Abstract
BACKGROUND
Transfusion-associated circulatory overload is characterized by new respiratory distress and hydrostatic pulmonary edema within 6 hours after blood transfusion, but its risk factors and outcomes are poorly characterized.
METHODS
Using a case control design, we enrolled 83 patients with severe transfusion-associated circulatory overload identified by active surveillance for hypoxemia and 163 transfused controls at the University of California, San Francisco (UCSF) and Mayo Clinic (Rochester, Minn) hospitals. Odds ratios (OR) and 95% confidence intervals (CI) were calculated using multivariable logistic regression, and survival and length of stay were analyzed using proportional hazard models.
RESULTS
Transfusion-associated circulatory overload was associated with chronic renal failure (OR 27.0; 95% CI, 5.2–143), a past history of heart failure (OR 6.6; 95% CI, 2.1–21), hemorrhagic shock (OR 113; 95% CI, 14.1–903), number of blood products transfused (OR 1.11 per unit; 95% CI, 1.01–1.22), and fluid balance per hour (OR 9.4 per liter; 95% CI, 3.1–28). Patients with transfusion-associated circulatory overload had significantly increased in-hospital mortality (hazard ratio 3.20; 95% CI, 1.23–8.10) after controlling for Acute Physiology and Chronic Health Evaluation-II (APACHE-II) score, and longer hospital and intensive care unit lengths of stay.
CONCLUSIONS
The risk of transfusion-associated circulatory overload increases with the number of blood products administered and a positive fluid balance, and in patients with pre-existing heart failure and chronic renal failure. These data, if replicated, could be used to construct predictive algorithms for transfusion-associated circulatory overload, and subsequent modifications of transfusion practice might prevent morbidity and mortality associated with this complication.
Keywords: Blood transfusion, Morbidity, Mortality, Pulmonary edema, Risk factors
Pulmonary complications of transfusion include transfusion-related acute lung injury, transfusion-associated circulatory overload (also known as TACO), and perhaps other types of acute lung injury. Transfusion-associated lung injury is a rare complication that is the subject of research on the roles of leukocyte antibodies or bioactive molecules in the transfused blood product;1 transfusion-associated circulatory overload has received less research attention but may account for greater overall morbidity due to its higher frequency.2,3 Although there is no consensus definition of transfusion-associated circulatory overload, the Centers for Disease Control and Prevention has proposed diagnostic criteria as part of their biovigilance surveillance program for adverse transfusion outcomes.4 A diagnosis of transfusion-associated circulatory overload is based upon the occurrence of symptoms and signs of acute pulmonary edema within 6 hours after blood transfusion.1 Differentiating transfusion-associated circulatory overload and transfusion-associated lung injury can be difficult, the distinction being primarily whether increased hydrostatic pressure versus capillary leak syndrome is responsible for the pulmonary edema.1,5,6
Although transfusion-associated circulatory overload represents the second most common cause of transfusion-related deaths reported to the Food and Drug Administration, and the number of annual deaths is increasing,7,8 the incidence of transfusion-associated circulatory overload is poorly defined.9 In retrospective or prospective cohorts with some degree of active surveillance, the incidence ranges from 1% to 8% of patients transfused.10–16 Data derived from surveillance of adverse transfusion outcomes or “biovigilance” tend to give much lower incidence rates, probably due to the predominantly passive nature of reporting in most of these systems.17 There also is underestimation of the severity of morbidity and mortality associated with transfusion-associated circulatory overload.8
Beyond the dogma that transfusion-associated circulatory overload is caused by the infusion of too much blood too quickly, risk factors for transfusion-associated circulatory overload are poorly defined. It has been suggested that infusion rate may be more important than total volume of blood transfused.8,18 Host factors are probably more important than infusion practice. Patients aged younger than 3 years and older than 60 years are reported to be at increased risk.5,14 Women predominate in several case series.14,19,20 A positive fluid balance and chronic renal failure also have been implicated.14,19,20 The role of underlying cardiac disease, although implicitly recognized, has not been well described.
There is a dearth of published research on this important and common clinical problem, and good quantitative studies of patient and procedural risk factors are lacking. In our case-control study using active surveillance, we define the risk factors associated with the development of transfusion-associated circulatory overload and also characterize the morbidity and mortality associated with transfusion-associated circulatory overload.
