Abstract
Neurogenesis continues throughout adulthood. The neurogenic capacity of the brain increases after injury by, e.g., hypoxia–ischemia. However, it is well known that in many cases brain damage does not resolve spontaneously, indicating that the endogenous regenerative capacity of the brain is insufficient. Neonatal encephalopathy leads to high mortality rates and long-term neurologic deficits in babies worldwide. Therefore, there is an urgent need to develop more efficient therapeutic strategies. The latest findings indicate that stem cells represent a novel therapeutic possibility to improve outcome in models of neonatal encephalopathy. Transplanted stem cells secrete factors that stimulate and maintain neurogenesis, thereby increasing cell proliferation, neuronal differentiation, and functional integration. Understanding the molecular and cellular mechanisms underlying neurogenesis after an insult is crucial for developing tools to enhance the neurogenic capacity of the brain. The aim of this review is to discuss the endogenous capacity of the neonatal brain to regenerate after a cerebral ischemic insult. We present an overview of the molecular and cellular mechanisms underlying endogenous regenerative processes during development as well as after a cerebral ischemic insult. Furthermore, we will consider the potential to use stem cell transplantation as a means to boost endogenous neurogenesis and restore brain function.
Keywords: hypoxia–ischemia, mesenchymal stem cells, neonatal, neurogenesis, neuroregeneration
Introduction
The intriguing discovery of proliferating cells in the mature rat brain by Altman and Das1, 2 in the mid-1960s was met with great skepticism by the scientific community. However, the development of proliferation markers such as [3H]-thymidine or 5-bromo-2′-deoxy-uridine (BrdU) during the past decades confirmed the discovery of the existence of proliferating cells in the mature mammalian brain. These studies have indisputably established that neural stem cells (NSCs) from the subgranular zone (SGZ) in the dentate gyrus of the hippocampus and the subventricular zone (SVZ) in the lateral ventricles continue to proliferate under normal conditions throughout mammalian adulthood3 (see review by Gould6). Evidence from studies in rodents suggests that every month ∼6% of proliferating cells in the dentate gyrus are functionally integrated into the hippocampus.7 However, aging decreases neurogenesis in the mammalian brain due to an increase in negative regulators of neurogenesis.8, 9, 10, 11 For instance, Wnt production was shown to decline with aging, which in turn decreases NSC proliferation.11
One might suppose that cerebral damage would lead to a molecular and cellular imbalance in the neurovascular niche favoring negative regulators and thus impair endogenous neurogenesis. However, studies in rodents provide accumulating evidence that the neurogenic capacity is preserved or even increases after injurious events such as seizures,12 stroke,13 or hypoxia–ischemia (HI).14 This intriguing discovery prompted a wave of studies addressing not only the more fundamental aspects of neurogenesis, but also its possible role in the development and treatment of several neurologic disorders, such as epilepsy,12 Parkinson's disease,15 and Alzheimer's disease.16 In this review, we will focus on the endogenous capacity of the neonatal brain to regenerate after a cerebral ischemic insult.
Currently, a growing number of studies focus on the development of strategies to protect and regenerate the ischemic-injured neonatal brain. Neonatal encephalopathy caused by perinatal cerebral ischemia remains a significant cause of neonatal mortality and leads to neurologic deficits such as cerebral palsy, mental retardation, and seizures.17, 18, 19, 20 At present, the only available therapy is hypothermia, which is only effective in babies born at term with mild to moderate brain damage.21, 22 Moreover, hypothermia has a short therapeutic window as it has to be applied within 6 hours after the ischemic event.23 Hence, there is an urgent need to unravel the mechanisms underlying neurogenesis in the immature brain to assist in the development of alternative therapeutic interventions that induce and/or support endogenous neurogenesis.
Several studies by our group and others have shown that pharmacological intervention aimed at preventing neuronal cell death or neuroinflammation can provide efficient neuroprotection when administered within the first 24 hours after HI neonatal brain damage in experimental animal models.24, 25, 26, 27, 28, 29, 30, 31 Additionally, there are a number of compounds that have a longer therapeutic window presumably because they promote neuronal migration, neurogenesis, and oligodendrogenesis.32, 33 We propose stem cell therapy as an additional strategy to regenerate the damaged brain areas with a potentially longer therapeutic time window. Recent work by our group and others support the concept that stem cell transplantation may have therapeutic potential with a relatively long time window by repairing the already damaged brain.34, 35, 36, 37, 38, 39
In this review, we will first give an overview of developmental events taking place in the normal postnatal mammalian brain with emphasis on neuronal migration, spine/axon pruning, synapse formation, and myelin formation. Subsequently, we will discuss recent findings showing the endogenous capacity of the neonatal brain to regenerate after HI insult and the molecular mechanisms underlying endogenous regenerative processes after brain damage. Finally, the potential to use stem cell transplantation as a means to promote endogenous repair and restore brain function will be discussed.
The Developing Mammalian Brain
Neural Stem Cells in the Postnatal Brain
Neural stem cells from the SVZ and SGZ are self-renewing and are capable of differentiating into neurons, astrocytes, and oligodendrocytes.40 In this review, the term lineage-specific progenitors or precursors refers to cells with restriction to one specific lineage (e.g., neuronal, astroglial, and oligodendroglial). There are three types of stem cells in the SVZ (viz., Type B, C, and A cells). Type B cells give rise to actively proliferating C cells,41 which in turn give rise to type A cells. Type A cells are immature neuroblasts that migrate in chains to the olfactory bulb (OB).42, 43 Evidence suggests that type B cells have an astrocytic nature as they show morphologic characteristics of astrocytes and express astroglial markers, such as glial fibrillary acidic protein (GFAP). The adult SGZ contains two types of stem cells (viz., type I and type II).44, 45 Type I progenitors are radial astrocytes that, in contrast to other astrocytes in the SGZ, express both GFAP and nestin.46 The lineage-specific type II progenitors (also called type D cells) are derived from type I cells.44, 45 Immature type II progenitors cells divide and will later show properties of neurons, e.g., express doublecortin (DCX), poly-sialylated neural adhesion molecule (PSA-NCAM), or neuronal nuclei (NeuN).7, 45, 47
Until recently, NSCs had only been observed in the SVZ and SGZ of the healthy mammalian brain. However, an intriguing study identified neural progenitor cells in the neocortical layer 1 of adult rats subjected to mild ischemia.48 These cells were shown to migrate radially into cortical layers 2–6 and differentiate into a subtype of GABAergic interneurons. The authors showed that proliferating cells in layer 1 are not derived from SVZ NSCs or progenitors, but rather from local progenitors that do not differentiate under normal conditions. However, the question remains whether these cortical progenitor cells are functionally integrated into cortical networks (see Fishell and Goldman49).
