Abstract
Objective
Humoral and cell-mediated immune responses to monovalent H1N1/2009 and seasonal trivalent influenza (TIV) vaccines were evaluated in healthy children and those with asthma, sickle cell disease (SCD), systemic lupus erythematosus (SLE), and solid organ transplantation (SOT).
Study design
Blood was collected from 112 subjects at the time of H1N1/2009 vaccination and 46±15 days later for hemagglutination inhibition (HI) titers and IFNγ ELISPOT responses to H1N1/2009 vaccine and TIV; unvaccinated children also received TIV at enrollment.
Results
A significant increase in the percentage of subjects with seroprotective HI titers to both vaccines was observed in all high risk groups. Children with asthma and SCD were most likely to achieve seroprotective titers to H1N1/2009, whereas fewer than 50% of subjects with SOT and SLE mounted a seroprotective response. The latter also had lower rates of seroprotection following TIV, and subjects with SLE had the lowest ELISPOT responses to both vaccines. Overall, 73% of healthy children exhibited protective responses to TIV; only 35% achieved seroprotection for H1N1/2009.
Conclusions
This evaluation of immune responses to H1N1/2009 in high risk children suggests suboptimal responses for SOT and SLE, but not subjects with SCD or asthma. Higher antigen dose and/or additional dose regimens for immunocompromised children warrant further investigation.
The emergence of 2009 pandemic influenza A (H1N1/09) prompted the rapid development of vaccines to protect against this new strain. H1N1/09 disproportionately impacted children and was associated with higher rates of hospitalization and nearly four times the number of influenza-associated pediatric deaths [1, 2]. These alarming characteristics, coupled with the emergence of oseltamivir resistance [3], underscored the urgency to optimize prevention strategies.
Four monovalent H1N1/2009 vaccines were expeditiously approved by the U.S. Food and Drug Administration (FDA). Early clinical studies conducted among healthy pediatric populations indicated response rates following one dose of vaccine ranging from 19%–93%, with children 6–35 months of age having the weakest response [4–7]. The biology behind the inconsistent responses observed in different studies is unclear, and none of these early trials focused on the highest risk pediatric poulations.
Immune responses to seasonal trivalent influenza vaccine (TIV) in immunocompromised patients have been evaluated in a small number of studies, and results have indicated attenuated responses. Specifically, poor vaccine immunogenicity in patients with SCD and SLE has been reported, and impaired CMI responses were observed in a recent study of pediatric liver transplantation recipients [8–11]. In contrast, prior studies suggest that children with asthma have antibody responses similar to those of healthy children, even in the setting of steroid therapy [12–14].
Influenza vaccine studies typically define a serum hemagglutination inhibition (HI) antibody titer ≥ 1:40 (seroprotection) or a four-fold increase in antibody titers from baseline (seroconversion) as correlates of vaccine efficacy. However, post-vaccine protection against influenza virus may occur in the absence of a detectable antibody response [15] and, conversely, disease has been observed in those with protective titers [16]. Other potential immunologic correlates of protection include assessment of cell-mediated immune (CMI) responses with ELISPOT or flow cytometry-based assays that measure γ-interferon production by peripheral blood mononuclear cells (PBMCs) or specific T cell populations [17, 18]. However few studies have evaluated CMI responses, and threshold responses predictive of protection have not been established.
Given the paucity of data among high risk pediatric populations, the primary objective of this study was to evaluate the humoral and CMI responses to H1N1/2009 in children with moderate/severe persistent asthma, SCD, SLE, and SOT. We also included a cohort of young pediatric patients. For patients who received both H1N1/2009 and TIV, a secondary objective was to study immune responses to TIV. Determining responses in these cohorts will inform vaccination recommendations for future influenza seasons and will provide insight into how best to protect the most vulnerable children.
METHODS
High risk or healthy pediatric subjects between the ages of six months and 22 years who were scheduled to receive H1N1/2009 vaccine as part of their routine medical care at the Children’s Hospital at Montefiore were recruited from their respective clinics between November 2009 and March 2010, prior to administration of the first dose of vaccine. The high risk cohorts included subjects with moderate/severe persistent asthma, SCD, SLE and SOT. Recipients of SOT were recruited primarily from the pediatric renal transplantation clinic. Exclusion criteria included medical conditions contraindicating receipt of inactivated influenza vaccine or anemia that precluded study blood sample collection. The protocol was approved by the Albert Einstein College of Medicine Committee for Clinical Investigation (Institutional Review Board), and written informed consent was obtained from parents and/or subjects. Subjects < 18 years of age provided assent, when appropriate. Blood samples for serum and PBMC isolation were obtained at enrollment and at 4–16 weeks following vaccination. Subjects’ medical insurance determined whether they received vaccine produced by Sanofi Pasteur or Novartis. Vaccination doses were chosen in accordance with recommendations of the Advisory Committee on Immunization Practices (7.5 ug of hemagglutinin antigen for < 36 months and 15 μg of hemagglutinin antigen for ≥ 36 months). Date(s) of prior TIV vaccination were recorded, and children who had not yet received TIV for the 2009/2010 season received both H1N1/2009 and TIV at the enrollment visit.
