Abstract
In the largest sample studied to date, we measured cognitive functioning in children and adolescents with pediatric multiple sclerosis (n =187) as well as those with clinically isolated syndrome (n =44). Participants were consecutively enrolled from six United States PediatricMultiple Sclerosis Centers of Excellence. Participants had amean of 14.8 ± 2.6 years of age and an average disease duration of 1.9 ± 2.2 years. A total of 65 (35%) children with multiple sclerosis and 8 (18%) with clinically isolated syndrome met criteria for cognitive impairment. The most frequent areas involved were fine motor coordination (54%), visuomotor integration (50%), and speeded information processing (35%). A diagnosis of multiple sclerosis (odds ratio = 3.60, confidence interval = 1.07, 12.36, P = .04) and overall neurologic disability (odds ratio = 1.47, confidence interval = 1.10, 2.10, P = .03) were the only independent predictors of cognitive impairment. Cognitive impairment may occur early in these patients, and prompt recognition is critical for their care.
Keywords: multiple sclerosis, cognition, clinically isolated syndrome, demyelination
As many as 5% of individuals with multiple sclerosis develop symptoms during childhood or adolescence.1–3 Among disorders in childhood, pediatric multiple sclerosis is uniquely challenging. In the context of childhood development, it attacks the central nervous system with acute waves of inflammation and demyelination and progresses with a neurodegenerative course. Understanding the influence of pediatric multiple sclerosis on cognitive functioning is critical for these patients.
Available data suggest that approximately one-third of children and adolescents with multiple sclerosis experience cognitive impairment. As with adult multiple sclerosis, areas of cognitive deficit can vary but often include attention and speeded processing, visuomotor functions, memory, and language.4–9
Because of small sample sizes and limited racial or ethnic diversity in prior studies, demographic and disease-related predictors of cognitive impairment remain unclear. Analyses of pediatric multiple sclerosis samples from Canada, Europe, and the United States have variably noted impairment to be associated with a range of factors, including age at disease onset,5,6 disease duration,6 Expanded Disability Status Scale score,4 Intelligence Quotient,5 and thalamic volume.7
This study leveraged the geographic diversity of the Pediatric Multiple Sclerosis Centers of Excellence, a network designated in 2006 by the National Multiple Sclerosis Society, and enrolled children from six different regions within the United States. Evaluating a large and diverse sample with a standardized clinical and neurologic assessment can clarify the associations between clinical and demographic features associated with cognitive impairment. In addition, this is the first study to include children with clinically isolated syndrome as well as those with multiple sclerosis.
Methods
Study Population
This cross-sectional study analyzed the neuropsychological performance of 231 participants evaluated between 2006 and 2011 and drawn from a multicenter study of patients with pediatric onset multiple sclerosis. Participants were seen at one of six Pediatric MS Centers of Excellence located in San Francisco, California (University of California, San Francisco); Rochester, Minnesota (Mayo Clinic); Birmingham, Alabama (University of Alabama at Birmingham); Buffalo, New York (State University of New York, Buffalo); Stony Brook, New York (State University of New York, Stony Brook); and Boston, Massachusetts (Harvard University, Massachusetts General Hospital, and Partners Health- Care). The network of centers is supported by the National Multiple Sclerosis Society and prospectively collects neuropsychological and other clinical data regarding children with multiple sclerosis, clinically isolated syndrome, and other demyelinating disorders. Each site received approval by its respective institutional review board for human subject data collection and sharing.
Eligibility criteria for this analysis included those participants who were younger than 18 years of age at disease onset (meeting criteria for a diagnosis of pediatric multiple sclerosis10 or clinically isolated syndrome10) with no other neurologic diagnosis. Participants were also required to be younger than age 18 years at the time of neuropsychological testing, to have completed at least 9 of the 11 neuropsychological tests from the battery, to be English speaking and reading, and to have sufficient visual or motor ability to complete the neuropsychological tests. Patients in the midst of a multiple sclerosis relapse, or who had received treatment with glucocorticoids 1 month or less prior to the time of cognitive assessment were excluded.