Thus, although some impressive experimental data suggest that neurogenesis can also take place in neocortical layer 1, indisputable proof is still lacking because of discrepancies between studies. Hence, the general theory is that under normal conditions, neurogenesis is restricted to the SVZ and SGZ in the adult brain, although progenitors may be present in other areas of the brain. This suggests that specific factors have to be present in the environment surrounding the NSCs, allowing the formation of new neurons. This concept is clearly shown in a study where progenitor cells from the spinal cord, a known nonneurogenic region, differentiate into neurons when transplanted into the SGZ, illustrating that the SGZ and SVZ provide an environment that supports neurogenesis.50 This environment is known as the neurovascular niche, which comprises progenitor cells, different types of neurons (e.g., granule cells), astrocytes, and oligodendrocytes in close proximity to blood vessels (see Figure 1). These cells not only express membrane-associated factors, but also secrete mitogenic factors, growth factors, and neurotrophic factors that play a role in promoting cell proliferation, fate determination, neuronal survival, and maturation.51 Therefore, we would like to propose that progenitors may be present outside the known stem cell niches in the brain, but that the environment of the progenitors will dictate differentiation and proliferation. The neurovascular niche will be described in more detail in the section ‘Molecular and cellular processes in the neurovascular niche after HI brain damage'.
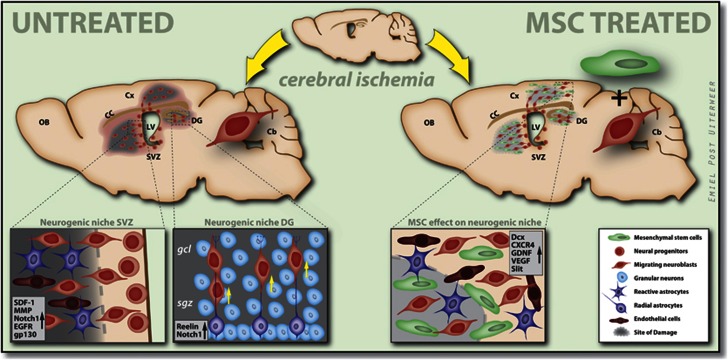
Figure 1.
Neurogenesis and migration in the subventricular zone (SVZ) and subgranular zone (SGZ) after hypoxia–ischemia (HI) and mesenchymal stem cell (MSC) treatment. Schematic overview of the neurogenic niche in the SVZ and SGZ. Neural progenitors in the SVZ differentiate into neuroblasts (Dcx+), which not only migrate toward the damaged striatum, but also through radial migration along the corpus callosum toward cortical regions. Neuroblasts in the SGZ migrate along radial astrocytes toward the granular cell layer GCL in the dentate gyrus (DG). HI insult induces the production of several factors that promote neurogenesis and migration in the damaged areas. MSC administration increases the production of several factors that are involved in cell proliferation, differentiation, and migration. Cb, cerebellum; CC, corpus callosum; Cx, cortex; Dcx, doublecortin; EGFR, epidermal growth factor receptor; gcl, granular cell layer; GDNF, glial cell-derived neurotrophic factor; gp130, glycoprotein 130; LV, lateral ventricle; MMP, matrix metalloproteinase; OB, olfactory bulb; sgz, subgranular zone; VEGF, vascular endothelial growth factor.
Postnatal Development of the Brain
The mammalian brain is not yet fully developed at the time of birth and thus several developmental processes are still taking place postnatally. It is during this period that neurons will form synapses, which in turn develop into neural networks. Hence, an injurious event at this period will hinder maturational processes that may lead to life-long detrimental effects on, e.g., cognitive and motor modalities.
During the first postnatal weeks, a substantial amount of migrating cells can be found in the SGZ and SVZ of the rodent brain. The majority of these cells are glial progenitors, as gliogenesis persists in the SVZ for several weeks. These progenitors migrate in two distinct ways depending on their target area in the brain. Although some progenitors migrate radially toward the dorsal cortex, others first migrate tangentially along the white matter and then radially toward the lateral cortex. The mechanisms that regulate neuronal migration from the neurogenic regions, SVZ and SGZ in the postnatal brain, are comparable to those in the adult SVZ. In both cases, progenitors from the SGZ migrate toward the hippocampal granule cell layer and those from the SVZ migrate tangentially toward the OB through the rostral migratory stream (RMS). The progenitors migrating through the RMS, which consists of astrocyte tubes, form chains of cells that, when reaching the olfactory bulb, migrate radially into the granule cell layer and glomerular layer of the olfactory bulb (see review by Cayre et al52). One major difference between migration from the postnatal and adult SVZ is that the astrocyte tubes only appear 2 to 3 weeks after birth.53 Therefore, migration to other brain regions, such as the striatum, may be facilitated in the first 3 postnatal weeks, as glial tubes may restrict the migration of progenitors within the RMS.
Neuronal network formation involves substantial reorganization of existing neuronal circuits, which is mediated by events such as spine/axon pruning and cell death. Spine/axon pruning and cell death are important opposing mechanisms establishing the patterning of the neural networks within the mammalian brain, and are essential for normal development and functioning of neural circuits. Indeed, abnormalities of spine structure and dynamics have been correlated to several diseases including Fragile X syndrome,54, 55 Alzheimer,56 and ischemia.57, 58, 59 Spine/ axon pruning is characterized by the removal of inappropriate connections in different regions of the mammalian brain. Axon pruning can either involve the elimination of certain axon terminals within the same target area by competition or the removal of collateral branches targeting functionally inappropriate areas.60, 61 This event is tightly regulated by intrinsic factors, such as transcription factors,62 the ubiquitin–proteosome system,63 and the Fragile X mental retardation protein (FMRP),64 that are triggered in response to differentiation or maturation of the neuron. Pruning can also be triggered by extrinsic factors such as axon repulsion molecules,65, 66, 67 hormones,68, 69 and trophic factors (see review by Vanderhaeghen and Cheng70).
Synapse formation, a process that also takes place in the developing brain, shares several similarities with axon guidance. Dendritic filopodia, like growth cones, search the proximal environment for a potential site to form a synapse. Furthermore, axon guidance cues also play a role in synaptogenesis as they promote or inhibit the formation of presynaptic terminals.60 After the formation of axon–dendritic complexes, some contacts will become stabilized. At present, the molecular mechanisms underlying the stabilization of synapses remain poorly understood. This process may be mediated by interactions between proteins found on the cell surfaces of axon–dendritic complexes, such as cadherins, neurexin, and neuroligin.71 Synaptic maturation is characterized by recruitment of post- and presynaptic proteins such as ion channels, scaffolding proteins, and presynaptic vesicles.72, 73
Axon myelination is essential for neurons to function. The majority of myelin production by mature oligodendrocytes takes place early in life and continues until adolescence. Myelination depends on differentiation of oligodendrocytes and factors secreted by axons (see review by Emery74). Mature oligodendrocytes will wrap their own cell membrane around axons, forming sheaths of compact multilayered membranes that function as an isolating membrane because of their rich lipid content. Axon myelination is a crucial step in the development of the central nervous system as it reduces energy consumption during the restoration of ion gradients by Na+/K+-ATPases. The restriction of action potential and ion currents combined with the isolating properties of myelin increase the conduction velocity, enabling saltatory signal propagation in the nervous system.75, 76 Thus, axon myelination is of critical importance for normal brain function. This is clearly illustrated in debilitating disorders such as leukodystrophies, in which oligodendrocytes fail to assemble or to maintain myelin, leading to impaired motor-sensory and cognitive development.75
Neurogenesis After a Hypoxic–Ischemic Insult
Hypoxic–Ischemic Induction of Neurogenesis in the Subgranular Zone and Subventricular Zone Region
A HI insult, which can occur during or after delivery, will lead to cerebral injury despite the endogenous neurogenic capacity of the brain. Accumulating evidence suggests that HI injury promotes extensive cell proliferation in the SVZ of the rodent brain.14, 77, 78, 79, 80, 81, 82, 83 Several studies in which P6 and P7 rats and P10 mice were subjected to moderate HI showed that the SVZ expands in size, illustrated by increased cresyl-violet staining and nestin-positive cells in the ipsilateral SVZ.78, 79, 83 Furthermore, an increase in BrdU+ cells was observed in the affected SVZ from 1 week to 3 weeks after HI, implying that cell proliferation is stimulated in this region.14, 77, 78, 79, 81, 83, 84 Interestingly, BrdU+ cells were also shown in the striatum14, 77, 78, 81, 83 and cortical regions14, 77, 82 from 1 to 4 weeks after the insult. This finding suggests that either proliferating cells in the SVZ migrate to these regions or that local progenitors proliferate because of molecular changes in the cell environment. Overall, these results indicate that the SVZ maintains the capacity to promote cell proliferation, that the progenitor cells are capable of migrating to damaged brain regions, and that the striatal and cortical environments support proliferating cells after HI injury.
