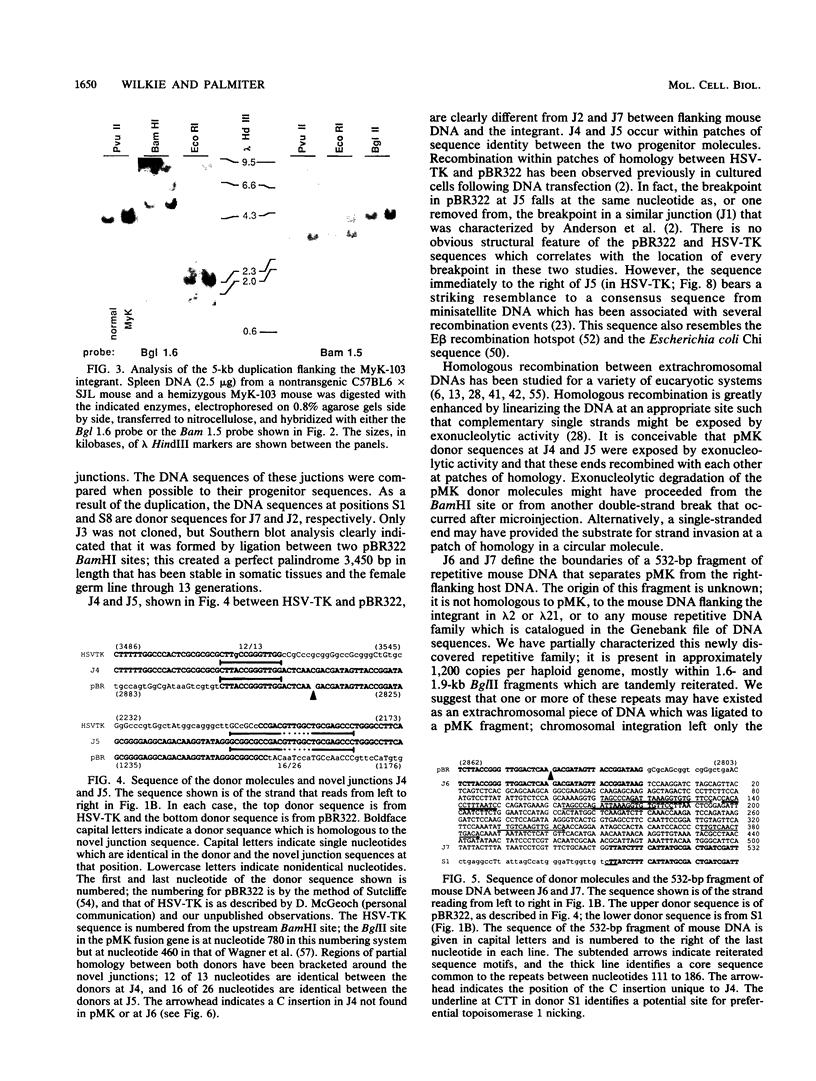
Abstract
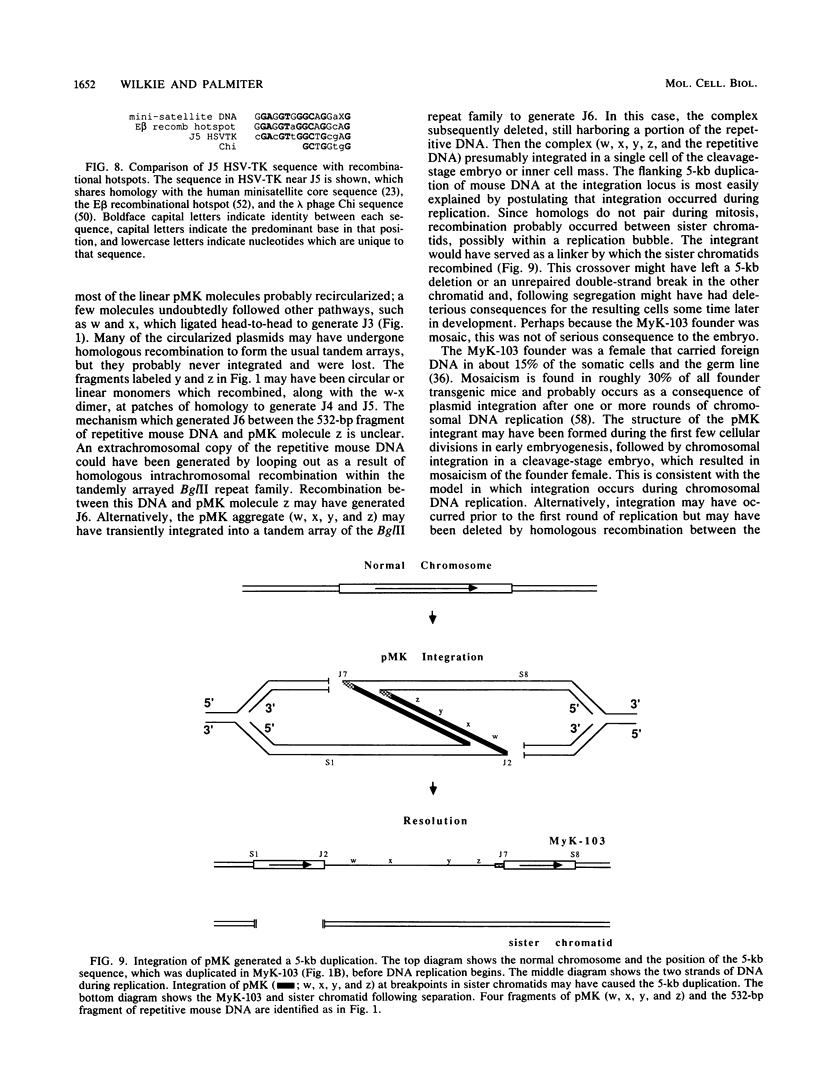
Males in the transgenic mouse pedigree MyK-103, although fertile, do not transmit the integrant to offspring. The integrant is on chromosome 6 near the T-cell receptor beta-chain locus. It contains four fragments of the plasmid pMK (a metallothionein-thymidine kinase fusion gene) and a 532-base-pair fragment of displaced mouse DNA originating from a previously uncharacterized repetitive DNA family. The integration complex is flanked on either side by a 5-kilobase duplication of mouse DNA normally found in a single copy at this locus. Sequence analysis of the six novel junctions and their donor sequences shows that plasmid-plasmid junctions occurred at patches of limited homology, whereas chromosome-plasmid junctions were nonhomologous.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. A., Kato S., Camerini-Otero R. D. A pattern of partially homologous recombination in mouse L cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):206–210. doi: 10.1073/pnas.81.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Barth R. K., Kim B. S., Lan N. C., Hunkapiller T., Sobieck N., Winoto A., Gershenfeld H., Okada C., Hansburg D., Weissman I. L. The murine T-cell receptor uses a limited repertoire of expressed V beta gene segments. Nature. 1985 Aug 8;316(6028):517–523. doi: 10.1038/316517a0. [DOI] [PubMed] [Google Scholar]
- Been M. D., Burgess R. R., Champoux J. J. Nucleotide sequence preference at rat liver and wheat germ type 1 DNA topoisomerase breakage sites in duplex SV40 DNA. Nucleic Acids Res. 1984 Apr 11;12(7):3097–3114. doi: 10.1093/nar/12.7.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. A., Kato S., Anderson R. A., Smigocki A. C., Camerini-Otero R. D. The recombination and integration of DNAs introduced into mouse L cells. Cold Spring Harb Symp Quant Biol. 1984;49:151–160. doi: 10.1101/sqb.1984.049.01.018. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M., Senear A. W., Warren R., Palmiter R. D. Somatic expression of herpes thymidine kinase in mice following injection of a fusion gene into eggs. Cell. 1981 Nov;27(1 Pt 2):223–231. doi: 10.1016/0092-8674(81)90376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock P., Champoux J. J., Botchan M. Association of crossover points with topoisomerase I cleavage sites: a model for nonhomologous recombination. Science. 1985 Nov 22;230(4728):954–958. doi: 10.1126/science.2997924. [DOI] [PubMed] [Google Scholar]
