Pathogens and bacteria that normally live in the gut induce different immune responses.
The human intestinal mucosa is exposed to a complex ecosystem of harmless bacteria (commensals) that are excluded from the sterile environment of the body by an antibody isotype called immunoglobulin A (IgA) (1). Characterizing the dynamics of this immune response has been problematic because of constant immune stimulation by such bacteria. On page 1705 of this issue, Hapfelmeier et al. (2) use a reversible system of gut bacterial colonization in mice to show that responses to commensals lack cardinal features of systemic (extramucosal) IgG responses to pathogenic bacteria.
Commensal microbes form a diverse community in the gut, estimated to exceed the host’s eukaryotic cell number by an order of magnitude (3). Commensals break down otherwise indigestible food components, generate essential nutrients, and “educate” the local immune system. Intestinal B cells maintain this mutualistic relationship by producing IgA, an isotype that induces a weak infl ammatory response compared to IgG in blood (1).
The poor inflammatory activity of IgA permits the intestinal mucosa to impede the entry of bacteria into the body without inducing inflammatory damage of the epithelial barrier (1). If invading pathogens breach this barrier, circulating IgG rapidly recruits innate immune cells with phagocytic function (granulocyetes, monocytes) through the activation of an infl ammatory reaction. With the help of IgG, these innate effector cells clear invading bacteria in a matter of hours.
Systemic IgG responses emerge 5 to 7 days after the immune system encounters a pathogen. IgG provides protection against secondary challenges by generating long-lived memory B cells that circulate like sentinels and produce massive amounts of IgG upon reencountering bacteria (4), and plasma cells, which release IgG from the bone marrow into the circulation (5). Key features of IgG memory by these cells are the quick increase and higher affinity of secondary responses, and the synergistic effect of repeated exposures. Bacterial products also enhance the half-life of serum IgG by activating memory B cells (6). Does intestinal IgA follow the same dynamics as systemic IgG?
To address this question, Hapfelmeier et al. directly introduced a mutant strain of the bacteria Escherichia coli into the intestine of otherwise germ-free mice. This bacterial strain (HA107) cannot divide and therefore provides a highly controlled and “reversible” situation in which a bacterial presence is initiated but cannot persist, and thus eventually disaappears. Strikingly, reversible colonization with HA107 induced as much total IgA production as irreversible colonization with multiple strains of other commensal bacteria. This IgA response was specifi c to HA107, indicating that intestinal IgA dynamics could be effectively examined without the background of continuous immune stimulation by commensal bacteria.
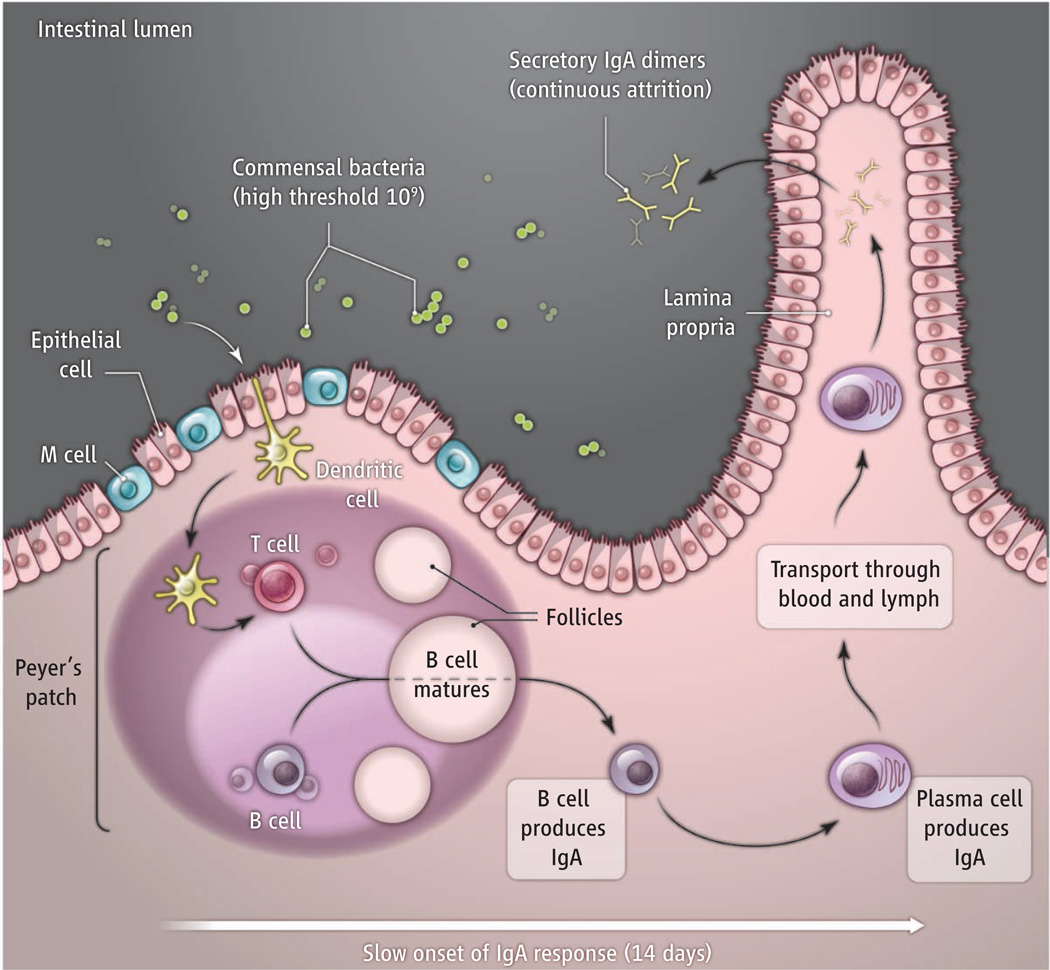
Intestinal IgA responses to HA107 had a slow onset (more than 14 days), which may reflect the time needed for specific IgA-producing plasma cells to move from inductive sites (follicles) of intestinal lymphoid tissues (Peyer’s patches) to the effector site of the intestinal lamina propria (see the figure) (1). Intestinal IgA responses also had a very high threshold for induction (109 bacteria), which was independent of preexisting natural IgA antibodies or competition with other endogenous commensal bacteria. This may be afforded by specialized microfold epithelial cells (M cells) and transepithelial dendritic cells that sample only tiny amounts of commensal bacteria from the intestinal lumen to initiate local immunity (7).
