Abstract
Protective responses to microorganisms involve the nonspecific but rapid defence mechanisms of the innate immune system, followed by the specific but slow defence mechanisms of the adaptive immune system. Located as sentinels at the interface between the circulation and lymphoid tissue, splenic marginal zone B cells rapidly respond to blood-borne antigens by adopting ‘crossover’ defensive strategies that blur the conventional boundaries of innate and adaptive immunity. This Review discusses how marginal zone B cells function as innate-like lymphocytes that mount rapid antibody responses to both T cell-dependent and T cell-independent antigens. These responses require the integration of activation signals from germline-encoded and somatically recombined receptors for microorganisms with helper signals from effector cells of the innate and adaptive immune systems.
The innate and adaptive immune systems are functionally interconnected by mechanisms that were originally predicted by Charles Janeway Jr1. In his unified model of the immune response, dendritic cells (DCs) and macrophages of the innate immune system instruct specific lymphocytes of the adaptive immune system to initiate protective responses after sensing conserved microbial molecular signatures via germline-encoded pattern-recognition receptors (PRRs), including Tolllike receptors (TLRs)1. Unlike DCs and macrophages, lymphocytes recognize discrete antigenic epitopes in a specific but temporally delayed manner through somatically recombined T cell receptors (TCRs) or B cell receptors (BCRs)2.
Most lymphocytes express specific antigen receptors encoded by highly diversified V(D)J genes. However, some subsets of B and T cells express less specific BCRs and TCRs encoded by semi-invariant or poorly diversified V(D)J genes that recognize multiple highly conserved microbial determinants3. These ‘innate-like’ lymphocytes are strategically positioned in ‘sensitive’ front-line areas that are continually exposed to microbial antigens, including the skin and mucosal surfaces3. A major population of innate-like lymphocytes comprises B cells from the marginal zone (MZ) of the spleen, a unique lymphoid area located at the interface between the circulation and the immune system4.
Unlike follicular B cells, which primarily express monoreactive BCRs, many MZ B cells express polyreactive BCRs that bind to multiple microbial molecular patterns1,3,5. In some cases, the recognition profile of these polyreactive BCRs is broadly similar to that of TLRs. In addition, MZ B cells express high levels of TLRs (similarly to DCs, macrophages and granulocytes), which allows them to cross over the conventional boundaries between the innate and adaptive immune systems6,7. Indeed, dual engagement of BCR and TLR molecules by conserved microbial molecules such as lipopolysaccharide (LPS) or peptidoglycan stimulates MZ B cells to initiate low-affinity antibody responses that bridge the temporal gap required for the induction of high-affinity antibody production by conventional follicular B cells3,4,8. B-1 cells from the spleen and coelomic cavities also have very pronounced innate functional features and indeed often cooperate with MZ B cells in the response to bloodborne microorganisms3,4, but these cells are not discussed in detail here.
This Review summarizes recent advances on the complex interplay of MZ B cells with different components of the innate and adaptive immune systems that lead to the initiation of rapid antibody responses. We describe the nature of the cellular and signalling pathways required for the diversification and production of antibodies by MZ B cells, and the species-specific differences in these pathways. In addition, we discuss evidence suggesting that MZ B cells take advantage of their unique innate properties not only to ‘repel’ invading pathogens, but also to communicate with mucosal commensal bacteria. This communication may be important for maintaining viable MZ B cells over time and for the generation of an innate layer of humoral protection against common microbial determinants.
Antigen capture in the MZ
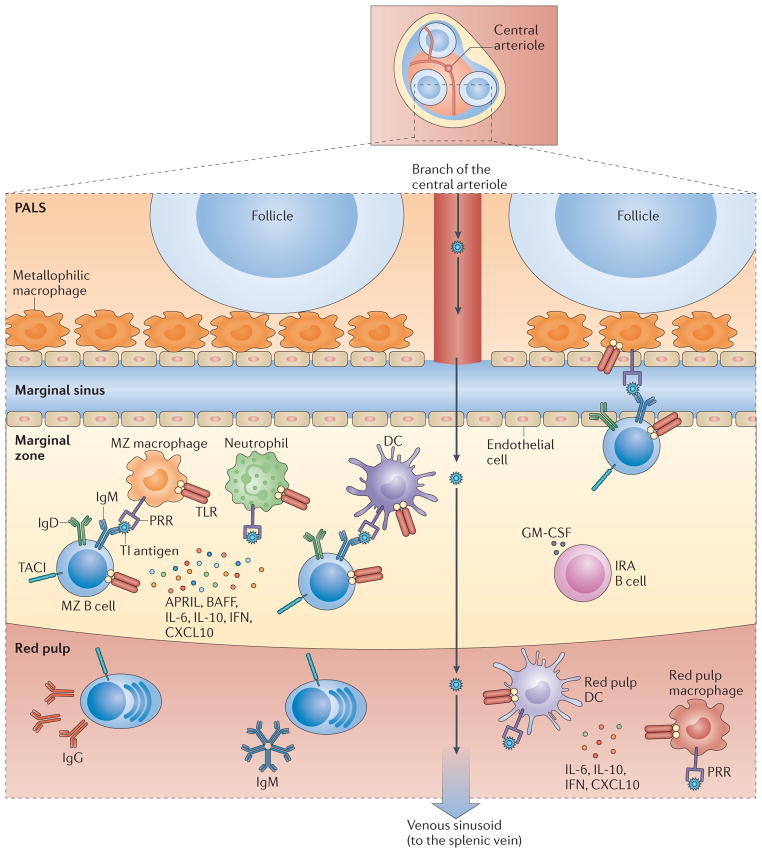
The spleen has an important role in host defence against blood-borne pathogens9. In humans, the spleen receives about 5% of the cardiac output, which constitutes a large blood supply for an organ that does not have a high oxygen consumption under steady-state conditions9. The elevated perfusion of the spleen permits this organ, through the MZ, to provide efficient immune surveillance of the circulatory system. Strategically interposed between the lymphoid tissue of the white pulp and the circulation, the splenic MZ contains B cells enmeshed with macrophages, DCs and granulocytes in a stromal reticular cell network9. All of these cells readily interact with circulating antigens as a result of the low flow rate of the blood passing through the MZ.
In mice, the blood flowing in splenic central arterioles encounters an area of decreased resistance after entering the wider spaces of the marginal sinus (BOX 1). The fenestrated nature of the marginal sinus facilitates the entry of blood-borne antigens into the MZ9. At this site, metallophilic macrophages and MZ macrophages capture antigens through various PRRs (FIG. 1), including scavenger receptors and C-type lectin receptors (CLRs)9–11. These and other uptake receptors may convey antigens to non-degradative intracellular vesicular compartments that subsequently recycle to the cell surface, potentially permitting macrophages to expose native antigens for the activation of MZ B cells4,12,13. In an alternative pathway, blood-borne antigens are captured by DCs and granulocytes in the circulation and then transported to the MZ via the marginal sinus14,15. At least some of the circulating DCs involved in this process express low levels of CD11c and therefore may correspond to plasmacytoid DCs rather than conventional DCs14. In humans, there is no marginal sinus and the MZ lacks specific macrophages16,17. One possibility is that antigens come into contact with human MZ B cells through the perifollicular zone16, an area with open circulation that contains neutrophils with powerful B cell helper functions (see below)18.
Box 1. MZ circulation.
The marginal zone (MZ) is a highly transited area that receives large amounts of blood from the general circulation. Remarkably, the splenic microvasculature shows striking differences in mice and humans.
Mouse microvasculature
In mice, the spleen is connected with the general circulation through the splenic artery9,17. This vessel branches into central arterioles that are surrounded by the periarteriolar lymphoid sheath (PALS). Central arterioles further branch into smaller follicular arterioles that traverse the PALS and follicles of the white pulp to end as capillaries in the marginal sinus and red pulp9,17. The marginal sinus is a low-resistance vessel with a fenestrated structure that separates the MZ from the PALS and follicles of the white pulp9,17. The blood contained in the marginal sinus slowly flows through the MZ and subsequently drains into the splenic cords and venous sinuses of the red pulp to enter the general circulation9,17. As a result of this microcirculation, the mouse MZ can be considered to be an open compartment.
Human microvasculature
In humans, the spleen receives blood from the splenic artery, which branches into central arterioles and penicillary arterioles9,17. These vessels are usually covered by the PALS, but not as often as in rodents. Owing to the absence of a histologically defined marginal sinus, the blood flowing in penicillary arterioles directly drains into capillaries of the red pulp and perifollicular zone9,17. The perifollicular zone is a well-defined area of decreased resistance that separates the MZ from the red pulp. Both the perifollicular zone and the red pulp consist of an open circulatory system of blood-filled spaces known as splenic cords, which have no defined endothelial delimitation and are in close contact with the venous sinusoidal vessels of the red pulp9,17.
Figure 1. T cell-independent responses by mouse MZ B cells.
T cell-independent (Tl) antigens are captured by metallophilic macrophages and marginal zone (MZ) macrophages after entering the splenic MZ via the marginal sinus. Alternatively, Tl antigens are captured by dendritic cells (DCs) and neutrophils in the circulation. Innate response activator (IRA) B cells may enhance the survival and activation of these antigen-capturing cells by releasing granulocyte–macrophage colony-stimulating factor (GM-CSF). Antigen-sampling cells stimulate MZB cells via the B cell receptor (BCR), Toll-like receptors (TLRs) and transmembrane activator and CAML interactor (TACI). TACI delivers signals that induce class-switch recombination and antibody production after ligating B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL), which are released by antigen-capturing cells in response to microbial TLR ligands. Antigen-capturing cells, including red pulp DCs and macrophages, also secrete interleukin-6 (IL-6), IL-10, type I interferons (IFNs) and CXC-chemokine ligand 10 (CXCL10), which cooperate with BAFF and APRIL to promote the differentiation and survival of plasmablasts secreting IgM or class-switched IgG. Arrows indicate the path followed by antigens through the spleen. PALS, periarteriolar lymphoid sheath; PRR, pattern-recognition receptor.
In mice, MZ B cells are confined to the MZ of the spleen5. In humans, MZ B cells are also located in the inner wall of the subcapsular sinus of lymph nodes, the epithelium of tonsillar crypts and the subepithelial area of mucosa-associated lymphoid tissues, including the subepithelial dome of intestinal Peyer’s patches18–21. These MZ-equivalent areas are poorly understood, but they have a cellular composition similar to that of the splenic MZ and may therefore provide alternative functional niches for MZ B cells, particularly in splenectomized individuals22. B cells identical to splenic MZ B cells are also present in human peripheral blood, which suggests that human MZ B cells recirculate23,24.
After interacting with antigens exposed on macrophages, DCs or neutrophils, MZ B cells rapidly differentiate into plasmablasts that produce large amounts of IgM4,14,18,25. In addition, MZ B cells produce IgG and some IgA via class-switch recombination (CSR)18,26. In humans, MZ B cells also undergo somatic hypermutation (SHM)22, and both CSR and SHM contribute to MZ B cell responses to pathogens and commensal antigens.
Role of MZ B cells in infections
Individuals that lack the spleen as a result of surgery or congenital asplenia are at a higher risk of pneumonitis, meningitis and fulminant septic syndrome caused by encapsulated bacteria such as Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis27–29. These infections are generally attributed to an impaired production by MZ B cells of IgM and IgG specific for capsular polysaccharides27–29. However, these B cell defects seem to be less severe in children, suggesting that the pathogenesis of post-splenectomy infections may actually be more complex30.
