Abstract
The Rare Diseases Clinical Research Network (RDCRN) Contact Registry has grown in size and scope since it was first reported in this journal in 2007. In this paper, we reflect on our seven years’ experience developing and expanding the RDCRN Contact Registry to include many more rare diseases. We present the functional and data requirements that motivated this registry, and the new features and policies that have been developed since. Given the high costs and long-term commitment required to build patient registries, the RDCRN Contact Registry experience represents a reasonable approach for identifying and cultivating potential research populations, with minimal resources and patient burden. The basic model of a patient-reported registry has not changed since our 2007 report, but the number of diseases has grown from 42 to 201, and the types of information that are exchanged with participants has expanded. A patient-directed information-sharing feature has been added to reduce barriers to communication between investigators and patients affected by rare and genetic diseases. As specific data and research needs arise, the Contact Registry can be leveraged to access needed data or to solicit patients for particular research opportunities. This multiple-disease registry is scalable, expandable, and standards-driven, and has become a model for clinical and translational research across rare and many other diseases.
Keywords: Rare disease, multiple-disease registry, patient-reported, registry, observational research, study recruitment
INTRODUCTION
In 2007, we presented our approach for enhancing enrollment in rare disease studies using a Contact Registry for the Rare Diseases Clinical Research Network (RDCRN).[1] That Contact Registry utilized a shared application to collect basic demographic data from patients who self-reported a diagnosis of one of over 40 rare diseases. These data were used to provide each participant with customized information on relevant clinical trials. This report provides an update on the progress of the RDCRN Contact Registry, including new functionalities and approaches for different diseases. Our discussion highlights specific challenges in operating a multi-disease, network-governed patient registry – specifically those challenges that have emerged from changes in network structure and membership. Other challenges surfaced from changes in data standards and the desire for a variety of models for registry implementation across clinical and research collaborations in different diseases. All of these components have contributed to a robust and responsive registry design that is applicable to a broad spectrum of clinical and translational research. Given recent emphasis on the potential contribution of patient registries as a shared resource, our experience is relevant to multiple stakeholders across many diseases and promotes increasingly visible and active patient roles in research.
BACKGROUND
Patient Registries
A patient registry is an organized program for the collection, storage, retrieval, and use of a clearly defined set of data on identifiable individuals for a specified purpose(s).[2] There are many types of registries for different purposes, including public health surveillance, epidemiologic and longitudinal research, patient education, research recruitment, and population safety monitoring for post-marketed drugs and devices. There is no single funder or regulator of patient registries. At present, there are no consensus standard practices for developing registries to support research. However, a recent AHRQ report, although focused on chronic disease registries designed for Comparative Effectiveness Research, provides sound and generalizable advice for registry development. [3]
Patient registries play an important role in the lifespan of disease-focused research and drug development. A registry can be a means for observational research in a population, supporting the generation of new hypotheses and preliminary data to be used in future clinical trials. A registry can be a means to estimate the number of available patients to guide study planning. (Note that registries in the public health context are carefully designed to support population surveillance, and often multiple data sources must be used to ensure completeness of case ascertainment if population-level impact of the disease is to be reported.) Although patient registries typically are not sufficient for population-based estimates of disease, they can be and are used to estimate the numbers of affected patients (potentially available for research) and enable the mobilization of disease-specific communities and advocacy action.
Because of the expense and long-term commitment required to develop patient registries, potential registry developers should clearly define their registry objectives and carefully consider models that are flexible and cost-effective. [1–3] Multi-disciplinary knowledge and shared resources and infrastructure combined with realistic expectations can provide benefit at reasonable costs. Our efforts to build a multiple-disease registry to support a network of diverse investigators forced us to initially adopt minimal data collection and to focus on the registry as a targeted communications resource, rather than as a data repository. We believe that our experience will be helpful to stakeholders interested in establishing registries, conducting translational research, and developing strategic research plans.
The Rare Diseases Clinical Research Network (RDCRN)
The RDCRN was launched in 2003 to facilitate research in rare diseases. (defined as those affecting 200,000 or fewer Americans). The RDCRN network model established by the NIH includes a single Data Management and Coordinating Center (DMCC) interfacing with multiple disease-specific consortia, each typically composed of a lead academic institution, multiple affiliated clinical/academic sites, and one or more patient advocacy groups. In 2009, with oversight from the Office of Rare Diseases Research (ORDR), seven NIH institutes and centers agreed to fund 14 new research consortia, expanding the network from 10 to 19 total consortia, that collectively study more than 140 rare disorders. Current RDCRN Consortia include more than 80 Patient Advocacy Groups, over 160 clinical sites, and hundreds of research staff. Since establishment of the RDCRN, the central DMCC for the network has been based at the University of South Florida, providing systems and data collection infrastructure, research design, and analysis support. The Principal Investigators of each of the 19 consortia and the DMCC, as well as representatives from multiple NIH sponsors and the network’s Coalition of Patient Advocacy Groups, serve on a Steering Committee to guide the strategic growth of the network, and to define research efficiencies and issues that will advance the understanding and treatment of rare diseases in general. [5]
The RDCRN Contact Registry
The RDCRN Contact Registry was developed in 2004 by the authors at the USF DMCC in response to the Request for Applications (RFA) set forth by the NIH funding center National Center for Research Resources (NCRR) when the RDCRN was established. The intent of the registry was threefold: 1) to provide relevant information regarding clinical research activities (specifically those sponsored by the RDCRN) to persons affected by rare diseases, 2) to identify potential participants for rare disease research activities, and 3) to facilitate enrollment in RDCRN clinical studies.