Accumulating knowledge has been gained on the capacity of the hippocampus of the adult brain to regenerate after an injurious event. Yet, only a few studies have investigated the proliferative capacity of the SGZ in the injured neonatal brain. Qiu et al85 compared neurogenesis after HI injury in immature (P9) and juvenile (P21) C57Bl/6 mice by injecting BrdU during the first 7 days after the insult. After 4 weeks, BrdU+ cells were quantified in the dentate gyrus (DG) and the cornu ammonis (CA) region individually, thus making it possible to determine whether these regions differ in proliferative capacity. Interestingly, HI injury did not affect cell proliferation in the DG, whereas in the CA region a significant increase in proliferation rate was detected in the immature (P9) brain. However, HI injury in juvenile (P21) mice induces a substantial increase in the proliferation rate in both the DG and the CA regions, showing the potential capacity of cells in the DG to increase proliferation in response to an insult. The apparent lack of cell proliferation in the immature (P9) DG may be explained by normal developmental processes; i.e., under baseline conditions, proliferation is substantially higher in P9 mice compared with P21 mice and may already have a maximal rate in the immature P9 mice.85
In contrast to the results discussed above, recent work by Kadam et al14 showed decreased cell proliferation in the DG region of CD-1 mice subjected to ischemia alone at P12. This discrepancy may be explained by the fact that different strains were used in the two studies and/or that there were differences in the severity of the insult. The CD-1 mice are more sensitive to HI injury than other mice strains, like, e.g., C57Bl/6.86 Even though only unilateral carotid artery ligation was performed in the model used by Kadam et al,14 histologic data show that the brain damage observed in their study was more severe than that experienced by the more resilient C57Bl/6 strain. Nevertheless, one should keep in mind that hypoxia might function as an additional trigger for cell proliferation. Another explanation could be the different BrdU injection protocols that were used. Qiu et al85 administered BrdU from P10 to P17 once every day, whereas in the study by Kadam et al14 BrdU was administered every 9 hours from P18 to P20. Therefore, the study by Qiu et al85 detected cells that were proliferating just after the insult, i.e., during a period when the DG is still developing in the P9 mouse, whereas the study by Kadam et al14 injected the BrdU during a phase in which DG was almost fully developed. Thus, the study paradigm may account for the lower levels of proliferating cells detected in the DG region by Kadam et al14 and emphasizes the importance of standardizing experimental set-ups. One of the major challenges encountered when comparing literature results is to correctly identify and account for differences in the selection of ischemia paradigms and experimental protocols.
Cell-Fate Commitment of Proliferating Cells in the Subgranular Zone and Subventricular Zone
The next question to be addressed is the cell-fate commitment of BrdU+ cells found in the SGZ and SVZ. At present, studies suggest that glial cell fate commitment increases after HI in the dentate gyrus, whereas neuronal cell fate commitment remains surprisingly unchanged.87, 88 This shows that although progenitor cells are able to proliferate after a HI insult, the damaged dentate gyrus is incapable of increasing neurogenesis and compensating for lost neurons and instead there is a trend toward astrocytic cell-fate commitment. Studies in adult rodents also suggest a limited regenerative capacity as only a few or no new neurons have been detected after HI injury.87, 88 Furthermore, studies by Miles and Kernie89 and Nakatomi et al87 show that HI leads to extensive loss of neurons and that only a few newly formed neurons survive up to 6 months after HI.87, 89 It is also unclear whether the newborn neuronal cells integrate into the local circuitry and become functional in the immature brain, and whether this effect is long-term. It has been described in studies using adult rodents that newborn neuronal cells show attenuated electrophysiological properties, such as field excitatory postsynaptic potentials (fEPSPs), which means that stimulation of a presynaptic terminal, e.g., the Schaffer collaterals, evokes a decreased postsynaptic response in the regenerated hippocampus.7 These data indicate that the hippocampal environment no longer supports neuronal cell-fate commitment and functionality after HI. More systematic studies are necessary to clarify the mechanisms underlying impaired neuronal cell-fate commitment in the HI-injured brain.
A growing number of studies address cell-fate commitment in the SVZ, striatum, and cortex after HI. Several studies observed a significant increase in Dcx/BrdU double-positive cells in the SVZ, striatum, and cortex from 1 to 4 weeks after injury.77, 79, 81, 82, 83 These double-positive cells were clustered in chains in the striatum and cortex and displayed the morphology of migrating neuroblasts.82, 83, 84 Studies investigating the differentiation rate of proliferating cells into neurons and their survival show contradicting results. Current data show a decrease,14, 78 increase,83, 84 or no change (cortical area)14 in NeuN/BrdU double-labeled cells compared with healthy brains. This discrepancy is possibly due to the use of different strains in the studies, which may affect the extent of brain damage after HI. It is striking that only the studies that used a neonatal HI model in Wistar rats observed increased neurogenesis in the striatum and cortex. This suggests that Wistar rats are more resilient to HI-induced cerebral damage than other rat or mice strains (e.g., Sprague-Dawley rats or CD-1 mice). For instance, in CD-1 mouse pups, a decrease in neuronal committed cells in the striatum and cortex was detected, despite substantial cell proliferation in the HI-affected SVZ.79 Conversely, a substantial increase in GFAP/BrdU double-labeled cells was observed at P24 and P31, suggesting that proliferating cells tend to differentiate toward an astrocytic cell fate.79 This finding is supported by all studies in rodents, independent of the strain used, as in all cases there is a substantial increase in glial cell-fate commitment in the striatum and cortex, implying that the environment after injury shows a predisposition toward gliogenesis. An alternative conclusion would be that this predisposition toward glial cell fate could be expected, as astrocytes form the largest glial cell population in the brain; they outnumber neurons by 5 times and comprise ∼50% of human brain volume.90, 91
Investigations on the differentiation of neuron-committed cells in the striatum has surprisingly shown that most proliferating cells were calretinin (CR) positive, a marker for an interneuron subtype that encompasses only a small percentage of striatal neurons.80, 82 Furthermore, no BrdU+ cells were shown to differentiate into striatal medium-sized spiny projection neurons, cholinergic neurons or parvalbumin (PV), calbindin (CB), or somatostatin (SOM) interneuron subtypes. Also, in the cortex, BrdU+ cells seem to differentiate into CR-expressing interneurons, which is unexpected because the majority of the cortical interneurons are of the PV subtype. These findings suggest that the progenitors from the SVZ are predisposed to differentiate into CR-expressing interneurons.