METHODS
Study Design and Subjects
A case-control study of patients with transfusion-associated circulatory overload and transfused controls was conducted in conjunction with a case-control study of transfusion-associated lung injury conducted at the University of California, San Francisco Medical Center (UCSF) and the Mayo Clinic, Rochester, Minnesota.21 Members of Transfusion Medicine Specialized Center of Clinically Oriented Research (SCCOR) TRALI Study Group are listed in the Appendix. Cases of transfusion-associated circulatory overload were identified by active surveillance using a real-time electronic method that screened arterial blood gas results in patients older than 6 months who received blood transfusion products, as previously described.22
Trained study nurses screened all alerts for potential cases of pulmonary edema. An expert panel of 3 board-certified critical care physicians adjudicated diagnoses of transfusion-associated lung injury, transfusion-associated circulatory overload, acute lung injury, overlapping transfusion-associated circulatory overload/transfusion-associated lung injury, or other causes. We defined transfusion-associated circulatory overload according to the criteria of the Centers for Disease Control Biovigilance System,4 namely, pulmonary edema occurring within 6 hours of transfusion and characterized by the new onset or exacerbation of at least 3 symptoms or signs. We excluded as cases patients who were classified into the transfusion-associated circulatory overload/transfusion-associated lung injury category. Surveillance via arterial blood gas results meant that detection was more likely for severe cases of transfusion-associated circulatory overload for which clinicians would order blood gas testing instead of simply treating the patient empirically.
Controls without hypoxemia were selected from among all transfused patients using a stratified sampling scheme (1–2, 3–9, and 10 or more blood products) at the same hospital and during the same time period as enrollment of cases. Only apheresis platelets were used; each was counted as one unit. Because this stratified control sampling procedure oversampled controls with more transfused blood products, all risk factor analyses adjusted for this oversampling. Estimated blood volume was calculated using the Nadler formula.23 The protocol was approved, including a waiver of consent, by the UCSF and Mayo Clinic institutional review boards.
Statistical Analysis
Analyses of risk factors accounted for the sampling fractions of controls from strata using the SAS Survey Logistic procedure (SAS Institute, Cary, NC). Logistic regression was initially performed with adjustment only for number of units transfused and hospital (UCSF vs Mayo Clinic). A multivariable model of risk factors was then developed by including variables significant in the minimally adjusted analysis as well as other variables deemed physiologically important, with backwards stepwise elimination. Because of the unequal time frames for selection of controls and cases, a sensitivity analysis was performed using the subset of controls enrolled contemporaneous with the cases.
Duration of hospital and intensive care unit (ICU) stays, and survival, were summarized using Kaplan-Meier survival analysis. Lengths of stay were calculated from the episode of pulmonary edema (cases) or from 6 hours following the index transfusion (controls); deaths were excluded from this analysis. Controls with no ICU stay were excluded from analysis of length of ICU stay. Medians were calculated and differences tested by generalized Wilcoxon tests. Proportional hazards models were used to obtain estimated effects of transfusion-associated circulatory overload while controlling for Acute Physiology and Chronic Health Evaluation-II (APACHE-II) score, and to generate Kaplan-Meier curves adjusted to the median overall APACHE-II score. An additional proportional hazards model was developed to estimate the odds ratio for in-hospital mortality for subjects with transfusion-associated circulatory overload. All statistical analyses were performed using SAS software 9.1 (Cary, NC).
RESULTS
A total of 47,783 patients who received blood products were monitored for abnormal arterial blood gas results during the term of the study. Electronic surveillance generated 14,472 hypoxemia alerts on 11,612 patients. Study nurses excluded 13,747 alerts for a variety of reasons, including, for example, no evidence of pulmonary edema, pre-existing pulmonary edema, no chest radiograph performed, or lung transplant. The expert panel reviewed 561 alerts with new or worsening bilateral pulmonary opacities and diagnosed 166 cases of transfusion-associated circulatory overload, 94 cases of transfusion-associated lung injury, 153 cases of acute lung injury or possible transfusion-associated lung injury, 47 cases of transfusion-associated circulatory overload/transfusion-associated lung injury, and 101 cases of miscellaneous causes of bilateral pulmonary opacities such as atelectasis or pleural effusions. Of the 166 cases of transfusion-associated circulatory overload, relevant clinical information was available for the last 83 consecutive cases, from February 2008 through August 2009, and these formed the basis of this publication. A total of 163 controls were selected from 36,171 patients who received blood products from May 2006 through August 2009 and did not have hypoxemia.
Table 1 shows the characteristics of the transfusion-associated circulatory overload cases and controls. Cases and controls had a similar distribution of number of blood units transfused due to the stratified control sampling procedures that oversampled controls with more blood products. Transfusion-associated circulatory overload cases were more likely to have been enrolled at Mayo Clinic than at UCSF, but controls were evenly distributed between the 2 hospitals. The same imbalance by site was seen in the 83 transfusion-associated circulatory overload cases not included in this analysis. Location within the hospital differed between cases and controls, with cases more likely to be identified in the intensive care unit and operating/recovery room. Only Mayo Clinic is a level I trauma center; both sites included surgical intensive care unit patients, but only 8 trauma patients (4 cases and 4 controls) were included.
Table 1.
