Abstract
Measurements of cerebral blood flow (CBF) show large variability among healthy subjects. The aim of the present study was to investigate the relative effect of established factors influencing CBF on the variability of resting CBF. We retrospectively analyzed spontaneous variability in 430 CBF measurements acquired in 152 healthy, young subjects using 133Xe single-photon emission computed tomography. Cerebral blood flow was correlated positively with both end-tidal expiratory PCO2 (PETCO2) and female gender and inversely with hematocrit (Hct). Between- and within-subject CO2 reactivity was not significantly different. Including PETCO2, Hct and gender in the model reduced between-subject and within-subject variance by 14% and 13.5%, respectively. Within-subject variability was mainly influenced by PETCO2 and between-subject variability mostly by Hct, whereas gender appeared to be of little added value when Hct was also accounted for. The present study confirms large between-subject variability in CBF measurements and that gender, Hct, and PETCO2 explain only a small part of this variability. This implies that a large fraction of CBF variability may be due to unknown factors such as differences in neuron density or metabolism that could be subject for further studies.
Keywords: carbon dioxide partial pressure, cerebral blood flow, gender, hematocrit, single-photon emission tomography
Introduction
Measurement of cerebral blood flow (CBF) is of great importance for both clinical and scientific use. During the past six decades, numerous studies have investigated the normal CBF using a range of techniques. Although CBF estimates vary between the various methods, most studies have reported a significant variability in normal subjects, with about a factor of two between the highest and the lowest 2.5% fractiles.1, 2, 3 Studies distinguishing within- and between-subject variability have shown that the between-subject variability is very similar across methods,1, 4 but the factors underlying this variability are not well established.
Cerebral blood flow may depend on various physiologic factors, in particular arterial PCO2, but also other factors such as age and gender are often considered to influence CBF.3, 5, 6 Most studies have been too small to quantify the relative influence on CBF and possible interactions of these factors. It is particularly unclear if the gender difference in CBF is merely explained by gender-related differences in oxygen transport capacity as measured by hemoglobin or hematocrit (Hct) or if additional gender-related factors may be of importance. Also, the effect of PCO2 has primarily been investigated using induced PCO2 changes, whereas knowledge on the influence of spontaneous variations in PCO2 is sparse.
In this study, we report on a unique set of data consisting of dynamic 133Xe single-photon emission computed tomography (SPECT) CBF measurements, with both short-term (hours) and long-term (weeks) repeated measurements. Data were obtained from studies investigating the effect of various vasoactive substances on CBF in healthy subjects over a total period of 9 years. From these studies, we have accumulated a very large number of CBF measurements in normal subjects during resting conditions. As the imaging protocol has not been changed, the data set provides a unique possibility to investigate the sources of variability of CBF.
The aims of the present study were to estimate between- and within-subject variability of resting CBF in healthy subjects using 133Xe SPECT and to investigate the relative importance of physiologic factors underlying this variability.
Materials and methods
Cerebral Blood Flow Measurements
The study is a retrospective analysis of 133Xe SPECT CBF measurements in young healthy subjects obtained in 14 (13 previously published) studies conducted within a 9-year period (1998 to 2007).7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 All measurements were performed using the same gamma camera and identical acquisition and processing protocols.
All subjects were interviewed for medical history and underwent both a neurologic and a general physical examination to exclude serious somatic or psychiatric disease. Subjects using prescription drugs (other than oral contraceptives which were required of female participants) were excluded from the study.
The general study design involved multiple measurements on a single day or on two or three different days at least 7 days apart. On each day, the subject was randomly allocated to receive either placebo or active substance. On all study days, an initial baseline measurement was followed by two measurements during administration of either placebo or active substance. In subjects studied only on 1 day, the baseline scan was followed by active substance scans only.
For each measurement, subject identity, scan date and time, and measurement type (baseline, placebo or active substance) were identified. Only baseline and placebo measurements were included for analysis. Subjects older than 40 years at first measurement and measurements performed >1 year from the first measurement were excluded. Measurements without a corresponding PETCO2 (end-tidal expiratory PCO2) or lung curve file and measurements deemed inadmissible by the primary investigators were also excluded from analysis.
A total of 430 CBF measurements (262 baseline and 168 placebo) from 152 subjects (85 males/67 females) were included for analysis. Population characteristics are presented in Table 1. Some covariates were only available for a subset of the subjects: mean arterial blood pressure (MAP, 331 observations in 112 subjects), Hct (265 observations in 77 subjects), and body mass index (BMI, 40 subjects).