Survival of newborn neurons has been shown to be impaired after HI insult. Several TUNEL+/BrdU+ cells were found at P31 in the striatum, showing that proliferating cells undergo cell death.79 Furthermore, only 15% of newly generated neurons in the cortex survived 5 weeks after HI.82 The authors proposed that newborn neurons mature slowly,83 raising the possibility that the failure to detect NeuN/BrdU-positive cells in previous studies78, 79 may be because of the relatively short period of time elapsed (2 to 3 weeks) after BrdU injection. Yet, prolonging the interval after BrdU administration may also lead to negative results, as the low number of newborn neurons could be the consequence of both slow maturation and impaired survival of neuroblasts.
Little is known regarding the functionality of newborn neurons in the brain after HI injury. A recent study by our group has shown for the first time that a HI insult induces changes in neuronal connectivity in the corticospinal tract.92 To visualize the axonal projections from the damaged (ipsilateral) motor cortex to the lateral corticospinal tract (contralateral side), biotinylated dextran amine (BDA) was injected into the damaged (ipsilateral) motor cortex. The BDA is actively taken up by neurons and transported anterogradely toward the axon terminals, thus allowing the detection of neuronal connectivity. Our data showed that axons from the damaged (ipsilateral) motor cortex rewire toward the contralateral (undamaged) motor cortex by crossing the corpus callosum instead of projecting to the spinal cord through the corticospinal tract. Furthermore, injection of the retrograde transsynaptic tracer pseudorabies into the left forelimb muscles (labeled with monomeric red fluorescent protein) and right forepaw muscles (labeled with enhanced green fluorescent protein) showed a significant decrease in monomeric red fluorescent protein-positive neurons, indicating a loss of neurons projecting from the impaired cortex along the corticospinal tract. Interestingly, monomeric red fluorescent protein-positive neurons were detected in the contralateral (undamaged) cortex, providing evidence of adaptive functional processes taking place in the neonatal brain after HI.
Although the postnatal brain has the capacity to initiate regenerative processes after an insult, cell proliferation, neuronal differentiation, and possibly functionality of these newborn neurons are impaired. For this reason it seems that the complex network of molecular and cellular processes that orchestrate neurogenesis and long-term survival of newborn cells are disrupted by HI injury. It would be interesting to establish which factors determine postnatal cell-fate commitment and to which degree the molecular mechanisms overlap with early developmental programs. The mechanisms underlying this impairment are yet to be clarified and will be shortly addressed in the next section.
Molecular and Cellular Processes in the Neurovascular Niche After HI Brain Damage
Factors Involved in Regulating Neurogenesis in the Healthy Brain
As mentioned before, the neurovascular niche plays a crucial role in regulating proliferation, differentiation, and survival of newborn neurons. Astrocytes provide structural support for proliferating cells besides producing several factors that are required to sustain neurogenic processes. Astrocytes comprise almost half of the cells in the dentate gyrus and are in direct contact with proliferating cells and in proximity to blood vessels.93, 94 Endothelial cells play an important role in neurogenesis as they produce several factors (e.g., FGF2) that are essential for promoting cell proliferation, neuronal fate commitment, and supporting both projection neuron and interneuron cell fate.95
The precise role of proteins involved in neurogenesis in the healthy adult brain has still to be unraveled, but during the past decade several studies using different approaches, including in vivo loss and gain of gene function, have shed some light on the role of some of these factors. Cell proliferation is mostly regulated by growth factors and neurotrophins, e.g., brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), but also by morphogens, such as Wnt3 and Shh. The latter class of proteins is also involved in neuronal cell-fate commitment. Most factors regulate more than one process, e.g., the morphogen Noggin that plays a role in neuronal cell differentiation and survival, and BDNF, which is among others involved in cell proliferation and survival (see review by Zhao et al51).
Besides growth and neurotrophic factors, the local neuronal network in the neurovascular niche is of pivotal importance in regulating neurogenesis in the adult brain. Dopaminergic signaling was shown to promote proliferation, GABAergic innervations are important for synapse formation, and glutamatergic innervations play a role in neuroblasts survival, dendritic development, and synaptogenesis (see review by Pathania et al96). More studies are necessary to determine the role that neurotransmitters play in regulating neuronal commitment, dendritic development, synaptic integration, and survival of newborn neurons in the postnatal brain after HI injury.
Factors Involved in the Modulation and Maintenance of Neurogenesis After HI
Until now, only a few proteins that play a role in neurogenesis have been correlated to neurogenic processes after HI. However, growing evidence shows that a HI insult alters the cellular and molecular composition of the neurovascular niche.96, 97, 98, 99 Therefore, although cell proliferation is maintained in the SVZ and SGZ, detrimental changes in protein expression in the brain can lead to impairments in neuronal fate commitment, survival, and functionality.
Results from microarray studies in the neonatal HI model show up- and down-regulation of several genes, e.g., growth factors and inflammatory proteins.84, 97 Recent studies are starting to unveil some of the proteins that may play a role in mediating neurogenesis after HI cerebral damage. Here, we will discuss some genes that were upregulated in studies investigating neurogenesis after HI in the neonatal brain.
Increased mRNA levels of membrane receptors Notch1, epidermal growth factor receptor (EGFR), and glycoprotein 130 (gp130) were observed in the ipsilateral SVZ at 48 hours after HI injury. These changes in mRNA levels coincide with increased cell proliferation in the ipsilateral SVZ, suggesting a possible role for these membrane receptors in regulating neurogenesis after HI brain damage.84 Notch1 was shown to be enriched in the SVZ and SGZ areas.84, 100 Interestingly, recent data show that ablation of Notch1 expression in GFAP-expressing stem cells in postnatal mice results in a substantial decrease in proliferating cells and an increased preference for neural cell fate in the SGZ.100 Alternatively, overexpression of the intracellular portion of Notch1 (called NICD), which initiates transcription of target genes,101, 102, 103 increases proliferation and maintains GFAP-expressing stem cells. Notch1 ablation also leads to less complex arborization and branching of newly generated neurons, which is modulated in a dosage-dependent way. Together, these findings suggest that Notch1 may promote cell proliferation after HI injury and that its function may change during the different stages of neurogenesis.