Clinical Characteristics of Transfusion-associated Circulatory Overload Cases and Controls
| Characteristic | Transfusion-associated Circulatory Overload (n = 83) | Controls (n = 163) |
|---|---|---|
| Age, years | ||
| <20 | 5 (6.0%) | 9 (5.5%) |
| 20–29 | 2 (2.4%) | 12 (7.4%) |
| 30–39 | 2 (2.4%) | 14 (8.6%) |
| 40–49 | 5 (6.0%) | 14 (8.6%) |
| 50–59 | 13 (15.7%) | 37 (22.7%) |
| 60+ | 56 (67.5%) | 77 (47.2%) |
| Sex | ||
| Female | 49 (59.0%) | 73 (44.8%) |
| Male | 34 (41.0%) | 90 (55.2%) |
| Race | ||
| White | 61 (73.5%) | 122 (74.8%) |
| Nonwhite | 5 (6.0%) | 19 (11.7%) |
| Missing/not reported | 17 (20.5%) | 22 (13.5%) |
| Mean body mass index* (kg/m2 ± SD) | 28.2 ± 6.48 | 27.6 ± 7.29 |
| Mean estimated blood volume† (L ± SD) | 4.030 (+/−1.190) | 4.510 (+/−1.090) |
| Comorbidities | ||
| Congestive heart failure | 29 (34.9%) | 13 (8.0%) |
| Chronic renal failure | 15 (18.1%) | 14 (8.6%) |
| Severe liver disease‡ | 9 (10.8%) | 15 (9.2%) |
| Surgery within past 48 hours | 62 (74.7%) | 71 (43.6%) |
| Transfusions | ||
| 1–2 units | 31 (37.3%) | 52 (31.9%) |
| 3–9 units | 30 (36.1%) | 63 (38.7%) |
| 10+ units | 22 (26.5%) | 48 (29.4%) |
| Patient location | ||
| Ward | 9 (10.8%) | 73 (44.8%) |
| ICU | 22 (26.5%) | 29 (17.8%) |
| Operating room/Recovery | 52 (62.7%) | 53 (32.5%) |
| Outpatient | 0 | 8 (4.9%) |
| Study location | ||
| Mayo Clinic | 63 (75.9%) | 84 (51.5%) |
| UCSF | 20 (24.1%) | 79 (48.5%) |
ICU = intensive care unit; UCSF = University of California, San Francisco Medical Center.
Available for 80 cases and 158 controls.
Nadler formula.
Mention in the medical record of one or more of the following: biopsy-proven cirrhosis, portal hypertension, or past episodes of upper gastrointestinal bleeding attributed to portal hypertension, prior episodes of hepatic failure/encephalopathy/coma.
Transfusion-associated circulatory overload was positively associated with the number of blood products transfused (odds ratio [OR] 1.23; 95% confidence interval [CI], 1.17–1.30). We next analyzed individual associations between potential risk factors and the occurrence of transfusion-associated circulatory overload, adjusting only for number of blood units transfused and hospital. Female sex was associated with transfusion-associated circulatory overload (OR 2.1; 95% CI, 1.03–4.1). Several cardiac conditions were associated with transfusion-associated circulatory overload, including a history of congestive heart failure (especially New York Heart Association class IV congestive heart failure), a history of coronary artery disease, previous coronary bypass surgery, and atrial fibrillation (Table 2). Current therapy with amiodarone and aspirin carried increased odds of transfusion-associated circulatory overload. Several aspects of the patient’s current hospital course were informative. Surgery within the previous 48 hours, and specifically cardiac, vascular, or liver surgery, was strongly associated with transfusion-associated circulatory overload. Similarly, mechanical ventilation before the onset of transfusion-associated circulatory overload therapy and ventilator variables indicative of more severe pre-existing pulmonary disease carried higher odds of transfusion-associated circulatory overload. Finally, several variables indicative of the patient’s volume status or renal function were associated with transfusion-associated circulatory overload. A history of chronic renal failure or hemodialysis as well as shock (especially hemorrhagic shock) before transfusion was associated with transfusion-associated circulatory overload. Similarly, physiologic measurements indicative of pre-existing volume overload, including lower estimated blood volume, a positive fluid balance, and elevated central venous and pulmonary artery pressures, were all associated with transfusion-associated circulatory overload.
Table 2.