Table 1. Characteristics of subjects.
| All (n=152) | Male (n=85) | Female (n=67) | P value for difference | |
|---|---|---|---|---|
| Age (years) | 25.0 (3.5) | 25.2 (3.1) | 24.7 (4.0) | 0.353 |
| BMI (kg/m2) (331 obs in 112 subjects) | 23.3 (3.1) | 24.1 (3.2) | 22.1 (2.5) | 0.047 |
| MAP (mm Hg) (331 obs in 112 subjects) | 80 (8.6) | 80.7 (8.9) | 79.2 (8.3) | 0.347 |
| Hct (%) (265 obs in 77 subjects) | 40.8 (3.9) | 43 (2.9) | 37.4 (2.5) | <0.001 |
| PETCO2 (kPa) | 5.17 (0.58) | 5.34 (0.41) | 4.95 (0.48) | <0.001 |
| CBF (mL/100 g per minute) | 52.6 (8.6) | 50.2 (7.4) | 55.7 (9.1) | <0.001 |
Abbreviations: BMI, body mass index; CBF, cerebral blood flow; Hct, hematocrit; MAP, mean arterial pressure; PETCO2, end-tidal expiratory PCO2.
All values are mean (s.d.) of average of values from each subject.
The mean number of measurements per subject was 2.8 (range 1 to 5). Sixty-one subjects were studied on 1 day only, 82 on 2 different days, and 9 on 3 different days. The median time interval from the first to the second study day was 17 days (range 6 to 151 days) and between measurements on the same day was 49 minutes (range 21 to 135 minutes).
Ethics
All studies were approved by the regional ethics committee (Ethics Committee of Copenhagen County/Region Hovedstaden) following the standards of The National Committee on Health Research Ethics. All experiments were conducted in accordance with the Helsinki Declaration of 1975 and all subjects gave written informed consent.
133Xe 133Xe Single-Photon Emission Computed Tomography
Acquisition
All CBF measurements were acquired using a dedicated brain gamma camera Ceraspect (Digital Scintigraphics, Inc., Waltham, MA, USA) equipped with an annular NaI crystal and a fast rotating collimator system. Spatial resolution (FWHM) is ∼15 mm. During 5 minutes acquisition 30 frames (3 background, 9 inhalation, 18 washout) of 10 seconds each were acquired in a 64 × 64 matrix. Images were acquired in the orbitomeatal plane covering 10.67 cm. A lung probe was placed over the right lung to measure the input function.
Xenon gas was administered using a Ceretronic (Xenon Administration System, XAS SM 32C, Randers, Denmark) except from the first two studies where a Xenamatic 4000 system (Diversified Diagnostic products, Houston, TX, USA) was used.
Reconstruction
Each frame was reconstructed into 32 slices (voxel size 0.33 cm × 0.33 cm × 0.33 cm). A Butterworth filter (1.5 cm/order 6.0) and Chang attenuation correction (μ=0.05/cm) were applied. Nose artifact correction was performed. Eight transaxial slices were produced by adding four slices together to a total slice thickness of 1.33 cm.
Quantitation of cerebral blood flow
Cerebral blood flow was quantified using the Kanno-Lassen algorithm with software supplied by the camera vendor (Ceraspect Display Program).20 Standard hemisphere regions of interest were assigned to the resulting maps of Ki (CBF divided by blood–brain partition coefficient of Xenon, λ) and adjusted manually. To avoid the influence of 133Xe in the nasal cavity and sinuses, only the five upper slices were included for analysis. If available, then Ki values generated by each primary investigator were used. If Ki values were unavailable, then original data were reprocessed by an experienced technologist. To obtain CBF values in mL/100 g per minute the mean Ki of the left right and left hemispheres was multiplied by a standard gray matter λ of 0.85.
Lung curve analysis
Accumulation of xenon in tissues overlying the lung during the measurement will add a background to the lung curve causing flattening of the input function and consequently to overestimation of CBF.21 To correct for this effect, the relative lung background was calculated as the mean value of the tail of the lung curve (last 1.5 minutes) divided by peak value (the 95th percentile) for each measurement and entered as a covariate in the regression analysis.
Physiologic Measurements
Body mass index was calculated in units of kg/m2 using height and weight stated in the study record of each subject. Hematocrit was measured in a venous blood sample collected at the day of the measurement. Mean arterial blood pressure was calculated from blood pressure measurements performed before and during each CBF measurement. Breathing rate and PETCO2 were monitored during acquisition with a Datex Normocap 200 (Dameca, Rødovre, Denmark) and stored in the acquisition computer.
Statistical Analysis
Each project was performed within one of five separate time intervals with 64, 57, 130, 95, and 88 measurements within each interval, respectively. To correct for between-period variations, PETCO2 and CBF in each period were initially normalized to the mean of all measurements.
Applying a linear mixed model to the data allows separation of within-subject from between-subject variability. The model is explained in more details in a previous publication.4 Within-subject variability (or test–retest variability) includes both random method imprecision and true within-subject variability, whereas between-subject variability reflects the true variability of CBF and systematic (or subject-specific) errors. These two variance components were entered as random effects in the model. The covariates of interest (COIs) PETCO2, Hct, and gender were entered as fixed effects along with other physiologic covariates (BMI, MAP, breathing rate, and age) and methodological factors of relevance (relative lung background, study day, and measurement number on same day).
