Abstract
Objective To determine the effectiveness of an intervention to enhance self management support for patients with chronic conditions in UK primary care.
Design Pragmatic, two arm, cluster randomised controlled trial.
Setting General practices, serving a population in northwest England with high levels of deprivation.
Participants 5599 patients with a diagnosis of diabetes (n=2546), chronic obstructive pulmonary disease (n=1634), and irritable bowel syndrome (n=1419) from 43 practices (19 intervention and 22 control practices).
Intervention Practice level training in a whole systems approach to self management support. Practices were trained to use a range of resources: a tool to assess the support needs of patients, guidebooks on self management, and a web based directory of local self management resources. Training facilitators were employed by the health management organisation.
Main outcome measures Primary outcomes were shared decision making, self efficacy, and generic health related quality of life measured at 12 months. Secondary outcomes were general health, social or role limitations, energy and vitality, psychological wellbeing, self care activity, and enablement.
Results We randomised 44 practices and recruited 5599 patients, representing 43% of the eligible population on the practice lists. 4533 patients (81.0%) completed the six month follow-up and 4076 (72.8%) the 12 month follow-up. No statistically significant differences were found between patients attending trained practices and those attending control practices on any of the primary or secondary outcomes. All effect size estimates were well below the prespecified threshold of clinically important difference.
Conclusions An intervention to enhance self management support in routine primary care did not add noticeable value to existing care for long term conditions. The active components required for effective self management support need to be better understood, both within primary care and in patients’ everyday lives.
Trial registration Current Controlled Trials ISRCTN90940049.
Introduction
The increasing prevalence of long term conditions and rising costs of care mean that health organisations worldwide need effective solutions to make future service delivery effective and sustainable. Primary care is increasingly seen as the optimal context to deliver care for people with long term conditions because it is accessible, is efficient, and can tackle inequalities related to socioeconomic deprivation.1 2 The chronic care model has been proposed as a guide to organise primary care for patients with long term conditions.3 In UK primary care, features of the model (such as clinical information systems and decision support) have been implemented through the Quality and Outcomes Framework, a pay for performance contract that has improved quality of care.4 Interventions for self management support are also critical to improving quality of care in the chronic care model.5 6 However, commensurate improvements in self management support have not been prioritised.
Self management support has been defined as “increasing the capacity, confidence, and efficacy of the individual” for self management and is widely seen as critical to ensure sustainability of services in terms of costs. There are two core models of self management support: a provider based model where support is embedded into the clinical practice of doctors and a patient based model that seeks to enable patients through individual or group based education7 8 and use of telehealth and telecare.9 10 Although potentially effective, patient based interventions can be considerably limited in their “reach”—that is, the numbers of patients able or willing to access and engage with the intervention.11 12 Interventions providing support aimed directly at patients (such as the UK expert patients programme) can struggle to recruit, and there is evidence that participants in expert patients programmes are more affluent and educated than the wider population of patients with long term conditions.13 14 15 Arguably, embedding self management support programmes into everyday clinical practice can more effectively deal with problems of reach and sustainability.16 17 18 In the United Kingdom, most patients access primary care regularly, practitioners have unrivalled knowledge of the needs of individual patients, and continuity of care means that self management support can be maintained over time and delivered according to need. Nevertheless, achieving the potential of primary care as a platform for self management support means overcoming known barriers, including competing clinical priorities, limited time, and lack of skills and confidence among professionals.19 20 21 22
We assessed a “whole systems” quality improvement intervention, which attempts to implement provider led self management support for long term conditions in primary care and provide an effective and sustainable model for the future. The theory of implementation drawn on for this trial was the normalisation process theory.23 24
Methods
We carried out a pragmatic, two arm, practice level cluster randomised controlled trial to test whether the adoption of a whole systems model of self management support compared with routine primary care leads to improved health outcomes and cost effective management of patients with long term conditions. Full details of the protocol have been published elsewhere.25
Population and setting
At the time of the study, primary care trusts were responsible for the delivery of primary care services to the population in a geographical area. This study took place between 2009 and 2012 within a primary care trust in the north west of England serving a predominantly white, socioeconomically deprived population, where primary care trust managers had a strong institutional commitment to improving self management support. In terms of health deprivation, 65% of the primary care trust population lived in areas classed in the most deprived fifth nationally.26 Patients with diabetes, chronic obstructive pulmonary disease, or irritable bowel syndrome were eligible for inclusion. We identified eligible patients from electronic health records and clinical staff checked the patients for exclusion criteria (under 18, insufficient English language, receiving palliative care, or insufficient capacity to give written consent) before being sent a postal invitation. The intervention was designed to be applicable to all long term conditions. We chose exemplar conditions theoretically amenable to self management interventions and where there was published evidence of effectiveness.27 28 Each condition was of high prevalence but to enhance generalisability the conditions had important differences in symptomatology, management, and priority—that is, care for diabetes and chronic obstructive pulmonary disease is financially incentivised in the United Kingdom but not irritable bowel syndrome.
Intervention
The intervention (whole system informing self management engagement, WISE) is based on accumulated evidence from multiple randomised controlled trials and an ongoing programme of work grounded in primary care.7 28 29 30 31 The core aim of the current trial was to take several components found to be effective in these previous studies and to deliver them as a comprehensive package under naturalistic conditions and using routine care providers to maximise real world applicability. The supplementary file outlines the elements of the WISE approach.