Infections by encapsulated bacteria are also more frequent in individuals with inflammatory bowel disease and correlate with a loss of MZ B cells that is possibly secondary to poorly understood inflammatory alterations of the spleen31. One possibility is that pro-inflammatory cytokines such as tumour necrosis factor (TNF) impair the generation of bone marrow precursors of MZ B cells by increasing myelopoiesis at the expense of lymphopoiesis32. An alternative but not mutually exclusive possibility is that the splenic MZ undergoes functional exhaustion and eventually atrophy as a result of an abnormally high influx of microbial antigens from the inflamed intestine to the general circulation.
Primary and secondary immunodeficiencies that involve the impaired survival or defective function of MZ B cells are also associated with an elevated risk of invasive pneumococcal disease and impaired antibody responses to capsular polysaccharides33–35. Similarly to splenectomized or immunodeficient humans, rodents that lack the spleen or have genetic manipulations that alter the survival, maintenance and/or activation of MZ B cells are more susceptible to infections by encapsulated bacteria25,36. In addition to combating blood-borne bacteria, MZ B cells may provide protection against some blood-borne viruses, at least in mice37,38.
Role of MZ B cells in homeostasis
In mice, MZ B cells produce antibodies not only after infection, but also under homeostatic conditions3,5. Some of these natural antibodies recognize molecular signatures shared by both foreign and autologous cells (including certain nucleic acids and membrane phospholipids) and may therefore facilitate the clearance of both intruding microorganisms and host apoptotic cells3,5. In addition to interacting with antigenic epitopes expressed by pathogenic bacteria, natural antibodies produced by mouse MZ B cells bind to antigenic determinants associated with commensal bacteria inhabiting the intestinal tract (including microbial α-1,3-galactosyl residues) and may therefore provide a secondary line of systemic defence against microorganisms that breach the mucosal barrier3,5,39,40. But how do MZ B cells come into contact with intestinal antigens? Despite being segregated from the immune system by physical and biological barriers, mucosal commensal bacteria can influence the development and function of the immune system41–45. Although not essential for splenic MZ B cell development, commensal bacteria may enhance the function of MZ B cells. Indeed, germ-free mice have impaired natural and post-immune antibody responses to artificial and microbial carbohydrates that usually activate MZ B cells, including levan, dextran, peptidoglycan-associated polysaccharides and 3-fucosyllactosamine45–47.
The mechanism by which the intestinal microbiota modulates the function of MZ B cells remains unclear, but studies in rodents show that commensal microbial products (such as LPS and peptidoglycan) can translocate from the intestinal lumen to the circulation and eventually reach systemic lymphoid organs, including the spleen and bone marrow44,48. This process could account for the presence of LPS and peptidoglycan in the blood of healthy individuals and probably leads to the capture of these and other microbial molecules by organs actively engaged in blood filtration (such as the spleen)18,48–50. The trapping of these commensal molecules in the spleen MZ would cause innate ‘priming’ of resident immune cells via TLRs. MZ B cells may thus build a ready-to-use natural antibody repertoire while acquiring a state of active readiness against pathogens (see below)18,39,40,51.
Basic features of MZ B cells
Considerable progress has been made regarding the mechanisms that underpin the ontogeny and homing of mouse MZ B cells. It is now clear that innate signals from stromal cells, endothelial cells and macrophages are crucial for the development of MZ B cells and their retention in the MZ.
Innate signals involved in MZ B cell ontogeny
In mice, MZ and follicular B cells belong to the B-2 cell lineage and arise from bone marrow precursors via transitional B cells. IgMhiIgDlowCD21lowCD23−CD1dlow transitional stage 1 (T1) B cells colonize a T cell-rich area of the spleen known as the periarteriolar lymphoid sheath (PALS)36,52. After this population has undergone negative selection to remove strongly self-reactive cells, the remaining T1 B cells differentiate into IgMhiIgDhiCD21medCD23+CD1dlow T2 B cells that colonize the follicle36. The survival of T2 B cells requires signals from the BCR, as well as signals from a receptor that binds the TNF family member B cell-activating factor (BAFF; also known as BLYS)36,53. This BAFF receptor (BAFFR; also known as BR3) activates an alternative nuclear factor-κB (NF-κB)- dependent pathway that cooperates with the classical NF-κB-dependent pathway downstream of the BCR to promote the survival of T2 B cells and their differentiation into IgMhiIgDlowCD21hiCD23−CD1dhi MZ B cells or IgMlowIgDhiCD21medCD23+CD1dlow follicular B cells36,53.
According to the signal-strength model, this fate decision may be dictated by the reactivity of the BCR to self antigens36,54–56. An intermediate BCR reactivity is thought to trigger the differentiation of mouse T2 B cells into follicular B cells through a pathway dependent on Bruton’s tyrosine kinase (BTK)36,54–56. Indeed, robust signals from BTK may block the differentiation of mouse T2 B cells into MZ B cells by inhibiting inductive signals from the receptor NOTCH2 (REFS 36,54–57). Insufficient BTK-mediated inhibitory signals in mouse T2 B cells with poorer BCR reactivity would elicit the differentiation of these cells into IgMhiIgDhiCD21hiCD23+CD1dhi T2–MZ precursor cells, which become highly receptive to inductive signals from NOTCH2 (REF. 36). Indeed, engagement of NOTCH2 on T2–MZ precursor cells by the ligand Delta-like 1 (DLL1) on endothelial cells leads to the emergence of MZ B cells57. Although consistent with in vivo data, the signal-strength model is not supported by evidence indicating that NOTCH2 governs the differentiation of mouse MZ B cells independently of the BCR36,58. Of note, recent studies indicate that NOTCH2 also promotes the development of human MZ B cells59.
Signals from TLRs may also contribute to the development of human MZ B cells. TLR ligands can promote the differentiation of transitional B cells into MZ-like B cells, and patients with defective TLR signalling have reduced numbers of MZ B cells35,60. However, this reduction may reflect a central role of TLRs in providing survival signals rather than differentiation signals to MZ B cells35. Patients with defective TLR signalling also have an alteration of the peripheral negative-selection checkpoints that remove autoreactive B cells, including autoreactive MZ B cells61,62. In general, the nature and origin of the TLR ligands involved in the development, survival and censoring of human MZ B cells remain poorly understood.
Innate signals involved in MZ B cell homing
In mice, the blood passing through the MZ contains sphingosine-1- phosphate (S1P), a lysophospholipid that binds to the S1P receptors S1P1 (also known as S1PR1) and S1P3 (also known as S1PR3), which are expressed by MZ B cells but not by follicular B cells63. Signals from these receptors interfere with the powerful attraction of B cells towards the follicles that is exerted through CXC-chemokine receptor 5 (CXCR5) in response to CXC-chemokine ligand 13 (CXCL13) produced by follicular dendritic cells (FDCs)64. Thus, mouse MZ B cells stall in the MZ, whereas follicular
B cells home to the follicle along the CXCL13 gradient63,64. The retention of mouse B cells in the MZ also requires the interaction of αLβ2 integrin (also known as LFA1) and α4β1 integrin (also known as VLA4) on MZ B cells with intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1), respectively65, on stromal cells (BOX 2). The key role of integrins in the positioning of MZ B cells is exemplified by the phenotype of mice lacking the integrin signalling molecules PYK2, LSC (also known as ARHGEF1), DOCK2 or RAC2 (REFS 25,66–68). These mice have normal numbers of follicular B cells but low numbers of MZ B cells, and they have poor antibody responses to blood-borne antigens. Mouse MZ B cells receive further retention signals from MZ macrophages via the scavenger receptor MARCO (macrophage receptor with collagenous structure)69. It is unknown whether similar signals govern the homing and retention of human MZ B cells.
Box 2. Stromal cells of the MZ.
The spleen requires various subsets of stromal cells for its correct development and function.
Mouse stromal cells
In the developing spleen, lymphoid tissue organizer (LTo) cells expressing intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1) and mucosal addressin cell adhesion molecule 1 (MADCAM1) promote the accumulation of lymphoid tissue inducer (LTi) cells around splenic blood vessels through a mechanism involving interleukin-7 (IL-7)9,146. LTi cells lack common lineage markers, but they express the transcription factor retinoic acid receptor-related orphan receptor-γt and secrete lymphotoxin9,146. Lymphotoxin stimulates LTo cells to secrete lymphocyte- and dendritic cell-attracting chemokines such as CC-chemokine ligand 19 (CCL19) and CCL21, which drive the development of the white pulp9,146. Moreover, lymphotoxin regulates the positioning of the MADCAM1-expressing endothelial cells that delimit the marginal sinus147. Additional splenic stromal cells include marginal reticular cells (MRCs), fibroblast reticular cells (FRCs) and follicular dendritic cells (FDCs)9,146,148. MRCs are located in the marginal zone (MZ) and express the ER-TR7 antigen, ICAM1, VCAM1 and the TNF family member RANKL (receptor activator of NF-κB ligand), whereas FRCs are located in the periarteriolar lymphoid sheath and red pulp and express the ER-TR7 antigen, ICAM1, VCAM1 and podoplanin together with typical fibroblast molecules, such as desmin, α-smooth muscle actin, fibrillin, laminin and fibronectin148. MRCs and FRCs regulate immune responses by producing collagen-rich reticular fibres and chemokines that guide the trafficking of antigens and lymphocytes via dynamic conduit networks148. Finally, FDCs provide a structural scaffold for splenic follicles and regulate the trafficking and survival of B cells by releasing CXC-chemokine ligand 13 (CXCL13) and B cell-activating factor (BAFF)148. All of these stromal cells may further enhance immunity by releasing antiviral cytokines such as type I interferons in response to lymphotoxin produced by B cells149.
Human stromal cells
In humans, stromal cells expressing ICAM1, VCAM1, MADCAM1, smooth muscle actin, CD90 and thrombomodulin divide the MZ into inner and outer regions16,17. These stromal cells may interact with LTi cells during splenic organogenesis and could regulate the trafficking of antigens to the MZ16,17. Another subset of stromal cells — known as sinus-lining cells (or littoral cells) — expresses unique molecules such as FH1/FH2 domain-containing protein 1 (FHOD1) and signal-regulatory protein-α (SIRPα; also known as CD172a), as well as CD8, the mannose receptor (also known as CD206), the adhesion molecules ICAM1 and VCAM1, and the endothelial molecules PECAM1 (platelet endothelial cell adhesion molecule 1), factor VIII and von Willebrand factor16–18,98,99. The function of sinus-lining cells is not clear, but they may mediate antigen capture, neutrophil reprogramming and B cell activation, including class switching and antibody production18,98,99.
Functional properties of MZ B cells
In mice, large blood-borne antigens activate MZ B cells after being captured by DCs and granulocytes circulating in the peripheral blood or by MZ macrophages in the spleen10,14. Smaller blood-borne antigens may directly interact with mouse MZ B cells with the help of complement opsonins25,70,71. In the presence of co-stimulatory signals from innate or adaptive immune cells, antigen-activated MZ B cells rapidly differentiate into antibody-secreting plasmablasts via either a T cell-independent pathway (TI pathway) or a T cell-dependent pathway (TD pathway)4,72,73. In addition to inducing IgM production, both mouse and human MZ B cells can undergo class switching to IgG or IgA18,25,26, a process that requires the enzyme activation-induced cytidine deaminase (AID). In humans, MZ B cells also undergo SHM in the absence of immunization or infection through a poorly understood pathway that becomes active at a very early developmental phase18,23,59,74,75. Although SHM is only one of the several species-specific properties of MZ B cells, human and mouse MZ B cells also have several similarities (TABLE 1).