- Bullock P., Miller J., Botchan M. Effects of poly[d(pGpT).d(pApC)] and poly[d(pCpG).d(pCpG)] repeats on homologous recombination in somatic cells. Mol Cell Biol. 1986 Nov;6(11):3948–3953. doi: 10.1128/mcb.6.11.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia N., Kronenberg M., Saxe D., Haars R., Bruns G. A., Goverman J., Malissen M., Willard H., Yoshikai Y., Simon M. The T cell receptor beta chain genes are located on chromosome 6 in mice and chromosome 7 in humans. Cell. 1984 Jul;37(3):1091–1099. doi: 10.1016/0092-8674(84)90443-4. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Carroll D., Wright S. H., Wolff R. K., Grzesiuk E., Maryon E. B. Efficient homologous recombination of linear DNA substrates after injection into Xenopus laevis oocytes. Mol Cell Biol. 1986 Jun;6(6):2053–2061. doi: 10.1128/mcb.6.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S. Activation of cellular oncogenes in hemopoietic cells by chromosome translocation. Adv Cancer Res. 1986;47:189–234. doi: 10.1016/s0065-230x(08)60200-6. [DOI] [PubMed] [Google Scholar]
- Courage-Tebbe U., Döring H. P., Fedoroff N., Starlinger P. The controlling element Ds at the Shrunken locus in Zea mays: structure of the unstable sh-m5933 allele and several revertants. Cell. 1983 Sep;34(2):383–393. doi: 10.1016/0092-8674(83)90372-0. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Nishida Y., Mintz B. Early developmental mutations due to DNA rearrangements in transgenic mouse embryos. Cold Spring Harb Symp Quant Biol. 1985;50:447–452. doi: 10.1101/sqb.1985.050.01.056. [DOI] [PubMed] [Google Scholar]
- Cox D. R., Sawicki J. A., Yee D., Appella E., Epstein C. J. Assignment of the gene for beta 2-microglobulin (B2m) to mouse chromosome 2. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1930–1934. doi: 10.1073/pnas.79.6.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristensky B. Improving the efficiency of dot-matrix similarity searches through use of an oligomer table. Nucleic Acids Res. 1986 Jan 10;14(1):597–610. doi: 10.1093/nar/14.1.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. DNA-mediated genetic transformation of mouse embryos and bone marrow--a review. Gene. 1985;33(2):121–136. doi: 10.1016/0378-1119(85)90087-3. [DOI] [PubMed] [Google Scholar]
- Hutchison K. W., Copeland N. G., Jenkins N. A. Dilute-coat-color locus of mice: nucleotide sequence analysis of the d+2J and d+Ha revertant alleles. Mol Cell Biol. 1984 Dec;4(12):2899–2904. doi: 10.1128/mcb.4.12.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kato S., Anderson R. A., Camerini-Otero R. D. Foreign DNA introduced by calcium phosphate is integrated into repetitive DNA elements of the mouse L cell genome. Mol Cell Biol. 1986 May;6(5):1787–1795. doi: 10.1128/mcb.6.5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W., Patel M. D., Lobel L. I., Goff S. P., Nguyen-Huu M. C. Insertion mutagenesis of embryonal carcinoma cells by retroviruses. Science. 1985 May 3;228(4699):554–558. doi: 10.1126/science.3838595. [DOI] [PubMed] [Google Scholar]
- Leach D. R., Stahl F. W. Viability of lambda phages carrying a perfect palindrome in the absence of recombination nucleases. 1983 Sep 29-Oct 5Nature. 305(5933):448–451. doi: 10.1038/305448a0. [DOI] [PubMed] [Google Scholar]