PBMCs were isolated from whole blood by Ficoll gradient centrifugation according to standard procedures, resuspended in RPMI containing 10% fetal bovine serum, divided into aliquots of 5×106 cells, and stored in liquid nitrogen until the time of analysis.
Hemagglutination inhibition (HI) and ELISPOT assays
Humoral immune responses to H1N1/2009 and TIV were evaluated by performing HI assays for A/California/04/2009-H1N1 (H1N1/2009) and A/Brisbane/59/2007-H1N1 (2009/2010 TIV) strain components of the vaccines by microtiter technique [19]. Recombinant viruses bearing the six internal genes from A/Puerto Rico/8/1934 (PB2, PB1, PA, NP, M, NS) and the HA and NA genes from A/California/04/2009 (PR8:Cal/09) or from A/Brisbane/59/2007 (PR8:BB/07) were used for these experiments. Serum samples were incubated overnight with receptor-destroying enzyme (RDE) from Vibrio cholerae (Sigma-Aldrich, St. Louis, MO) at 37° C and then diluted 1:10 with phosphate-buffered saline (PBS). HI assays for both H1N1/2009 and TIV were performed for all subjects using a standard protocol with washed turkey red blood cells [20]. Seroprotective antibody response was defined as an HI titer ≥ 1:40, and seroconversion was defined as a 4-fold rise in antibody titers from baseline to follow-up.
Cell mediated immune response was measured by IFNγ ELISPOT assay, according to the manufacturer’s protocol (ELISPOTPRO for Human Interferon-γ, Mabtech AB, Sweden). Briefly, 1×105 PBMCs were stimulated with with PR8:Cal/09 or PR8:BB/07 at a multiplicity of infection (MOI) of 3, CD3 monoclonal antibody (positive control reflective of generalized T lymphocyte response), or media (negative control). ELISPOT results were expressed as the number of spot forming units (SFU) per 105 PBMC.
Study outcomes and statistical analysis
Fisher exact test was used for categorical data. Continuous variables within groups were compared from baseline to follow up by paired t test or the nonparametric equivalent, where appropriate. One-way ANOVA or the nonparametric equivalent was used to compare continuous variables between groups. Spearman’s correlation coefficients were calculated to address possible associations between continuous variables. All tests for statistical significance were two-sided, and p < 0.05 was considered statistically significant. Data analyses were performed with GraphPad Prism Software version 5.0.
Results
112 subjects were enrolled, and 106 subjects completed the study. Subject characteristics, including maintenance immunosuppressive medications (Table). Although time to follow up after administration of H1N1 vaccine ranged from 30 to 112 days, mean days to follow up ± standard deviation (SD) was 46 ± 15, and there was no significant difference in time to follow up between groups (p=0.06). Overall mean age ± standard deviation (SD) was 11.9 years ± 6.3. Healthy subjects were significantly younger than subjects with SCD, SLE, and SOT (p=0.04, < 0.0001, and 0.0037, respectively) but not subjects with asthma (Table). All SOT subjects were renal transplant recipients, with the exception of a single liver transplant recipient. SOT recipients were a median of 36 months from transplant (range 1–136). All SOT subjects who were fewer than 12 months post transplantation had received induction therapy with antithymocyte globulin. All subjects with asthma received inhaled steroids, and 10 received short course oral prednisone (1–2 mg/kg/day for a maximum of 5 days) during the study. Three subjects with SLE received methotrexate, two received solumedrol for 2–3 days during the course of the study, and one received cyclophosphamide. Two SOT recipients (renal) were treated for rejection in the six months prior to enrollment; one received solumedrol for two days with IVIG and plasmaphersis, and one received plasmapheresis and IVIG. Fourteen of the 20 subjects with SCD received hydroxyurea.
Table.