Table 1.
Species-specific features of MZ B cells
| Parameter | Mouse | Human | Refs |
|---|---|---|---|
| Phenotype | IgMhiIgDlowCD21hiCD23−CD1dhi | IgMhiIgDlowCD1c+CD21hiCD23−CD27+ | 5,22,36 |
| Mutational status and reactivity | Non-mutated, polyreactive | Mutated, monoreactive, probably also polyreactive | 3,5,22 |
| Localization | Splenic MZ | Splenic MZ, subcapsular sinus in lymph nodes, tonsillar epithelium, subepithelial dome of Peyer’s patches | 5,9,22 |
| Site of development | Postnatal spleen | Fetal liver, fetal mesenteric lymph nodes, postnatal spleen | 22,36 |
| Precursors | T2B cells, some T1B cells | Fetal B cells, transitional B cells, follicular B cells | 22,36 |
| Recirculation | Limited to follicular shuttling | Extensive | 5,22 |
| Homing receptors | S1P1, S1P3, CXCR5, αLβ2 integrin, α4β1 integrin | Unclear | 61–65 |
| Antibodies produced in T cell-dependent responses | IgG1 | IgM, IgG1, IgG2, IgA1 | 5,22, 138,139 |
| Antibodies produced in T cell-independent responses | IgM, IgG2b, IgG3, IgA | IgM, IgG1, IgG2, IgA2 | 5,22, 138,139 |
| Helper cells | DCs, MZ macrophages, marginal metallophilic macrophages, iNKT cells, TFH cells | NBH cells, Tcells | 5,9,148 |
| Activating signals | Capsular polysaccharides, TLR ligands, BAFF, APRIL, CD40L, complement proteins, IFNγ | Capsular polysaccharides, TLR ligands, BAFF, APRIL, CD40L, IL-21 | 5,9,148 |
APRIL, a proliferation-inducing ligand; BAFF, B cell-activating factor; CD40L, CD40 ligand; CXCR5, CXC-chemokine receptor 5; DC, dendritic cell; IFNγ, interferon-γ; iNKT, invariant natural killer T; MZ, marginal zone; NBH cell, B cell-helper neutrophil; S1P1 sphinqosine-1-phosphate receptor 1; T1, transitional stage 1; TFH, T follicular helper; TLR, Toll-like receptor.
Mouse MZ B cells
In mice, MZ B cells are ontogenetically distinct from follicular B cells and B-1 cells, selectively occupy the MZ, have an IgMhiIgDlowCD21hiCD23− CD1dhi phenotype and primarily express non-mutated immunoglobulin variable (IgV) genes, some of which encode polyreactive BCRs5,36. These BCRs recognize conserved molecular signatures that are often shared by foreign and autologous antigens3,5. As a result of this promiscuous reactivity, antibodies from mouse MZ B cells can recognize and clear bacteria from the external environment and also damaged cells from the host3,5.
Mouse MZ B cells also express high levels of TLRs, similarly to myeloid cells of the innate immune system1,6,76,77. This dual expression pattern permits mouse MZ B cells to uniquely integrate signals from both clonally distributed and germline-encoded microorganism-recognition receptors8,78. Signals generated via dual BCR and TLR engagement induce the extensive production of low-affinity antibodies by mouse MZ B cells, which bridges the temporal gap required for the production of high-affinity antibodies by follicular B cells8. In mice, similar signals regulate B cell tolerance; indeed, dysregulated co-engagement of BCR and TLR molecules by self antigens contributes to the onset of autoimmunity through the pathogenic activation of autoreactive B cells (including MZ B cells)78.
Mouse MZ B cells are also specialized in the recognition of complement opsonins via the complement receptors CD21 and CD35 and in the recognition of microbial lipids via the MHC class I-like molecule CD1d25,79,80. After recognizing a microorganism, mouse MZ B cells rapidly give rise to non-mutated antibody-secreting plasmablasts via extrafollicular TI or TD pathways (see below)4,14,79,81. However, mouse MZ B cells can also generate long-lived plasma cells that secrete high-affinity antibodies via a canonical follicular TD pathway that involves the presentation of peptide–MHC class II complexes to CD4+ T helper (TH) cells, including T follicular helper cells (TFH cells)72. Indeed, mouse MZ B cells have a high expression level of MHC class II, CD80 and CD86 molecules, which are required for the activation of TFH cells5,72. Thus, mouse MZ B cells are functionally versatile, as they respond to multiple types of microbial challenge, including TI carbohydrate antigens and TD protein antigens.
Human MZ B cells
In humans, the splenic MZ contains B cells that have an IgMhiIgDlowCD1c+CD21hiCD23− CD27+ phenotype17,18,23,82,83. As mentioned above, these B cells also inhabit the inner wall of the subcapsular sinus of lymph nodes, the epithelium of tonsillar crypts and the subepithelial dome of intestinal Peyer’s patches18–21. Similarly to the splenic MZ, these MZ-like sites are highly exposed to antigens and contain antigen-capturing macrophages and DCs, which re inforces the notion that human MZ B cells serve as front-line sentinels involved in the induction of innate-like antibody responses at the interface between the host and the environment.
Similarly to mouse MZ B cells, human MZ B cells may be able to follow a TI pathway to produce antibodies specific for microbial polysaccharides23,28,61. Accordingly, MZ B cells and polysaccharide-specific antibodies are present in patients with hyper-IgM syndrome (HIGM syndrome), an immunodeficiency syndrome that impairs TD antibody responses24,74.
However, there is also evidence pointing to the ability of human MZ B cells to participate in TD antibody responses. Indeed, IgMhiIgDlowCD1c+CD21hiCD23− CD27+ B cells recirculate, express the TNF receptor family member CD27, and contain mutated V(D)J genes, and at least some of them show molecular footprints of a past germinal centre experience84–86. All of these traits are hallmarks of classical class-switched IgM−IgD−CD1c− CD21medCD23−CD27+ memory B cells, suggesting that human MZ B cells may be equivalent to memory B cells that have retained IgM expression by exiting the germinal centre before the induction of CSR87. However, unlike human class-switched (IgG- or IgA-expressing) or unswitched (IgM-expressing) memory B cells, human MZ B cells express IgD, and they also have a distinct mechanism of IgV gene repertoire diversification during ontogeny, a different pattern of IgV gene usage, fewer IgV gene mutations, a slower rate of accumulation of IgV gene mutations, a lesser dependence on germinal centres and CD40L-expressing CD4+ T cells for the generation of IgV gene mutations, and fewer past cell divisions compared with memory B cells24,74,75. Additional studies indicate that human MZ B cells may develop during fetal life in the absence of germinal centres and undergo antigen-independent SHM by following a TI pathway59,75,88.
Compared with canonical memory B cells, the human MZ B cell population also shows a slower recovery after B cell depletion therapy, a lesser dependence on CD40 signals and a stronger dependence on TLR signals35,74,89. In this regard, patients lacking TLR signalling molecules — such as myeloid differentiation primary-response protein 88 (MYD88), TIR domain-containing adaptor protein (TIRAP) or interleukin-1 receptor-associated kinase 4 (IRAK4) — have a selective loss of MZ B cells35. This finding implies that MZ and memory B cells have distinct requirements for their development and/or survival. In particular, MZ B cells appear to be more dependent on TLR signals, which could be triggered by either self or commensal antigens.
One way to reconcile all of the divergent phenotypical, molecular and functional findings in humans is to consider IgMhiIgDlowCD1c+CD21hiCD23−CD27+ B cells as a heterogeneous population that is composed of at least two distinct subsets, including MZ B cells that develop outside the germinal centre and unswitched memory B cells that develop inside the germinal centre. These subsets probably reflect the versatile nature of human MZ B cells, which are now viewed as a population capable of proficiently mounting antibody responses to both TI and TD antigens.
Regulation of MZ B cell responses
Although they are generally thought to mount innatelike TI responses against microbial carbohydrates, MZ B cells can also initiate adaptive TD responses to microbial proteins25,72. In mice, this functional plasticity could reflect the presence in the spleen of multiple MZ B cell precursors that give rise to functionally heterogeneous subsets of MZ B cells55,56,90. Remarkably, mouse follicular B cells show a similar functional flexibility, as they can accelerate the kinetics and enhance the magnitude and quality of TI responses to microbial carbohydrates in addition to mediating conventional TD responses to protein antigens91. Thus, despite their divergent ontogeny, MZ and follicular B cells can establish a protective alliance to combat blood-borne pathogens.
Innate signals involved in MZ B cell responses to TI antigens
TD IgG and IgA responses by follicular B cells promote immune protection and memory, but they usually require 5–7 days to develop, a delay that can prove fatal in the presence of fast-replicating blood-borne pathogens, including encapsulated bacteria. In mice, MZ B cells compensate for this limitation by entering a faster TI pathway (FIG. 1), which generates short-lived plasmablasts that secrete low-affinity IgM (as well as IgG and some IgA) as early as 1–3 days after exposure to blood-borne microorganisms4,15,92. These plasmablasts are located in the red pulp of the spleen, and their rapid formation is facilitated by an elevated baseline expression in MZ B cells of transcripts encoding the plasma cell-inducing trans criptional regulator B lymphocyte-induced maturation protein 1 (BLIMP1; also known as PRDM1)4.
The mechanism underlying the state of active readiness of MZ B cells remains poorly understood, but exposure to small amounts of complement-decorated commensal antigens may play a part. Consistent with this possibility, mouse MZ B cells respond more robustly to complement proteins than follicular B cells do, partly because the NOTCH2-mediated signalling programme required for the differentiation of MZ B cells induces the robust expression of complement receptors in these cells6,93. Owing to their constitutive partial activation, MZ B cells rapidly produce antibodies in response to either type 1 TI antigens (such as LPS), which bind to the BCR and often co-engage TLRs, or type 2 TI antigens (such as polysaccharides), which induce extensive BCR crosslinking and co-engage multiple PRRs8,11,12,94.
In mice, MZ B cells become exposed to large TI antigens after interacting with metallophilic macrophages, which are strategically positioned along the marginal sinus, or with MZ macrophages, which are scattered throughout the MZ (BOX 3). These MZ-specific macrophages capture antigens through various PRRs of the scavenger receptor and CLR families10,11. Particulate TI antigens are also conveyed to mouse MZ B cells by circulating DCs that weakly express CD11c and therefore may correspond to plasmacytoid DCs14. Such DCs home to the MZ, together with neutrophils, in mice exposed to blood-borne microorganisms and, similarly to macrophages, capture whole microorganisms or fragments thereof and make them available to MZ B cells as antigenic ligands14,15. In mice, DCs further sustain TI antibody responses by providing survival signals to extrafollicular plasmablasts via BAFF and its homologue APRIL (a proliferation-inducing ligand)14,95,96.
Box 3. MZ macrophages.
Marginal zone (MZ) B cells cooperate with distinct subsets of antigen-capturing macrophages to mount protective antibody responses to blood-borne pathogens.