The RDCRN Contact Registry includes a web-based enrollment application linked from various RDCRN-hosted consortia and disease-specific public web sites.[1] The Contact Registry application is designed to allow interested individuals to enter data about themselves or family members who have been diagnosed with a rare disorder that is studied as part of the RDCRN. This information is stored at the DMCC, and is used to facilitate contact and communications between RDCRN investigators and registrants. Typical communications include new study announcements, new clinical site openings, periodic consortia newsletters, and information about consortia-sponsored events. USF, as the registry host, has obtained all required regulatory and privacy approvals, and the investigators and institutions associated with the RDCRN do not require additional approval for standard operations of the Contact Registry.
The RDCRN Contact Registry was specifically designed to function as a single scalable and expandable registry that would address the needs of many rare diseases. Enrollment is open to anyone affected by the conditions under study in the RDCRN. At the time of our initial paper describing the registry in 2007, there were over 4,000 patients enrolled (representing 10 consortia and 42 rare diseases). [1] That evaluation of the registry demonstrated that for consortia with studies recruiting subjects, the number of individuals enrolled in the Contact Registry who also participated in network clinical studies ranged from 6–27% across consortia. When considering only Contact Registry enrollees who lived within 100 miles of a clinical research study site, study participation rates were over 40% for some diseases.
METHODS
The RDCRN was significantly transformed in 2009, when after a competitive funding process, five consortia left the network and 14 new research consortia (representing 113 new diseases, 147 new clinical sites, and 67 new PAGS) were added (for a total of 19 network consortia). These consortia brought new requirements and requests for the Contact Registry.
The existing Contact Registry design and planned enhancements were examined (by authors representing the DMCC) in light of network needs, nature of the diseases in the network, and new developments with regard to standards, regulations, and recommendations/initiatives from PAG and rare disease communities. The objective of this paper is to characterize the stakeholders and users of the RDCRN registry, describe types of usage, and articulate progress and needs for patient registries – from the perspective of a multi-disciplinary central data center at the hub of a large and diverse clinical and translational research network. As developers and hosts of the RDCRN registry, the authors report data on the volume and utilization of the Contact Registry, and describe variations on how it is used by different network consortia. The network needs and registry requirements that are described in the next section were compiled internally by a group of DMCC investigators, technical developers, registry administrators, and regulatory experts. These requirements were then reviewed with stakeholders and have been vetted through individual consortia and the network’s Registry Committee as part of the implementation process.
RESULTS
In this section, we present: (1) the RDCRN network features, including a characterization of the diseases under study, (2) data on the characteristics of participants in the Contact Registry, (3) type and number of communications distributed to registry participants; as well as (4) new features and functions, and (5) specific research applications of the Contact Registry.
1.) RDCRN Consortia and Diseases
The current RDCRN includes studies in more than 140 diseases, listed in Appendix A. Table 1 presents an overview of the 19 clinical research consortia of the network, the types of diseases they study, and the registry enrollment for the consortia. It is important to note that these are simplifications intended to illustrate differing needs for studies of different disease types, which are described further in the discussion section. For example, disorders known to have a genetic etiology might collect genetic testing or family history data, and pediatric studies will have issues of guardianship and parent-directed communications. For many rare diseases, the etiology is poorly understood, and the first types of studies are natural history studies. An unfortunate reality for many of the diseases in the RDCRN is that they are under-studied – most with only a few relevant studies – if any. To illustrate the small amount of research activity for the various diseases, we include the number of diseases with at least three open studies (observational or interventional) on ClinicalTrials.gov.
Table 1.
Number and Characteristics of Rare Diseases in the RDCRN Contact Registry, by Consortium, and number of Registrants.