Characteristics of study subjects.
| Characteristic | Healthy n=20 |
Asthma n=26 |
SCD n=20 |
SLE n=20 |
SOT n=24 |
|---|---|---|---|---|---|
|
| |||||
| Age* | |||||
| Mean ± SD (years) | 7.9 ± 6.4 | 9.8 ± 5.5 | 12.1 ± 5.8 | 12.7 ± 3.4 | 13.5 ± 5.9 |
| Number (n) < 3 yrs | 7 | 1 | 2 | 0 | 1 |
|
| |||||
| Ethnicity | |||||
| Asian/Indian | 1 | 5 | 0 | 3 | 4 |
| Black | 5 | 11 | 15 | 9 | 7 |
| Hispanic | 11 | 9 | 5 | 7 | 8 |
| White | 3 | 1 | 0 | 1 | 5 |
|
| |||||
| Sex | |||||
| Female | 10 | 17 | 4 | 16 | 8 |
| Male | 10 | 9 | 16 | 4 | 16 |
|
| |||||
| Time to follow up | |||||
| Mean ± SD (days) | 44.6 ± 2.3 | 42.2 ± 9.8 | 41.3 ± 2.8 | 47.1 ± 3.8 | 53.2 ± 4.4 |
|
| |||||
| Tacrolimus (n) | 24 | ||||
| Mean level ± SD, ng/ml | 5.36 ± 2.23 | ||||
|
| |||||
| Daily prednisone (n) | 3 | 16 | 21 | ||
| Median mg/kg/day (range)** | 0.2(0.09–2.2) | 0.3(0.1–0.9) | 0.1(0.03–0.8) | ||
|
| |||||
| Hydroxyurea (n) | 14 | ||||
| Mean mg/kd/day ± SD | 23.5 ± 6.6 | ||||
|
| |||||
| Mycophenolate mofetil (n)*** | 7 | 18 | |||
Immunosuppressant medications reflect doses and levels at the time of enrollment.
Healthy subjects were significantly younger than all groups, except children with asthma.
Subjects with SLE received significantly higher doses of prednisone compared with recipients of SOT(p<0.001)
Recipients of SOT were more likely to receive mycophenolate mofetil relative to subjects with SLE (p=0.02).
One recipient of SOT received azathioprine in addition to tacrolimus and prednisone.
Antibody responses to monovalent H1N1/2009 and TIV vaccines by HI titer
At enrollment, 5–14% of subjects in each group had seroprotective antibody titers to H1N1/2009, with no significant difference between groups (p=0.16) (Figure 1, A). Children with asthma and those with SCD exhibited the most vigorous responses to H1N1/2009 vaccination, with 73% and 70% achieving seroprotective titers, respectively. However only 39% and 45% of subjects with SOT and SLE, respectively, achieved HI titers ≥ 1:40. Healthy children had the least robust response; only 35% mounted seroprotective antibody responses, and only 30% seroconverted following a single dose of H1N1/2009 (Fig 1B).
Figure 1.
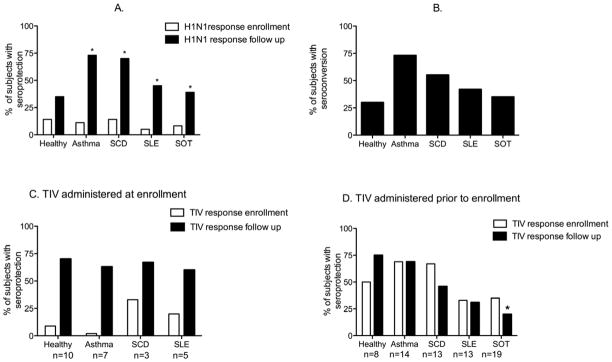
A) Percentage of subjects with seroprotective titers (≥1:40) to H1N1/2009 at enrollment and follow-up; asterisk indicates a significant increase from enrollment to follow up in the percentage of subjects with seroprotective titers. B) Percentage of subjects in each cohort who seroconverted, as evidenced by a 4-fold rise in HI antibody titers to H1N1/2009 at follow-up. C) Percentage of subjects (n=25) who received TIV at enrollment and had seroprotective titers to TIV at enrollment and follow-up. D) Percentage of subjects (n=67) who had received TIV within 3 months prior to enrollment and had seroprotective titers to TIV at enrollment and follow-up. Asterisk indicates significantly lower responses for recipients of SOT relative to healthy subjects.
In contrast, for the 25 participants who received TIV at the time of enrollment, antibody responses following a single dose of TIV were more consistent among healthy and immunocompromised subjects. 73%, 63%, 67% and 60% of healthy children and subjects with asthma, SCD, and SLE, respectively, had seroprotective HI titers to the H1N1 component of TIV (A/Brisbane/59/2007) following a single dose of vaccine (4–6 weeks post-immunization) (Figure 1, C). The remaining 67 subjects had received one dose of TIV within three months prior to enrollment, including all 24 SOT recipients. Within this subset, approximately 70% of healthy or subjects with asthma who received TIV prior to enrollment had seroprotective HI titers at study conclusion (after one dose of vaccine) (mean ± SD 109 ± 59 days post vaccination) (Figure 1, D), whereas subjects with SCD, SLE, and SOT had less sustained rates of seroprotection, with only 50%, 39%, and 20%, respectively, having seroprotective HI titers at the end of the study. This may reflect both a diminished response to vaccine and a more rapid decline in antibody titers. Overall SOT recipients were the least likely to achieve and maintain seroprotective titers for TIV relative to healthy subjects (p=0.003).