Mouse macrophages
In mice, there are two specific subsets of MZ macrophages. Metallophilic macrophages express the sialoadhesin CD169 (also known as SIGLEC1) and lie as sentinels in proximity of mucosal addressin cell adhesion molecule 1 (MADCAM1)-positive endothelial cells lining the marginal sinus5,9,147. Metallophilic macrophages form a ring between the MZ and the white pulp and have an important role in the clearance of blood-borne particulate antigens5,9,147. Similarly to CD169-expressing subcapsular macrophages from lymph nodes, metallophilic macrophages might activate CD1d-restricted invariant natural killer T (iNKT) cells to promote rapid antibody responses via extrafollicular B cells106,117,137. MZ macrophages are found throughout the MZ and lack CD169, but express the scavenger receptor MARCO (macrophage receptor with collagenous structure) and the endocytic receptor SIGN R1 (DC-SIGN-related protein l)9–11. These pattern-recognition receptors bind to blood-borne antigens with the help of complement and enable MZ macrophages to interact with antigens and present them to MZ B cells9–11.
Human macrophages
In humans, the MZ lacks metallophilic macrophages and MZ macrophages but contains dispersed nonspecific macrophages that express the scavenger receptor CD68 and the haemoglobin receptor CD163 (REFS 16,17). Similar nonspecific macrophages are present in the red pulp16,17. Macrophages expressing CD169 form pericapillary sheaths in the perifollicular zone, an area of low resistance adjacent to the MZ16,17. Together with neutrophils, sinus-lining cells and dendritic cells expressing CD11c and the endocytic receptor DEC205 (also known as CD205), perifollicular macrophages may capture antigens to activate MZ B cells16,18,97–99.
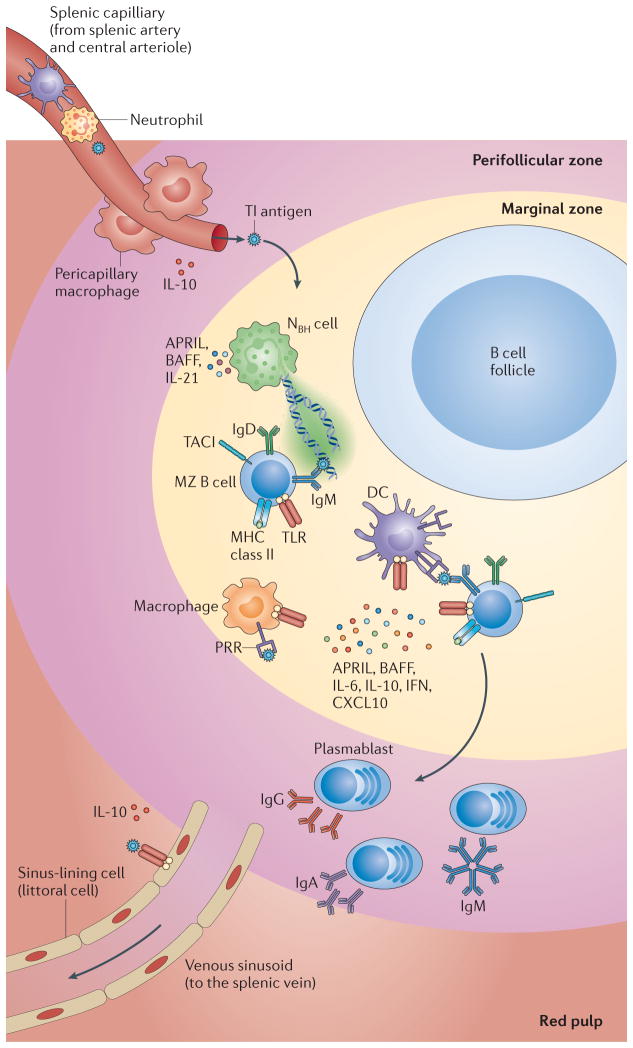
In humans, the lack of metallophilic macrophages and MZ macrophages raises questions as to how MZ B cells interact with blood-borne antigens (FIG. 2). Neutrophils, some DCs and perhaps sinus-lining cells (known as littoral cells) may compensate for the lack of MZ-specific macrophages18,97–99. Similarly to macrophages and DCs, neutrophils and sinus-lining cells release BAFF and APRIL, which promote B cell and plasma cell survival, antibody production and CSR through a TFH cell-independent pathway7,18,96,99,100.
Figure 2. T cell-independent responses by human MZ B cells.
T cell-independent (Tl) antigens are thought to enter the marginal zone (MZ) through the perifollicular zone. Once in the MZ, they may be captured by neutrophil extracellular trap (NET)-like structures emanating from B cell-helper neutrophils (NBH cells). These cells may differentiate from circulating neutrophils as a result of the production of interleukin-10 (IL-10) by perifollicular sinus-lining cells and macrophages in response to microbial Toll-like receptor (TLR) ligands. Antigen capture may also involve reticular cells, macrophages, sinus-lining cells and dendritic cells (DCs). In addition to making Tl antigens available to the B cell receptor (BCR) and TLRs on MZ B cells, antigen-capturing cells release B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL), which engage transmembrane activator and CAML interactor (TACI) on MZ B cells. NBH cells also release IL-21, thereby inducing class-switch recombination, somatic hypermutation and antibody production in MZ B cells. The generation of plasmablasts secreting IgM or class-switched IgG and IgA involves the production of IL-6, IL-10, IL-21 and CXC-chemokine ligand 10 (CXCL10) by antigen-capturing cells. Arrows indicate the putative path followed by antigens through the spleen. IFN, interferon; PALS, periarteriolar lymphoid sheath; PRR, pattern-recognition receptor.
In both mice and humans, antigen-mediated engagement of BCR and TLR molecules activates MZ B cells by cooperating with transmembrane activator and CAML interactor (TACI), which binds to both BAFF and APRIL7,8,14,18,96,101. TACI is highly expressed by MZ B cells and is further upregulated in response to microbial TLR ligands6,7,102. TACI also cooperates with TLRs to induce CSR and antibody production via a TLR-like pathway involving MYD88, IRAK1, IRAK4 and TNF receptor-associated factor (TRAF) adaptor proteins7,102. Moreover, TACI cooperates with the BCR and CD40 (REFS 7,103), suggesting that MZ B cells integrate classical innate and adaptive signalling pathways to mount rapid antibody responses.
In mice, complement opsonins decorating TI antigens deliver additional activating signals to MZ B cells via CD21 and CD35, two complement receptors that enhance BCR signalling by interfering with the expression of the inhibitory receptor programmed cell death protein 1 (PD1)11,25,80. Complement opsonins may further activate mouse MZ B cells via CD40 (REF. 104). CD40 may also be engaged by CD40L on neutrophils, natural killer (NK) cells or invariant natural killer T cells (iNKT cells)18,105,106, which provides an additional layer of complexity in the interplay between the innate and adaptive immune systems during MZ B cell responses. In general, currently available mouse and human studies indicate that MZ B cells initiate rapid responses to TI antigens by integrating adaptive signals from antigenic BCR ligands with innate helper signals from microbial TLR ligands, opsonins, TNF-like factors, cytokines and chemokines.
Innate signals involved in MZ B cell responses to TD antigens
The composition of the immunizing antigen is likely to be crucial for determining whether MZ B cells produce antibodies via the TI or the TD pathway107. In general, blood-borne bacteria express not only TI antigens, such as TLR ligands and conventional poly saccharides, but also TD antigens, such as outer-membrane proteins and zwitterionic polysaccharides4,8,15,25,42,108. Protein antigens and zwitterionic polysaccharides are processed to peptides and low-molecular- weight carbohydrates, respectively, and both are presented to CD4+ T cells through the MHC class II endocytic pathway42,109. Unlike follicular B cells, MZ B cells constitutively express elevated levels of surface MHC class II, CD80 and CD86 molecules and therefore display a robust antigen-presenting activity, at least in mice110,111. This antigen-presenting capability may partly relate to the continuous exposure of MZ B cells to microbial or self TLR ligands, which are powerful inducers of T cell co-stimulatory molecules1.
After capturing an antigen, mouse MZ B cells migrate to the PALS of the spleen to establish cognate interactions with antigen-specific naive CD4+ TH cells72,111 (FIG. 3). The ensuing germinal centre reaction probably involves the differentiation of MZ B cell-primed naive CD4+ TH cells into canonical TFH cells, with or without the help of local DCs72,112. By expressing CD40L and cytokines such as interleukin-4 (IL-4), IL-21 and interferon-γ (IFNγ), TFH cells stimulate antigen-specific B cells to undergo CSR and SHM through a CD40-dependent pathway that leads to the emergence of long-lived memory B cells and plasma cells expressing class-switched high-affinity IgG antibodies72,112. In mice, MZ B cells may further enhance follicular TD antibody responses by continuously depositing complement-decorated blood-borne antigens in the follicle. The interaction of antigens with CD21 and CD35 on MZ B cells causes the downregulation of S1P receptor expression, followed by the temporary interruption of MZ-retaining signals63,64. The resulting predominance of CXCR5 signals induces the displacement of antigen-transporting MZ B cells to the follicle63,64. After depositing antigens on FDCs, MZ B cells re-express S1P receptors and return to the MZ63,64. This shuttling involves a large proportion of mouse MZ B cells and occurs under homeostatic conditions independently of BCR engagement by antigens63,64. An important implication of these findings is that mouse MZ B cells are continuously exposed to complement-opsonized antigens, which may include commensal antigens from mucosal surfaces. By delivering microbial antigens to FDCs, MZ B cells may inform follicles as to the antigenic composition of the blood flowing through the marginal sinus, thereby facilitating the selection of germinal centre B cells that have a high affinity for those antigens.
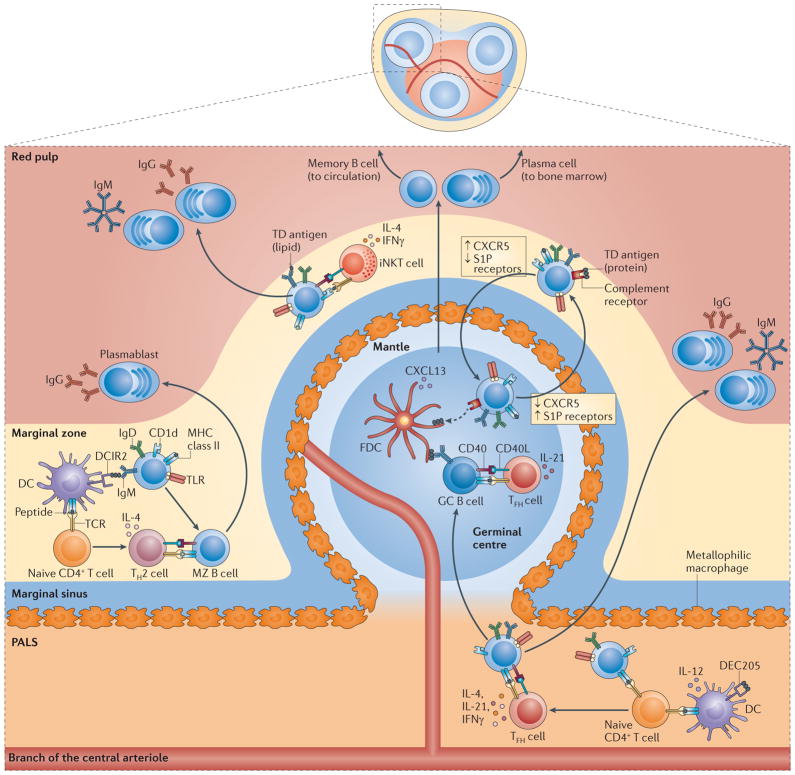
Figure 3. T cell-dependent responses by mouse MZ B cells.