Neuropsychological Battery
The Pediatric Multiple Sclerosis Centers of Excellence used a comprehensive network battery consisting of 11 tests whose scores addressed the following cognitive domains: (1) general ability level—Wechsler Abbreviated Scale of Intelligence–2 subtest battery11 including Vocabulary and Matrix Reasoning subtests; (2) reading and language— Wechsler Individual Achievement Test II Pseudoword Decoding,12 Expressive One Word Picture Vocabulary Test ,13 and the Wechsler Abbreviated Scale of Intelligence Vocabulary subtest; (3) attention, working memory, and speeded processing—Digit Span test from the Wechsler Adult Intelligence Scale IV (for age 16 years and above) or the Wechsler Intelligence Scale for Children IV (for younger than age 16 years), Wechsler Adult Intelligence Scale or Wechsler Intelligence Scale for Children coding test; (4) executive functioning—Contingency Naming Test,14 Delis Kaplan Executive Function System Trail Making Test15; (5) verbal episodic learning and recall—California Verbal Learning Test–Child version or II (as age appropriate)16,17; (6) visuospatial functioning—Beery-Buktenica Developmental Test of Visual-Motor Integration–sixth edition18 and the Wechsler Abbreviated Scale of Intelligence Matrix Reasoning; (7) fine motor speed and coordination— Grooved Pegboard Test19 and Delis Kaplan Executive Function System Trail Making Test–Motor Speed Condition.
The testing battery was administered by a clinical neuropsychologist or trained and supervised psychometrician and took approximately 2.5 hours to complete. Participants were given breaks as needed.
We estimated impairment ratings for each of the 25 test scores (from the 11 neuropsychological tests) using published, age-stratified normative data. We adopted the traditional benchmark definition for mild impairment as a score falling at least 1 standard deviation below the normative mean, with moderate impairment requiring scores 2 standard deviations or more below population norms.20–23 Participants were classified with general cognitive impairment using the sum of the number of impaired test scores divided by the total number of completed test scores (out of 25 total). A participant was considered to be impaired when this proportion of impaired scores was greater than one-third.
Sociodemographic and Clinical Variables
Sociodemographic variables included age at symptom onset, age at neuropsychological evaluation, gender, race, and ethnicity (Hispanic or not Hispanic). Clinical variables available for analyses included disease duration (years), age at symptom onset (years), diagnostic status (multiple sclerosis vs clinically isolated syndrome), and disability as measured by the Expanded Disability Status Scale.24
Data Analyses
Descriptive characteristics of the group and impairment ratings of neuropsychological scores were presented either as percentages (%) or using a mean ± standard deviation. Next, we ran a multivariate logistic regression determining predictors of general cognitive impairment using sociodemographic and clinical variables. Only patients with complete sociodemographic and clinical data (n =164) were included in the multivariate analysis.
Results
Table 1 shows the demographic and clinical characteristics for the 231 children and adolescents with multiple sclerosis or clinically isolated syndrome. The majority (80%) of individuals met diagnostic criteria for multiple sclerosis within 2 years of symptom onset (mean ± standard deviation =1.9 ± 2.2).
Table 1.
Demographic and Clinical Characteristics
| Total group (n = 231) | RRMS (n = 187) | CIS (n = 44) | |
|---|---|---|---|
| Age, M ± SD (years) | 14.8 ± 2.6 | 14.8 ± 2.6 | 14.8 ± 2.6 |
| Female, n (%) | 129 (56) | 105 (56) | 24 (55) |
| Race | |||
| Caucasian, n (%) | 168 (73) | 133 (71) | 35 (80) |
| African American, n (%) | 35 (15) | 31 (17) | 4 (9) |
| Asian, n (%) | 8 (3) | 6 (3) | 2 (4) |
| Mixed/other, n (%) | 20 (9) | 17 (9) | 3 (7) |
| Hispanic ethnicity, n (%) | 66 (29) | 51 (27) | 15 (34) |
| Disease duration, M ± SD (years) | 1.9 ± 2.2 | 2.2 ± 2.3 | 0.8 ± 1.7 |
| Age at symptom onset, M ± SD (years) | 12.9 ± 3.5 | 12.7 ± 3.6 | 14.0 ± 3.0 |
| EDSS score, median (range) | 1.5 (0–6.5) | 1.5 (0–5.5) | 1.0 (0–6.5) |
| NP indices ≤ –1 SD, median (range) | 4 (0–20) | 5 (0–20) | 4 (0–17) |
| One-third of NP indices ≤ –1 SD, n (%) | 73 (32) | 65 (35) | 8 (18) |
Abbreviations: CIS, clinically isolated syndrome; EDSS, Expanded Disability Status Scale; NP, neuropsychological; RRMS, relapsing remitting multiple sclerosis; SD =Standard Deviation.