| Consortia Name / Disease Grouping | Year joined the RDCRN | # RDCRN diseases included in the Contact Registry | # of genetic | # pediatric disorders | # diseases with ≥ 3 active studies on ClinicalTrials.gov | Total Registrants as of December 31, 2011 |
|---|---|---|---|---|---|---|
| Angelman, Rett, & Prader-Willi Syndromes Consortium⋄ | 2003 | 3 | 3 | 3 | 3 | 1037 |
| Autonomic Rare Diseases Clinical Research Consortium | 2009 | 6 | 0 | 0 | 3 | 354 |
| Brain Vascular Malformation Consortium | 2009 | 3 | 3 | 3 | 0 | 33 |
| Chronic Graft Versus Host Disease Consortium⋄⋄ | 2009 | 4 | 0 | 4 | 4 | 0 |
| Clinical Research Consortium for Spinocerebellar Ataxias (CRC-SCA) | 2009 | 48 | 38 | 6 | 17 | 327 |
| Consortium for Clinical Investigation of Neurologic Channelopathies⋄ | 2003 | 3 | 2 | 2 | 0 | 297 |
| Genetic Disorders of Mucociliary Clearance Consortium⋄ | 2003 | 3 | 3 | 3 | 3 | 372 |
| Inherited Neuropathies Consortium | 2009 | 7 | 3 | 3 | 2 | 715 |
| Lysosomal Disease Network | 2009 | 47 | 40 | 40 | 29 | 16 |
| Molecular and Epidemiologic Characterization of Salivary Gland Carcinomas | 2009 | 3 | 0 | 2 | 3 | 5 |
| Nephrotic Syndrome Rare Disease CR Network | 2009 | 4 | 0 | 4 | 4 | 1091 |
| North American Mitochondrial Diseases Consortium | 2009 | 29 | 19 | 19 | 12 | 435 |
| Porphyria Rare Disease Clinical Research Consortium | 2009 | 8 | 1 | 5 | 0 | 636 |
| Primary Immune Deficiency Treatment Consortium | 2009 | 3 | 3 | 3 | 3 | 8 |
| Rare Kidney Stone Consortium | 2009 | 4 | 4 | 4 | 2 | 3 |
| Sterol and Isoprenoid Diseases Consortium | 2009 | 7 | 4 | 5 | 2 | 34 |
| Urea Cycle Disorders Consortium⋄ | 2003 | 8 | 8 | 8 | 3 | 347 |
| Vasculitis Clinical Research Consortium⋄ | 2003 | 11 | 2 | 2 | 9 | 3151 |
| Total | 201** | 133 | 116 | 99 | 8861 | |
Note: Only 18 of the 19 RDCRN consortia utilize the RDCRN Contact Registry.
The RDCRN Contact Registry includes more disorders (n=201) than the number of disorders (n=140) that are represented in RDCRN Consortia research studies and activities.
Represents consortium that were part of the first RDCRN funding cycle (2004). Other consortia joined RDCRN in 2009.
The RDCRN Contact Registry was only made available to these patients in late 2011, a short time before this manuscript was submitted.
2.) Registrant Characteristics
As shown in Table 1, there are over 8,800 persons enrolled in the RDCRN Contact Registry. Overall, most registrants are from the United States (86%) and Canada (5%). This is not surprising given that all the consortia are lead by U.S. institutions. Communications at this point are largely in English and there have been no formal efforts to market the Contact Registry internationally. Nevertheless, current participants represent 106 countries from six continents. Patient-reported data shows that almost half (40%) learned of the registry via the internet, although (7%) and (41%) learned of the Contact Registry through their physician or a Patient Advocacy or Support Group. The majority (66%) report that they prefer to be contacted by email. These proportions of patient referral source and contact preference have been consistent since the implementation of the Contact Registry in 2005.
3.) Activity of the Registry
Each new registrant in the Contact Registry receives a customized welcome email introducing them to the consortium and the registry. Since the start of the Contact Registry in 2004, over 80,000 customized communications have been sent, approximately 13,000 in 2010 alone. The Contact Registry allows consortium-specific configurations for automated communications that pull from standardized pre-approved information. The Contact Registry can be configured to send customized, periodic (e.g., quarterly, semi-annual, or annual) consortium updates/newsletters, event-based communications (e.g., the opening of a new protocol or clinical site can trigger an automated communication to appropriate participants in the Registry), or ad-hoc communications that are developed by the research consortia and pushed out to appropriate participants. Each of these communications types, when customized and targeted to a group of registrants (by reported disease, and/or other factors, such as age or location), is referred to as a campaign. In 2010, there were 46 different campaigns, including eight new protocol announcements, 25 announcements of new clinical sites for existing RDCRN protocols, four consortium updates, and nine special communications (announcements of specific consortium activities, educational event, or customized study promotion).
4.) New features of the RDCRN Contact Registry
The USF DMCC works closely with the 19 consortia to support all studies and network operations, and the Contact Registry complements other established communications mechanisms. Each participating consortium has designated a contact person who interacts with staff supporting the Contact Registry, and there is also a network-wide Registry Committee. The Registry Committee initially worked to address themes that were identified as part of the transition to the second funding cycle. These have included how to coordinate the Contact Registry with other registries (existing physician-directed registries and PAG registries), how to market and brand it, ownership of data, and planning for potential close-out of the registry (for example, if funding ends). These issues demonstrate the role of this multi-disease, multi-stakeholder registry as one part of a complex system of resources supporting the research progress of a specific disease and, ultimately, dissemination of better information and treatments. Below, we describe three notable features of the Contact Registry that have emerged since our initial registry report in 2007. In the Discussion section we reflect on future directions and impact of the registry.
4A.) Enhanced information for patients and RDCRN members
In the first five years of the Contact Registry, communications were limited (per NIH sponsor request) to describe only RDCRN-funded studies. Moving forward, based upon policies recently approved by the RDCRN Steering Committee, the Contact Registry will be able to address information regarding research studies funded outside of the RDCRN. We expect more communication campaigns to be developed in close collaboration with consortia investigators and patient groups. Though the intended purpose of the Contact Registry is to provide patients with information on rare disease research opportunities, it is an unfortunate reality that there are few opportunities for patient participation in rare diseases research, owing to the small number (if any) of active trials in a given rare disease at a given time. (As shown in Table 1, the majority of diseases studied in the RDCRN consortia have fewer than three active studies from all funding sources.) At any given time, many diseases included within the scope of the RDCRN do not have any open studies.