Intestinal IgA dynamics.
Dendritic cells sample commensal bacteria from the intestinal lumen and migrate to follicles, where they present antigen to T cells. T cells and dendritic cells in the follicles stimulate B cells to mature and produce IgA. B cells then enter lymphatic and blood vessels. After about 14 days, they enter the lamina propria and differentiate into long-lived (more than 16 weeks) plasma cells that release IgA dimers. Dimers enter the intestinal lumen and function as secretory IgA. Exposure to a different commensal rapidly attenuates an ongoing response (attrition).
Similar to systemic IgG responses (5), intestinal IgA responses had a sustained half-life (more than 16 weeks), in contrast to the short duration of germinal centers (where B cells mature) in intestinal follicles. One possibility is that commensal bacteria “hidden” within these follicles stimulate B cells through signals that do not require germinal centers (8), such as T cell–independent signals from primary follicular dendritic cells (9). Alternatively, epithelial, dendritic, and stromal cells lodged in the lamina propria may provide maturation and survival signals to IgA-secreting plasma cells (10).
Unlike systemic memory IgG responses, intestinal memory IgA responses did not show a synergistic increase in strength (prime-boost effect), but displayed additive increases after each challenge. Moreover, exposure of mice colonized with HA107 to bacteria different from HA107 limited the persistence of HA107-specific IgA memory. This attrition suggests that the intestine adapts its memory IgA response to the predominant commensal species present in the lumen at any given time, perhaps to compensate for the space constraints of plasma cells in the lamina propria.
The mechanism underlying IgA attrition remains unknown, but may relate to properties specific to intestinal B cells and/or T cells. In this regard, intestinal IgA responses require a subset of regulatory T cells usually involved in the negative regulation of other T cells (11, 12). The signals from this subset of T cells that help B cells in intestinal follicles may be qualitatively different from those signals delivered by helper T cells to B cells in nonintestinal follicles during systemic IgG responses. Intestinal IgA responses may also involve a larger component of T cell–independent signals from innate immune cells and stromal cells (13, 14), which could account for the limited diversity in the IgA antibodies observed by Hapfelmeier et al.
The lack of canonical IgG memory characteristics such as the prime-boost effect in intestinal IgA responses has important implications for developing effective vaccines against mucosal pathogens, including HIV. One prediction is that induction of long-lasting IgA-mediated protection will require the development of creative vaccine-delivery strategies to ensure sustained stimulation of intestinal B cells. These strategies could include embedding appropriate immunogens in stable components of our microbiota, edible probiotic bacteria, or genetically modifying foods, such as transgenic plants, that express the appropriate stimulatory antigen (15).
References
- 1.Cerutti A, Rescigno M. Immunity. 2008;28:740. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hapfelmeier S, et al. Science. 2010;328:1705. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macpherson AJ, Harris NL. Nat. Rev. Immunol. 2004;4:478. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 4.McHeyzer-Williams LJ, McHeyzer-Williams MM. Annu. Rev. Immunol. 2005;23:487. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 5.Radbruch A, et al. Nat. Rev. Immunol. 2006;6:741. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 6.Bernasconi NL, Traggiai E, Lanzavecchia A. Science. 2002;298:2199. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 7.Chieppa M, Rescigno M, Huang AY, Germain RN. J. Exp. Med. 2006;203:2841. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obata T, et al. Proc. Natl. Acad. Sci. U.S.A. 2010;107:7419. [Google Scholar]
- 9.El Shikh ME, El Sayed RM, Szakal AK, Tew JG. J. Immunol. 2009;182:3482. doi: 10.4049/jimmunol.0802317. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor BP, et al. J. Exp. Med. 2004;199:91. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. Proc. Natl. Acad. Sci. U.S.A. 2009;106:19256. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuji M, et al. Science. 2009;323:1488. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji M, et al. Immunity. 2008;29:261. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 14.He B, et al. Immunity. 2007;26:812. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Tokuhara D, et al. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8794. doi: 10.1073/pnas.0914121107. [DOI] [PMC free article] [PubMed] [Google Scholar]