The median number of tests classified as “impaired” was 4 (range =0–20), and 32% of the entire sample met criteria for general impairment. Thirty-five percent of participants with pediatric multiple sclerosis and 18% of those with clinically isolated syndrome were classified as impaired. For the combined total sample, performances varied across the individual neuropsychological tests (Table 2). Overall, rates of mild impairment on any individual test score ranged from 9% (Language) to 34% (Visuomotor). Moderate-to-severe impairment was relatively less common, ranging from 0.4% impairment (Language) to 43%(Finemotor speed/manipulation).
Table 2.
Patient Performance on the Neuropsychological Battery (n = 231)
| No impairment, n (%) | Mild impairment,a n (%) | Moderate to severe impairment,b n (%) | Total cell size | |
|---|---|---|---|---|
| General ability | ||||
| WASI Full 2c | 198 (86) | 21 (9) | 4 (2) | 223 |
| Verbal functioning/reading/language | ||||
| WASI Vocabulary | 180 (78) | 23 (10) | 7 (3) | 210 |
| WIAT-II Pseudoword Decoding | 203 (88) | 23 (10) | 2 (0.9) | 228 |
| EOWPVT | 196 (85) | 20 (9) | 1 (0.4) | 217 |
| Attention/working memory/speeded processing | ||||
| Digit Span Test | 168 (73) | 50 (22) | 11 (5) | 229 |
| Coding/Digit Symbol | 122 (53) | 56 (24) | 27 (11) | 205 |
| Executive functioning | ||||
| CNT–Time | 163 (71) | 30 (13) | 22 (10) | 215 |
| CNT–Efficiency | 140 (61) | 62 (27) | 8 (4) | 210 |
| DKEFS TMT–Visual Scanning | 165 (71) | 25 (11) | 29 (13) | 219 |
| DKEFS TMT–Number Sequencing | 148 (64) | 38 (17) | 36 (16) | 222 |
| DKEFS TMT–Letter Sequencing | 144 (62) | 41 (17) | 37 (16) | 222 |
| DKEFS TMT–Switching | 148 (64) | 36 (16) | 37 (16) | 221 |
| Verbal learning and recall | ||||
| CVLT Learning Trials 1–5 | 193 (84) | 28 (12) | 9 (4) | 230 |
| CVLT List B Recall | 164 (71) | 57 (25) | 9 (4) | 230 |
| CVLT Short Delay Free Recall | 184 (80) | 25 (11) | 21 (9) | 230 |
| CVLT Short Delay Cued Recall | 179 (78) | 31 (13) | 20 (9) | 230 |
| CVLT Long Delay Free Recall | 182 (79) | 27 (12) | 21 (9) | 230 |
| CVLT Long Delay Cued Recall | 186 (81) | 24 (10) | 20 (9) | 230 |
| CVLT Recognition Hits | 201 (87) | 20 (9) | 8 (4) | 229 |
| CVLT Discriminability | 181 (78) | 23 (10) | 13 (6) | 217 |
| Visuospatial Functioning | ||||
| Beery VMI | 78 (33) | 79 (34) | 37 (16) | 194 |
| WASI Matrix Reasoning | 182 (79) | 23 (10) | 4 (2) | 209 |
| Fine motor speed/coordination | ||||
| Grooved Pegboard–Dominant Hand | 123 (53) | 46 (20) | 53 (23) | 222 |
| Grooved Pegboard–Nondominant Hand | 88 (38) | 33 (14) | 100(43) | 221 |
| DKEFS TMT–Motor Speed | 187 (81) | 24 (10) | 10 (4) | 221 |
Abbreviations: CNT, Contingency Naming Test; CVLT, California Verbal Learning Test (Childs version for ages 5–15; CVLT-II for Ages 16 and above); DKEFS, Delis Kaplan Executive Function System; EOWPVT, Expressive One Word Picture Vocabulary Test; TMT, Trail Making Test; VMI, Visual-Motor Integration; WASI, Wechsler Abbreviated Scale of Intelligence; WIAT, Wechsler Individual Achievement Test.
Mild impairment: scores between 1 and 1.99 standard deviations below population normative data.
Moderate to severe impairment = scores at or below 2 standard deviations below population normative data.
WASI Full 2 is a composite index of WASI Vocabulary and WASI Matrix Reasoning.