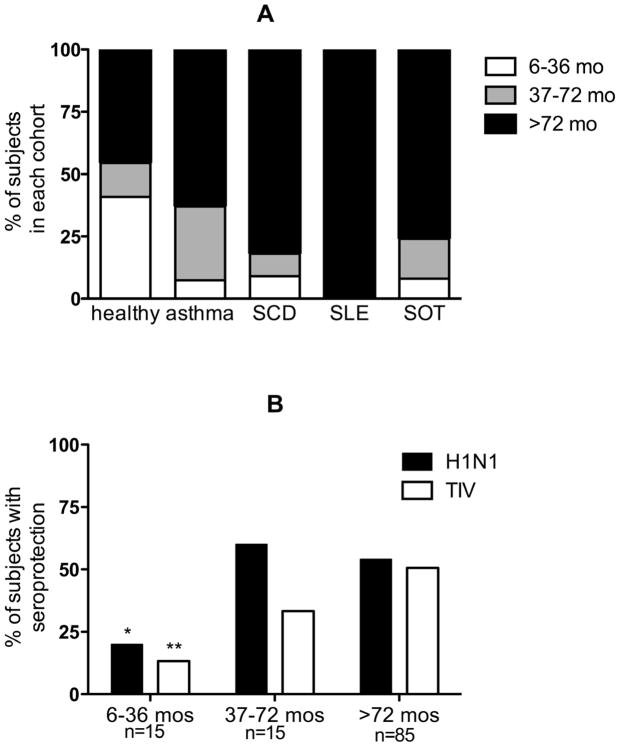
The observation that healthy participants had the least robust response to H1N1/2009 suggested the possibility that young age and prior influenza vaccination may impact the ability to respond to this vaccine. To assess this hypothesis, humoral immune responses for all subjects were stratified by the following age cohorts: < 36 months, 37–72 months, and > 72 months (Figure 2). The proportion of subjects that responded to H1N1/2009 and TIV increased significantly with rising age (Chi square test for trend; p=0.04 H1N1/2009, p=0.005 TIV). However, only five of the ten healthy subjects > 72 months achieved seroprotective titers to H1N1/2009 compared with 12 of 15 subjects with asthma > 72 months of age, suggesting that age does not fully account for the lower response rate to H1N1/2009 in the healthy cohort.
Figure 2.
A) The percentage of healthy subjects, those with asthma, subjects with SCD, those with SLE, and recipients of SOT in each age cohort. B) The percentage with seroprotective HI titers stratified by age. Asterisk denotes significant decrease in seroprotection for H1N1/2009 (*) and TIV (**) as age decreases (Chi square test for trend).
The potential for prior seasonal influenza vaccine exposure to boost the response to H1N1/2009 or TIV was examined by comparing seroprotection among subjects with documented receipt of the seasonal influenza vaccination during the 2007–2008 and/or 2008–2009 seasons (n=57) to response rates among the remainder without prior seasonal influenza immunization. The proportions of subjects with seroprotective titers for H1N1/2009 were similar between those with or without documented prior influenza vaccination (p=0.6, 0.6, 1.0, 0.15, 1.0 for healthy children and those with asthma, SCD, SLE, and SOT, respectively). However, there was a nonsignificant trend towards higher rates of seroprotection to TIV in healthy subjects who had received at least one prior seasonal influenza vaccination compared with healthy subjects who were influenza vaccine naïve (p=0.07). No such trend was seen in the other cohorts (p=0.6, 1, 1, 0.3 for children with asthma, SCD, SLE, and SOT, respectively).
Cell-mediated immune responses as measured by IFNγ ELISPOT assay
Vaccine-induced CMI responses may also play a direct role in protection and promote B cell antibody production. Therefore, we evaluated the IFNγ ELISPOT response to H1N1/2009 and TIV at enrollment and at 4–6 weeks post-H1N1/2009 vaccination. Baseline H1N1/2009 ELISPOT responses (SFU/105 PBMC) were highest in subjects with SCD and lowest in healthy and subjects with SLE (p<0.05 for SCD vs. healthy and SCD vs. SLE) (Figure 3, A). Following vaccination, subjects with SCD continued to have the highest ELISPOT responses, and subjects with SLE had the least robust responses (78.8 SFU/105 PBMC vs. 9.6, p<0.05). In contrast, responses to CD3 monoclonal antibody were vigorous and similar among all groups (Figure 3, A).