The intervention was designed to be feasible to implement widely in primary care, which put practical limitations on the intensity of the intervention. Training (developed and piloted with two non-trial practices) was delivered in each practice over two sessions, which we estimated through informed feedback was the maximum feasible in UK primary care using current educational structures. Session 1 involved all practice staff (doctors, nurses, technicians, and administration staff) and session 2 focused on clinical staff. Fidelity checks and reinforcement sessions with trainers were scheduled after training. Details of the training content are outlined in the supplementary file and described elsewhere.32 Two facilitators employed by the primary care trust delivered the training and also provided access to self management support activities and resources in the primary care trust. The practices were provided with resources to support self management, including a tool to assess patient support needs and priorities (PRISMS).33 In session 1, practices worked on ways to embed self management tools in their systems; in session 2, clinicians practised ways to use core self management skills in consultations and ensure patients received, or were directed to, appropriate resources. Assessment of patient need was linked to appropriate support, including self help guidebooks based on published development methods,34 access to relevant community groups and programs via a web based directory of local self management resources, and for patients with severe and enduring irritable bowel syndrome, enhanced access to psychological therapists. Existing information and primary care trust support resources were available to those in the comparator group, only the specific WISE guidebooks and irritable bowel syndrome psychological therapies were accessed through the trial.
Comparator
We used a wait list comparator group. Using a minimisation procedure based on practice size, area deprivation (the area index of multiple deprivation), and contractual status (contracted either to the National Health Service or to the local primary care trust) we allocated practices 1:1 to intervention or control groups.
Sample size
This study was planned to take place within a single primary care trust, with 43 eligible practices. Sample size calculations based on previous studies35 indicated that to detect a standardised effect size of 0.2 (intraclass correlation coefficient 0.05, α 5%, 80% power) on the primary outcomes within each of the three conditions, we would need to recruit 40 practices (20 per trial arm) and 48 patients per condition per practice. This effect size represents a group difference in mean outcome score of 4.8 points (out of 100) on shared decision making, 4.6 (out of 100) on self efficacy, and 0.07 (out of 1) on health related quality of life. Since the required sample represented most of the eligible patients, we opted to undertake a “whole population” study and invited all practices and eligible patients. Practices are required to create registers for patients with chronic obstructive pulmonary disease and diabetes but not for patients with irritable bowel syndrome, therefore the sample with irritable bowel syndrome is likely to under-represent the actual population with the condition.
Outcomes
Practice staff completed questionnaires on their views of the WISE training immediately after training, and on its application in their everyday work at six months after training.
We collected patient level outcomes by postal questionnaire at baseline and at six and 12 months. The trial had three primary outcomes, all at 12 months: shared decision making (using the short form healthcare climate questionnaire),36 37 self efficacy (confidence to undertake the management of chronic disease),38 and generic health related quality of life (EQ-5D).39 Secondary outcomes were general health, social or role limitations, energy and vitality, psychological wellbeing, self care activity, and enablement (see supplementary file for full details). We treated the six month scores on the three main outcome measures and on self care as additional secondary outcomes. Since outcome measures varied widely in scale and direction, to aid interpretability we rescaled all outcomes on a 0 to 100 scale, with a positive score indicating a better outcome; an exception was the EQ-5D which, as a standard economic measure, we kept on its original scale (maximum value 1.0).
Self reported resource use was collected through the questionnaires. Healthcare utilisation was based on patient self reports at each follow-up (using the same postal questionnaire), including visits to primary health practitioners and community based health and social care services and use of specialist healthcare services.40
Statistical analysis
Analysis followed a prespecified plan (see supplementary file). We subjected each outcome to analysis of covariance within a multilevel (patients within practices) regression framework, following intention to treat principles and with the analyst (DR) blind to practice allocation. Although we powered the study to detect effects for separate conditions, we maximised power and minimised multiple testing in the analysis by testing for a treatment effect across all three condition groups combined, and for an interaction between trial arm and condition group (controlled for the main effects of condition group). This analysis also controlled for baseline values of each outcome, design factors (practice list size, deprivation, and contractual type), and additional covariates.
In the case of a non-significant (P>0.05) interaction between trial arm and condition group, no further condition specific analyses would be conducted; if the interaction term was significant this would imply that the effect varied by condition, and we would conduct further analyses for each separate condition group.
We applied multiple imputation (five imputed datasets) to baseline variables with missing values (all <5%), using chained equations and all variables in the model. We did not impute missing follow-up data but used multivariate logistic regression to identify baseline covariates predictive of missing data and included these (disease, age, general health, deprivation index, and home ownership) as covariates.41 Additional prespecified covariates included sex, count of comorbid conditions, education, and primary care visits six months before baseline.
Sensitivity analyses assessed the stability of the results to the model specification (see supplementary file). All analyses used Stata v12 and an α value of 5%. For outcome variables with skewness or kurtosis values ≥1.0, we derived confidence intervals and P values using standard errors based on 100 bootstrapped samples.
Results
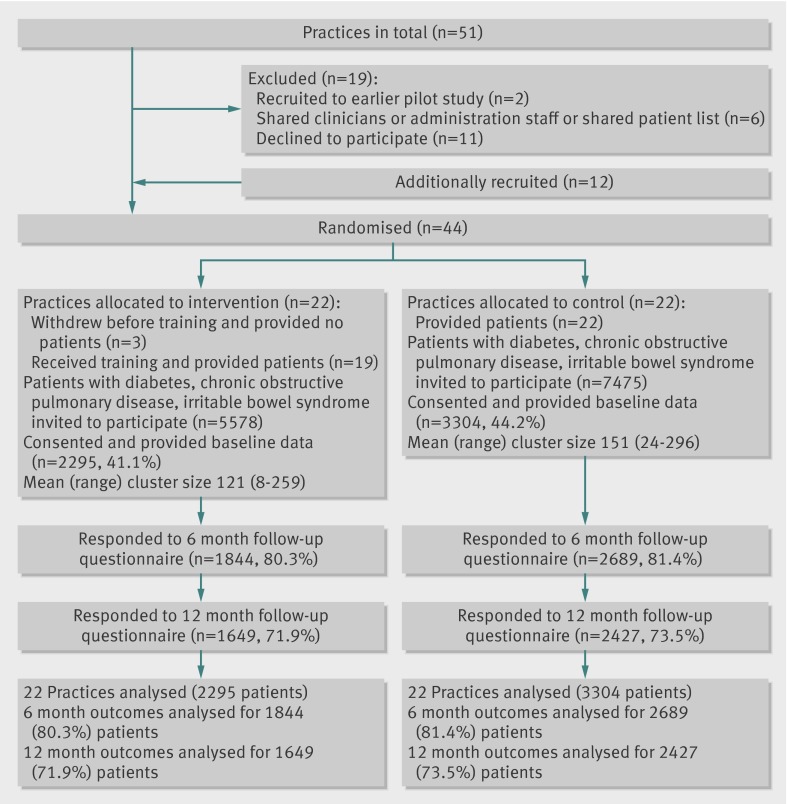
Practice recruitment from the main primary care trust (32 practices) fell short of the 40 required to ensure full power. We therefore included additional practices from an adjoining primary care trust with a similar demographic profile, resulting in a final total of 44 practices randomised (fig 1). Three practices randomised to the intervention group withdrew before data collection, leaving 19 intervention and 22 control practices.