While the Contact Registry staff can manually “push” a communication on behalf of any consortium at any time, most communication campaigns are designed to be automated and data driven. As new studies and clinical sites open within a network consortium, Contact Registry communications can be automatically populated with information about the protocol (e.g., title, lay description) and sites (e.g., location, contact information). Because the number of RDCRN-funded studies is limited, externally maintained and regularly updated databases can be used as sources of study- or disease-specific information. Pointers to these resources can be re-used in Contact Registry design, therefore more frequent communications can be automated and provide up-to-date information to patients. Using this strategy, we use standardized, searchable (indexed), sources of information about rare diseases that are authoritative, current and patient-focused.
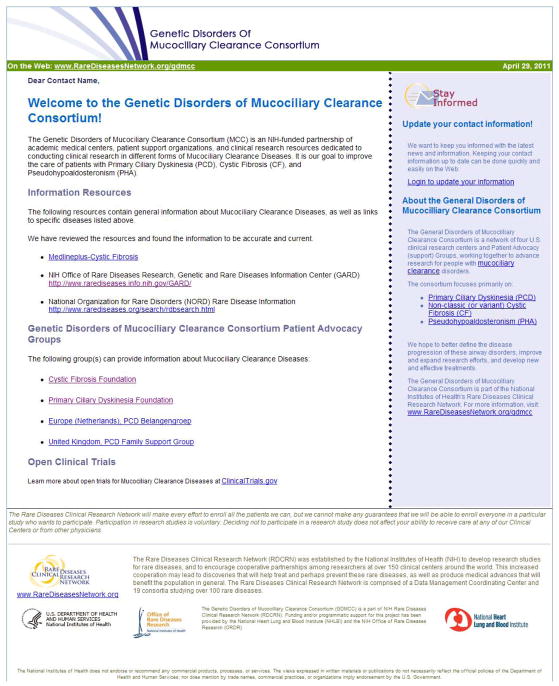
An example of enhanced information is presented in Figure 1. Each communication contains links to patient-directed, disease-specific articles from sources such as MedLinePlus®, the Genetic and Rare Diseases Information Center (GARD) from the Office of Rare Diseases Research, and the National Organization for Rare Disorders (NORD) Rare Disease Database. The communication also links to consortium-affiliated PAG patient information pages. There is also a section containing a link to a preformatted query (by disease) of ClinicalTrials.gov. These information sources were chosen because they are regularly maintained and authoritative. They were also selected for stability and scalability in order that the same query can be reused over time and the same approach can be used for most diseases.
Figure 1.
An example of an email communication and links to external information sources.
The communication shown in Figure 1 provides specific disease information, rather than information about the activities of the consortium. Such communications are especially helpful for consortia with no activity or open studies; providing updated disease information communications serves as a means to keep participants engaged and informed. The information newsletters can be easily customized to each disease, but the information sources and communication layout are re-used, creating a scalable approach for customized, meaningful, current, and disease-specific information across all diseases. A consumer health information specialist provides expertise to customize and monitor communications. The communications are preformatted for layout and presentation of content using a standard and consistent “branding” for the consortium that includes logos, headings, and graphics that have been previously approved by the consortium. Their re-use saves time in developing and approving new communications and creates consistency in branding and presentation.
The RDCRN Contact Registry has successfully informed rare disease investigators about new patient populations. For example, RDCRN investigators had targeted their study recruitment efforts for one type of heritable or familial form of Cerebral Cavernous Malformations (estimated population prevalence of 15/100,000, typically seen in Hispanic families of the Southwest) within the state of New Mexico, because of the large Hispanic population, large family sizes affected with the disease, and relatively little outmigration. [This rare genotype has been traced back to early Hispanic settlers in New Mexico.[6] It also exists in families from Chihuahua, Mexico. Study participants seen at the University of New Mexico (UNM) are rarely from geographical areas outside of Chihuahua or New Mexico.] The RDCRN Contact Registry, however, alerted investigators to two families from California affected by the disease. After initial screening, both families meet the study criteria and will be traveling to the UNM to be consented and screened for study participation.
4B.) Patient-Driven Data Sharing
The model for the Contact Registry involves central administration of the registry by USF DMCC, who acts as a broker for communications between researchers and patients (i.e., the DMCC will push information about studies to participants, but the onus has been on the participant to contact the researcher for further information or to enroll in those studies.) Since the initiation of the Contact Registry in 2004, several investigators requested the ability to contact patients directly regarding specific research opportunities and eligibility screening, and some patients expressed the desire to be contacted by researchers as well. A recently added functionality, offered in August 2010, allows registrants to consent to share their contact information directly with relevant consortia investigators. New registrants can do this directly from a check box on the enrollment form as they register. A similar opportunity to opt to share information directly is offered to existing registrants in conjunction with new electronic communications sent periodically announcing particular studies. At the time a registrant opts to share their information with the clinical site, the information is sent via email to the designated consortium, and the registrant is also sent a notification specifying the contact information for the recipient consortium. The DMCC has acquired all necessary regulatory (IRB and HIPAA) approvals for this function; which required documentation and registry design provisions that clearly inform patients about their choice to share information directly with researchers and that highlights which data they opt to share. The centralization of these approvals spared significant resources for multiple investigators and institutions, and illustrates the benefits of a centralized multi-disease approach to patient registries. This patient-driven data sharing enhancement facilitates participant decision-making regarding involvement in the research activities of the network. Because the implementation of this feature is dependent upon the individual consortia having the resources and capacity to respond directly to interested patients, this is an optional feature, implemented at the discretion of each consortium Principal Investigator.