Table 3 shows a multivariate analysis of predictors of general cognitive impairment, controlling for sociodemographic factors. Diagnostic status (multiple sclerosis vs clinically isolated syndrome) was significantly associated with the presence of poorer cognitive functioning (odds ratio =3.60, confidence interval =1.07, 12.36, P =.04). Neurologic disability, measured by the Expanded Disability Status Scale, was the only significant clinical predictor (odds ratio =1.47, confidence interval =1.05, 2.06, P =.03). Post hoc bivariate analyses of the proportion of test scores considered to be impaired across tertiles of Expanded Disability Status Scale scores showed increasing disability associated with an increasing burden of cognitive impairment (Figure 1). Both Hispanic ethnicity (odds ratio =2.05, confidence interval =0.93, 4.56, P =.08) and total years of parent education (odds ratio =1.02, confidence interval =0.99, 1.05, P =.06) trended toward significance as predictors of cognitive impairment.
Table 3.
Logistic Regression: Demographic and Disease-Related Predictors of Cognitive Impairment in Pediatric Multiple Sclerosis and Clinically Isolated Syndrome (n = 164)a
| Demographics + disease- related OR (95% CI) | Significance | |
|---|---|---|
| Age | 0.92 (0.80–1.06) | 0.25 |
| Race nonwhite | 0.87 (0.36–2.10) | 0.87 |
| Hispanic ethnicity | 2.05 (0.93–4.56) | 0.08 |
| Female | 1.10 (0.52–2.35) | 0.80 |
| Parent education | 1.02 (0.99–1.05) | 0.06 |
| Disease duration | 1.08 (0.92–1.30) | 0.35 |
| Diagnosis RRMS or CIS | 3.60 (1.07–12.36) | 0.04 |
| EDSS | 1.47 (1.05–2.06) | 0.03 |
Abbreviations: CI, confidence interval; OR, odds ratio; CIS, clinically isolated syndrome; EDSS, Expanded Disability Status Scale; RRMS, relapsing remitting multiple sclerosis.
The sample (n = 164) were included in the regression based on having complete clinical data (eg, expanded disability status scale, parent education) and 9 of 11 neuropsychological tests complete on the neuropsychological battery.
Figure 1.
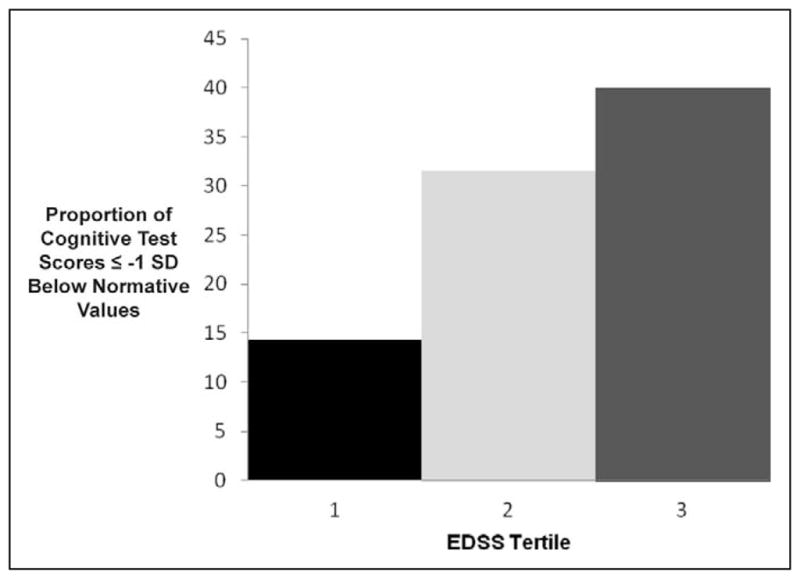
Bivariate Analyses: Cognitive Impairment Increases with Neurologic Disability
Discussion
We found that approximately one-third of pediatric patients with multiple sclerosis or clinically isolated syndrome met criteria for general cognitive impairment, defined here as having at least one-third of completed test scores falling 1 standard deviation or more below published normative data. The proportion of impairment in our sample is generally consistent with previous reports from much smaller studies,4,25 although direct comparisons are difficult across studies because of important differences in the assessment battery and criterion for cognitive impairment.