Fig 1 Flow of practices through trial
Baseline characteristics of study participants
A total of 5599 patients (2546 with diabetes, 1634 chronic obstructive pulmonary disease, 1419 with irritable bowel syndrome) were recruited, representing 43% of the eligible population. Just over half the sample were women (n=2990, 53.5%) and around half (n=2824, 50.8%) were aged 65 or more (table 1). Few (3.4%) were non-white. Most (n=4061, 72.5%) had more than one chronic condition and 23% (n=1232) had visited their general practitioner five or more times in the six months before the study.
Table 1.
Baseline characteristics of participants. Values are number (percentage) unless stated otherwise
| Characteristics | Usual care (n=3304) | WISE intervention (n=2295) | Total (n=5599) |
|---|---|---|---|
| Main chronic condition: | |||
| Diabetes | 1486 (45.0) | 1060 (46.2) | 2546 (45.5) |
| Chronic obstructive pulmonary disease | 1009 (30.5) | 625 (27.2) | 1634 (29.2) |
| Irritable bowel syndrome | 809 (24.5) | 610 (26.6) | 1419 (25.3) |
| Sex: | |||
| Female | 1728 (52.4) | 1262 (55.1) | 2990 (53.5) |
| Male | 1573 (47.7) | 1030 (44.9) | 2603 (46.5) |
| Age group (years): | |||
| <50 | 540 (16.4) | 431 (18.9) | 971 (17.5) |
| 50-64 | 1039 (31.6) | 730 (32.0) | 1769 (31.8) |
| 65-74 | 948 (28.9) | 627 (27.5) | 1575 (28.3) |
| ≥75 | 757 (23.1) | 492 (21.6) | 1249 (22.5) |
| No of chronic conditions: | |||
| 0 or 1 | 909 (27.5) | 628 (27.4) | 1537 (27.5) |
| 2 | 999 (30.3) | 709 (30.9) | 1708 (30.5) |
| 3 | 780 (23.6) | 532 (23.2) | 1312 (23.4) |
| ≥4 | 615 (18.6) | 426 (18.6) | 1041 (18.6) |
| Accommodation: | |||
| Owner-occupier | 2164 (66.2) | 1498 (66.2) | 3662 (66.2) |
| Renting | 1106 (33.8) | 765 (33.8) | 1871 (33.8) |
| Education: | |||
| No qualifications | 1044 (31.6) | 699 (30.5) | 1743 (31.1) |
| School level qualifications | 362 (11.0) | 250 (11.0) | 612 (10.9) |
| Professional or vocational | 949 (28.7) | 649 (28.3) | 1598 (28.5) |
| Bachelor’s degree or higher | 198 (6.0) | 157 (6.84) | 355 (6.3) |
| Missing | 751 (22.7) | 540 (23.5) | 1291 (23.1) |
| Mean (SD) index of multiple deprivation | 30.7 (20.0) | 28.9 (18.1) | 30.0 (19.3) |
| Visits to doctor in past 6 months: | |||
| 0 | 407 (12.9) | 265 (12.1) | 672 (12.6) |
| 1 or 2 | 1215 (38.4) | 881 (40.3) | 2096 (39.2) |
| 3 or 4 | 808 (25.5) | 545 (24.9) | 1353 (25.3) |
| 5 or 6 | 448 (14.2) | 264 (12.1) | 712 (13.3) |
| ≥7 | 287 (9.1) | 233 (10.7) | 520 (9.7) |
| Ethnicity: | |||
| White | 3167 (96.4) | 2207 (97.0) | 5374 (96.7) |
| Non-white | 117 (3.6) | 69 (3.0) | 186 (3.4) |
| Mean (SD) shared decision making* | 76.7 (24.0) | 75.7 (24.4) | 76.3 (24.1) |
| Mean (SD) self efficacy score† | 71.1 (23.0) | 70.5 (23.5) | 70.8 (23.2) |
| Mean (SD) health related quality of life‡ | 0.6 (0.3) | 0.6 (0.3) | 0.6 (0.3) |
| Mean (SD) general health§ | 41.4 (23.7) | 41.2 (24.2) | 41.3 (23.9) |
| Practice variables: | |||
| No of practices | 22 | 19 | 41 |
| Mean (SD) practice list size | 4528 (2591) | 4003 (2211) | 4285 (2407) |
| Mean (SD) practice index of multiple deprivation | 37.9 (21.9) | 40.6 (19.6) | 39.1 (20.6¶) |
| Contract type: | |||
| General medical services | 14 (63.6) | 11 (57.9) | 25 (61.0) |
| Personal medical services | 8 (36.4) | 8 (42.1) | 16 (39.0) |
WISE=whole system informing self management engagement.
*Six item short form health care climate questionnaire (see supplementary file).
†Five item scale of confidence to undertake chronic disease management, from Medical Outcomes Survey (see supplementary file).
‡EuroQol EQ-5D (see supplementary file).
§General health rated on a five point scale ranging from excellent to poor, from Medical Outcomes Survey (see supplementary file).
¶Compared with an average for all practices nationally of 26.3 (SD 17.5).61
The two trial arms were well balanced on all variables at the patient level, although practices in the intervention group were on average slightly smaller (mean list size 4003 v 4528 patients).