In the extrafollicular T cell-dependent (TD) pathway, marginal zone (MZ) dendritic cells (DCs) expressing DC inhibitory receptor 2 (DCIR2) capture protein antigens and present them to naive CD4+ T cells, which subsequently differentiate into T helper 2 (TH2) cells expressing CD40 ligand (CD40L) and interleukin-4 (IL-4). DCIR2+ DCs also expose antigens to MZ B cells, and signals from the B cell receptor (BCR) convert MZB cells into antigen-presenting cells. By establishing cognate interactions with TH2 cells, activated MZ B cells rapidly produce low-affinity IgGl but not IgM. ln an alternative pathway, lipid-reactive MZ B cells activate invariant natural killer T (iNKT) cells by presenting CDld-loaded glycolipids. The expression of CD40L, IL-4 and interferon-γ(IFNγ) by activated iNKT cells induces MZ B cells to differentiate into plasma blasts that secrete low-affinity IgM or IgG. In the follicular TD pathway, MZB cells downregulate receptors for sphin-gosine-1-phosphate (SIP) after capturing complement-decorated protein antigens via CD21 and CD3B. The interruption of MZ-retaining signals from SIP receptors stimulates MZ B cells to enter the follicle in response to CXC-chemokine receptor 5 (CXCRB) signals. In the follicle, MZ B cells deposit antigens on follicular dendritic cells (FDCs) and thereafter upregulate SI P receptor expression to exit the follicle and return to the MZ. Protein antigens displayed by FDCs enhance TD antibody responses by promoting the selection of follicular germinal centre (GC) B cells expressing a high-affinity BCR. This selection also involves cognate interactions between GC B cells and T follicular helper (TFH) cells expressing CD40L and IL-21. Ultimately, selected GC B cells differentiate into long-lived memory B cells or plasma cells that produce high-affinity IgG. Alternatively, MZ B cells capture protein antigens with or without the help of metallophilic macrophages and migrate to the border between the periarteriolar lymphoid sheath (PALS) and the follicle to initiate a GC reaction after establishing a cognate interaction with TFH cells expressing CD40L, IL-4 or interferon-γ(IFNγ). These TFH cells probably emerge from the priming of naive CD4+T cells by either MZ B cells or DCs expressing DEC205. A subset of these TFH cells can also promote the generation of low-affinity extrafollicular IgM and IgG plasmablasts. CXCL13, CXC-chemokine ligand 13; TCR. T cell receptor; TLR, Toll-like receptor.
In mice, MZ B cells also engage in extrafollicular TD responses that rapidly give rise to short-lived plasmablasts secreting low-affinity IgM and/or IgG antibodies73,108 (FIG. 3). Recent findings suggest that the isotypic composition of this extrafollicular response is regulated by distinct subsets of DCs73. MZ-based DCs expressing the CLR DC inhibitory receptor 2 (DCIR2; also known as CLEC4A4) selectively stimulate rapid IgG1 but not IgM production by mouse MZ B cells73. DCIR2+ DCs make protein antigens available to MZ B cells as BCR ligands, which activate MZ B cells and induce them to become potent antigen-presenting cells. Such MZ B cells establish cognate interactions with CD4+ T cells, which subsequently undergo robust proliferation in response to DCIR2+ DCs. The TH2 cells emerging from these interactions selectively induce IgG1 production by stimulating MZ B cells via CD40L and cytokines (including IL-4)73. Remarkably, DCIR2+ DCs fail to induce affinity maturation through a TFH cell-controlled germinal centre reaction, unless they are exposed to certain microbial TLR ligands73. Unlike MZ-localized DCIR2+ DCs, DCs in the PALS express the CLR DEC205 (also known as LY75) and may stimulate low-affinity extrafollicular or high-affinity follicular IgM and IgG responses by activating MZ B cells through a pathway involving IL-12-induced TFH cells73,108,113–115.
In mice, rapid extrafollicular TD antibody responses may also involve the cognate interaction of lipid-specific MZ B cells with iNKT cells106,116,117. These cells express an invariant Vα14+ TCR that binds to microbial glycolipids presented by CD1d on MZ B cells106,117. The ensuing activation of iNKT cells causes the upregulation of CD40L expression and the secretion of IFNγ, which elicit the production of low-affinity IgM and IgG antibodies by MZ B cells106,116,117. Activated iNKT cells have recently been shown to modulate the T cell-suppressive activity of IL-10-secreting neutrophils, at least in mice118. Perifollicular neutrophils from human spleens also have T cell-suppressive activity in addition to MZ B cell helper function (see below)18, which raises the possibility that iNKT cells establish a dialogue with splenic neutrophils to modulate the differentiation of MZ B cells along TD or TI pathways. Overall, these findings indicate that MZ B cells interact with multiple cells of the innate immune system to mount TD antibody responses.
Innate signals involved in MZ B cell differentiation to plasmablasts
In mice, antigen-activated MZ B cells differentiate into plasmablasts that migrate to the red pulp in response to CXCL12, which is a CXCR4 ligand expressed by stromal cells, macrophages, neutrophils and DCs in the red pulp and perifollicular zone18,119,120. The homing of mouse MZ B cell-derived plasmablasts to the red pulp also requires the downregulation of their expression of integrins, S1P receptors, CXCR5 (a receptor for CXCL13) and CC-chemokine receptor 7 (CCR7; a receptor for CCL19 and CCL21) through a mechanism that involves the stimulation of these cells by microbial TLR ligands63,119. Once in the red pulp, plasma blasts receive trophic signals from multiple innate cells, including red-pulp DCs, macrophages, neutrophils, stromal cells and sinus-lining cells. In both mice and humans, these innate cells release BAFF and APRIL, which support the maturation and survival of plasmablasts by engaging TACI and B cell maturation antigen (BCMA)7,14,18,95,99,121.
In humans, DCs upregulate their release of BAFF and APRIL in response to microbial TLR ligands and cytokines such as type I IFNs and IFNγ96,100,122. In mice, IFNγ stimulates DCs to produce BAFF through a signal transducer and activator of transcription 1 (STAT1)-dependent pathway that is negatively regulated by autoimmune regulator (AIRE), a transcription factor mostly known for its role in thymic negative selection123. In humans, plasmablasts receive additional maturation and survival signals from IL-6, type I IFNs and CXCL10 (REFS 124,125). CXCL10 is a CXCR3 ligand that stimulates activated B cells to release IL-6, which in turn enhances CXCL10 production by a subset of macrophages expressing the haemoglobin receptor CD163 (REF. 125). Thus, MZ B cell-derived plasmablasts require a multicomponent survival niche to deliver their effector functions in the red pulp. Inhibitory signals restricting the lifespan of these plasmablasts must also be involved, but their nature remains obscure.
MZ B cell diversification via CSR
Although usually associated with potent IgM responses, MZ B cells also produce class-switched IgG and IgA under steady-state conditions and after immunization. Whereas IgM is mostly associated with the neutralization and complement-mediated killing of microorganisms, IgG and IgA amplify the phagocytosis and killing of opsonised microorganisms and thereby provide MZ B cells with additional effector functions. But how do MZ B cells undergo CSR?
Neutrophils trigger CSR in MZ B cells
A fraction of mouse and human MZ B cells produce class-switched antibodies under steady-state conditions, possibly in response to TI antigens from mucosal surfaces, including the intestine18,44,48,126. In humans, this process is associated with the induction of CSR by a unique subset of neutrophils that interact with MZ B cells through a non-inflammatory pathway that begins during fetal life and accelerates after birth, a time that coincides with the colonization of mucosal surfaces by commensal bacteria18. Crosstalk between neutrophils and MZ B cells may seem surprising, but it is consistent with studies showing that neutrophils release BAFF and APRIL127. Unlike circulating conventional neutrophils, splenic neutrophils deliver powerful antibody-inducing signals to human MZ B cells, and therefore we have termed them B cell-helper neutrophils (NBH cells)18.
Compared with conventional neutrophils, NBH cells have similar morphological and ultrastructural features, but they have a distinct phenotype, gene expression profile and function. In particular, human NBH cells express higher levels of BAFF, APRIL, CD40L, IL-6 and IL-21, produce more plasmablast-attracting factors (such as CXCL12) and spontaneously activate MZ B cells18. These unique features probably reflect the activation and reprogramming of NBH cells by local microenvironmental signals18. Consistent with this possibility, the accumulation of NBH cells in peri-MZ areas coincides with postnatal deposition of discrete amounts of microbial products of probable mucosal origin (such as LPS) in the peri-MZ18,48. In addition to activating NBH cells, LPS and other microbial TLR ligands stimulate perifollicular sinus-lining cells to express neutrophil-attracting chemokines (such as CXCL1, CXCL2, CXCL3, CXCL6 and CXCL8), which may contribute to the recruitment of NBH cells18. Accordingly, a lack of TLR signals leads to a dramatic reduction in the number of NBH cells and MZ B cells18,35.
Microbial TLR ligands may also stimulate the reprogramming of human conventional neutrophils into NBH cells by eliciting the release of non-inflammatory STAT3-inducing cytokines such as IL-10 from perifollicular sinus-lining cells and macrophages18. Considering that IL-10 provides regulatory signals to neutrophils128, this cytokine may be instrumental in generating antibody-inducing NBH cells devoid of inflammatory activity18. The generation and/or maintenance of NBH cells may also require granulocyte–macrophage colony-stimulating factor (GM-CSF), a neutrophil-activating STAT3- and STAT5-inducing cytokine produced by innate response activator B cells129.
In humans, NBH cells induce AID expression, class switching to IgG and IgA, and the generation of antibody-secreting plasmablasts by activating MZ B cells through a TI pathway involving BAFF, APRIL and IL-21 (REF. 18). In agreement with these in vitro findings, patients with neutropenia, defective TACI signalling or impaired STAT3 signalling have fewer MZ B cells and reduced steady-state production of IgG and IgA specific for various TI antigens, including LPS18. By contrast, neutropenic patients show normal steady-state production of IgG and IgA specific for TD antigens18; such antibodies are usually derived from long-lived plasma cells located in the bone marrow. In mice, these plasma cells require APRIL-mediated signals from eosinophils to survive in the bone marrow130. Thus, there may be a division of labour among different granulocyte subsets for the maintenance of antibody-secreting cells in distinct lymphoid districts.
Optimal stimulation of human MZ B cells by NBH cells may involve neutrophil extracellular trap (NET)-like structures18. By capturing commensal antigens, NETs could enhance the provision of BCR and TLR ligands to MZ B cells18. Moreover, NETs may further stimulate MZ B cells by delivering endogenous TLR ligands, including self DNA131,132. The stimulation of MZ B cells by NBH cells could involve a largely TI pathway, because spleens from adults contain neither TFH cells nor active germinal centres75,114. This possibility is further suggested by the observation that human NBH cells strongly suppress CD4+ T cell proliferation in a contact-independent manner, at least in vitro18. However, there is also the possibility that, in the presence of appropriate pro-inflammatory signals, NBH cells acquire antigen-presenting cell function to enhance TD antibody production.