We report the first neuropsychological data from children and adolescents with clinically isolated syndrome. Cognitive impairment is well recognized in adults with clinically isolated syndrome. Cognitive impairment was identified in 8 of 44 (18%) children with clinically isolated syndrome and 69 (35%) children with multiple sclerosis. Identification of impairment at the early clinically isolated syndrome stage of disease is worrisome. The relative increased frequency of impairment among participants with multiple sclerosis compared to clinically isolated syndrome is consistent with the observation that cognitive impairments in children with multiple sclerosis progress over time.26,27
The test with the most frequent rate of impairment was the grooved pegboard, a measure of fine motor speed and coordination. We recognize that this finding may be attributable to the disease’s effects on motor as well as cognitive functioning. Future studies might specifically address the separate contributions of motor and cognitive functioning to multiple sclerosis patients’ performance on this measure.
Frequent impairment was also found on the Beery-Buktenica Developmental Test of Visual-Motor Integration, a measure that is dependent in part on fine motor coordination as well. In contrast, performances on a measure of visuospatial functioning that is not dependent on motor functioning (Wechsler Abbreviated Scale of Intelligence–Matrix Reasoning) were relatively normal. Previous studies of adults and children have documented visuomotor functions as vulnerable to impairment, both with respect to visuospatial functioning and visuospatial learning and recall. Therefore, assessments of this domain should include multiple measures, both with and without dependence on upper-extremity motor functioning.
Another area of impairment was identified by tests requiring speeded processing (Coding/Digit Symbol and Delis Kaplan Executive Function System trail making test). Slowed information processing is one of the most commonly observed domains of cognitive impairment in both adult and pediatric multiple sclerosis patients, and the results of this study confirm that this cognitive domain is highly susceptible to the effects of multiple sclerosis across the lifespan.
Other than the diagnosis of multiple sclerosis, neurologic disability as measured by the Expanded Disability Status Scale was the only variable significantly and independently associated with reduced cognitive function. Some studies,4 but not others,5,6 have found similar associations and it is likely that cognitive impairment progresses as does the neurologic burden of disease. This interpretation is also supported by the higher frequency of impairment in multiple sclerosis than in clinically isolated syndrome. There were also trends suggesting an association between reduced cognitive functioning with both fewer years of parent education as well as Hispanic ethnicity. Although race has been previously reported to be associated with cognitive impairment,28 we did not find it to be a significant predictor.
This study had several limitations. First, we used a relatively liberal cut-off point of 1 standard deviation below published norms to determine impairment on individual test scores. We chose this traditional benchmark in order to include children with even milder degrees of impairment that are likely clinically meaningful for academic performance and other aspects of functioning.20,22 However, we used a more stringent cut-off point to classify participants as cognitively impaired, with impaired performances on at least one-third of test scores from the completed battery (eg, at least 9 of 25 possible scores) in contrast to three impaired test scores used in prior studies.5 Second, although our neuropsychological battery was quite comprehensive, we excluded some tests in order to keep the battery brief; some excluded tests would have assessed domains that have been found to be sensitive indicators of cognitive status in both children and adults with multiple sclerosis, including measures of visuospatial learning and recall and measures of reading comprehension.4,8,9 Third, our multivariate analyses did not include detailed variables available regarding parent language status and socioeconomic status descriptors known to influence neuropsychological performance in children. In addition, fatigue and the presence of psychiatric distress were not included in these analyses but represent important characteristics influencing cognitive functions in multiple sclerosis.29,30 Aspects of disease burden as shown on magnetic resonance imaging (MRI)7,31,32 can also influence cognitive functions in pediatric multiple sclerosis. Finally, this study is cross-sectional; associations among sociodemographic and clinical variables in relation to cognitive function cannot be interpreted as causal relationships.
Despite these limitations, this is the largest study to date that uses a comprehensive neuropsychological battery to describe neuropsychological function in children and adolescents with multiple sclerosis. This study draws from a diverse catchment area across the United States and shows that cognitive impairment is a major feature of pediatric multiple sclerosis that can occur at the earliest stages of the disease. Cognitive impairment represents an important clinical problem for all patients with multiple sclerosis and confers specific challenges when it occurs during the context of childhood development. Further research is needed to develop strategies for prompt identification of children with multiple sclerosis at risk for cognitive problems so that treatment can be initiated.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by the National Multiple Sclerosis Society.