Engagement with training
Attendance rates for the practice staff at the training sessions were generally high: 90% of eligible staff attended session 1 (n=179) and 82% (n=85) attended session 2. Training was rated positively (mean score >2.5 on a 5 point scale) by 76% of session 1 participants and by 89% of session 2 participants.
Implementation of training
Questionnaire data (a low response rate 48%) obtained from clinicians showed varying levels of implementation in routine practice: information guidebooks were readily used (88% of clinicians reporting use, 51% “regularly”) whereas the PRISMS tool was least used (42% reporting no use).
Patient report data showed that referral to specialist psychological therapy (only available to the patients with irritable bowel syndrome in the intervention practices) was rare (2.1%). Across the 12 months of the study, similar percentages of intervention and control patients reported on each type of support, including receiving a guidebook (25% v 24%) and encouragement to use community programmes (19% v 20%) and patient support groups (11% v 12%).
Analysis of outcomes
Overall, 4533 patients (81.0%) completed the six month follow-up and 4076 (72.8%) the 12 month follow-up.
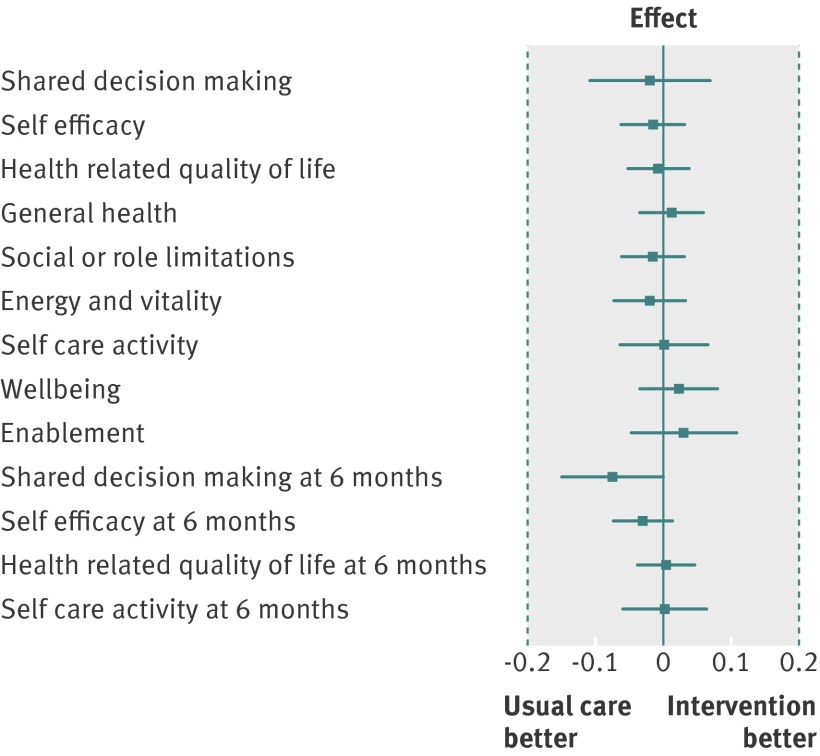
With one exception, patients attending intervention practices and those attending control practices did not differ significantly on any primary or secondary outcome (table 2). The exception was shared decision making at the six month follow-up (P=0.05), with the difference favouring the control group (see supplementary file). All effect size estimates were small with narrow 95% confidence intervals and well below the minimally important difference of 0.2 that the trial was powered to detect (fig 2). The lack of effect applied equally to the intermediate outcomes of shared decision making, self efficacy, enablement, and self care activity—which might reasonably be expected to be most directly affected by increased support for self management—as it did to health related outcomes. Furthermore, none of the interactions between intervention group and condition group were significant; therefore we conducted no condition specific analyses. Sensitivity analyses provided no evidence for the results being substantively influenced by model assumptions (see supplementary file).
Table 2.
Summary of analyses of covariance
| Outcomes* | Mean (SD) unadjusted scores, No of patients | Adjusted mean difference (95% CI)† | Effect size (95% CI)‡ | P value | P value for interaction with condition group§ | |
|---|---|---|---|---|---|---|
| Control group | WISE intervention group | |||||
| Primary outcomes: | ||||||
| Shared decision making | 69.1 (26.3), n=2379 | 67.7 (27.7), n=1626 | −0.47 (−2.55 to 1.61) | −0.02 (−0.11 to 0.07) | 0.66 | 0.70 |
| Self efficacy score | 71.2 (22.5), n=2394 | 70.4 (22.8), n=1611 | −0.35 (−1.42 to 0.71) | −0.02 (−0.06 to 0.03) | 0.52 | 0.21 |
| Health related quality of life | 0.6 (0.3), n=2382 | 0.6 (0.3), n=1609 | −0.00 (−0.02 to 0.01) | −0.01 (−0.05 to 0.04) | 0.72 | 0.31 |
| Secondary outcomes: | ||||||
| General health | 41.7 (24.8), n=2413 | 42.2 (25.8), n=1643 | 0.28 (−1.37 to 0.82) | 0.01 (−0.03 to 0.06) | 0.62 | 0.88 |
| Social or role limitations | 63.3 (31.1), n=2408 | 62.8 (32.3), n=1638 | −0.49 (−1.95 to 0.96) | −0.02 (−0.06 to 0.03) | 0.51¶ | 0.44¶ |
| Energy and vitality | 46.8 (20.9), n=2411 | 46.2 (21.8), n=1638 | −0.42 (−1.53 to 0.69) | −0.02(−0.07 to 0.03) | 0.46 | 0.33 |
| Self care activity | 42.4 (14.6), n=2382 | 42.5 (14.9), n=1613 | 0.01 (−0.95 to 0.97) | 0.00 (−0.06 to 0.07) | 0.98 | 0.96 |
| Psychological wellbeing | 64.7 (21.9), n=2412 | 64.7 (22.2), n=1640 | 0.49 (−0.75 to 1.73) | 0.02 (−0.03 to 0.08) | 0.44 | 0.30 |
| Enablement | 78.6 (28.8), n=2365 | 80.7 (28.3), n=1624 | 0.85 (−1.36 to 3.06) | 0.03 (−0.05 to 0.11) | 0.45¶ | 0.95¶ |
| Shared decision making (6 months) | 70.3 (26.1), n=2658 | 68.3 (27.3), n=1818 | −1.77 (−3.53 to 0.0) | −0.07 (−0.15 to 0.0) | 0.05** | 0.07†† |
| Self efficacy (6 months) | 71.1 (22.5), n=2659 | 70.4 (23.1), n=1816 | −0.70 (−1.69 to 0.29) | −0.03 (−0.07 to 0.01) | 0.17 | 0.32 |
| Health related quality of life (6 months) | 0.6 (0.3), n=2646 | 0.6 (0.3), n=1803 | 0.00 (−0.01 to 0.01) | 0.00 (−0.04 to 0.05) | 0.86 | 0.82 |
| Self care activity (6 months) | 42.5 (14.6), n=2645 | 42.7 (15.0), n=1813 | 0.03 (−0.88 to 0.93) | 0.00 (−0.06 to 0.06) | 0.96 | 0.78 |
WISE=whole system informing self management engagement.