Associations between Various Risk Factors and Case versus Control Status
| Risk Factor | Case (n = 83) | Controls (n = 163) | OR | (95% CI) |
|---|---|---|---|---|
| Cardiac | ||||
| History of congestive heart failure (n) | 29 | 13 | 4.1 | (1.68–10.1) |
| NYHA class 4 congestive heart failure (n) | 6 | 2 | 13.9 | (2.6–74.0) |
| History of coronary disease (n) | 34 | 29 | 2.6 | (1.23–5.6) |
| Atrial fibrillation (n) | 20 | 13 | 2.9 | (1.11–7.8) |
| History bypass surgery (n) | 38 | 11 | 13.4 | (3.2–57.0) |
| Amiodarone use (n) | 12 | 3 | 4.0 | (0.87–18.6) |
| Aspirin use (n) | 40 | 41 | 2.1 | (1.04–4.3) |
| Surgery and ICU | ||||
| Surgery before transfusion-associated circulatory overload (n) | 63 | 81 | 3.2 | (1.59–6.6) |
| Duration of surgery (h) | 5.1 | 2.5 | 1.28 | (1.16–1.41) |
| Cardiac surgery (n) | 41 | 14 | 10.2 | (3.2–32.0) |
| Vascular surgery (n) | 6 | 5 | 6.6 | (1.17–38.0) |
| Liver surgery (n) | 4 | 5 | 7.5 | (1.31–43.0) |
| Mechanical ventilation (n)* | 62 | 79 | 3.7 | (1.83–7.6) |
| Duration of ventilation (h)* | 10.9 | 4.6 | 1.02 | (1.00–1.03) |
| Peak airway pressure (mm Hg)† | 28.6 | 25.0 | 1.1|| | (1.01–1.26) |
| PaO2/FiO2 ratio* | 320 | 390 | 0.9|| | (0.87–0.99) |
| Volume status | ||||
| On hemodialysis (n) | 14 | 14 | 3.3 | (1.25–8.4) |
| Chronic renal disease (n) | 15 | 14 | 3.2 | (0.99–10.4) |
| Hemorrhagic shock (n)† | 7 | 1 | 31 | (3.1–311) |
| Other types shock (n)† | 25 | 29 | 3.6 | (1.26–10.2) |
| Body weight (kg) | 74.4 | 77.5 | 0.98 | (0.96–1.00) |
| Estimated blood volume (L)‡ | 3.83 | 4.25 | 0.52 | (0.33–0.83) |
| Blood products transfused (units)* | 6.3 | 6.0 | 1.23 | (1.17–1.3) |
| Fluid balance (L)*§ | +4.77 | +2.75 | 1.18 | (1.08–1.28) |
| Fluid balance/hour (L)*§ | +0.38 | +0.26 | 3.6 | (1.52–8.5) |
| Central venous pressure* (mm Hg) | 13.3 | 9.7 | 1.19 | (1.04–1.36) |
| Pulmonary artery diastolic pressure* (mm Hg) | 19.9 | 16.7 | 1.57 | (1.01–2.5) |
ICU = intensive care unit; NYHA = New York Heart Association.
Counts (n) are given for binary variables and means for numeric variables, with units as indicated. Odds ratios (ORs) and 95% confidence intervals (CI) adjusted only for center and transfusion intensity.
Before onset of edema.
Before transfusion.
Nadler formula.
Fluid intake minus urine output, in Liters, total and hourly average over previous 24 hours.
Odds ratio per 10 units.
The final multivariable model (Table 3) showed statistically significant associations between transfusion-associated circulatory overload and a diagnosis of chronic renal failure (OR 27.0), a past history of congestive heart failure (OR 6.6), hemorrhagic shock (OR 113 with wide confidence intervals), blood products transfused (OR 1.11 per unit), fluid balance per hour (OR 9.4 per liter), age (inversely; OR 0.78 per 10 years) and hospital (OR 11.2 for Mayo Clinic vs. UCSF). A borderline positive association was seen with “other types of shock” and a borderline inverse association was seen with peripheral blood volume. A sensitivity analysis (data not shown) performed with the 78 controls enrolled during the same time period as the cases yielded overall similar results for most variables, with the exception that the OR for a past history of congestive heart failure decreased to 2.7, the OR for age increased to 0.93 per 10 years, the OR for “other types of shock” decreased to 1.24, and the OR for male sex increased to 2.2.
Table 3.
Risk Factors for Transfusion-associated Circulatory Overload from the Final Multivariable Logistic Regression Model
| Variable | Odds Ratio (95% CI) |
|---|---|
| Chronic renal failure | 27.0 (5.2–143) |
| History of congestive heart failure | 6.6 (2.1–21) |
| Shock before transfusion | |
| Hemorrhagic shock | 113 (14.1–903) |
| Other shock | 2.5 (0.8–7.5) |
| Estimated blood volume* (per liter) | 0.51 (0.23–1.1) |
| Blood products transfused (per unit) | 1.11 (1.01–1.22) |
| Fluid balance per hour (per liter) | 9.4 (3.1–28) |
| Age (per 10 years) | 0.78 (0.62–0.99) |
| Male sex | 1.11 (0.35–3.5) |
| Hospital (Mayo Clinic vs UCSF) | 11.2 (1.98–63) |
CI = confidence interval; UCSF = University of California, San Francisco Medical Center.
Nadler formula.