Figure 3. ELISPOT responses To H1N1/2009, TIV and CD3 expressed as SFU/105 PBMC.
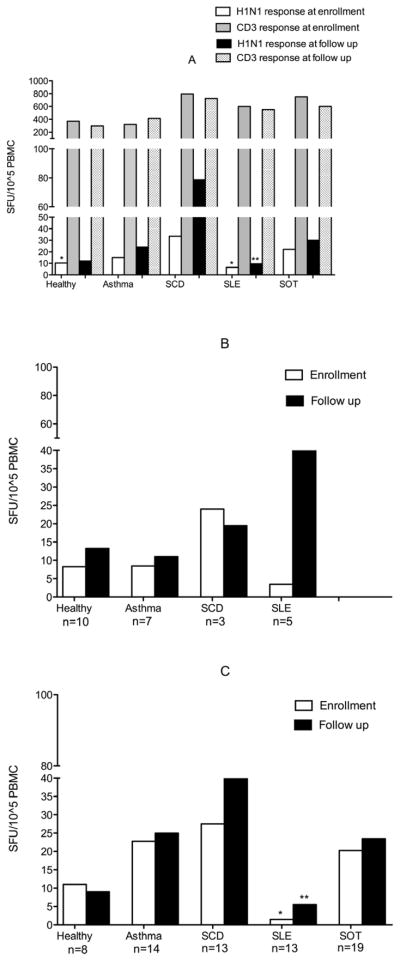
A) ELISPOT responses to H1N1/2009 and CD3 monoclonal antibody. CD3 responses were similar from enrollment to follow up, thus only responses at enrollment are indicated. Asterisk indicates significantly lower baseline responses for SLE and healthy relative to SCD (*) and significantly lower follow up responses for SLE relative to SCD (**). B) ELISPOT responses to TIV at enrollment and follow up for the 25 subjects who received TIV at enrollment. C) ELISPOT responses to TIV at enrollment and follow up for the 67 subjects who received TIV before study enrollment. Asterisk denotes significantly lower responses at enrollment for SLE subjects relative to all groups except healthy children (*) and at follow up relative to SCD subjects (**).
ELISPOT responses to TIV were similar to those observed for H1N1/2009. For the 25 subjects who received TIV at enrollment (10 healthy, 7 asthma, 3 SCD, 5 SLE), there was no significant difference in ELISPOT responses between groups at enrollment or follow up. (Figure 3, B). The remaining majority of subjects (8 healthy, 14 asthma, 13 SCD, 13 SLE, 19 SOT) had received TIV prior to enrollment (Figure 3, C). No significant change in ELISPOT responses was observed for any group from enrollment to follow up, and subjects with SLE had the lowest responses at both time points.
Correlation of immune responses with clinical parameters
For the entire study cohort, H1N1/2009 antibody titers correlated modestly and inversely with time from vaccination (r=−0.22, p=0.02) and positively with baseline titers (r=0.25, p=0.01). Following vaccination, ELISPOT responses for H1N1/2009 strongly correlated positively and significantly with TIV ELISPOT responses (r=0.88, p< 0.001). However, baseline ELISPOT responses for H1N1/2009 were not predictive of responses following vaccination (r=0.15, p=0.13), and post vaccination HI titers for H1N1 did not correlate with ELISPOT results (r=0.17, p=0.08).
For subjects with SCD receiving hydroxyurea therapy, a direct correlation was observed between hydroxyurea dose (mg/kg/day) and HI antibody response (r=0.7, p=0.006) but not between hydroxyurea dose and ELISPOT response. Otherwise type and dose of immunosuppressive drug, time from transplantation, and need for red blood cell or exchange transfusion did not significantly impact immune responses. Subjects with asthma ability to achieve seroprotection or seroconversion was not impacted by use of oral steroids compared with inhaled steroids alone, nor by use of short-course (10 subjects) versus continuous (3 subjects) oral steroid therapy.
Prior to study enrollment, eight subjects (1 healthy, 2 subjects with asthma, 1 with SCD, and 4 recipients of SOT) had a history of documented influenza A infection within the past nine months (mean ± SD 5.3 ± 2.2 months). Only two of these eight (1 healthy, 1 SOT) had baseline seroprotective titers for H1N/2009 despite a history of natural disease, and only five achieved seroprotective titers following vaccination.