*Outcome at 12 months unless otherwise stated.
†Difference in group means after adjustment for model factors and covariates.
‡Adjusted mean difference (intervention minus control) divided by within practice standard deviation.
§P value for test of whether intervention effect varies by disease condition group (intervention by condition group interaction).
¶P value based on boot strapped variance estimates.
**Non-significant (P=0.1) in analysis of sensitivity to exposure.
††Significant in analysis of sensitivity to covariates (p=0.04) and sensitivity to exposure (P=0.018).
Fig 2 Forest plot of standardised effect sizes by outcome measure, with vertical bars indicating minimally important differences
Service utilisation
Analysis of complete data showed that the utilisation of services did not differ substantially in association with the intervention. The supplementary file shows the mean levels of the major resources used by each group, and reports 95% confidence intervals around the mean difference for these complete cases.
Discussion
An intervention to enhance self management support for patients with chronic conditions in UK primary care (WISE, whole system informing self management engagement) had no significant effects on patient outcomes or on service use. This has important implications for primary care. This report focuses on trial results, but a separate process evaluation will explore why practitioners were not able to implement the intervention.
Strengths and limitations of this study
Strengths of the study included a large practice and patient sample size and an intervention based on published trials and delivered at an intensity feasible in primary care. A patient recruitment rate of 43% is relatively high for a community based trial in UK primary care,42 43 and we achieved good levels of follow-up. We also achieved high levels of participation by the practices.
A response rate of 43% could mean that the participating patients were not fully representative of the practice populations, and we lacked data by which to compare participants with those who did not return our questionnaires. Although it could be argued that effects might have been shown in different long term conditions or outcomes, we are confident that the lack of effect is robust owing to our inclusion of a range of conditions and our comprehensive outcome assessment. We cannot rule out the possibility that the approach might work more effectively in a more affluent population.
The nature of the intervention required a cluster trial. The loss of three practices in the intervention arm introduced the possibility of baseline imbalance. Another important threat to cluster trial validity is recruitment bias, where professionals recruit differently depending on the trial arm to which they are allocated.44 We intended to recruit patients before allocation, but this proved logistically impractical. Recruitment was through electronic health records rather than by professional invitation, but practitioners could exclude patients after identification.25 These exclusions represented a relatively small proportion of patients (11% control and 15% intervention patients with chronic obstructive pulmonary disease, 10% and 11% with diabetes, and 18% and 11% with irritable bowel syndrome). The proportions excluded were broadly similar, and there was no consistent pattern of higher or lower rates of exclusion in the intervention or control practices. One other limitation was that utilisation outcomes were based on self report and such measures may not always agree with other sources, such as service records.
We set out to implement a practice based training programme to improve outcomes through enhanced self management, which involved several steps:
(1) Engaging a high proportion of practices with the programme
(2) Delivering training to a high proportion of clinicians and other staff
(3) Ensuring training was relevant and acceptable
(4) Encouraging implementation of the training in routine practice
(5) Enhancing shared decision making and self management
(6) Improving outcomes.
Our data show that steps 1-3 were largely achieved, but we suggest that the intervention failed at step 4 and consequently failed to generate changes at steps 5 and 6. Ensuring that training was acceptable at step 3 required considerable compromise in restricting the length and content of training to match the time that practices were willing to devote. This necessarily limited the intensity of the intervention and our ability to subsequently add reinforcement and ensure fidelity. More success may have been achieved if that had not been the case, but that could have led to lower levels of practice engagement.
A common problem in health services research is that effective interventions are often not feasible and feasible interventions are often not effective. Many published trials on self management are conducted in atypical contexts with selected, volunteer samples. Our study took proved components of self management support and tested whether we could implement these as a comprehensive package in routine primary care practice using existing educational structures, applied to an entire local health economy. We sought to sensitise our intervention to the particular nature of primary care, providing a structure and tools to allow practitioners to introduce self management support into time limited consultations, to enhance partnerships with patients, and to encourage behaviour change.
The local context included strong institutional commitment from the host health management organisation. This was reflected in the high level of practice engagement.45 46 Data from practice staff suggest training facilitation was successful, with high levels of attendance and acceptability.
However, staff self report data suggest that implementation was variable and the low response to this questionnaire meant that even reported rates could overestimate the actual impact. The time available for training was limited but was based on our pilot studies and negotiations with practices and was judged the maximum acceptable to clinical staff, given the demands on time and the high costs of providing cover for staff. We allowed practices flexibility in how they implemented self management support at the practice level, and flexibility can lead to attenuated outcomes. Although a more standardised approach may have enhanced effectiveness, this may have jeopardised our high levels of recruitment and engagement. Also, despite our best efforts and the full support of the primary care trust, no practice was prepared to free up further staff time for additional reinforcement sessions and only one practice allowed access for fidelity checks. The study took place during a period of major upheaval for primary care trusts, with the introduction of Primary Care Clinical Commissioning Groups,47 and the influence of the primary care trust over the practices was quite limited.