Footnotes
Author Contributions
LJ, LC, JA, RHBB, EB, TB, EHOD, JP, TP, MZ, and LBK were responsible for the design and conceptualization of the study. LJ, DS, LC, and LBK analyzed and interpreted the data. LJ, DS, LC, AB, TC, MG, JN, MP, MR, EW, BWG, AY, and LBK drafted and revised the manuscript.
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LJ is currently employed by Genentech. RHBB has served as a consultant for Actelion, Biogen Idec, Bayer, and Novartis; received grant/research support from Accorda, Biogen Idec, and Shire; and also receives royalties from Psychological Assessment Resources. TC has served as a consultant for Biogen-Idec, Sanofi Aventis, Novartis, EMD-Serono, and Teva Neurosciences, and has received grant support from Merck-Serono for unrelated activities. MG has research funding from NIH and Actelion. He has done consulting for Actelion, Shire HGT and Orphazyme and DMSB for Stem cells, Inc; Amicus and Shire HGT. EWhas acted as an advisor/consultant/advisory board member or speaker for Actelion, Roche, Sanofi Aventis, and Teva, and has received research support from Biogen Idec, Roche, and Sanofi-Aventis. BWG has participated in speakers bureaus and served as a consultant for Biogen Idec, Teva Neurosciences, EMD Serono, Pfizer, Novartis, Genzyme, and Acorda; excluding Genzyme, she also has received grant/research support from the agencies listed above as well as ITN, Questcor, and Shire. LBK serves as a consultant and/or on the speakers’ bureau for Teva Neurosciences, Biogen Idec, EMD Serono,MEDA Corp, Acorda, Betaseron/Bayer Healthcare Pharmaceuticals, Gerson Lehrman Group, Guidepoint Global, Adler, Cohen, Harvey, Wakeman & Guekguezian, and Daniel J. Edelman; receives royalties from Genzyme, Zymogenetics, Ortho-McNeill Pharmaceutical, and ER Squibb & Sons; and receives research support from Genentech, BiogenIdec, Teva Neurosciences, Celgene Corporation, Garnett McKeen Laboratory Incorporated, NIH, Slomo and Cindy Silvian Foundation, MS Foundation, and the Lourie Foundation. Rest of the authors report no disclosures.
Ethical Approval
Each site received approval by its respective institutional review board for human subject data collection and sharing. For those sites whose institutions did not provide a waiver, informed consent procedures were followed.
References
- 1.Duquette P, Murray TJ, Pleines J, et al. Multiple sclerosis in childhood: clinical profile in 125 patients. J Pediatr. 1987;111:359–363. doi: 10.1016/s0022-3476(87)80454-7. [DOI] [PubMed] [Google Scholar]
- 2.Ghezzi A, Deplano V, Faroni J, et al. Multiple sclerosis in childhood: clinical features of 149 cases. Mult Scler. 1997;3:43–46. doi: 10.1177/135245859700300105. [DOI] [PubMed] [Google Scholar]
- 3.Chitnis T, Glanz B, Jaffin S, Healy B. Demographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United States. Mult Scler. 2009;15:627–631. doi: 10.1177/1352458508101933. [DOI] [PubMed] [Google Scholar]
- 4.MacAllister WS, Belman AL, Milazzo M, et al. Cognitive functioning in children and adolescents with multiple sclerosis. Neurology. 2005;64:1422–1425. doi: 10.1212/01.WNL.0000158474.24191.BC. [DOI] [PubMed] [Google Scholar]
- 5.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features of childhood and juvenile MS. Neurology. 2008;70:1891–1897. doi: 10.1212/01.wnl.0000312276.23177.fa. [DOI] [PubMed] [Google Scholar]
- 6.Banwell BL, Anderson PE. The cognitive burden of multiple sclerosis in children. Neurology. 2005;64:891–894. doi: 10.1212/01.WNL.0000152896.35341.51. [DOI] [PubMed] [Google Scholar]
- 7.Till C, Ghassemi R, Aubert-Broche B, et al. MRI correlates of cognitive impairment in childhood-onset multiple sclerosis. Neuropsychology. 2011;25:319–332. doi: 10.1037/a0022051. [DOI] [PubMed] [Google Scholar]
- 8.Smerbeck AM, Parrish J, Serafin D, et al. Visual-cognitive processing deficits in pediatric multiple sclerosis. Mult Scler. 2011;17:449–456. doi: 10.1177/1352458510391689. [DOI] [PubMed] [Google Scholar]
- 9.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features in childhood and juvenile MS: two-year follow-up. Neurology. 2010;75:1134–1140. doi: 10.1212/WNL.0b013e3181f4d821. [DOI] [PubMed] [Google Scholar]