As of April 2011, ten network consortia have opted to activate this Data Sharing Feature. Overall, 1341 (46%) registrants in these ten consortium have requested that their information be sent directly to RDCRN study investigators. Of these ten consortia using the data sharing feature, five were new to the network in 2009 and had not heavily utilized or promoted the Contact Registry (and hence have fewer numbers of registrants compared with consortia participating in the network for many years). One legacy consortium (UCDC) had 340 registrants (accumulated over the previous five years) who were re-contacted and offered a hyperlink to update their information profile, including the opportunity to share information directly with investigators. Only 15% of existing UCDC registrants who were re-contacted actually updated their information and opted for this feature. The new data sharing feature was also offered to new registrants at the time they first joined the Contact Registry, with much higher rates of uptake (ranging from 29–90% across consortia). When only “new” registrants are considered (i.e., those for whom the data sharing feature was available at their initial registration), overall 72% (511 of 713) opted to share their information with researchers.
5.) New Connections to research tools
The potential of the Contact Registry to assist with study enrollment was recently demonstrated by its use as the sole recruitment tool for an online study of reproductive health outcomes in patients with various forms of vasculitis. The Reproductive Health in Men and Women with Vasculitis Study opened on Feb. 1, 2011 (after approval from NIH sponsor and USF and Duke IRBs). The online survey was developed (over a period of approximately three months) and an announcement inviting registrants to join the study was sent. The announcement contained a hyperlink to an overview of the study and an online informed consent form. Once consent was indicated, patients completed their surveys remotely online and submitted them to the data center. Within 45 days of opening the survey, nearly 500 (approximately 25%) of eligible registrants responded and completed the questionnaire. Data was ready for analysis immediately. Within 24 hours after study closing, the data and data dictionary were provided to investigators. Because online validity checks were used, no data cleaning was necessary. Abstracts were developed, submitted, and accepted for presentation at professional scientific conference within two months.[7, 8] Since an interface for research staff was unnecessary, development costs for the online survey were limited to development of a questionnaire database for the study. Additionally, the resources required for promotion of the project and patient recruitment were minimal, involving disease and survey research experts designing a single promotional communication that the DMCC sent to all eligible Contact Registrants.
Despite obvious sampling issues, this study was initiated to collect preliminary data regarding patient-reported reproductive issues (e.g., fertility, birth outcomes, etc.) and conduct qualitative analysis to inform new hypotheses and future study in this poorly understood area. Because patients were the best source of data, the use of the Contact Registry and an online patient-directed survey tool was logical. The results are informing a new prospective Pregnancy Registry for vasculidities that will allow investigators to identify, evaluate, and refine data collection via patient-directed questions. Certainly, the Reproductive Health in Men and Women with Vasculitis Study illustrates that an active registry can collect a limited number of baseline data elements, and then collect new data as needed in a timely and secure manner. (This is less expensive than collecting all of these data continuously and indefinitely, and is also less costly than retrospective chart review.)
DISCUSSION
The RDCRN Contact Registry has served to amass thousands of patients across the world with rare diseases that can be potential research participants. The numbers and geographic distribution of rare disease patients and their families can inform the design of research studies and the location of clinical sites. These patients are finding the registry largely on their own through the internet, or by referral from patient advocacy and support groups. The majority if new registrants have opted to share their information with investigators and ask to be contacted directly regarding specific research opportunities. The RDCRN Contact Registry has also served as a conduit to more extensive protocol-based data collection efforts.
Efficient Model
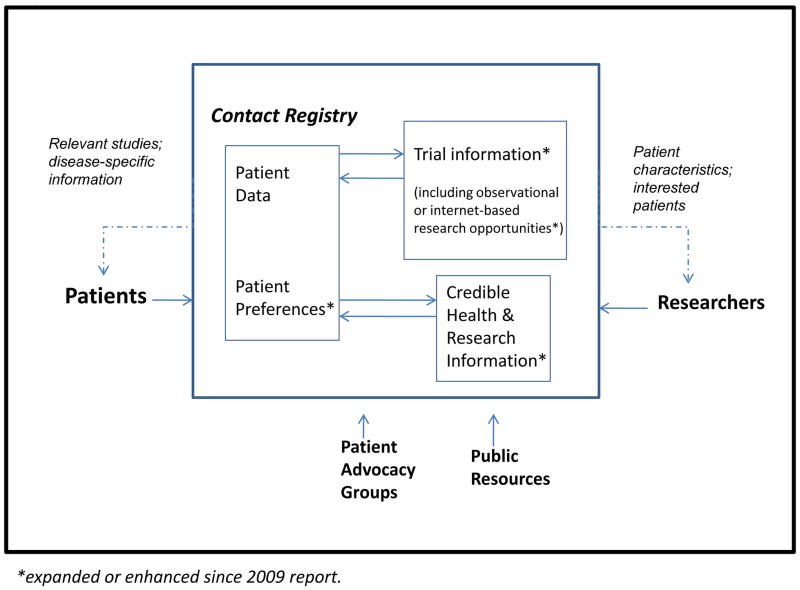
Shared resources and centralized management, technical support, and regulatory approvals afford a Contact Registry model that is stable and efficient. Figure 2 illustrates the role of the Contact Registry as a conduit between patients and researchers. Patients share data regarding their condition, preferences, and information needs. Researchers can provide information on current or future protocols, including patient eligibility characteristics. The system can match patients to studies and resources, and also provide timely and useful disease-specific information to attract patients to the service and keep them engaged. The Contact Registry can collect different data elements, at the discretion of consortium investigators, to support the automated matching of patients to relevant trials. The Contact Registry managed by the USF DMCC acts as an honest broker between researchers and patients, and system automation creates efficiencies across many diseases. The registry is operated on the principle that its communications should be useful, understandable, relevant, and accurate. Within some boundaries, the content and frequency of Contact Registry communications to participants are at the discretion of each research consortium, but under the network principle that the frequency and content of communication should be such as to ensure that registrants feel their participation is meaningful and useful, while remaining relevant and unobtrusive.