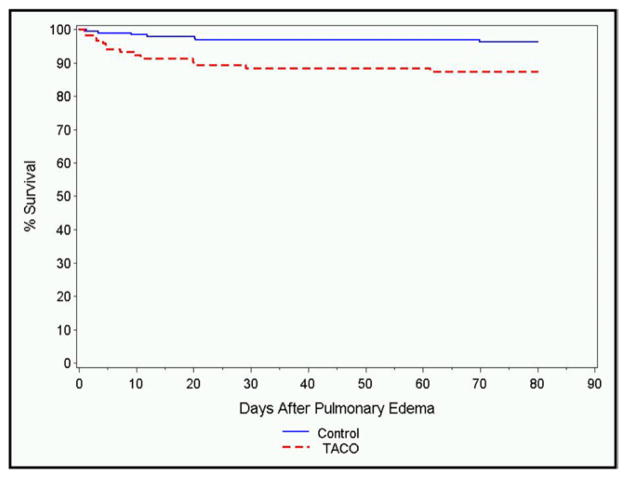
Among the transfusion-associated circulatory overload cases, there were 14 in-hospital deaths, while there were 7 deaths within the control group (Figure 1). In multivariable survival analysis, in-hospital mortality was associated significantly with transfusion-associated circulatory overload (hazard ratio [HR] 3.20; 95% CI, 1.23–8.10), APACHE-II score (HR 1.087; 95% CI, 1.015–1.164), and enrollment in the ICU (HR 3.2; 95% CI, 1.29–7.8).
Figure 1.
In-hospital mortality among transfusion-associated circulatory overload cases (dotted line) compared with transfused controls without pulmonary edema (solid line).
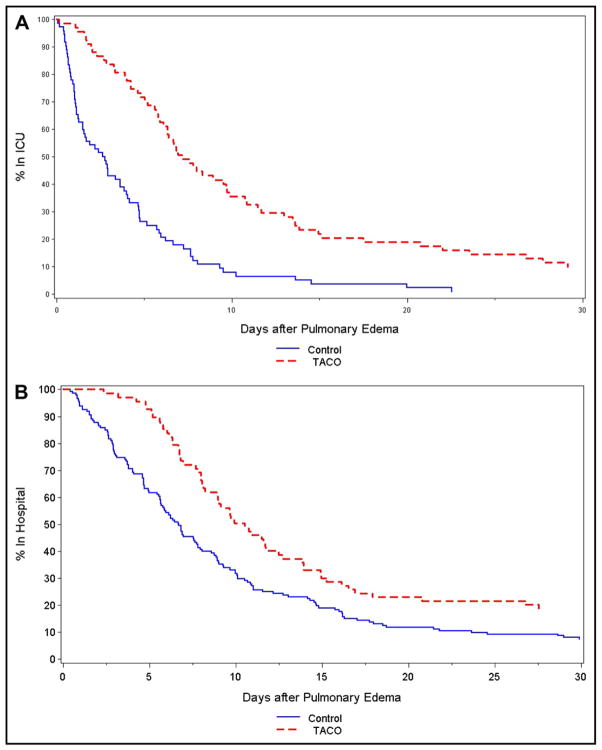
Both ICU and hospital lengths of stay were increased in transfusion-associated circulatory overload cases compared with controls (Figure 2A and B). These analyses counted length of stay from the time of the development of pulmonary edema (cases) or 6 hours after transfusion (controls) until discharge from the ICU and hospital, respectively. Median ICU length of stay after pulmonary edema was 7.03 days in transfusion-associated circulatory overload cases compared with 2.62 days among controls (P <.0001), and median hospital length of stay after pulmonary edema was 10.5 days in transfusion-associated circulatory overload cases compared with 6.8 days among controls (P <.0001). Increased length of stay in the ICU was significantly associated with transfusion-associated circulatory overload (HR for discharge 0.37; 95% CI, 0.26–0.53), hemorrhagic shock (HR for discharge 0.31; 95% CI, 0.13–0.75) and nonhemorrhagic shock (HR for discharge 0.63; 95% CI, 0.43–0.94), and Mayo Clinic (UCSF HR for discharge 1.89; 95% CI, 1.24–2.90). Increased hospital length of stay was significantly associated with transfusion-associated circulatory overload (HR for discharge 0.64; 95% CI, 0.48–0.86), with none of the other examined factors being statistically significant.
Figure 2.
Length of stay in the (A) intensive care unit (ICU) and (B) hospital, in days, for transfusion-associated circulatory overload (TACO) cases (dotted line) and transfused controls without pulmonary edema (solid line). These analyses counted length of stay from the time of the development of pulmonary edema (transfusion-associated circulatory overload) or 6 hours after transfusion (controls) until discharge from the ICU and hospital, respectively.
DISCUSSION
Our results show that the recent clinical management of the patient, including number of blood products transfused and positive fluid balance, was positively associated with transfusion-associated circulatory overload. Associations with patient-related factors, including pre-existing congestive heart failure and chronic kidney disease, also are consistent with clinical experience with transfusion-associated circulatory overload. Importantly, we also provide data supporting increased morbidity, as measured by length of stay in the ICU and hospital, and increased mortality in patients with transfusion-associated circulatory overload.
Our findings about the number of blood products transfused and positive fluid balance are consistent with both the experience of physicians and with recent literature on transfusion-associated circulatory overload. These findings are physiologically plausible, in that both blood product transfusion and infusion of other fluids increase intravascular volume and cardiac filling pressures, leading to pulmonary edema in patients with limited cardiovascular reserve. In fact, the very definition of transfusion-associated circulatory overload includes a requirement for recent blood transfusion. A recent study reported that transfusion dose and infusion rate were associated with transfusion-associated circulatory overload.24 Unfortunately, data on infusion rates were not available in the current study.