DCs, macrophages and iNKT cells trigger CSR in MZ B cells
In mice, MZ B cells are thought to receive TLR-mediated CSR-inducing signals from soluble microbial products or whole microorganisms captured by MZ macrophages or DCs. Consistent with this possibility, mouse MZ B cells produce IgG3 but also IgG2 in response to infection-associated LPS8,93. This type 1 TI antigen induces CD40-independent CSR through an extrafollicular pathway that involves dual BCR and TLR engagement8. Mouse MZ B cells also produce IgG3 and possibly IgA in response to polysaccharides92. In humans, these type 2 TI antigens may trigger CD40-independent CSR through an extrafollicular pathway involving co-engagement of the BCR and carbohydrate receptors such as CLRs133. In addition to BAFF and APRIL, TI CSR may require macrophage- and DC-derived cytokines such as type I IFNs and transforming growth factor-β1 (TGFβ1) in both mice and humans96,134–136.
Mouse MZ B cells also produce IgG1 and IgG2 in response to microbial proteins72,73,108. These TD antigens trigger CSR through follicular and extrafollicular pathways that involve CD40L-expressing TFH or TH2 cells8,72,73,108. Mouse MZ B cells also produce IgG2 and IgG3 in response to CD1d-binding glycolipids106,116. These molecules induce CD40-dependent CSR through an extrafollicular pathway involving CD1d-restricted iNKT cells106,117. In this pathway, splenic MZ B cells function as antigen-presenting cells in a similar manner to CD169-expressing subcapsular macrophages from lymph nodes137. In all of these TD pathways, the induction of CSR in MZ B cells requires the cooperation of CD40L with IL-4, IL-21 and IFNγ from TFH, TH2 or iNKT cells73,106,114,116.
Although not formally proven, human MZ B cells may also undergo CSR following the immunization of the host with TI or TD antigens. In particular, type 2 TI antigens (such as polysaccharides) induce the production of IgG1, as well as that of IgG2 and IgA2, two subclasses preferentially produced by B cells in response to TI signals18,24,122,138,139. Remarkably, human MZ B cells secrete large amounts of IgA after undergoing CSR in response to BAFF, APRIL and IL-21 from NBH cells18. The functional advantage of systemic IgA production by MZ B cells remains poorly understood, but this response could provide a secondary line of non-inflammatory IgA-mediated defence against commensal bacteria that breach the mucosal barrier18.
MZ B cell diversification via SHM
Mouse MZ B cells seem to primarily express non-mutated immunoglobulin V(D)J genes3,5, whereas some rat MZ B cells harbour small numbers of mutations126,140. Low-level SHM has also been reported in B cells from BCR-transgenic mice exposed to TI antigens such as (4-hydroxy-3-nitrophenyl)acetyl–Ficoll or to self antigens such as IgG–nucleic acid complexes141,142. In humans, most MZ B cells contain mutations of the more limited extrafollicular variety, but the underlying mechanism is unclear23,24. One line of thought is that human MZ B cells correspond to memory B cells that exited the germinal centre after accumulating some mutations but before undergoing CSR87. This suggestion explains the follicular mutational pattern of some MZ B cells86, but is inconsistent with the observation that patients with HIGM syndrome, who have impaired germinal centres, retain mutated MZ B cells24,74. The unconventional nature of SHM in human MZ B cells is also suggested by the finding that these cells have molecular footprints of past proliferation in an extrafollicular environment and accumulate mutations during fetal life in the absence of germinal centres and independently of antigenic stimulation24,59,75.
Innate SHM in fetal MZ B cells
Human MZ B cells undergo SHM at a very early developmental stage through a mechanism that does not require the presence of an anatomical MZ59. Indeed, MZ B cells producing mutated antibodies can be detected in fetuses and infants that physiologically lack the MZ59,75. Studies in humanized mice and human fetal tissues indicate that MZ B cells undergo SHM in the fetal liver and mesenteric lymph nodes through a TI pathway involving AID but not antigens59,88. Remarkably, this innate pathway requires NOTCH2 (REF. 59), which is also necessary for the development of MZ B cells in mice57. The implication of these findings is that human MZ B cells may be generated through innate post-rearrangement immuno globulin gene diversification events similar to those present in birds and some mammals (including rabbits)22. In these species, the IgV genes of B cell precursors located in gut-associated lymphoid tissues undergo post-rearrangement SHM and/or gene conversion to generate a ready-to-use pre-immune antibody repertoire capable of rapidly recognizing microbial antigens22,143. The challenge ahead lies in the identification of the innate signals that diversify the human MZ B cell repertoire before birth.
Innate SHM in adult MZ B cells
The SHM machinery of human MZ B cells remains functional after birth, as MZ B cells accumulate more mutations through antigen-dependent and antigen-independent mechanisms in both children and adults23,75. The splenic MZ may be involved in the induction of these mutations, because it contains foci of proliferating and clonally related B cells, some of which express AID18,20. These MZ B cells may undergo limited SHM via a TI pathway involving the recognition of commensal antigens trapped on NETlike structures formed by NBH cells18. Consistent with this possibility, SHM is decreased in MZ B cells from neutropenic patients, whereas SHM is normal in MZ B cells from patients with HIGM syndrome, who have impaired TD antibody responses18,74. In agreement with findings indicating that microbial TLR ligands control the number of splenic NBH cells with SHM-inducing activity18, TLR ligation induces SHM in MZ B cell precursors60,144,145.
However, signals from non-TLR PRRs may have an even more important role, because MZ B cells from patients with defective TLR signalling have normal levels of mutations in their immunoglobulin genes35. In principle, a slow postnatal induction of SHM via an extrafollicular TI pathway involving MZ B cell helper signals from commensal microbial products and innate immune cells may be instrumental for increasing the diversity of the pre-immune antibody repertoire of MZ B cells after birth. Immunization or infection would introduce additional mutations via extrafollicular or follicular TD pathways that probably involve MZ B cell helper signals from TFH cells86. The combination of these TI and TD pathways would ensure the development of a highly diversified repertoire of ready-to-use MZ B cells in both children and adults.
Concluding remarks
Follicular B cells generate protective responses and long-term immune memory by producing highly specific antibodies. The price for this specificity is that rousing follicular B cells takes time, as plasma cells emerging from the germinal centre reaction do not produce high-affinity antibodies until several days after infection. MZ B cells fill this gap through their ability to deploy numerous innate immune defensive strategies, including the rapid sensing of microorganisms via both germline-encoded and somatically recombined receptors and robust antigen-presenting cell activity. As a result of their prominent innate immune functions, MZ B cells rapidly generate low-affinity antibodies not only via TI pathways, but also via TD pathways that were previously ascribed solely to follicular B cells.
Despite recent advances, there are several questions that remain to be answered regarding MZ B cells. In humans, at least some MZ B cells develop during fetal and early postnatal life, but the nature of the innate signals that regulate this process remains unclear59,75. During adulthood, innate immune signals enhance the survival and diversification of MZ B cells, but the origin of these signals is also poorly understood. Self antigens and/or microbial TLR ligands from the commensal microbiota may have a role18,35,60,145. The latter possibility echoes earlier studies suggesting that microbial TLR signals are central to the establishment of long-term serological memory and natural antibody production40,131,143.
Of note, some natural antibodies target key microbial epitopes, blood-group antigens and self antigens, and a better understanding of the innate signals underpinning their production by MZ B cells may have a beneficial impact in multiple clinical settings, including immunodeficiencies, autoimmunity, transplantation and transfusion medicine. More generally, a deeper knowledge of the innate signals involved in the ontogeny, reactivity, diversification and activation of MZ B cells will be important for fully elucidating the protective role of MZ B cells against infections. This information could be used to develop novel MZ B cell-harnessing strategies aimed at improving the protective activity of vaccines.
Acknowledgments
The authors are supported by European Research Council 2011 Advanced Grant 20110310 (A.C.); US National Institutes of Health research grants AI074378, AI61093, AI95613 and AI96187 (A.C.); Ministerio de Ciencia e Innovación grant SAF 2008–02725 (A.C.); and the Juan de la Cierva programme (I.P.).
Glossary
- Toll-like receptors (TLRs)
A large family of germline-encoded pattern-recognition receptors that initiate innate and adaptive immune responses after recognizing conserved molecular signatures associated with microorganisms
- B cell receptors (BCRs)
A term that refers to transmembrane immunoglobulin molecules irrespective of their heavy-chain constant region
- B-1 cells
IgMhiIgDlowMAC1+B220lowCD23− cells that are dominant in the peritoneal and pleural cavities. Their precursors develop in the fetal liver and omentum, and in adult mice the size of the B-1 cell population is kept constant owing to the self-renewing capacity of these cells. B-1 cells recognize self components, as well as common bacterial antigens, and they secrete antibodies that tend to have a low affinity and broad specificity
- Metallophilic macrophages
A subset of macrophages that is located at the border of the white pulp and the marginal zone of the spleen. These cells are stained by silver impregnation, which explains the name
- C-type lectin receptors (CLRs)
A large family of germline-encoded pattern-recognition receptors that initiate innate and adaptive immune responses after recognizing specific conserved molecular signatures associated with microorganisms
- Peyer’s patches
Groups of lymphoid nodules present in the small intestine that are massed together on the intestinal wall, opposite the line of attachment of the mesentery. Peyer’s patches consist of a subepithelial dome area, B cell follicles and interfollicular T cell zones
- Class-switch recombination (CSR)
A switch in the DNA that encodes the constant region of the immunoglobulin heavy chain, from Cμ (which encodes the constant region of IgM) to Cγ, Cα or Cε, which encode the constant regions of IgG, IgA and IgE, respectively. This is accomplished through an intrachromosomal deletional rearrangement
- Somatic hypermutation (SHM)
A unique mutation mechanism that is targeted to the variable regions of rearranged immunoglobulin gene segments. Combined with selection for B cells that produce high-affinity antibodies, SHM leads to the affinity maturation of B cells in germinal centres
- Congenital asplenia
A genetic deficiency of unknown origin in humans that results in the absence of splenic development and is frequently associated with cardiovascular malformations
- Natural antibodies
Antibodies spontaneously released by B-1 cells and marginal zone B cells in the absence of immunization or infection. These antibodies have a low affinity and broad specificity for conserved self and microbial antigens and mostly belong to the IgM class, but also include IgG and IgA, particularly in humans
- Transitional B cells
Refers to transitional stage 1 (T1) and transitional stage 2 (T2) B cells, which are immature, newly formed B cells that are present in the bone marrow, blood and spleen and give rise to marginal zone and follicular B cells
- Follicular dendritic cells (FDCs)
Cells with a dendritic morphology that are present in lymph nodes. These cells display on their surface intact antigens that are held in immune complexes, and B cells in the lymph node can interact with these antigens. FDCs are of non-haematopoietic origin and are not related to dendritic cells
- T cell-independent pathway (TI pathway)
A pathway followed by B cells to produce antibodies specific for TI antigens such as microbial LPS and capsular polysaccharides. The pathway usually involves extrafollicular marginal zone B cells and B-1 cells and does not require help from T cells
- T cell-dependent pathway (TD pathway)
A pathway followed by B cells to produce antibodies specific for TD antigens such as microbial proteins. The pathway usually involves follicular B cells and requires help from T cells expressing CD40 ligand. However, extrafollicular marginal zone B cells can also respond to TD antigens
- AID (Activation-induced cytidine deaminase)
A DNA cytidine deaminase that mediates somatic hypermutation and class-switch recombination
- T follicular helper cells
(TFH cells). CD4+ T helper cells that are essential for the induction of class switching in the germinal centres of secondary follicles during antibody responses to T cell-dependent antigens
- Hyper-IgM syndrome (HIGM syndrome)
A congenital immunodeficiency characterized by defective immunoglobulin heavy-chain class switching and increased IgM production. The underlying molecular defect involves CD40 ligand (in HIGM1), activation-induced cytidine deaminase (in HIGM2), CD40 (in HIGM3), uracil DNA glycosylase (in HIGM4) or other unknown B cell proteins (in HIGM5)
- Germinal centre