Notch1 expression is increased by activation of gp130, which is a membrane receptor important for NSC survival and proliferation.104 Importantly, Felling et al84 showed that gp130 is upregulated in the SVZ where neurogenesis takes place after HI. Hence, it seems likely that gp130 may play a role in ensuring an environment that supports neurogenesis. Additionally, EGFR is a receptor for several ligands, including Egf1 and TGFα, that acts on a plethora of signaling pathways including mitogen-activated protein kinase (MAPK) and Rac pathways, and therefore plays a crucial role in promoting proliferation, migration, differentiation, and survival.105
Another protein that appears to play a role in neurogenic processes after HI is the fibroblast growth factor receptor 1 (Fgfr1), which promotes progenitor proliferation106 and neuronal fate commitment in proliferating cells.107, 108 A recent study showed that ablation of the Fgfr1 gene from GFAP-expressing cells in mice leads to attenuated cell proliferation in the SVZ and decreased cortical pyramidal neuron production after HI.109 Interestingly, Fgfr1 knockout does not increase apoptosis when compared with wild-type hypoxic mice, suggesting that Fgfr1 may be involved in mediating neurogenesis rather than neuroprotection after hypoxic injury. These results can therefore be interpreted as evidence that 48 hours after the insult, the SVZ environment supports proliferation, migration, and cell survival.84
Besides an increase in neurogenic factors, some proinflammatory cytokines have also been shown to be upregulated after HI injury. Indeed, the expression levels of the cytokines interleukin-1β (Il1β) and transforming growth factor β-1 (Tgfβ-1) increase after HI.97 The Il1-β is expressed by activated microglia and promotes a proapoptotic and inflammatory environment.110 The Tgf1β is not only expressed by activated microglia, but also by astrocytes. Conversely, Tgf1β is a proneurogenic factor, as downregulation of this factor reduces neurogenesis and impairs cognitive behavior.111 Interestingly, the growth factor, colony stimulation factor 1 (Csf1), was also upregulated at 24 hours after HI.97 The Csf1, which is expressed in astrocytes, promotes microglial development and survival, which in turn play an important role in promoting neurogenesis after HI injury.112 Microglia are involved in the uptake of cell debris and dying cells after injury, which is essential for neuronal cell survival. Thereby, microglia can either be harmful or neuroprotective after HI (see Figure 1).
Future studies need to assess the level of neurogenic factors at later stages during neurogenesis to understand how changes in the expression patterns of these proteins relate to neurogenic processes and cell survival.
Migration of Immature Neurons in the Neonatal Hypoxia–Ischemia-Injured Brain
A recent study followed the migration of NSCs in the SVZ in healthy and HI-injured brains by in situ labeling the cells with micron sized iron oxide particle (MPIO), and measuring with magnetic resonance imaging. The results clearly showed that these cells no longer migrate toward the OB through the RMS, but instead migrate toward the damaged cortical areas.113
The mechanisms regulating neuroblast migration toward the striatum in the neonatal HI brain are still unclear (see review by Cayre et al52). However, there is evidence that in the rodent adult brain SDF-1/CXCR-4 signaling mediates migration of neuroblasts from the SVZ to the striatum. Results show that reactive astrocytes produce SDF-1, whereas immature neurons express the receptor CXCR-4.114 Furthermore, migration of immature neurons could be inhibited by the specific CXCR-4 inhibitor AMD-3100, proposing that these molecules are important regulators of migration of immature neurons in this brain region. Another study showed that DCX/BrdU-positive neuroblasts colocalize with matrix metalloproteinases (MMPs), a family of zinc endopeptidases that modulate all components of extracellular matrix in the brain, thus allowing the neuroblasts to migrate through axonal extension.115, 116 Interestingly, inhibiting MMPs significantly suppressed migration of neuroblasts from the SVZ to striatum.116 Furthermore, several studies have shown that MMPs are upregulated after ischemia.117, 118, 119
Evidence suggests that migrating neuroblasts in the hippocampus use radial glial fibers as a scaffold because these cells extend their axons from the SGZ and traverse the granule layer perpendicularly (see Figure 1). Another cell type that regulates migration in the SGZ are the Cajal–Retzius cells that secrete reelin, which is an extracellular matrix serine protease that seems to function as a stop or detachment signal for migrating neurons. The pivotal role of this protein is illustrated in the so-called reeler mice, as lack of reelin results in an inverted cortex due to aberrant migration of neurons to the superficial plate.120 It remains unclear exactly how reelin regulates neuronal migration. So far, it seems to be mediated by very-low-density lipoprotein receptor (VLDLR) and apolipoprotein E type 2 receptor (ApoER2), which leads to tyrosine phosphorylation of the adaptor protein Disabled-1 (Dab-1).121
Hypoxia is a known trigger of angiogenesis. Several studies have shown that blood vessels play a role in progenitor cell migration in the adult brain. Thored et al114 established that HI triggers angiogenesis in the SVZ and striatum and that Dcx+ cells and blood vessels are closely associated. However, different numbers of progenitor cells were observed in areas with similar vascular density, suggesting that vessels are not sufficient to regulate migration efficiently.
In conclusion, it appears that the neurovascular niche in neonatal mice does not support full repair after HI damage and thus does not provide the structural and humoral support necessary for inducing and maintaining long-term neurogenesis (see Figure 1). The precise balance and gradient of proteins may be tightly regulated through the different stages of neurogenesis. Furthermore, cell migration and network formation is still taking place in the neonatal brain. It is therefore crucial to assist the neonatal brain in the regeneration process after cerebral ischemia, allowing it to repair the cellular and structural damage and to keep up with the developmental phase.
Stem-Cell Based Therapy: Enhancing the Neurogenic Potential of Neural Stem Cells
Until now, no definitive proof has been obtained that the primary goal of neurogenesis is regeneration of the brain after an injurious event. Enriched odor exposure increases the survival of newborn neurons in the rodent OB, thereby correlating neurogenesis to olfactory experience and learning in rodents.47, 122 Methyl-CpG binding proteins (Mbd1) are highly expressed in progenitor cells and neurons, and are possibly involved in regulating DNA methylation in the adult rodent brain.123 Studies show that mbd1 knockout mice have decreased SGZ neurogenesis and impaired learning and memory,123, 124 suggesting a functional significance for neurogenesis in the SGZ of the rodent brain. Nevertheless, the fact that neurogenesis is restricted to specific brain regions implies that it plays a role in regulating the function of the hippocampus and olfactory system. Thus, current data raise the interesting possibility that neurogenesis may be important for the rodent brain to function normally. Nevertheless, evidence suggests that neurogenesis decreases after an injurious event, thereby proposing that regeneration may not be the primary goal of neurogenesis.
A growing number of studies suggest that mesenchymal stem cell (MSC) transplantation may be a promising tool to boost endogenous neurogenesis. Recent data show that administration of MSC after a HI insult significantly reduces lesion volume, improves behavioral performance, and promotes neurogenesis.35, 125, 126, 127 Furthermore, studies show that MSCs migrate to the ischemic boundary zone where they induce changes in brain environment that promote and support neurogenesis.128, 129, 130 A study from our group showed that intracranial MSC treatment at 3 and 10 days after HI-induced injury changes the expression of genes involved in regenerative processes. Some key functions associated with an increased gene expression are cell growth, cell proliferation, nervous system development, and cell migration. Our findings are further supported by studies in which human NSCs were transplanted intracranially at 24 hours after HI inducing an increase in the expression of genes involved in neurogenesis (e.g., doublecortin), migration (e.g., CXCR4), and survival (e.g., glial-derived neurotrophic factor)34 (see Figure 1). Furthermore, studies show that MSCs130 and human NSCs improve axonal sprouting and neurite plasticity by increasing the expression of factors like VEGF and Slit.131 Results from our group show that transplanted MSCs do not differentiate into neurons and oligodendrocytes, suggesting that stimulation of endogenous NSCs by MSC is mainly responsible for restoring tissue damage.132 Hence, current data strongly suggest that stem cells secrete factors that promote neurogenic processes and boost regenerative processes in the HI-injured neonatal brain.