- 10.Krupp LB, Banwell B, Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16 suppl 2):S7–S12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 11.The Psychological Corporation. The Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 12.Wechsler Individual Achievement Test. 2. San Antonio, TX: Harcourt Assessment; 2005. [Google Scholar]
- 13.Gardner MF. Expressive One-Word Picture Vocabulary Test. Novato, CA: Academic Therapy Publications; 1979. [Google Scholar]
- 14.Anderson P, Anderson V, Northam E, Taylor HG. Standardization of the Contingency Naming Test: A measure of reactive flexibility. Clin Neuropsychol Assess. 2000;1:247–273. [Google Scholar]
- 15.Delis DC, Kaplan E, Kramer JH, Ober BA. Delis-Kaplan Executive Function Scale (D-KEFS) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 16.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test Manual. 2. San Antonio, TX: Psychological Corporation; 2000. Adult Version. [Google Scholar]
- 17.Delis JH, Kramer JH, Kaplan E, Ober B. California Verbal Learning Test—Children’s Version. San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- 18.Beery KE, Beery NA. The Beery-Buktenica Developmental Test of Visual-Motor Integration. 5. Minneapolis, MN: NCS Pearson; 2006. [Google Scholar]
- 19.Matthews CG, Klove H. Instruction Manual for the Adult Neuropsychological Test Battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- 20.Trittschuh EH, Crane PK, Larson EB, et al. Effects of varying diagnostic criteria on prevalence of mild cognitive impairment in a community based sample. J Alzheimers Dis. 2011;25:163–173. doi: 10.3233/JAD-2011-101821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achiron A, Barak Y. Cognitive impairment in probable multiple sclerosis. J Neurol Neurosurg Psychiatry. 2003;74:443–446. doi: 10.1136/jnnp.74.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lezak M, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 23.Rao S. Neurobehavioral Aspects of Multiple Sclerosis. New York, NY: Oxford University Press; 1990. [Google Scholar]
- 24.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 25.Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008;8:1585–1596. doi: 10.1586/14737175.8.10.1585. [DOI] [PubMed] [Google Scholar]
- 26.MacAllister WS, Christodoulou C, Milazzo M, Krupp LB. Longitudinal neuropsychological assessment in pediatric multiple sclerosis. Dev Neuropsychol. 2007;32:625–644. doi: 10.1080/87565640701375872. [DOI] [PubMed] [Google Scholar]
- 27.Amato MP, Portaccio E, Goretti B, et al. Relevance of cognitive deterioration in early relapsing-remitting MS: a 3-year follow-up study. Mult Scler. 2010;16:1474–1482. doi: 10.1177/1352458510380089. [DOI] [PubMed] [Google Scholar]
- 28.Ross KA, Schwebel DC, Rinker J, 2nd, Ness J, Ackerson J. Neurocognitive sequelae in African American and Caucasian children with multiple sclerosis. Neurology. 2010;75:2097–2102. doi: 10.1212/WNL.0b013e318200d7b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacAllister WS, Boyd JR, Hollans NJ, Milazzo MC, Krupp LB. The psychosocial consequences of pediatric multiple sclerosis. Neurology. 2007;68(16 suppl 2):S66–S69. doi: 10.1212/01.wnl.0000259420.54635.63. [DOI] [PubMed] [Google Scholar]
- 30.Goretti B, Portaccio E, Ghezzi A, et al. Fatigue and its relationships with cognitive functioning and depression in paediatric multiple sclerosis. Mult Scler. 2012;18:329–334. doi: 10.1177/1352458511420846. [DOI] [PubMed] [Google Scholar]
- 31.Fuentes A, Collins DL, Garcia-Lorenzo D, et al. Memory performance and normalized regional brain volumes in patients with pediatric-onset multiple sclerosis. J Int Neuropsychol Soc. 2012;18:471–480. doi: 10.1017/S1355617711001913. [DOI] [PubMed] [Google Scholar]
- 32.Till C, Deotto A, Tipu V, et al. White matter integrity and math performance in pediatric multiple sclerosis: a diffusion tensor imaging study. Neuroreport. 2011;22:1005–1009. doi: 10.1097/WNR.0b013e32834dc301. [DOI] [PubMed] [Google Scholar]