A lymphoid structure that arises in follicles after exposure to T cell-dependent protein antigens. This structure fosters the induction of B cell clonal expansion, class-switch recombination and somatic hypermutation, followed by the development of memory B cells and long-lived plasma cells that express high-affinity antibodies
- Invariant natural killer T cells (iNKT cells)
A subset of innate-like lymphocytes expressing an invariant Vα14+ T cell receptor that recognizes soluble glycolipids loaded on the non-polymorphic MHC class I-like molecule CD1d. In humans, CD1c might serve the same function that CD1d has in mice
- Innate response activator B cells
B-1 cell-derived plasmablasts that populate perifollicular areas of both mouse and human spleens and secrete the cytokine GM-CSF
- Neutrophil extracellular trap (NET)
A set of extracellular fibres produced by an activated neutrophil to ensnare invading microorganisms. NETs enhance neutrophil-mediated killing of extracellular pathogens while minimizing damage to host cells
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Andrea Cerutti’s homepage 1:
http://www.icrea.cat/Web/ScientificStaff/Andrea-Cerutti-452
Andrea Cerutti’s homepage 2:
http://www.mountsinai.om/profiles/andrea-cerutti
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nature Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 4.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 5.Martin F, Kearney JF. Marginal-zone B cells. Nature Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. References 3 and 5 describe the function, ontogeny and reactivity of MZ B cells. [DOI] [PubMed] [Google Scholar]
- 6.Treml LS, et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 7.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nature Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. This was the first demonstration that BAFF and APRIL activate B cells through a signalling pathway involving the TLR-associated protein MYD88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pone EJ, et al. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-κB pathway. Nature Commun. 2012;3:767. doi: 10.1038/ncomms1769. This was the first demonstration that TI antigens trigger B cell activation and class switching by co-engaging BCRs and TLRs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mebius RE, Kraal G. Structure and function of the spleen. Nature Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. This article provides an overview of the structure, vascularization, cell composition and function of the spleen in mice and humans. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, et al. Defective microarchitecture of the spleen marginal zone and impaired response to a thymus-independent type 2 antigen in mice lacking scavenger receptors MARCO and SR-A. J Immunol. 2005;175:8173–8180. doi: 10.4049/jimmunol.175.12.8173. [DOI] [PubMed] [Google Scholar]
- 11.Kang YS, et al. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. This work shows that MZ macrophages generate innate immune responses against encapsulated bacteria via an antibody-independent mechanism involving the uptake receptor SIGNR1 and an unusual complement activation pathway. [DOI] [PubMed] [Google Scholar]
- 12.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nature Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. This article provides an overview of the localization and mechanisms of antigen recognition by B cells. [DOI] [PubMed] [Google Scholar]
- 14.Balázs M, Martin F, Zhou T, Kearney JF. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 15.Colino J, Shen Y, Snapper CM. Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein- and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J Exp Med. 2002;195:1–13. doi: 10.1084/jem.20011432. References 14 and 15 demonstrate that DCs interact with MZ B cells to induce antibody responses against blood-borne microorganisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiniger B, Barth P, Hellinger A. The perifollicular and marginal zones of the human splenic white pulp: do fibroblasts guide lymphocyte immigration? Am J Pathol. 2001;159:501–512. doi: 10.1016/S0002-9440(10)61722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steiniger B, Timphus E, Barth P. The splenic marginal zone in humans and rodents: an enigmatic compartment and its inhabitants. Histochem Cell Biol. 2006;126:641–648. doi: 10.1007/s00418-006-0210-5. [DOI] [PubMed] [Google Scholar]
- 18.Puga I, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nature Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. This paper indicates that neutrophils provide unconventional help to human MZ B cells to generate antibodies specific for conserved microbial structures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer J, Finn T, Pulford KA, Mason DY, Isaacson PG. The human gut contains a novel population of B lymphocytes which resemble marginal zone cells. Clin Exp Immunol. 1985;62:607–612. [PMC free article] [PubMed] [Google Scholar]
- 20.Tierens A, Delabie J, Michiels L, Vandenberghe P, De Wolf-Peeters C. Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood. 1999;93:226–234. [PubMed] [Google Scholar]
- 21.Dono M, et al. Heterogeneity of tonsillar subepithelial B lymphocytes, the splenic marginal zone equivalents. J Immunol. 2000;164:5596–5604. doi: 10.4049/jimmunol.164.11.5596. [DOI] [PubMed] [Google Scholar]
- 22.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. This article provides an updated overview of the differences between human and mouse MZ B cells. [DOI] [PubMed] [Google Scholar]
- 23.Weller S, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. This was the first demonstration that MZ B cells recirculate in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkowska MA, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118:2150–2158. doi: 10.1182/blood-2011-04-345579. This work provides molecular evidence that human MZ B cells are distinct from canonical memory B cells and may originate outside the germinal centre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nature Immunol. 2000;1:31–36. doi: 10.1038/76882. This work demonstrates the essential role of MZ B cells in early antibody responses to TI antigens in mice. [DOI] [PubMed] [Google Scholar]
- 26.MacLennan IC, et al. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 27.Amlot PL, Hayes AE. Impaired human antibody response to the thymus-independent antigen, DNPFicoll, after splenectomy. Implications for post-splenectomy infections. Lancet. 1985;1:1008–1011. doi: 10.1016/s0140-6736(85)91613-7. [DOI] [PubMed] [Google Scholar]
- 28.Kruetzmann S, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castagnola E, Fioredda F. Prevention of life-threatening infections due to encapsulated bacteria in children with hyposplenia or asplenia: a brief review of current recommendations for practical purposes. Eur J Haematol. 2003;71:319–326. doi: 10.1034/j.1600-0609.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 30.Wasserstrom H, Bussel J, Lim LC, Cunningham-Rundles C. Memory B cells and pneumococcal antibody after splenectomy. J Immunol. 2008;181:3684–3689. doi: 10.4049/jimmunol.181.5.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Sabatino A, et al. Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am J Gastroenterol. 2005;100:1788–1795. doi: 10.1111/j.1572-0241.2005.41939.x. References 27–31 indicate that human MZ B cells mediate protective antibody responses to encapsulated bacteria. [DOI] [PubMed] [Google Scholar]
- 32.Traggiai E, et al. Selective preservation of bone marrow mature recirculating but not marginal zone B cells in murine models of chronic inflammation. PLoS ONE. 2010;5:e11262. doi: 10.1371/journal.pone.0011262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carsetti R, et al. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. 2005;115:412–417. doi: 10.1016/j.jaci.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 34.Hart M, et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol. 2007;178:8212–8220. doi: 10.4049/jimmunol.178.12.8212. [DOI] [PubMed] [Google Scholar]
- 35.Weller S, et al. IgM+IgD+CD27+ B cells are markedly reduced in IRAK-4-, MyD88- and TIRAP- but not UNC-93B-deficient patients. Blood. 2012;120:4992–5001. doi: 10.1182/blood-2012-07-440776. This was the first demonstration that the maintenance of human MZ B cells is highly dependent on signals from TLRs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nature Rev Immunol. 2009;9:767–777. doi: 10.1038/nri2656. This article provides an overview of the ontogeny of MZ B cells. [DOI] [PubMed] [Google Scholar]
- 37.Szomolanyi-Tsuda E, Welsh RM. T-cell-independent antiviral antibody responses. Curr Opin Immunol. 1998;10:431–435. doi: 10.1016/s0952-7915(98)80117-9. [DOI] [PubMed] [Google Scholar]
- 38.Gatto D, Ruedl C, Odermatt B, Bachmann MF. Rapid response of marginal zone B cells to viral particles. J Immunol. 2004;173:4308–4316. doi: 10.4049/jimmunol.173.7.4308. [DOI] [PubMed] [Google Scholar]
- 39.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-α-galactosyl specificity. J Exp Med. 1984;160:1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ochsenbein AF, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 41.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nature Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 42.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Wei B, et al. Resident enteric microbiota and CD8+ T cells shape the abundance of marginal zone B cells. Eur J Immunol. 2008;38:3411–3425. doi: 10.1002/eji.200838432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarke TB, et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nature Med. 2010;16:228–231. doi: 10.1038/nm.2087. This was the first demonstration that molecules from the commensal microbiota enhance innate immunity after translocating from the intestine to the general circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamouse-Smith ES, Tzeng A, Starnbach MN. The intestinal flora is required to support antibody responses to systemic immunization in infant and germ free mice. PLoS ONE. 2011;6:e27662. doi: 10.1371/journal.pone.0027662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bos NA, et al. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol. 1989;19:2335–2339. doi: 10.1002/eji.1830191223. [DOI] [PubMed] [Google Scholar]
- 47.Butler JE, et al. Antibody repertoire development in fetal and neonatal piglets. II Characterization of heavy chain complementarity-determining region 3 diversity in the developing fetus. J Immunol. 2000;165:6999–7010. doi: 10.4049/jimmunol.165.12.6999. [DOI] [PubMed] [Google Scholar]
- 48.Kool J, et al. Detection of intestinal flora-derived bacterial antigen complexes in splenic macrophages of rats. J Histochem Cytochem. 1994;42:1435–1441. doi: 10.1177/42.11.7930525. [DOI] [PubMed] [Google Scholar]
- 49.Krueger JM, et al. Peptidoglycans as promoters of slow-wave sleep. II Somnogenic and pyrogenic activities of some naturally occurring muramyl peptides; correlations with mass spectrometric structure determination. J Biol Chem. 1984;259:12659–12662. [PubMed] [Google Scholar]
- 50.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 51.Haas A, et al. Systemic antibody responses to gut commensal bacteria during chronic HIV-1 infection. Gut. 2011;60:1506–1519. doi: 10.1136/gut.2010.224774. [DOI] [PubMed] [Google Scholar]
- 52.Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends Immunol. 2003;24:343–349. doi: 10.1016/s1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 53.Mackay F, Schneider P. Cracking the BAFF code. Nature Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 54.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 55.Cariappa A, et al. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 56.Wen L, et al. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23:297–308. doi: 10.1016/j.immuni.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Tanigaki K, et al. Notch–RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nature Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 58.Hampel F, et al. CD19-independent instruction of murine marginal zone B-cell development by constitutive Notch2 signaling. Blood. 2011;118:6321–6331. doi: 10.1182/blood-2010-12-325944. References 54–58 show that signals from the BCR and NOTCH2 are important for the development of MZ B cells. [DOI] [PubMed] [Google Scholar]
- 59.Scheeren FA, et al. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J Exp Med. 2008;205:2033–2042. doi: 10.1084/jem.20070447. This works show that human MZ B cells develop during fetal life and undergo SHM through an antigen-independent process that does not require T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Capolunghi F, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 61.Tsuiji M, et al. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Isnardi I, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cinamon G, et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nature Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 64.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster J. G Follicular shuttling of marginal zone B cells facilitates antigen transport. Nature Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. References 63–65 elucidate the mechanisms by which MZ B cells localize in the splenic MZ and transport antigens to the follicle. [DOI] [PubMed] [Google Scholar]
- 66.Fukui Y, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 67.Croker BA, et al. The Rac2 guanosine triphosphatase regulates B lymphocyte antigen receptor responses and chemotaxis and is required for establishment of B-1a and marginal zone B lymphocytes. J Immunol. 2002;168:3376–3386. doi: 10.4049/jimmunol.168.7.3376. [DOI] [PubMed] [Google Scholar]