The intervention also faced competing demands in care for long term conditions. Within UK primary care, practice nurses are increasingly responsible for managing patients with long term conditions. This workload shift has been in response to the introduction of the Quality and Outcomes Framework within the new General Medical Services contract.48 Assessment and recording for pay for performance has come to dominate interactions between patients and clinicians in the United Kingdom, leading to a focus on biomedical work.49 50 This may leave little time to develop the skills required to support patient self management, which is neither audited nor rewarded. Training (even when underlined by institutional and professional commitment) may be insufficient when faced with more powerful incentives such as income generation. Overcoming this might be achieved by making self management support part of the pay for performance scheme, although such an approach is fraught with difficulties concerning measurement.51
Engaging patients in behaviour change can be difficult and the amount of time that patients spend in contact with primary care is only a tiny fraction of the active day. Even where patients did receive good self management support this may not have translated into their everyday activities.14 Patients from deprived areas, such as in our study, often feel distanced from professional notions of participation and shared decision making, particularly when ill and feeling least competent.52
Comparison with other studies
Significantly, our results concur with a growing body of evidence that highlights the limited ability of self management support interventions, of all kinds, to deliver real benefits for patients.53 54 55 56 57 McCall et al reported on a randomised study of eight commercial disease management programmes involving over 240 000 patients.54 Improvements in processes of care were patchy and at best modest, and no programmes were cost saving. Trials of educational self management interventions for patients with heart failure from multiple research groups have consistently failed to find any substantive benefits.55 A systematic review of self management education programmes concluded that effects were small to moderate and limited to specific chronic diseases—diabetes and hypertension.53 A more recent review of trials of self management support for type 2 diabetes found only “trivial” effects on biochemical outcomes; patchy data meant that conclusions on other kinds of outcomes could not be drawn.56 Although some individual interventions have shown a strong and sustained impact,58 59 it is unclear what the active components in these interventions are that differentiate them from the majority of largely ineffective interventions, and whether their effects can be replicated outside their local context. Detailed investigations across a wide range of studies of varying interventions, to help determine the active ingredients through metaregression techniques, may be useful.60 We also note the changing nature of evidence during the span of this trial. All three conditions were amenable to self management in principle, but evidence varied at baseline and more negative evidence was published during the lifetime of the trial. As a point of context, support for pulmonary rehabilitation classes was withdrawn by the primary care trust during the course of the trial.
Conclusions
Embedding self management support into routine primary care practice cannot be achieved within existing educational structures and may require considerable additional incentives to encourage practices to engage with a self management agenda. The challenge is to show how a different intervention (for example, of greater intensity or duration) might enhance effectiveness without compromising “reach.”
One possibility is that most forms of intervention, whether provider based or patient based, are outside patients’ workaday and social activities, so fail to embed themselves into their everyday lives. It may be that greater efforts to integrate support for self management into patients’ personal social networks (family, friends, and other social groups) or using means that are more pervasive in people’s lives, such as mobile technology, would prove a more effective approach to engaging patients with self management and the behaviour changes necessary to that end.
What is already known on this topic
Self management support interventions are potentially effective but do not reach many of the people with long term conditions who might benefit
Enhancing the ability of primary care practitioners to provide self management support could provide a way to improve outcomes among the wider population of patients with long term conditions, because of their knowledge about individual patients and the continuity of care they provide
A whole systems approach, which integrates self management support at the level of the patient, practitioner, and service organisation, has proved effective in improving outcomes for patients
What this study adds
Short training interventions (even when combined with local managerial support and additional resources) are ineffective for enhancing self management support in routine primary care
A need exists to better understand the active components required for effective self management support, how these might be delivered within primary care, and the training and system changes that would subsequently be needed
We thank the participating practices, staff, and patients for their contribution. Members of the Salford National Institute for Health Research Gastrointestinal programme Grant Research Group are: Karen Armstrong, David Backhouse, Paula Beech, Peter Bower, Carolyn Chew-Graham, Andrew Clough/Karen Proctor (chair), Anne Kennedy, Karina Lovell, Jim Nuttall, Sarah O’Brien, David Reeves, Gerry Richardson, Anne Rogers, David Thompson, and Peter Whorwell.
Contributors: AK designed the trial, managed and monitored the trial, developed the intervention, designed data collection tools, analysed the data, and drafted and revised the paper. DR designed the trial, wrote the statistical analysis plan, designed data collection tools, analysed the data, and drafted and revised the paper. PB designed the trial, designed data collection tools, analysed the data, and drafted and revised the paper. TB designed the trial, developed the intervention, designed data collection tools, and revised the draft paper. RB collected data and revised the draft paper. CCG designed the trial, developed the intervention, designed data collection tools, and revised the draft paper. ME collected, cleaned, and analysed the data and revised the draft paper. CF and HG cleaned and analysed the data and revised the draft paper. CG managed and monitored the trial; designed data collection tools; collected, cleaned, and analysed the data; and revised the draft paper. VL recruited practices, collected data, and revised the draft paper. RM designed data collection tools and revised the draft paper. JP designed the trial, developed the intervention, designed data collection tools, and revised the draft paper. GR designed the trial, wrote the cost effectiveness analysis plan, designed data collection tools, analysed the data, and revised the draft paper. CS designed the trial, designed data collection tools, and revised the draft paper. AS collected, cleaned, and analysed the data and revised the draft paper. DT initiated and led the National Institute for Health Research Gastrointestinal programme, designed the trial, and revised the draft paper. AR initiated and led the National Primary Care Research and Development centre side of the project, designed the trial, designed data collection tools, and revised the draft paper. All members of the Salford National Institute for Health Research Gastrointestinal programme Grant Research Group monitored the trial and commented on the draft paper. All authors had full access to all of the data in the study. AK and DR are guarantors for the study.