In our study, patients with pre-existing congestive heart failure or chronic kidney disease were more likely to develop transfusion-associated circulatory overload. These strong independent associations are biologically plausible. Patients with cardiac dysfunction may not tolerate the increased preload associated with transfusion and would be more likely to experience hydrostatic pulmonary edema. Patients with renal failure would be unable to mount an appropriate diuresis in the face of increased blood volume and would be more susceptible to hydrostatic pulmonary edema even with relatively preserved cardiac function. In addition, uremia may increase capillary permeability, thus rendering the patient more susceptible to fluid extravasation in response to a given hydrostatic pressure.25
We find it harder to explain a couple of other findings from the final model. The inverse association of transfusion-associated circulatory overload with age was unexpected, especially because our transfusion-associated circulatory overload cases were often elderly and female. Perhaps physicians are more conservative in their transfusion practice with the oldest patients, younger patients had more severe comorbidity, or the finding is due to chance. Because few pediatric patients were included in the study, the results should not be extrapolated to children, and further studies in that population are warranted. The association of transfusion-associated circulatory overload with Mayo Clinic probably reflects a different case mix between the 2 hospitals, such as a higher proportion of cardiac disease patients at Mayo Clinic.
In our minimally adjusted analyses, several variables associated with mechanical ventilation were associated with increased risk of transfusion-associated circulatory overload, but these associations did not persist in the final multivariable model. Mechanical ventilation may alter pulmonary hemodynamics or increase intrathoracic pressure and render pulmonary capillaries more permeable in the setting of increased hydrostatic pressures. It is interesting that mechanical ventilation also has been associated with transfusion-associated lung injury and acute lung injury,21,26 supporting the hypothesis that lung damage predisposes to transfusion-associated circulatory overload. On the other hand, the fact that the association did not persist after controlling for confounding variables suggests that mechanical ventilation may simply be a surrogate for more seriously ill patients with less cardiopulmonary reserve who may be predisposed to pulmonary edema.
Similarly, surgery within the previous 48 hours, notably cardiac or liver surgery, was associated with transfusion-associated circulatory overload in the minimally adjusted analysis, but had little association in the final model controlling for congestive heart failure, renal failure, and other variables in the multivariable model. Intraoperative or postoperative fluid management also could have produced a positive fluid balance and increased risk for pulmonary edema. Alternatively, postoperative patients may have dilutional anemia with a normal fluid volume, which may be misinterpreted as hemorrhage. Although based upon few patients, our finding that hemodynamic shock was associated with transfusion-associated circulatory overload also is intriguing, and may be related to increased pulmonary capillary permeability following hypotension.
We found increased in-hospital mortality following an episode of transfusion-associated circulatory overload, as well as prolonged ICU and hospital length of stay after pulmonary edema for patients who developed transfusion-associated circulatory overload compared with controls. Li et al3 found increased mortality following both transfusion-associated lung injury and transfusion-associated circulatory overload, although only the former association was statistically significant. Another study with a very small number of cases also suggested that patients with transfusion-associated circulatory overload had increased mortality and morbidity.15 Hebert et al27 found similar survival but higher risk of pulmonary edema in patients transfused with a liberal versus restrictive transfusion strategy. Transfusion-associated circulatory overload also is one of the most common causes of transfusion-associated deaths reported to the Food and Drug Administration. Among patients who survived, the increased ICU and hospital length of stay carry substantial increased morbidity and health care cost implications, and argue strongly for better predictive and prevention measures for transfusion-associated circulatory overload.
Strengths of the study include its patient population composed of both medical and surgical inpatients with and without intensive care. The use of an electronic surveillance system to identify all transfused patients with abnormal arterial blood gas measurements allowed a broader spectrum of transfusion-associated circulatory overload cases to be enrolled.14,20 Finally, our study also was able to perform a limited analysis of clinical outcomes of transfusion-associated circulatory overload, including length of stay and mortality, controlling for comorbidity by the calculation of APACHE-II scores.3,14,20
Several limitations of the study, some of which derive from its origins within the earlier transfusion-associated lung injury study,21 should be noted. First, we adjusted for relevant confounders in our analyses, but residual confounding could exist. Second, relevant clinical information for transfusion-associated circulatory overload, such as blood product infusion rates and diuretic use, were not obtained as they were deemed less relevant for the transfusion-associated lung injury study. Third, the parent study did not collect extended clinical information on the first half of the 166 cases of transfusion-associated circulatory overload identified, so our comparison of the last half of the transfusion-associated circulatory overload cases to noncontemporaneous controls could have created bias. However, a sensitivity analysis using only contemporaneous controls showed generally similar results. Finally, by virtue of its surveillance system, our study only captured more severe cases of transfusion-associated circulatory overload for which a clinician found respiratory distress significant enough to obtain an arterial blood gas.