DISCUSSION
Our study evaluated humoral and CMI response to the H1N1/2009 monovalent vaccine in high risk pediatric patients and indicates that patients with SLE or SOT mount suboptimal responses, whereas children with moderate to severe asthma or SCD have a vigorous response to immunization. We also found, perhaps unexpectedly, that only 35% of healthy subjects achieved a seroprotective response following a single dose of vaccine. This is a significantly lower response rate than that reported in an early pediatric study of H1N1 vaccine immunogenicity, in which 74.5% and 97.1% of 3–11 and 12–17 year old healthy children, respectively, had seroprotective titers 21 days after a single dose of vaccine [21]. However, earlier experiences are consistent with the results of our study; only 34% of healthy children ages 3–5 years had seroprotective antibody responses following a single dose of the killed swine influenza vaccine developed following the 1976 outbreak [22]. Our cohort’s ELISPOT responses to TIV were low compared with those observed in healthy children in our previous study [10] (approximately 30 SFU/105 PBMC after a single dose of TIV), although the mean age for healthy children in this prior study was 9.2 years. A combination of young age and limited prior exposure to influenza A/H1N1 may contribute to the reduced vaccine immunogenicity we observed in our healthy subjects.
This study only assessed vaccine responses after a single dose of vaccine. Prior studies of TIV immunogenicity suggest that a second dose may result in significantly higher HI titers, especially when children have limited prior exposure to the vaccine strains [23]. We found that receipt of TIV within the past two influenza seasons led to higher rates of seroprotection in healthy children and those with asthma, but not in subjects with SCD, SLE, or SOT. These findings suggest that the boosting effect of prior seasonal vaccines may be attenuated in immuncompromised hosts. In our prior study of humoral and CMI response to TIV in 30 pediatric liver transplantation recipients who were at least one year post transplantation, we also failed to identify an increase in HI titer or ELISPOT response following a second dose of vaccine [10]. Moreover in the current study, even documented prior infection with influenza A/H1N1 did not guarantee a vigorous immune response in the small subset of participants who had symptomatic H1N1 infection in spring 2009; only four of the high-risk subjects with a history of H1N1 infection (57%) achieved seroprotective titers following vaccination.
If larger studies confirm that two doses yield inadequate protection in high risk children, alternative vaccination strategies, including adjuvants or the recently FDA-approved Fluzone® High-Dose TIV, should be evaluated. The Fluzone® High-Dose TIV formulation contains 60 mcg of HA antigen for each of the three viral strains included in the vaccine, is more immunogenic compared with standard formulation in individuals > 65 years, and could prove more immunogenic in high risk pediatric populations [24–26]. Studies are needed to evaluate the safety and immunogenicity of this vaccine formulation in children.
Children with SCD are at high risk for influenza-related complications, but there is little published literature investigating the immunogenicity of influenza vaccine in this population. We found that children with SCD mounted vigorous immune responses. Our results were comparable with those described in the single small prior study, which found that 54–84% of children with SCD mounted a seroprotective response following two doses of TIV vaccine [8]. Importantly, although prior work suggests that hydroxyurea may inhibit T cell proliferation, SCD subjects had the highest ELISPOT responses [27]. Interestingly, we identified a positive and significant correlation between hydroxyurea dose and HI titers, although the mechanisms that might contribute to this correlation are unclear.
Our results also suggest no impairment of humoral or CMI responses in children with asthma who received oral and inhaled steroids compared with those maintained on inhaled steroids alone, which is consistent with prior studies evaluating vaccine immunogenicity in patients with asthma receiving oral prednisone as short term (at the time of or shortly before vaccination) or chronic therapy [12, 28]. The vigorous immune responses observed in children with asthma could also reflect more frequent exposure to wild type influenza in this cohort. Collectively these data indicate that patients with asthma respond robustly to the influenza vaccine, regardless of oral steroid use, and that vaccination does not need to be delayed in the context of steroid therapy.
We observed the most diminished humoral and CMI responses in subjects with SLE, even relative to recipients of SOT. Poor CMI responses to influenza specific antigens were independent of responses to CD3 monoclonal antibody, which were vigorous in all groups. Diminished humoral and cell mediated responses to TIV have been documented in adult patients with SLE [11], although a recent study investigating immunogenicity of inactivated 2009/H1N1 vaccine in adult patients with SLE suggested similar seroprotection rates to those observed in healthy control subjects [29]. The poor vaccine immunogenicity observed in our subjects with SLE suggests that these patients may experience a higher “net state” of immunosuppression and a greater inability to respond to vaccine-specific antigens relative to subjects with SOT who are years past transplantation. Thus, patients with SLE, who often receive years of intensive immunosuppression, may be a population at greatest need for novel vaccine strategies.