- 68.Rubtsov A, et al. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 2005;23:527–538. doi: 10.1016/j.immuni.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 69.Karlsson MC, et al. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 71.Test ST, Mitsuyoshi J, Connolly CC, Lucas AH. Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide. Infect Immun. 2001;69:3031–3040. doi: 10.1128/IAI.69.5.3031-3040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J Exp Med. 2003;198:1923–1935. doi: 10.1084/jem.20031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chappell CP, Draves KE, Giltiay NV, Clark EA. Extrafollicular B cell activation by marginal zone dendritic cells drives T cell-dependent antibody responses. J Exp Med. 2012;209:1825–1840. doi: 10.1084/jem.20120774. References 72 and 73 demonstrate that MZ B cells respond to protein antigens by following follicular or extrafollicular TD pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weller S, et al. CD40–CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci USA. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weller S, et al. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med. 2008;205:1331–1342. doi: 10.1084/jem.20071555. References 74 and 75 provide evidence that human MZ B cells undergo somatic diversification via a CD40-independent pathway that may not require antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Genestier L, et al. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178:7779–7786. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- 77.Rubtsov AV, et al. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J Immunol. 2008;180:3882–3888. doi: 10.4049/jimmunol.180.6.3882. [DOI] [PubMed] [Google Scholar]
- 78.Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nature Rev Immunol. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belperron AA, Dailey CM, Bockenstedt LK. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J Immunol. 2005;174:5681–5686. doi: 10.4049/jimmunol.174.9.5681. [DOI] [PubMed] [Google Scholar]
- 80.Haas KM, Poe JC, Tedder TF. CD21/35 promotes protective immunity to Streptococcus pneumoniae through a complement-independent but CD19-dependent pathway that regulates PD-1 expression. J Immunol. 2009;183:3661–3671. doi: 10.4049/jimmunol.0901218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia de Vinuesa C, O’Leary P, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur J Immunol. 1999;29:1314–1323. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 82.Timens W, Boes A, Poppema S. Human marginal zone B cells are not an activated B cell subset: strong expression of CD21 as a putative mediator for rapid B cell activation. Eur J Immunol. 1989;19:2163–2166. doi: 10.1002/eji.1830191129. [DOI] [PubMed] [Google Scholar]
- 83.Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188:1691–1703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dunn-Walters DK, Isaacson PG, Spencer J. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J Exp Med. 1995;182:559–566. doi: 10.1084/jem.182.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seifert M, Kuppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+) CD27+ B cells and the dynamics of memory B cell generation. J Exp Med. 2009;206:2659–2669. doi: 10.1084/jem.20091087. This work provides molecular evidence that human MZ B cells originate in a germinal centre reaction and may therefore be considered to be bona fide IgM+ memory B cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or “memory” B cells? J Immunol. 2007;179:13–19. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- 88.Kuraoka M, et al. Activation-induced cytidine deaminase expression and activity in the absence of germinal centers: insights into hyper-IgM syndrome. J Immunol. 2009;183:3237–3248. doi: 10.4049/jimmunol.0901548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377–2386. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- 90.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. J Exp Med. 2008;205:2043–2052. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swanson CL, et al. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med. 2010;207:1485–1500. doi: 10.1084/jem.20092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Do RK, et al. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med. 2000;192:953–964. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 94.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 95.Garcia De Vinuesa C, et al. Dendritic cells associated with plasmablast survival. Eur J Immunol. 1999;29:3712–3721. doi: 10.1002/(SICI)1521-4141(199911)29:11<3712::AID-IMMU3712>3.0.CO;2-P. Together with references 14 and 81, this work demonstrates that DCs enhance antibody responses to TI antigens by supporting the survival of extrafollicular plasmablasts derived from MZ B cells. [DOI] [PubMed] [Google Scholar]
- 96.Litinskiy MB, et al. Antigen presenting cells induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nature Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pack M, et al. DEC-205/CD205+ dendritic cells are abundant in the white pulp of the human spleen, including the border region between the red and white pulp. Immunology. 2008;123:438–446. doi: 10.1111/j.1365-2567.2007.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ogembo JG, et al. SIRPα/CD172a and FHOD1 are unique markers of littoral cells, a recently evolved major cell population of red pulp of human spleen. J Immunol. 2012;188:4496–4505. doi: 10.4049/jimmunol.1103086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cols M, et al. Stromal endothelial cells establish a bidirectional crosstalk with chronic lymphocytic leukemia cells through the TNF-related factors BAFF, APRIL, and CD40L. J Immunol. 2012;188:6071–6083. doi: 10.4049/jimmunol.1102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu W, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nature Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 101.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–4491. doi: 10.4049/jimmunol.173.7.4479. References 96 and 99–101 show that BAFF and APRIL are produced by several innate cell types and induce CD40-independent class switching. [DOI] [PubMed] [Google Scholar]
- 102.Groom JR, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. This study provides an elegant demonstration that BAFF promotes antibody production through a mechanism involving the TLR-associated protein MYD88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Castigli E, et al. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J Allergy Clin Immunol. 2007;120:885–891. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brodeur SR, et al. C4b-binding protein (C4BP) activates B cells through the CD40 receptor. Immunity. 2003;18:837–848. doi: 10.1016/s1074-7613(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 105.Li S, et al. Rapidly induced, T-cell independent xenoantibody production is mediated by marginal zone B cells and requires help from NK cells. Blood. 2007;110:3926–3935. doi: 10.1182/blood-2007-01-065482. [DOI] [PubMed] [Google Scholar]
- 106.Leadbetter EA, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008;105:8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Snapper CM. Mechanisms underlying in vivo polysaccharide-specific immunoglobulin responses to intact extracellular bacteria. Ann NY Acad Sci. 2012;1253:92–101. doi: 10.1111/j.1749-6632.2011.06329.x. [DOI] [PubMed] [Google Scholar]
- 108.Cunningham AF, et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 109.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- 111.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol. 2004;172:803–811. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 112.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 113.Idoyaga J, Suda N, Suda K, Park CG, Steinman RM. Antibody to Langerin/CD207 localizes large numbers of CD8α+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2009;106:1524–1529. doi: 10.1073/pnas.0812247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schmitt N, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)- expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci USA. 2011;108:E488–E497. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barral P, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci USA. 2008;105:8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bialecki E, et al. Role of marginal zone B lymphocytes in invariant NKT cell activation. J Immunol. 2009;182:6105–6113. doi: 10.4049/jimmunol.0802273. Together with reference 106, references 116 and 117 indicate that MZ B cells undergo antibody production after interacting with iNKT cells. [DOI] [PubMed] [Google Scholar]
- 118.De Santo C, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nature Immunol. 2010;11:1039–1046. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hargreaves DC, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ellyard JI, Avery DT, Mackay CR, Tangye SG. Contribution of stromal cells to the migration, function and retention of plasma cells in human spleen: potential roles of CXCL12, IL-6 and CD54. Eur J Immunol. 2005;35:699–708. doi: 10.1002/eji.200425442. [DOI] [PubMed] [Google Scholar]
- 121.Avery DT, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.He B, et al. Intestinal bacteria trigger T cellindependent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 123.Lindh E, et al. AIRE regulates T-cell-independent B-cell responses through BAFF. Proc Natl Acad Sci USA. 2008;105:18466–18471. doi: 10.1073/pnas.0808205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jego G, et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 125.Xu W, et al. Macrophages induce differentiation of plasma cells through CXCL10/IP-10. J Exp Med. 2012;209:1813–1823. doi: 10.1084/jem.20112142. References 124 and 125 describe novel plasma cell differentiation pathways that involve an interplay between IL-6, type I IFNs and CXCL10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hendricks J, et al. Class-switched marginal zone B cells in spleen have relatively low numbers of somatic mutations. Mol Immunol. 2011;48:874–882. doi: 10.1016/j.molimm.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 127.Scapini P, et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Crepaldi L, et al. Up-regulation of IL-10R1 expression is required to render human neutrophils fully responsive to IL-10. J Immunol. 2001;167:2312–2322. doi: 10.4049/jimmunol.167.4.2312. [DOI] [PubMed] [Google Scholar]
- 129.Rauch PJ, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. This work demonstrates that a unique subset of innate B cells generates protection against sepsis by producing the pleiotropic cytokine GM-CSF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chu VT, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nature Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 131.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. This work suggests that the long-lived survival of antibody-producing MZ and memory B cells is highly dependent on innate microbial signals. [DOI] [PubMed] [Google Scholar]
- 132.Lande R, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.He B, et al. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 134.Castigli E, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaminski DA, Stavnezer J. Enhanced IgA class switching in marginal zone and B1 B cells relative to follicular/B2 B cells. J Immunol. 2006;177:6025–6029. doi: 10.4049/jimmunol.177.9.6025. [DOI] [PubMed] [Google Scholar]
- 136.Le Bon A, et al. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 137.Barral P, et al. CD169+ macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nature Immunol. 2010;11:303–312. doi: 10.1038/ni.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tarkowski A, et al. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgA2-subclass antibody responses. J Immunol. 1990;144:3770–3778. [PubMed] [Google Scholar]
- 139.Carson PJ, Schut RL, Simpson ML, O’Brien J, Janoff EN. Antibody class and subclass responses to pneumococcal polysaccharides following immunization of human immunodeficiency virusinfected patients. J Infect Dis. 1995;172:340–345. doi: 10.1093/infdis/172.2.340. References 122, 138 and 139 suggest that human IgG2 and IgA2 antibodies can be produced through TI pathways. [DOI] [PubMed] [Google Scholar]
- 140.Dammers PM, de Boer NK, Deenen GJ, Nieuwenhuis P, Kroese FG. The origin of marginal zone B cells in the rat. Eur J Immunol. 1999;29:1522–1531. doi: 10.1002/(SICI)1521-4141(199905)29:05<1522::AID-IMMU1522>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 141.Toellner KM, et al. Low-level hypermutation in T cell-independent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J Exp Med. 2002;195:383–389. doi: 10.1084/jem.20011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and Toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. References 141 and 142 show that B cells can undergo SHM in a TI manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lanning DK, Rhee KJ, Knight KL. Intestinal bacteria and development of the B-lymphocyte repertoire. Trends Immunol. 2005;26:419–425. doi: 10.1016/j.it.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 144.Mao C, et al. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 145.Aranburu A, et al. TLR ligation triggers somatic hypermutation in transitional B cells inducing the generation of IgM memory B cells. J Immunol. 2010;185:7293–7301. doi: 10.4049/jimmunol.1002722. References 144 and 145 provide additional evidence that some B cells activate the SHM machinery in response to innate signals without requiring T cells. [DOI] [PubMed] [Google Scholar]
- 146.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nature Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 147.Zindl CL, et al. The lymphotoxin LTα1β2 controls postnatal and adult spleen marginal sinus vascular structure and function. Immunity. 2009;30:408–420. doi: 10.1016/j.immuni.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nature Rev Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schneider K, et al. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe. 2008;3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]