Besides showing promising results as a therapeutic strategy, the use of MSCs as a treatment for HI injury holds a few more advantages over other strategies. One major appeal of using MSCs as a therapeutic tool is the considerably longer therapeutic window after HI, as administration of MSCs at 10 days after HI leads to improved motor and histologic outcome.132 MSC treatment also has some advantages over NSC or embryonic stem cell therapies because MSCs do not express HLA-DR antigens and these cells are low immunogenic, in contrast to NSCs, and can be used over the allogeneic barrier. Over the past decade, allogeneic MSCs have been widely used as a treatment for hematopoietic diseases.133, 134 Another major advantage is that they can be obtained easily and safely from placental tissue, umbilical cord stroma, and cord blood, whereas NSCs and embryonic stem cells can only be obtained from fetal tissue, thereby raising ethical issues. Transplantation of embryonic stem cells may have undesirable consequences as these cells can transdifferentiate into tumors besides differentiating into the desired tissue type. Although it has never been shown, the possibility remains that MSCs become HLA-DR+ after activation and thus lead to alloreactivity. However, MSCs only survive a few weeks after administration into the brain, which will limit the risk of inducing a host versus graft response in the brain.132 Nevertheless, autologous MSCs from the stroma or blood of the umbilical cord might be the safest treatment option.
Another important aspect of stem cell therapy is finding an efficient and noninvasive administration route. Recent studies show that intranasally administered MSCs migrate toward damaged brain regions.135 This method has some advantages over the more conventional administration routes, i.e., intracranial and intravenous injection, because it is less invasive and may reduce systemic exposure. Indeed, results from our own group show that intranasal administration of MSCs after neonatal HI in P9 mice is as effective as intracranial MSC transplantation leading to improved motor behaviour.136 Furthermore, efficacy of treatment may also depend on the administration route. Recently, it has been shown that intravenously injected mononuclear cells137 and NSCs138 are not only detected in the brain, but also in a substantial amount in the spleen,137 and liver and lungs, respectively.138 Hence, significantly less of the injected stem cells will end up in the brain after intravenous delivery as they will circulate throughout the body and may even have an undesired effect in other organs.
Conclusion and Future Prospects
It is increasingly evident that the neonatal brain has a limited capacity to adapt and regenerate after a deleterious event, such as cerebral ischemia. Although neurogenesis occurs under physiologic conditions and may even increase after an insult, the immature brain is incapable of fully regenerating after cerebral ischemia. This impairment may be because of the extent of cellular loss and also a possible disturbed expression of growth and differentiation factors in the neurovascular niche as a consequence of brain damage. Crucial questions to be answered are which factors play a pivotal role during the different phases of neurogenesis under normal conditions and, on top of that, how neurogenic processes within the brain are affected by an insult. Moreover, the molecular and cellular composition of the neurogenic niche that will favor regeneration has to be defined more precisely.
In the past years, significant progress has been made in optimizing stem cell treatment as a tool to boost the limited capacity of the neonatal brain to regenerate, especially by the use of MSCs. The intranasal administration route may represent the most optimal route in rodents, but has still to be verified in humans. Before translating the results to the clinic, more knowledge is also needed on the exact array of molecules that direct MSC migration toward the damaged regions.
Transplantation of MSCs induces regulation of the expression of many genes in the neonatal brain, but research is still scarce regarding the hierarchy or redundancy of the plethora of factors that are up- and down-regulated after transplantation. Although MSC treatment has a relatively long therapeutic window in animal models, one has to realize that the neonatal brain is still immature, being in a critical developmental phase. One of the most challenging issues to be solved in this area of research is to unravel the mechanism of how MSCs specifically adapt to the developmental and regenerative needs of the environment after injurious events in the neonate. We also propose here that MSCs do not integrate into the network but stimulate proliferation and differentiation of endogenous precursors. It will be intriguing to know to what extent these new (autologous) neurons and other cell types of the brain survive and integrate into existing functional networks. However, preclinical research has shown that MSC therapy has the potential to become an efficient therapy to treat neonatal brain damage by boosting the endogenous capacity of the immature brain to regenerate, thereby repairing the lesion and improving motor and cognitive behavior in the long term. The neonatal brain may even be a better ‘target' for stem cell therapy than the adult brain because of the higher availability of endogenous precursors in the neonatal brain.
Acknowledgments
The authors thank Emiel Post-Uiterweer, MD, for his contribution in designing the figure.
The authors declare no conflict of interest.
Footnotes
This review was supported by EU-7 Neurobid (HEALTH-F2-2009-241778) from the European Union and Zon-MW Project (no. 116002003).
References
- Altman J, Das GD. Autoradiography and histological evidence of postnatal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiography and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals. Nat Rev Neurosci. 2007;6:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci USA. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda CJ, Braun L, Jiang Y, Hester ME, Zhang L, Riolo M, et al. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt- mediated surviving signalling. Aging Cell. 2012;11:542–552. doi: 10.1111/j.1474-9726.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, McCloskey DP. Postnatal neurogenesis as a therapeutic target in temporal lobe epilepsy. Epilepsy Res. 2009;85:150–161. doi: 10.1016/j.eplepsyres.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SW, Kim SS, Lee SY, Lee HS, Kim HS, Lee YD, et al. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med. 2008;40:387–397. doi: 10.3858/emm.2008.40.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam SD, Mulholland JD, McDonald JW, Comi AM. Neurogenesis and neuronal commitment following ischemia in a new mouse model for neonatal stroke. Brain Res. 2008;1208:35–45. doi: 10.1016/j.brainres.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, et al. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci. 2006;3:e2935. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, et al. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS One. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7:56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Badr Zahr LK, Purdy I. Brain injury in the infant: the old, the new and the uncertain. J Perinat Neonatal Nurs. 2006;20:163–175. doi: 10.1097/00005237-200604000-00011. [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Ferriero DM. Molecular and biochemical mechanisms of perinatal brain injury. Semin Neonatol. 2001;6:99–108. doi: 10.1053/siny.2001.0041. [DOI] [PubMed] [Google Scholar]
- Dammann O, Ferriero D, Gressens P. Neonatal encephalopathy or hypoxic-ischemic encephalopathy? Appropriate terminology matters. Pediatr Res. 2011;70:1–2. doi: 10.1203/PDR.0b013e318223f38d. [DOI] [PubMed] [Google Scholar]
- Azzopardi DV, Strohm B, Edwards AD, Dyet l, Halliday HL, Jucszak E, et al. Moderate hypothermia to treat perinatal asphyxia encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28:1353–1365. doi: 10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Nijboer CHA, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. Strong neuroprotection by inhibition of NF{kappa}B after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke. 2008;39:2129–2137. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]