Funding: The trial was funded by the National Institute for Health Research and The National Primary Care Research and Development Centre (funded by the Department of Health). This paper presents independent research commissioned by the National Institute for Health Research under its programme grants for applied research funding scheme (RP-PG-0407-10136). The views expressed in this paper are those of the authors and not necessarily those of the National Health Service, National Institute for Health Research, or the Department of Health.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by Salford and Trafford local research ethics committee (reference 09/H1004/6).
Data sharing: No additional data available.
Cite this as: BMJ 2013;346:f2882
Web Extra. Extra material supplied by the author
Whole system informing self management engagement (WISE) training
Supplementary material
Results of service utilisation
References
- 1.Bodenheimer T. Transforming Practice. N Engl J Med 2008;359:2086-9. [DOI] [PubMed] [Google Scholar]
- 2.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. [DOI] [PubMed] [Google Scholar]
- 3.Stange K, Nutting P, Miller W, Jaén C, Crabtree B, Flocke S, et al. Defining and measuring the patient-centered medical home. J Gen Intern Med 2010;25:601-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doran T, Fullwood C, Gravelle H, Reeves D, Kontopantelis E, Hiroeh U, et al. Pay-for-performance programs in family practices in the United Kingdom. N Engl J Med 2006;355:375-84. [DOI] [PubMed] [Google Scholar]
- 5.Zwar N, Harris M, Griffiths R, Roland M, Dennis S, Powell Davies G, et al. A systematic review of chronic disease management. Australian Primary Health Care Research Institute, 2006.
- 6.Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag 2003;6:63-71. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy A, Reeves D, Bower P, Lee V, Middleton E, Richardson G, et al. The effectiveness and cost effectiveness of a national lay led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health 2007;61:254-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorig K, Sobel DS, Stewart AL, Brown BW, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care 1999;37:5-14. [DOI] [PubMed] [Google Scholar]
- 9.Bodenheimar T, MacGregor K, Shafiri C. Helping patients manage their chronic conditions. California Healthcare Foundation, 2005.
- 10.Steventon A, Bardsley M, Billings J, Dixon J, Doll H, Hirani S, et al. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012;344:e3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chodosh J, Morton SC, Mojica W, Maglione M, Suttorp MJ, Hilton L, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med 2005;143:427-38. [DOI] [PubMed] [Google Scholar]
- 12.Furler J, Harris M, Rogers A. Equity and long-term condition self-management. Chronic Illn 2011;7:3-5. [DOI] [PubMed] [Google Scholar]
- 13.Protheroe J, Nutbeam D, Rowlands G. Health literacy: a necessity for increasing participation in health care. Br J Gen Pract 2009;59:721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassilev I, Rogers A, Blickem C, Brooks H, Kapadia D, Kennedy A, et al. Social networks, the ‘work’ and work force of chronic illness self-management: a survey analysis of personal communities. PLoS One 2013;8:e59723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan A, Wilson S, Taylor A, Greenfield S. Factors associated with self-care activities among adults in the United Kingdom: a systematic review. BMC Public Health 2009;9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams AM, Dennis S, Harris MF. How effective are the linkages between self-management programmes and primary care providers, especially for disadvantaged patients? Chronic Illn 2011;7:20-30. [DOI] [PubMed] [Google Scholar]
- 17.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83:457-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy A, Rogers A, Bower P. Support for self care for patients with chronic disease. BMJ 2007;335:968-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parchman ML, Romero RL, Pugh JA. Encounters by patients with type 2 diabetes—complex and demanding: an observational study. Ann Fam Med 2006;4:40-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blakeman T, Macdonald W, Bower P, Gately C, Chew-Graham C. A qualitative study of GPs’ attitudes to self-management of chronic disease. Br J Gen Pract 2006;56:407-14. [PMC free article] [PubMed] [Google Scholar]
- 21.Effing T, Monninkhof EM, van der Valk PD, van der Palen J, van Herwaarden CL, Partidge MR, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007;(4):CD002990. [DOI] [PubMed] [Google Scholar]
- 22.Macdonald W, Rogers A, Blakeman T, Bower P. Practice nurses and the facilitation of self-management in primary care. J Advanced Nurs 2008;62:191-9. [DOI] [PubMed] [Google Scholar]
- 23.May C, Finch T. Implementation, embedding, and integration: an outline of Normalization Process Theory. Sociology 2009;43:535-54. [Google Scholar]
- 24.Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bower P, Kennedy A, Reeves D, Rogers A, Blakeman T, Chew-Graham C, et al. A cluster randomised controlled trial of the clinical and cost-effectiveness of a ‘whole systems’ model of self-management support for the management of long- term conditions in primary care: trial protocol. Implement Sci 2012;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partners in Salford. Our vision of a healthy city. 2013. www.partnersinsalford.org/content-healthy-city.htm.