- Lee N. E., D'Eustachio P., Pravtcheva D., Ruddle F. H., Hedrick S. M., Davis M. M. Murine T cell receptor beta chain is encoded on chromosome 6. J Exp Med. 1984 Sep 1;160(3):905–913. doi: 10.1084/jem.160.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Model for homologous recombination during transfer of DNA into mouse L cells: role for DNA ends in the recombination process. Mol Cell Biol. 1984 Jun;4(6):1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark W. H., Signorelli K., Lacy E. An insertional mutation in a transgenic mouse line results in developmental arrest at day 5 of gestation. Cold Spring Harb Symp Quant Biol. 1985;50:453–463. doi: 10.1101/sqb.1985.050.01.057. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Conrad B. Improved programs for DNA and protein sequence analysis on the IBM personal computer and other standard computer systems. Nucleic Acids Res. 1986 Jan 10;14(1):443–454. doi: 10.1093/nar/14.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Lai S. P., Van Quill K. R., Westphal H. Tissue-specific expression in transgenic mice of a fused gene containing RSV terminal sequences. Science. 1986 Mar 28;231(4745):1574–1577. doi: 10.1126/science.3006249. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Brinster R. L. Differential regulation of metallothionein-thymidine kinase fusion genes in transgenic mice and their offspring. Cell. 1982 Jun;29(2):701–710. doi: 10.1016/0092-8674(82)90186-6. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Wilkie T. M., Chen H. Y., Brinster R. L. Transmission distortion and mosaicism in an unusual transgenic mouse pedigree. Cell. 1984 Apr;36(4):869–877. doi: 10.1016/0092-8674(84)90036-9. [DOI] [PubMed] [Google Scholar]
- Quertermous T., Strauss W., Murre C., Dialynas D. P., Strominger J. L., Seidman J. G. Human T-cell gamma genes contain N segments and have marked junctional variability. Nature. 1986 Jul 10;322(6075):184–187. doi: 10.1038/322184a0. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Farabaugh P. J., Chaleff D. T., Fink G. R. The origins of gene instability in yeast. Science. 1980 Sep 19;209(4463):1375–1380. doi: 10.1126/science.6251544. [DOI] [PubMed] [Google Scholar]
- Roehm N. W., Carbone A., Kushnir E., Taylor B. A., Riblet R. J., Marrack P., Kappler J. W. The major histocompatibility complex-restricted antigen receptor on T cells: the genetics of expression of an allotype. J Immunol. 1985 Sep;135(3):2176–2182. [PubMed] [Google Scholar]
- Roth D. B., Porter T. N., Wilson J. H. Mechanisms of nonhomologous recombination in mammalian cells. Mol Cell Biol. 1985 Oct;5(10):2599–2607. doi: 10.1128/mcb.5.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz J., Subramani S. The minimum amount of homology required for homologous recombination in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2253–2258. doi: 10.1128/mcb.4.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnieke A., Harbers K., Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. 1983 Jul 28-Aug 3Nature. 