- Nijboer CHA, Heijnen CJ, van der Kooij MA, Zijlstra J, van Velthoven CT, Culmsee C, et al. Targeting the p53 pathway to protect the neonatal ischemic brain. Ann Neurol. 2011;70:255–264. doi: 10.1002/ana.22413. [DOI] [PubMed] [Google Scholar]
- Compagnoni G, Pogliani L, Lista G, Castoldi F, Fontana P, Mosca F. Hypothermia reduces neurological damage in asphyxiated newborn infants. Biol Neonate. 2002;82:222–227. doi: 10.1159/000065890. [DOI] [PubMed] [Google Scholar]
- Ishida A, Trescher WH, Lange MS, Johnston MV. Prolonged suppression of brain nitric oxide synthase activity by 7-nitroindazole protects against cerebral hypoxic-ischemic injury in neonatal rat. Brain Dev. 2001;23:349–354. doi: 10.1016/s0387-7604(01)00237-6. [DOI] [PubMed] [Google Scholar]
- Lin S, Zhang Y, Dodel R, Farlow MR, Paul SM, Du Y. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett. 2001;315:61–64. doi: 10.1016/s0304-3940(01)02324-2. [DOI] [PubMed] [Google Scholar]
- Han BH, Xu D, Choi J, Han Y, Xanthoudakis S, Roy S, et al. Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic-ischemic brain injury. J Biol Chem. 2002;277:30128–30136. doi: 10.1074/jbc.M202931200. [DOI] [PubMed] [Google Scholar]
- Leonardo CC, Eakin AK, Ajmo JM, Collier LA, Pennypacker KR, Strongin AY, et al. Delayed administration of a matrix metalloproteinase inhibitor limits progressive brain injury after hypoxia-ischemia in the neonatal rat. J Neuroinflammation. 2008;5:34–44. doi: 10.1186/1742-2094-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowper-Smith CD, Anger GJA, Magal E, Norman MH, Robertson GS. Delayed administration of a potent cyclin dependent kinase and glycogen synthase kinase 3 β inhibitor produces long-term neuroprotection in a hypoxia-ischemia model of brain injury. Neuroscience. 2008;155:864–875. doi: 10.1016/j.neuroscience.2008.05.051. [DOI] [PubMed] [Google Scholar]
- Han J, Pollak J, Yang T, Siddiqui MR, Doyle KP, Taravosh-Lahn K, et al. Delayed administration of a small molecule tropomyosin-related kinase B ligand promotes recovery after hypoxic-ischemic stroke. Stroke. 2012;43:1918–1924. doi: 10.1161/STROKEAHA.111.641878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Stetler A, Xing J, Hu X, Gao Y, Zhang W, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin following neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:1032–1037. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM, Davis AS, Arac A, Li Z, Maag A-L, Bhatnagar R, et al. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516–523. doi: 10.1161/STROKEAHA.109.573691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Velthoven CTJ, Kavelaars A, van Bel F, Heijnen CJ. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J Neurosci. 2010;30:9603–9611. doi: 10.1523/JNEUROSCI.1835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigaluppi M, Pluchino S, Jametti LP, Kilic U, Salani G, Brambilla E, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- Liao W, Xie J, Zhong J, Liu Y, Du l, Zhou B, et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation. 2009;87:350–359. doi: 10.1097/TP.0b013e318195742e. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodelling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- Pimentel-Coelho PM, Magalhaes ES, Lopes LM, de Azevedo LC, Santiago MF, Mendez-Otero R. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: functional outcome related to neuroprotection in the striatum. Stem Cells Dev. 2010;19:351–358. doi: 10.1089/scd.2009.0049. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:1–20. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23:9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci. 1993;13:2351–2358. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, et al. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 2010;13:173–179. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- Fishell G, Goldman JE. A silver lining to stroke: does ischemia generate new cortical interneurons. Nat Neurosci. 2010;13:145–146. doi: 10.1038/nn0210-145. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretto P, Giachino C, Aimar P, Fasolo A, Bonfanti L. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. J Comp Neurol. 2005;487:407–427. doi: 10.1002/cne.20576. [DOI] [PubMed] [Google Scholar]
- Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Meyer-Luehmann M, Osetek JD, Jones PB, Stern EA, Bacskai BJ, et al. Impaired spine stability underlies plaque-related spine loss in an Alzheimer's disease mouse model. Am J Pathol. 2007;171:1304–1311. doi: 10.2353/ajpath.2007.070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright LE, Zhang S, Murphy TH. Fine mapping of the spatial relationship between acute ischemia and dendritic structure indicates selective vulnerability of layer V neuron dendritic tufts within single neurons in vivo. J Cereb Blood Flow Metab. 2007;27:1185–2000. doi: 10.1038/sj.jcbfm.9600428. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Li P, Betts K, Liu R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci. 2008;28:1756–1772. doi: 10.1523/JNEUROSCI.5128-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Murphy TH. Livin' on the edge: imaging dendritic spine turnover in the peri-infarct zone during ischemic stroke and recovery. Neuroscientist. 2008;14:139–146. doi: 10.1177/1073858407309854. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- Parrish JZ, Emoto K, Kim MD, Jan YN. Mechanisms that regulate establishment, maintenance, and remodelling of dendritic fields. Annu Rev Neurosci. 2007;30:399–423. doi: 10.1146/annurev.neuro.29.051605.112907. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P, Cheng HJ. Guidance molecules in axon pruning and cell death. Cold Spring Harb Perspect Biol. 2010;2:a001859. doi: 10.1101/cshperspect.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Tessier CR, Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 2008;135:1547–1557. doi: 10.1242/dev.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphoring family. Cell. 2003;113:285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Plexin signalling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proc Natl Acad Sci USA. 2008;105:8136–8141. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Low LK, Jones EG, Cheng HJ. Stereotyped axon pruning via plexin signalling is associated with synaptic complex elimination in the hippocampus. J Neurosci. 2005;25:9124–9134. doi: 10.1523/JNEUROSCI.2648-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan WM, Fawcett JW, O'Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225:1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, et al. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11:649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocytes differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocytes-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Iwai M, Ikeda T, Jin G, Deguchi K, Nagotani S, et al. Neural precursor cells division and migration in neonatal rat brain after ischemic/hypoxic injury. Brain Res. 2005;1038:41–49. doi: 10.1016/j.brainres.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Ong J, Plane JM, Parent JM, Silverstein FS. Hypoxic-ischemic injury stimulates subventricular zone proliferation and neurogenesis in the neonatal rat. Pediat Res. 2005;58:600–606. doi: 10.1203/01.PDR.0000179381.86809.02. [DOI] [PubMed] [Google Scholar]
- Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM. Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol Dis. 2004;16:585–595. doi: 10.1016/j.nbd.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Yang Z, You Y, Levison SW. Neonatal hypoxic/ischemic brain injury induces production of calretinin-expressing interneurons in the striatum. J Comp Neurol. 2008;511:19–33. doi: 10.1002/cne.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Levison SW. Perinatal hypoxic/ischemic brain injury induces persistent production of striatal neurons from subventricular zone progenitors. Dev Neurosci. 2007;29:331–340. doi: 10.1159/000105474. [DOI] [PubMed] [Google Scholar]