In contrast, we observed relatively preserved vaccine immunogenicity in subjects with SOT, compared with healthy children and those with asthma. Our prior study of pediatric liver transplantation recipients found markedly decreased ELISPOT responses to TIV relative to healthy siblings [10], The SOT cohort described in the current study, however, was primarily limited to pediatric renal transplant recipients who were relatively older (13.5 ± 5.9 years) and were, on average, 36 months post transplantation, whereas in our prior study among liver transplant patients the mean age was 8.5 years. Thus our results for this subgroup may not extrapolate to other types of SOT or to the most vulnerable transplant populations that are younger and closer to the time of transplantation.
We noted a strong correlation between ELISPOT responses for H1N1/2009 and the H1N1 component of the seasonal TIV, both at baseline and follow-up. Our use of whole vaccine virus for PBMC stimulation, as opposed to a single antigen, may have reduced our ability to identify strain-specific T cell responses, as six of the eight proteins that comprise the H1N1/2009 and H1N1/TIV vaccine viruses are identical. However use of whole virus may more accurately replicate the cell-mediated response to in vivo infection than would single antigen stimulation. Consistent with our prior study of TIV immunogenicity in recipients of SOT and their healthy siblings [10], we found no significant correlation between HI and CMI responses. Boosting of HI titers without a concomitant increase in CMI responses may reflect a plateau-effect for CD4+ T-cell responses following repeated influenza infection and vaccination [30].
Our results may be limited by the small number of subjects in each subgroup and the lack of age-matched healthy control subjects. Enrollment of older healthy children was limited by preferential use of live attenuated influenza vaccine in this population within the community. In addition, we focused only on the response to a single dose of vaccine, which may reflect vaccine coverage achieved in many communities. However, the consistently low humoral and CMI responses in the highest risk groups highlight the need for novel vaccination strategies for the most vulnerable pediatric patients. We speculate that a higher dose inactivated vaccine, live attenuated influenza vaccines, or the addition of immunostimulatory adjuvants may prove more effective, although the safety of these alternative strategies requires extensive study. Larger and more detailed studies are also needed to define a truly protective CMI response to influenza vaccine in healthy and immunocompromised populations. Determining the parameters that predict an effective and safe response to influenza vaccine could lead to more effective strategies for protecting immunocompromised hosts against new influenza pandemic strains and could provide insights into optimal approaches to develop vaccines for emerging pathogens.
Acknowledgments
Supported in part by the CTSA, National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), (grant UL1RR025750, KL2RR025749, and TL1RR025748), NIH roadmap for Medical Research, and NIH Center of excellence for influenza research and surveillance (grant HHSN266200700010C to A.F.-S). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Recombinant influenza viruses for HI and ELISPOT assays were the kind gifts of John Steel and Adolfo Garcia-Sastre.
The authors thank Harish Rao, MD, Caroline Hu, MD, and Hridya Suman, MD, for their assistance in preparing the manuscript.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med. 2010;362:45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 2.Bundy DG, Strouse JJ, Casella JF, Miller MR. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics. 2010;125:234–43. doi: 10.1542/peds.2009-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang B, Dwyer DE, Blyth CC, Soedjono M, Shi H, Kesson A, et al. Detection of the rapid emergence of the H275Y mutation associated with oseltamivir resistance in severe pandemic influenza virus A/H1N1 09 infections. Antiviral Res. 2010;87:16–21. doi: 10.1016/j.antiviral.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 5.Arguedas A, Soley C, Lindert K. Responses to 2009 H1N1 vaccine in children 3 to 17 years of age. N Engl J Med. 2010;362:370–2. doi: 10.1056/NEJMc0909988. [DOI] [PubMed] [Google Scholar]
- 6.Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA. 2010;303:37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- 7.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–8. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 8.Glezen WP, Glezen LS, Alcorn R. Trivalent, inactivated influenza virus vaccine in children with sickle cell disease. Am J Dis Child. 1983;137:1095–7. doi: 10.1001/archpedi.1983.02140370055018. [DOI] [PubMed] [Google Scholar]