Figure 2. The Contact Registry as a Facilitator of Patient-Research Communications.
Patients provide data on their disease and their preferences for health information and research participation; in turn, they receive customized information and study invitations. Researchers provide data on research opportunities and can receive (aggregate) information on patient chracteristics, and detailed patient information if patients have authorized. Patient advocacy groups and public resources provide authoritative and useful information that can benefit patients and keep them engaged in the registry.
The frequency of the above types of communications, and the effort put into the development of customized and information-rich materials, has had to be balanced against the intended purpose of the Contact Registry, which is to promote participation in research studies, particularly those funded by the network. We are designing the information content for these communications to be automatically customized to various rare diseases, keeping our core Contact Registry design team small and focused. While these are fairly easy to generate using a single medical information specialist, it is important to recognize that some human effort indeed is required to design and monitor the appropriateness and utility of the communications to unique rare disease populations. As developers of a multi-disease contact registry we found it critical to maintain focus on the core registry functions so as to limit costs and enhance the scope of the Contact Registry only where there is value.
The multi-disciplinary staff (informatics, biostatistics, regulatory science, clinical research administration, and information technology) at the DMCC design, implement, and monitor the use of new registry features. The centralized multi-disciplinary approach enables feedback from researchers, federal research sponsors, and patient representatives to directly inform the prioritization, allocation of resources, and development of Contact Registry enhancements. The DMCC is formally advised by an open network Registry Committee, representing all network consortia, that is charged to generate new ideas and provide feedback on the Contact Registry, its system features, and its utility. The DMCC reports on status of the Contact Registry to the Steering Committee (consisting of consortium principal investigators, CPAG chair, and NIH science and program officers) quarterly.
A centralization of informatics and creative support allows many diseases and PAGs to benefit. Methods and tools to outreach to the rare disease community – including disease-specific and umbrella PAGs – can be re-used and shared. The RDCRN Steering Committee recently approved policies allowing externally-funded studies and educational events to be promoted using the RDCRN Contact Registry. These opportunities for expanded information will compel the registry to become more sophisticated at capturing and communicating patient, PAG and researcher preferences and needs, as well as influence the ongoing development of standards in this area.
Extensible
The RDCRN Contact Registry and automated targeted communications system provide a current, maintained, cultivated list of potential research participants for many rare disorders. The design of the Contact Registry can easily be extended to include more diseases or more communications within a particular disease area. These extensions require proportionally small amount of human and technical resources. Because the system is largely automated and email-based, there are no additional costs incurred by growing registry enrollments. The Contact Registry communications could be expanded to further facilitate participation in research studies. For example, they might include study brochures, videos of study procedures, consent forms, and medical record releases. The Contact Registry has been used to support enrollment in independent research efforts, and in the future may be used to direct patients to online, validated patient-directed instruments (for example, those available through PROMIS®)[9].
CONCLUSION
The RDCRN Contact Registry is a shared resource designed to increase recruitment in multi-disease networked research. Previous evaluation has shown that the RDCRN Contact Registry can facilitate enrollment in RDCRN clinical studies. More recent experience illustrates the Contract Registry remains an important means to identify new potential participants for rare disease research, and is a useful tool to support study planning and recruitment for traditional research and online patient-reported studies. The Contact Registry can collect and utilize specific disease information and patient preferences to provide targeted, timely, relevant, and automated information regarding clinical research activities to persons affected by rare diseases. The Contact Registry design provides an efficient and scalable model that can serve as an essential component of a comprehensive and strategic infrastructure for research in rare and genetic diseases, as well as supporting educational and advocacy efforts. This experience has relevance for networked, global and internet-based research.
Acknowledgments
The RDCRN Registry and Steering Committees are aware of and have approved the submission of this manuscript for publication in this journal.