- Yang Z, Covey MV, Bitel CL, Ni L, Jonakait GM, Levison SW. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann Neurol. 2007;61:199–208. doi: 10.1002/ana.21068. [DOI] [PubMed] [Google Scholar]
- Yang Z, Levison SW. Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience. 2006;139:555–564. doi: 10.1016/j.neuroscience.2005.12.059. [DOI] [PubMed] [Google Scholar]
- Felling RJ, Snyder MJ, Romanko MJ, Rothsein RP, Ziegler AM, Yang Z, et al. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci. 2006;26:4359–4369. doi: 10.1523/JNEUROSCI.1898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Zhu C, Wang X, Xu F, Eriksson PS, Nilsson M, et al. Less neurogenesis and inflammation in the immature than in the juvenile brain after cerebral hypoxia-ischemia. J Cereb Blood Flow Metab. 2007;27:785–794. doi: 10.1038/sj.jcbfm.9600385. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nozaki K, Takahashi J, Yodoi J, Ishikawa M, Hashimoto N. Proliferation of neuronal precursor cells in the dentate gyrus is accelerated after transient forebrain ischemia in mice. Brain Res. 1999;831:283–287. doi: 10.1016/s0006-8993(99)01411-0. [DOI] [PubMed] [Google Scholar]
- Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus. 2008;18:793–806. doi: 10.1002/hipo.20439. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Velthoven CTJ, van de Looij Y, Kavelaars A, Zijlstra J, van Bel F, Huppi PS, et al. Mesenchymal stem cells restore cortical rewiring after neonatal ischemia in mice. Ann Neurol. 2012;71:785–796. doi: 10.1002/ana.23543. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Pathania M, Yan LD, Bordey A. A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology. 2010;58:865–876. doi: 10.1016/j.neuropharm.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Velthoven CTJ, Heijnen CJ, van Bel F, Kavelaars A. Osteopontin enhances endogenous repair after neonatal hypoxic-ischemic brain injury. Stroke. 2011;42:2294–2301. doi: 10.1161/STROKEAHA.110.608315. [DOI] [PubMed] [Google Scholar]
- Zhao YD, Cheng SY, Ou S, Chen PH, Ruan HZ. Functional response of hippocampal CA1 pyramidal cells to neonatal hypoxic-ischemic brain damage. Neurosci Lett. 2012;10:5–8. doi: 10.1016/j.neulet.2012.02.067. [DOI] [PubMed] [Google Scholar]
- Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60:95–104. [PubMed] [Google Scholar]
- Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendritic morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci USA. 2007;51:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signalling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signaling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S. Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci. 2003;23:1730–1741. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Stavridis MP, Lunn JS, Collins BJ, Storey KG. A discrete period of FGF-induced Erk1/2 signaling is required for vertebrate neural specification. Development. 2007;134:2889–2894. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]
- Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Rhee J, Lyn-Cook R. Fibroblast growth factor signalling regulates growth and morphogenesis at multiple steps during brain development. Curr Top Dev Biol. 1999;46:179–200. doi: 10.1016/s0070-2153(08)60329-4. [DOI] [PubMed] [Google Scholar]
- Fagel DM, Ganat Y, Cheng E, Silbereis J, Ohkubo Y, Ment LR, et al. Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia. J Neurosci. 2009;29:1202–1211. doi: 10.1523/JNEUROSCI.4516-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green HF, Treacy E, Keohane AK, Sullivan AM, O'Keeffe GW, Nolan YM. A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 2012;3:311–321. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Graciarena M, Depino AM, Pitossi FJ. Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFβ1 downregulation. Brain Behav Immun. 2010;8:1301–1308. doi: 10.1016/j.bbi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Fedoroff S, Berezovskaya O, Maysinger D. Role of colony stimulating factor-1 in brain damage caused by ischemia. Neurosci Biobehav Rev. 1997;2:187–197. doi: 10.1016/s0149-7634(96)00009-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu J, Niu G, Chan KC, Wang R, Liu Y, et al. In vivo MRI of endogenous stem/progenitor cell migration from subventricular zone in normal and injured developing brains. Neuroimage. 2009;48:319–328. doi: 10.1016/j.neuroimage.2009.06.075. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Maddahi A, Chen Q, Edvinsson L. Enhanced cerebrovascular expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 via the MEK/ERK pathway during ischemia in the rat. BMC Neurosci. 2009;10:56. doi: 10.1186/1471-2202-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-R, Kim H-Y, Rogowska J, Zhao B-Q, Bhide P, Parent JM, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashe Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, et al. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Leonardo C, Eakin A, Ajmo J, Collier L, Pennypacker K, Strongin A, et al. Delayed administration of a matrix metalloproteinase inhibitor limits progressive brain injury after hypoxia-ischemia in the neonatal rat. J Neuroinflammation. 2008;5:34. doi: 10.1186/1742-2094-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;347:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Alonso M, Viollet C, Gabellec MM, Meas-Yedid V, Olivo-Marin JC, Lledo PM. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 2006;26:10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AM, Liang X, Luo Y, Pak C, Li X, Szulwach KE, et al. The loss of methyl-CpG binding protein 1 leads to autism-like behavioral deficits. Hum Mol Genet. 2008;17:2047–2057. doi: 10.1093/hmg/ddn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Ueba T, Christie BR, Barkho B, McConnell MJ, Nakashima K, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc Natl Acad Sci USA. 2003;100:6777–6782. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84:1495–1504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, et al. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:329–340. doi: 10.1038/sj.jcbfm.9600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N, Nakai Y, Seo TB, Yamada Y, Ohno T, Yamanaka A, et al. Characterization of conditioned medium of cultured bone marrow stromal cells. Neurosci Lett. 2010;483:57–61. doi: 10.1016/j.neulet.2010.07.062. [DOI] [PubMed] [Google Scholar]
- Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27:355–363. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Velthoven CTJ, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav Immun. 2011;25:1342–1348. doi: 10.1016/j.bbi.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res. 2010;68:419–422. doi: 10.1203/PDR.0b013e3181f1c289. [DOI] [PubMed] [Google Scholar]
- Heijnen CJ, Witt O, Wulffraat N, Kulozik AE. Stem cells in pediatrics: state of the art and future perspectives. Pediatr Res. 2012;71:407–409. doi: 10.1038/pr.2012.1. [DOI] [PubMed] [Google Scholar]
- Domen J, Gandy K, Dalal J. Emerging uses for pediatric hematopoietic stem cells. Pediatr Res. 2012;71:411–417. doi: 10.1038/pr.2011.55. [DOI] [PubMed] [Google Scholar]
- Danielyan L, Schafer R, von Ameln-Mayerhofer A, Buadze M, Geisler J, Klopfer T, et al. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88:315–324. doi: 10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Donega V, van Velthoven CT, Nijboer CH, van Bel F, Kas MJ, Kavelaars A, et al. Intranasal mesenchymal stem cell treatment for neonatal brain damage: long-term cognitive and sensorimotor improvement. PLoS One. 2013;8:e51253. doi: 10.1371/journal.pone.0051253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Strong R, Sharma S, Brenneman M, Mallikarjunarao K, Xi X, et al. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89:833–839. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendharkar AV, Chua JY, Andres RH, Wang N, Gaeta X, Wang H, et al. Biodistribution of neural stem cells after intravascular therapy for hypoxia-ischemia. Stroke. 2010;41:2064–2070. doi: 10.1161/STROKEAHA.109.575993. [DOI] [PMC free article] [PubMed] [Google Scholar]