What are the clinical implications of this study? Treatment of transfusion-associated circulatory overload is familiar to most medical interns, and includes elevation of the head of the bed, administration of oxygen, intravenous diuretics, and the use of nitrates or other vasodilators.18 Although most patients recover with appropriate treatment, in-hospital mortality may be increased3,17,20 and duration of ICU and hospital stays are prolonged.3,14 In this study, patients with pre-existing congestive heart failure and chronic renal failure, and who received more blood products or had a positive fluid balance, were at increased risk of transfusion-associated circulatory overload. Physicians should consider reduction in transfusion dose and infusion rate in such patients and should monitor them closely for the development of transfusion-associated circulatory overload post-transfusion, consistent with recent evidence that conservative fluid management improves survival in patients with acute renal failure.28
These findings, if replicated by others, also lend themselves to the creation of a predictive algorithm for the risk of development of transfusion-associated circulatory overload. Patients deemed at high risk by such an algorithm could be treated preventively with slower blood product infusion rates or prophylactic diuretic therapy. The algorithm also could identify patients at high risk of transfusion-associated circulatory overload for inclusion of clinical trials of novel preventive strategies. However if transfusion-associated circulatory overload is simply a marker for patients with more advanced cardiovascular disease, its mitigation might not prevent mortality nor reduce ICU and hospital length of stay.
In conclusion, this case-control study has identified several clinical management and patient-specific risk factors for transfusion-associated circulatory overload. The study confirms that transfusion-associated circulatory overload is associated with both increased mortality and substantially increased ICU and hospital lengths of stay, with attendant increased costs. Future studies should include more patients with transfusion-associated circulatory overload, include better measures of comorbidity, and assess both in-hospital and longer-term mortality.
CLINICAL SIGNIFICANCE.
Pulmonary edema following blood transfusion is a frequently encountered and potentially avoidable clinical complication that has received relatively little rigorous research.
The current study implicates several patient- and practice-specific risk factors that could form the basis for predictive algorithms that would allow modification of transfusion practice in susceptible patients.
It also documents the substantial cost in patient mortality and prolonged intensive care unit and hospital lengths of stay associated with this complication.
Acknowledgments
Funding: Funded by NHLBI SCCOR grant P50-HL-81027 (Dr. Toy), CTSA grant UL1-RR-024131 (Dr. Bacchetti), and NHLBI Mid-Career Award K24-HL-075036 (Dr. Murphy).
We are grateful to Erin Madden and Barbara Grimes for statistical programming, to Susan Yuen for manuscript preparation, and to the patients who participated in the study for allowing access to their medical data.
APPENDIX
Transfusion Medicine Specialized Center of Clinically Oriented Research (SCCOR) TRALI Study Group
Steering Committee: Pearl Toy, MD (Principal Investigator), University of California San Francisco (UCSF); Ognjen Gajic, MD, Mayo Clinic; Mark Looney, MD, UCSF; Rolf Hubmayr, MD, Mayo Clinic; Michael A. Gropper, MD, PhD, UCSF; Michael Matthay, MD, UCSF; Richard B. Weiskopf, MD, UCSF; Edward L. Murphy, MD, MPH, UCSF; and Clifford Lowell, MD, PhD, UCSF.
Advisors: Steve Kleinman, MD, Kleinman BioMedical Research; David Stroncek, MD, NIH/CC/DTM; Ram Kakaiya, MD, Institute for Transfusion Medicine; Thomas H. Price, MD, Puget Sound Blood Center; Michael P. Busch, MD, PhD, Blood Systems Research Institute; and Dean Sheppard, MD, UCSF.
Statistics: Peter Bacchetti, PhD, UCSF; and Barbara Grimes, PhD, UCSF.
Clinical Site Coordinators and Research Assistants
UCSF: Monique Koenigsberg, RN, Kelly Lang, RN, Christopher Chin, Deanna Lee, PhD, Lynda Bartek, RN
Mayo Clinic: Gregory Wilson, CCRC, Tami Krpata, Deborah Rasmussen, Cindy Medcalfe
Blood Banks and Investigators
Blood Centers of the Pacific: Nora Hirschler, MD
Blood Systems Research Institute: Rosa Sanchez Rosen, MD, Philip Norris, MD, Dan Hindes
University of California San Francisco: Pearl Toy, MD
Mayo Clinic: S. Breandan Moore, MD, Jeffrey L. Winters, MD, Manish Gandhi, MD
American Red Cross National Neutrophil Reference Laboratory: David Mair, MD, Randy Schuller
HLA Antibody Testing and Analysis (Mayo Clinic): S. Breandan Moore, MD, Manish Gandhi, MD, Steven DeGoey, Nancy Ploeger, Philip Norris, MD
Neutrophil Priming and Cytokine Laboratory (American Red Cross): Clifford Lowell, MD, PhD, Yong Mei Hu, Ping Wu
Lysophosphatidylcholine Laboratory (Mayo Clinic): Joseph McConnell, PhD
Administrator: Charlene Anderson, UCSF
Footnotes
Conflict of Interest: None.