The RDCRN Contact Registry is funded by the National Institute for Neurologic Disease and Stroke (NINDS), (NLM Grant Number 7U54-RR019259-02), a component of the NIH, and supported by the Office of Rare Diseases Research. The contents are solely the responsibility of the authors and do not necessarily represent the official views of NINDS or ORDR or NIH or RDCRN investigators. The views expressed in written materials or publications do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
The authors wish to thank Drs. Mark Batshaw and Mendel Tuchman and Jennifer Seminara (Children’s National Medical Center and the Urea Cycle Disorders Consortium) for their support of the Contact Registry and help defining requirements for the data sharing feature. We wish to thank Ken Young, Jennifer Lloyd, David Cuthbertson, Heather Guillette, Rajesh Adusumalli, and Kate Paulus for their significant contributions to the development of the Contact Registry, and Alice Graves for her editorial contributions to this paper. Our thanks to Dr. Leslie Morrison (University of New Mexico) for clarifying the registry experience of the Brain Vascular Malformation Consortium, and to Drs. Peter Merkel (Boston University) and Megan Clowse (Duke University) and Carol Mcalear (Boston University) of the Vasculitis Clinical Research Consortium (National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant Number U54AR057319). The VCRC Registry is supported by the Vasculitis Foundation, the Churg-Strauss Syndrome Association, and the PAN Research and Support Network.
We also express gratitude to Dr. Alan Percy (U. Alabama, Birmingham; Chair, RDCRN Steering Committee) for his assistance vetting policies and registry information through the network, and the current RDCRN Registry Committee for their efforts: Jessica Adsit, Karen Ball, Lada Beara-Lasic, Richard Buchsbaum, Henry Brehm, Dana Doheny, Cindy Dorminy, Debbie Gipson, Joyce Kullman, Magee Leigh, Matthew Lewis, Michele Manion, Yaffa Rubinstein, S. H. Subramony, Seamus Thompson and Steven Ytterberg.
Publisher's Disclaimer: this is the author's version of a work that was accepted for publication in Contemporary Clinical Trials. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Contemporary Clinical Trials, [Volume 33, ISSUE 4, (July 2012)] DOI# 101016/j.cct.2012.02.12.
Appendix A. Diseases under study in the RDCRN
Listed alphabetically.
Acute Intermittent Porphyria
Adenocarcinoma salivary duct carcinoma (ACC)
Adenoid cystic carcinoma (ACC)
AID: Aminoglycoside-Induced Deafness
Alpers syndrome
alpha-Mannosidosis types I / II
Aminolevulinate Dehydratase Deficiency Porphyria
Andersen-Tawil Syndrome (Periodic Paralysis)
Angelman Syndrome
Arginase Deficiency (Hyperargininemia)
Argininosuccinate Lyase Deficiency (Argininosuccinic Aciduria)
Argininosuccinate Synthetase Deficiency (Citrullinemia I)
Aspartylglucosaminuria
Autoimmune autonomic neuropathy
Baroreflex failure
Batten disease
beta-Mannosidosis
Blepharospasm Bronchiolitis Obliterans
Carbamyl Phosphate Synthetase (CPS) Deficiency
Cerebrotendinous Xanthomatosis
Cervical Dystonia
Cholesteryl Ester Storage Disease
Chronic Graft versus Host Disease
Chronic Granulomatous Disease
Churg-Strauss Syndrome (CSS)
Citrin Deficiency (Citrullinemia II)
CMT
CMT1
CMT2
CMT4
Complex I Deficiency
Complex II (SDH) Deficiency
Complex III Deficiency
Complex IV Deficiency
Complex V Deficiency
Congenital Porphyria
CoQ Deficiency
CPEO: Chronic Progressive External Ophthalmoplegia
Cutaneous Sclerosis
Cystic Fibrosis
Cystinuria
DAD: Diabetes and Deafness
Dent’s disease
Dihydroxyadeninuria
Dopamine beta hydroxylase deficiency (DBHD)
Dysarthria Ophthalmoplegia
Encephalomyopathy
Encephalopathy
Episodic Ataxias
Erythropoietic Protoporphyria and X-Linked Protoporphyria
Fabry Disease
Familial Cavernous Malformations (CCM) - Common Hispanic Mutation
Familial Dysautonomia
FBSN: Familial Bilateral Striatal Necrosis
Focal segmental glomerulosclerosis (FSGS)
Fucosidosis
Galactosialidosis types I / II
Giant Cell (Temporal) Arteritis (GCA)
Hepatocerebral disease
Hepatoerythropoietic Porphyria
Hereditary Coproporphyria
Hereditary Hemorrhagic Telangectasia (HHT) - Brain Arteriovenous Malformation (BAVM)
Hunter syndrome (Mucopolysaccharidosis type II)
Hyperimmunoglobulinemia D with Periodic Fever Syndrome
Hypovolemic postural tachycardia syndrome (hPOTS)
Krabbe disease
KSS: Kearns-Sayre syndrome