- 27.Deakin T, McShane C, Cade JE, Williams RDRR. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;(2):CD003417. [DOI] [PubMed] [Google Scholar]
- 28.Robinson A, Lee V, Kennedy A, Middleton E, Rogers A, Thompson DG, et al. A randomised controlled trial of self-help interventions in patients with a primary care diagnosis of IBS. Gut 2006;55:643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy AP, Robinson A, Hann M, Thompson DG, Wilkin D. A cluster-randomised controlled trial of a patient-centred guidebook for patients with ulcerative colitis: effect on knowledge, anxiety and quality of life. Health Soc Care Community 2003;11:64-72. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy AP, Nelson E, Reeves D, Richardson G, Robinson A, Rogers A, et al. A randomised controlled trial to assess effectiveness and cost of a patient orientated self-management approach to chronic inflammatory bowel disease. Gut 2004;53:1639-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson A, Wilkin D, Thompson DG, Roberts C. Guided self-management and patient-directed follow-up of ulcerative colitis: a randomised trial. Lancet 2001;358:976-81. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy A, Chew-Graham C, Blakeman T, Bowen A, Gardner C, Protheroe J, et al. Delivering the WISE (Whole Systems Informing Self-Management Engagement) training package in primary care: learning from formative evaluation. Implement Sci 2010;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Protheroe J, Blakeman T, Bower P, Chew-Graham C, Kennedy A. An intervention to promote patient participation and self-management in long term conditions: development and feasibility testing. BMC Health Serv Res 2010;10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy A, Robinson A, Rogers A. Incorporating patients’ views and experiences of life with IBS in the development of an evidence based self-help guidebook. Patient Education and Counseling 2003;50(3):303-310. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy A, Reeves D, Bower P, Lee V, Middleton E, Richardson G, et al. The effectiveness and cost effectiveness of a national lay led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health 2007;61:254-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams GC, McGregor HA, King D, Nelson CC, Glasgow RE. Variation in perceived competence, glycemic control, and patient satisfaction: relationship to autonomy support from physicians. Patient Educ Counsel 2005;57:39-45. [DOI] [PubMed] [Google Scholar]
- 37.Williams GC, McGregor HA, Zeldman A, Freedman ZR, Deci EL. Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychol 2004;23:58-66. [DOI] [PubMed] [Google Scholar]
- 38.Lorig K, Stewart AL, Ritter P, Gonzalez VM, Laurent D, Lynch J. Outcome measures for health education and other health care interventions. Sage, 1996.
- 39.Kind P. The EuroQol instrument: an index of health-related quality of life. In: Spilker B, ed. Quality of life and pharmacoeconomics in clinical trials. 2nd edn. Lippincott-Raven, 1996.
- 40.Ritter PL, Stewart AL, Kaymaz H, Sobel DS, Block DA, Lorig KR. Self-reports of health care utilization compared to provider records. J Clin Epidemiol 2001;54:136-41. [DOI] [PubMed] [Google Scholar]
- 41.Groenwold R, Donders A, Roes K, Harrell F Jr, Moons K. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol 2012;175:210-7. [DOI] [PubMed] [Google Scholar]
- 42.McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones R, Jones RO, McCowan C, Montgomery AA, Fahey T. The external validity of published randomized controlled trials in primary care. BMC Fam Pract 2009;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torgerson DJ. Contamination in trials: is cluster randomisation the answer? BMJ 2001;322:355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodyear-Smith F, York D, Petousis-Harris H, Turner N, Copp J, Kerse N, et al. Recruitment of practices in primary care research: the long and the short of it. Fam Pract 2009;26:128-36. [DOI] [PubMed] [Google Scholar]
- 46.Salmon P, Peters S, Rogers A, Gask L, Clifford R, Iredale W, et al. Peering through the barriers in GPs’ explanations for declining to participate in research: the role of professional autonomy and the economy of time. Fam Pract 2007;24:269-75. [DOI] [PubMed] [Google Scholar]
- 47.Department of Health. Equity and excellence: liberating the NHS. Stationery Office, 2010.
- 48.McDonald R, Campbell S, Lester H. Practice nurses and the effects of the new general practitioner contract in the English National Health Service: the extension of a professional project? Soc Sci Med 2009;68:1206-12. [DOI] [PubMed] [Google Scholar]
- 49.McGregor W, Jabareen H, O’Donnell CA, Mercer SW, Watt GCM. Impact of the 2004 GMS contract on practice nurses: a qualitative study. Br J Gen Pract 2008;58:711-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell SA, McDonald R, Lester H. The experience of pay for performance in english family practice: a qualitative study. Ann Fam Med 2008;6:228-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woolhandler S, Ariely D, Himmelstein D. Why pay for performance may be incompatible with quality improvement. BMJ 2012;345:e5015. [DOI] [PubMed] [Google Scholar]
- 52.Protheroe J, Brooks H, Chew-Graham C, Gardner C, Rogers A. ‘Permission to participate?’: a qualitative study of participation in patients from differing socio-economic backgrounds. J Health Psychol 2012; published online 26 Oct. [DOI] [PubMed]
- 53.Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease—a systematic review and methodological critique of the literature. Arch Internl Med 2004;164:1641-9. [DOI] [PubMed] [Google Scholar]
- 54.McCall N, Cromwell J. Results of the Medicare Health Support Disease-Management Pilot Program. N Engl J Med 2011;365:1704-12. [DOI] [PubMed] [Google Scholar]
- 55.Powell LH, Calvin JE, Richardson D, Janssen I, de Leon CFM, Flynn KJ, et al. Self-management counseling in patients with heart failure the heart failure adherence and retention randomized behavioral trial. JAMA 2010;304:1331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Egginton JS, Ridgeway JL, Shah ND, Balasubramaniam S, Emmanuel JR, Prokop LJ, et al. Care management for Type 2 diabetes in the United States: a systematic review and meta-analysis. BMC Health Serv Res 2012;12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Motheral BR. Telephone-based disease management: why it does not save money. Am J Manag Care 2011;17:e10-6. [PubMed] [Google Scholar]
- 58.Trento M, Gamba S, Gentile L, Grassi G, Miselli V, Morone G, et al. Rethink Organization to iMprove Education and Outcomes (ROMEO) A multicenter randomized trial of lifestyle intervention by group care to manage type 2 diabetes. Diabetes Care 2010;33:745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hornsten A, Stenlund H, Lundman B, Sandstrom H. Improvements in HbA1c remain after 5 years—a follow up of an educational intervention focusing on patients’ personal understandings of type 2 diabetes. Diabetes Res Clin Pract 2008;81:50-5. [DOI] [PubMed] [Google Scholar]
- 60.Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control—a meta-regression analysis. JAMA 2006;296:427-40. [DOI] [PubMed] [Google Scholar]
- 61.Communities and Local Government (CLG). Indices of deprivation 2007 data at Primary Care Trust (PCT) level. 2013. www.communities.gov.uk/communities/neighbourhood renewal/deprivation/deprivation07.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Whole system informing self management engagement (WISE) training
Supplementary material
Results of service utilisation