- 9.Ballester OF, Abdallah JM, Prasad AS. Impaired IgM antibody responses to an influenza virus vaccine in adults with sickle cell anemia. Am J Hematol. 1985;20:409–12. doi: 10.1002/ajh.2830200413. [DOI] [PubMed] [Google Scholar]
- 10.Madan RP, Tan M, Fernandez-Sesma A, Moran TM, Emre S, Campbell A, et al. A prospective, comparative study of the immune response to inactivated influenza vaccine in pediatric liver transplant recipients and their healthy siblings. Clin Infect Dis. 2008;46:712–8. doi: 10.1086/527391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holvast A, van Assen S, de Haan A, Huckriede A, Benne CA, Westra J, et al. Studies of cell-mediated immune responses to influenza vaccination in systemic lupus erythematosus. Arthritis Rheum. 2009;60:2438–47. doi: 10.1002/art.24679. [DOI] [PubMed] [Google Scholar]
- 12.Park CL, Frank AL, Sullivan M, Jindal P, Baxter BD. Influenza vaccination of children during acute asthma exacerbation and concurrent prednisone therapy. Pediatrics. 1996;98:196–200. [PubMed] [Google Scholar]
- 13.Bell TD, Chai H, Berlow B, Daniels G. Immunization with killed influenza virus in children with chronic asthma. Chest. 1978;73:140–5. doi: 10.1378/chest.73.2.140. [DOI] [PubMed] [Google Scholar]
- 14.Groothuis JR, Lehr MV, Levin MJ. Safety and immunogenicity of a purified haemagglutinin antigen in very young high-risk children. Vaccine. 1994;12:139–41. doi: 10.1016/0264-410x(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 15.Treanor J, Wright PF. Immune correlates of protection against influenza in the human challenge model. Dev Biol (Basel) 2003;115:97–104. [PubMed] [Google Scholar]
- 16.Liu W, de Vlas SJ, Tang F, Ma MJ, Wei MT, Liu LJ, et al. Clinical and immunological characteristics of patients with 2009 pandemic influenza A (H1N1) virus infection after vaccination. Clin Infect Dis. 2010;51:1028–32. doi: 10.1086/656588. [DOI] [PubMed] [Google Scholar]
- 17.He XS, Holmes TH, Mahmood K, Kemble GW, Dekker CL, Arvin AM, et al. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J Infect Dis. 2008;197:803–11. doi: 10.1086/528804. [DOI] [PubMed] [Google Scholar]
- 18.He XS, Holmes TH, Sasaki S, Jaimes MC, Kemble GW, Dekker CL, et al. Baseline levels of influenza-specific CD4 memory T-cells affect T-cell responses to influenza vaccines. PLoS One. 2008;3:e2574. doi: 10.1371/journal.pone.0002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sever JL. Application of a microtechnique to viral serological investigations. Journal of Virology. 1962;88:320–9. [PubMed] [Google Scholar]
- 20.Noah DL, Hill H, Hines D, White EL, Wolff MC. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol. 2009;16:558–66. doi: 10.1128/CVI.00368-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–23. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 22.Levine MM, Hughes TP, Simon P, O’Donnell S, Grauel S, Levine SG. Monovalent inactivated A/New Jersey/8/76 (Hsw1N1) vaccine in healthy children aged three to five years. J Infect Dis. 1977;136 (Suppl):S571–4. doi: 10.1093/infdis/136.supplement_3.s571. [DOI] [PubMed] [Google Scholar]
- 23.Englund JA, Walter EB, Gbadebo A, Monto AS, Zhu Y, Neuzil KM. Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers. Pediatrics. 2006;118:e579–85. doi: 10.1542/peds.2006-0201. [DOI] [PubMed] [Google Scholar]
- 24.Keitel WA, Dekker CL, Mink C, Campbell JD, Edwards KM, Patel SM, et al. Safety and immunogenicity of inactivated, Vero cell culture-derived whole virus influenza A/H5N1 vaccine given alone or with aluminum hydroxide adjuvant in healthy adults. Vaccine. 2009;27:6642–8. doi: 10.1016/j.vaccine.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007;25:7656–63. doi: 10.1016/j.vaccine.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. The Journal of infectious diseases. 2009;200:172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 27.Benito JM, Lopez M, Lozano S, Ballesteros C, Gonzalez-Lahoz J, Soriano V. Hydroxyurea exerts an anti-proliferative effect on T cells but has no direct impact on cellular activation. Clin Exp Immunol. 2007;149:171–7. doi: 10.1111/j.1365-2249.2007.03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairchok MP, Trementozzi DP, Carter PS, Regnery HL, Carter ER. Effect of prednisone on response to influenza virus vaccine in asthmatic children. Arch Pediatr Adolesc Med. 1998;152:1191–5. doi: 10.1001/archpedi.152.12.1191. [DOI] [PubMed] [Google Scholar]
- 29.Lu CC, Wang YC, Lai JH, Lee TS, Lin HT, Chang DM. A/H1N1 influenza vaccination in patients with systemic lupus erythematosus: safety and immunity. Vaccine. 2011;29:444–50. doi: 10.1016/j.vaccine.2010.10.081. [DOI] [PubMed] [Google Scholar]
- 30.Zeman AM, Holmes TH, Stamatis S, Tu W, He XS, Bouvier N, et al. Humoral and cellular immune responses in children given annual immunization with trivalent inactivated influenza vaccine. Pediatr Infect Dis J. 2007;26:107–15. doi: 10.1097/01.inf.0000253251.03785.9b. [DOI] [PubMed] [Google Scholar]