Laryngeal dystonia
Late Acute Graft versus Host Disease
Leigh Syndrome
Leukoencephalopathy
Lewy Body Disease
LHON: Leber Hereditary Optic Neuropathy
Limb Dystonia
Maroteaux-Lamy syndrome (Mucopolysaccharidosis type VI)
MELAS: Mitochondrial Encephalopathy Lactic Acidosis with Stroke-like Episodes
Membranous nephropathy (MN)
MERRF: Myoclonus Epilepsy Ragged-red Fibers
Mevalonic Aciduria
Microscopic Polyangiitis (MPA)
MILS: Maternally Inherited Leigh Syndrome
Minimal change disease (MCD)
Mitochondrial DNA Depletion Syndrome
MNGIE: Mitochondrial Neurogastrointestinal Encephalomyopathy
Mucoepidermoid carcinoma (MEC)
Mucolipidosis Type III (alpha/beta)
Mucolipidosis Type IV
Mucopolysaccharidosis type I (Hurler, Murler-Scheie, and Scheie)
Multiple Deletions of Mitochondrial DNA
Multiple Respiratory Chain Enzyme Deficiencies
Multiple system atrophy (MSA)
Muscle tension dysphonia (MTD)
N-Acetylglutamate Synthase (NAGS) Deficiency
NARP: Neuropathy, Ataxia and Retinitis Pigmentosa Syndrome
Nephrotic syndrome, other or unspecified cause
Neurally Mediated Syncope
Neuronal Ceroid Lipofuscinosis (Infantile, Late Infantile, Juvenile)
Niemann-Pick Disease Type C
Non-dystrophic Myotonic Disorders
Norepinephrine Transporter Dysfunction
Ornithine Transcarbamylase (OTC) Deficiency
Ornithine Translocase Deficiency (HHH Syndrome)
Oromandibular dystonia
Parkinsonism with Autonomic Failure
Pearson Syndrome
Pheochromocytoma
Polyarteritis Nodosa (PAN)
Pompe Disease
Porphyria Cutanea Tarda
Prader-Willi Syndrome
Primary Ciliary Dyskinesia (PCD)
Primary hyperoxaluria
Pseudohypoaldosteronism (PHA)
Psychogenic dystonia
Pure autonomic failure (PAF)
Rett Syndrome
Sandhoff disease
SANDO: Sensory Ataxia Neuropathy
Sanfilippo syndrome A (Mucopolysaccharidosis type III)
Sanfilippo syndrome B (Mucopolysaccharidosis type III)
Sanfilippo syndrome C (Mucopolysaccharidosis type III)
Sanfilippo syndrome D (Mucopolysaccharidosis type III)
SCA 1
SCA 2
SCA 3
SCA 6
Schindler disease
Severe Combined Immunodeficiency (SCID)
Sialidosis types I / II
Sitosterolemia
Sjögren-Larsson Syndrome
Smith-Lemli-Opitz Syndrome
Spasmodic Dysphonia
Sturge-Weber syndrome (SWS) - Leptomeningeal Angiomatosis
Takayasu’s Arteritis (TAK)
Takotsubo Syndrome
Tay-Sachs disease
Variegate Porphyria
Vocal fold paresis or paralysis
Wegener’s Granulomatosis (WG)
Wiskott-Aldrich Syndrome
Wolman Disease
Contributor Information
Rachel Richesson, Email: Rachel.Richesson@epi.usf.edu.
Rebecca Sutphen, Email: Rebecca.Sutphen@epi.usf.edu.
Denise Shereff, Email: Denise.Shereff@epi.usf.edu.
Jeff Krischer, Email: Jeff.Krischer@epi.usf.edu.
References
- 1.Richesson RL, Lee HS, Cuthbertson D, Lloyd J, et al. An Automated Communication System in a Contact Registry for Persons with Rare Diseases: Scalable Tools for Identifying and Recruiting Clinical Research Participants. Contemp Clin Trials. 2009;30:55–62. doi: 10.1016/j.cct.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richesson R, Vehik K. Patient registries: utility, validity and inference. Adv Exp Med Biol. 2010;686:87–104. doi: 10.1007/978-90-481-9485-8_6. [DOI] [PubMed] [Google Scholar]
- 3.Gliklich RE, Dreyer NA, editors. AHRQ. Registries for Evaluating Patient Outcomes: A User’s Guide. Agency for Healthcare Research and Quality; Rockville, MD: 2010. [PubMed] [Google Scholar]
- 4.Richesson R, Young K, Guillette H, Tuttle M, et al. Standard terminology on demand: facilitating distributed and real-time use of SNOMED CT during the clinical research process. AMIA Annu Symp Proc. 2006:1076. [PMC free article] [PubMed] [Google Scholar]
- 5.Griggs RC, Batshaw M, Dunkle M, Gopal-Srivastava R, et al. Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab. 2009;96(1):20–6. doi: 10.1016/j.ymgme.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzales NGJ. CCM1: A common Hispanic Gene Mutation? Journal of the New Mexico Genealogical Society. 2009;48:3. [Google Scholar]
- 7.Clowse MEB, Richesson RL, Pieper C, Merkel PA, et al. Infertility Among Patients with Vasculitis. American College of Rheumatology (ACR)/ARHP Annual Scientific Meeting; 2011; Chicago. [Google Scholar]
- 8.Clowse MEB, Richesson RL, Pieper C, Merkel PA, et al. Pregnancy in Men and Women with Vasculitis. American College of Rheumatology (ACR)/ARHP Annual Scientific Meeting; 2011; Chicago. [Google Scholar]
- 9.PROMIS. PROMIS Home Page. 2011 [cited 2011 April 14]; Available from: http://www.nihpromis.org/default.