304(5924):315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- Shani M. Tissue-specific and developmentally regulated expression of a chimeric actin-globin gene in transgenic mice. Mol Cell Biol. 1986 Jul;6(7):2624–2631. doi: 10.1128/mcb.6.7.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Buckler C. E. Statistical considerations for linkage analysis using recombinant inbred strains and backcrosses. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1423–1427. doi: 10.1073/pnas.83.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim G. K., Augustin A. A. V beta gene polymorphism and a major polyclonal T cell receptor idiotype. Cell. 1985 Aug;42(1):89–92. doi: 10.1016/s0092-8674(85)80104-5. [DOI] [PubMed] [Google Scholar]
- Skowronski J., Plucienniczak A., Bednarek A., Jaworski J. Bovine 1.709 satellite. Recombination hotspots and dispersed repeated sequences. J Mol Biol. 1984 Aug 15;177(3):399–416. doi: 10.1016/0022-2836(84)90292-4. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Kunes S. M., Schultz D. W., Taylor A., Triman K. L. Structure of chi hotspots of generalized recombination. Cell. 1981 May;24(2):429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Drosophila genome organization: conserved and dynamic aspects. Annu Rev Genet. 1981;15:219–264. doi: 10.1146/annurev.ge.15.120181.001251. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Stephan D., Fischer Lindahl K. Gene organization and recombinational hotspots in the murine major histocompatibility complex. Cell. 1986 Mar 28;44(6):895–904. doi: 10.1016/0092-8674(86)90012-7. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. Recombination between poly[d(GT).d(CA)] sequences in simian virus 40-infected cultured cells. Mol Cell Biol. 1985 Jun;5(6):1247–1259. doi: 10.1128/mcb.5.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Folger K. R., Capecchi M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986 Feb 14;44(3):419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Covarrubias L., Stewart T. A., Mintz B. Prenatal lethalities in mice homozygous for human growth hormone gene sequences integrated in the germ line. Cell. 1983 Dec;35(3 Pt 2):647–655. doi: 10.1016/0092-8674(83)90097-1. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie T. M., Brinster R. L., Palmiter R. D. Germline and somatic mosaicism in transgenic mice. Dev Biol. 1986 Nov;118(1):9–18. doi: 10.1016/0012-1606(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Fried M. Inverted duplication-transposition event in mammalian cells at an illegitimate recombination join. Mol Cell Biol. 1986 Jun;6(6):2179–2184. doi: 10.1128/mcb.6.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woychik R. P., Stewart T. A., Davis L. G., D'Eustachio P., Leder P. An inherited limb deformity created by insertional mutagenesis in a transgenic mouse. Nature. 1985 Nov 7;318(6041):36–40. doi: 10.1038/318036a0. [DOI] [PubMed] [Google Scholar]