Authorship: All authors had access to the data and a role in writing the manuscript.
References
- 1.Popovsky MA. Pulmonary consequences of transfusion: TRALI and TACO. Transfus Apher Sci. 2006;34(3):243–244. doi: 10.1016/j.transci.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Popovsky MA, Audet AM, Andrzejewski C., Jr Transfusion-associated circulatory overload in orthopedic surgery patients: a multi-institutional study. Immunohematology. 1996;12(2):87–89. [PubMed] [Google Scholar]
- 3.Li G, Kojicic M, Reriani MK, et al. Long-term survival and quality of life after transfusion-associated pulmonary edema in critically ill medical patients. Chest. 2010;137(4):783–789. doi: 10.1378/chest.09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention (CDC) The National Healthcare Safety Network (NHSN) Manual, Biovigilance Component. Atlanta, GA: CDC; Jul, 2010. [Google Scholar]
- 5.Skeate RC, Eastlund T. Distinguishing between transfusion related acute lung injury and transfusion associated circulatory overload. Curr Opin Hematol. 2007;14(6):682–687. doi: 10.1097/MOH.0b013e3282ef195a. [DOI] [PubMed] [Google Scholar]
- 6.Ozier Y, Mertes PM. TRALI and TACO: diagnostic and clinical management of patients [French] Transfus Clin Biol. 2009;16(2):152–158. doi: 10.1016/j.tracli.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. [Accessed November 27, 2012];Fatalities reported to FDA following blood collection and transfusion: annual summary for fiscal year. 2009 Available at: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/ucm204763.htm.
- 8.Popovsky MA. Transfusion-associated circulatory overload: the plot thickens. Transfusion. 2009;49(1):2–4. doi: 10.1111/j.1537-2995.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- 9.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108(3):759–769. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 10.Jessup M. Aldosterone blockade and heart failure. N Engl J Med. 2003;348(14):1380–1382. doi: 10.1056/NEJMe030030. [DOI] [PubMed] [Google Scholar]
- 11.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 12.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 13.Audet AM, Andrzejewski C, Popovsky MA. Red blood cell transfusion practices in patients undergoing orthopedic surgery: a multi-institutional analysis. Orthopedics. 1998;21(8):851–858. doi: 10.3928/0147-7447-19980801-08. [DOI] [PubMed] [Google Scholar]
- 14.Rana R, Fernandez-Perez ER, Khan SA, et al. Transfusion-related acute lung injury and pulmonary edema in critically ill patients: a retrospective study. Transfusion. 2006;46(9):1478–1483. doi: 10.1111/j.1537-2995.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- 15.Popovsky MA, Audet AM, Andrzejewski C., Jr Transfusion-associated circulatory overload in orthopedic surgery patients: a multi-institutional study. Immunohematology. 1996;12(2):87–89. [PubMed] [Google Scholar]
- 16.Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120–162. doi: 10.1053/tmrv.2003.50009. [DOI] [PubMed] [Google Scholar]
- 18.Renaudier P, Rebibo D, Waller C, et al. Pulmonary complications of transfusion (TACO-TRALI) [French] Transfus Clin Biol. 2009;16(2):218–232. doi: 10.1016/j.tracli.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Tobian AA, Sokoll LJ, Tisch DJ, Ness PM, Shan H. N-terminal pro-brain natriuretic peptide is a useful diagnostic marker for transfusion-associated circulatory overload. Transfusion. 2008;48(6):1143–1150. doi: 10.1111/j.1537-2995.2008.01656.x. [DOI] [PubMed] [Google Scholar]
- 20.Li G, Daniels CE, Kojicic M, et al. The accuracy of natriuretic peptides (brain natriuretic peptide and N-terminal pro-brain natriuretic) in the differentiation between transfusion-related acute lung injury and transfusion-related circulatory overload in the critically ill. Transfusion. 2009;49(1):13–20. doi: 10.1111/j.1537-2995.2008.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119(7):1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finlay HE, Cassorla L, Feiner J, Toy P. Designing and testing a computer-based screening system for transfusion-related acute lung injury. Am J Clin Pathol. 2005;124(4):601–609. doi: 10.1309/1XKQKFF83CBU4D6H. [DOI] [PubMed] [Google Scholar]
- 23.Nadler S, Hidalgo J, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–232. [PubMed] [Google Scholar]
- 24.Li G, Rachmale S, Kojicic M, et al. Incidence and transfusion risk factors for transfusion-associated circulatory overload among medical intensive care unit patients. Transfusion. 2011;51(2):388–343. doi: 10.1111/j.1537-2995.2010.02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faubel S. Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis. 2008;15(3):284–296. doi: 10.1053/j.ackd.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 27.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 28.Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6(5):966–973. doi: 10.2215/CJN.08781010. [DOI] [PMC free article] [PubMed] [Google Scholar]