The CBF values were log-transformed to fit an exponential effect of PETCO2 on CBF.
Regression coefficients were back-transformed using antilogarithmic transformation into relative effects and reported as per cent change in CBF per unit change in the covariate. Relative between- and within-subject variability was calculated as the antilogarithm of the standard deviation of the two random effects parameter estimates. The standard deviation of the random effects parameter estimates was also used to calculate between-subject, within-subject, and total variance.
Exploratory analyses showed that MAP, BMI, breathing rate, and age were not correlated with CBF. Two final models were investigated:
Fixed effects model A: PETCO2, gender, study day, measurement number, and relative background.
Fixed effects model B: identical to model A but also including Hct.
In both models A and B, between- and within-subject variability was entered as random effects. Model A was applied to the entire population to assess the effects of PETCO2 and model B only to the subsample where Hct was measured to assess the effects of gender and Hct.
The effect of COI's on variance component estimates was investigated by comparing model B with identical models without each COI.
The CBF values are reported as mL/100 g per minute assuming brain tissue density of 1 g/mL. Results in square brackets denote 95% confidence interval.
Results
Effects of Covariates of Interest
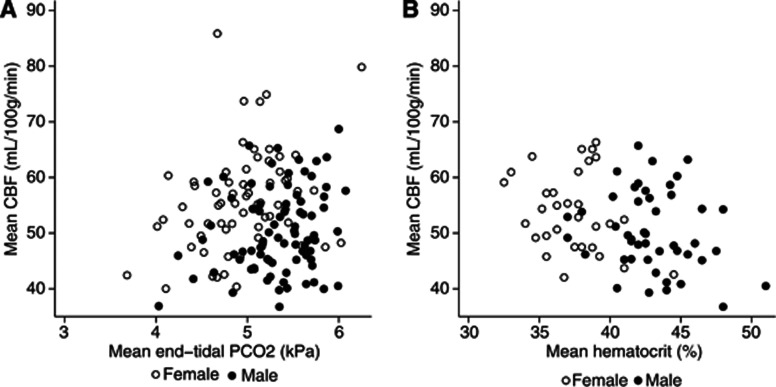
Cerebral Blood Flow was correlated positively with PETCO2 and inversely with Hct (Table 2). Figure 1 presents the between-subject effect of PETCO2 and Hct on CBF. Estimated between- and within-subject effect of PETCO2 was not significantly different in neither model A (9.4%/kPa [4.2% to 15%/kPa] vs. 10.3%/kPa [5.4% to 15.5%/kPa]) nor model B (9.3%/kPa [0.5% to 18.8%/kPa] vs. 15.2%/kPa [8.4 to 22.4]).
Table 2. Effects of covariates on cerebral blood flow.
|
Model A |
Model B |
|||||
|---|---|---|---|---|---|---|
| Regression coefficient | 95% Confidence interval | P value | Regression coefficient | 95% Confidence interval | P value | |
| PETCO2 (kPa) | 9.91% | 6.24%, 13.72% | <0.001 | 13.10% | 7.60%, 18.88% | <0.001 |
| Gender (male) | −14.14% | 17.95%, −10.15% | <0.001 | −6.53% | −13.02%, 0.45% | 0.066 |
| Hct (%) | — | — | — | −1.29% | −1.98%, −0.60% | <0.001 |
| Study day #2 | −2.82% | −4.62%, −0.98% | 0.003 | −2.32% | −4.43%, −0.16% | 0.035 |
| Study day #3 | 0.71% | −4.27%, 5.96% | 0.784 | −1.49% | −6.73%, 4.05% | 0.591 |
| Measurement #2 | 2.42% | 0.59%, 4.27% | 0.009 | 2.78% | 0.43%, 5.17% | 0.020 |
| Measurement #3 | −0.27% | −2.36%, 1.87% | 0.804 | 0.02% | −2.42%, 2.52% | 0.989 |
| Intercept (mL/100 g per minute)a | 52.82 | 50.69, 55.03 | — | 50.74 | 47.68, 54.00 | — |
Abbreviations: CBF, cerebral blood flow; Hct, hematocrit; PETCO2, end-tidal expiratory PCO2.
All regression coefficients (except the intercept) are reported as % change in CBF per unit change in covariate.
Baseline CBF at study day #1 in males and at mean PETCO2 and Hct.
Figure 1.
Effect of end-tidal PCO2 and hematocrit on CBF measurements. Mean CBF value of all measurements in each subject vs. mean end-tidal PCO2 (A) and mean hematocrit (B). Note that females have higher CBF, but lower hematocrit and end-tidal PCO2 than males. CBF, cerebral blood flow; PETCO2, end-tidal expiratory PCO2.
Cerebral blood flow was lower in males than in females, but the effect of gender was diminished and became just insignificant when Hct was added to the model (model B).
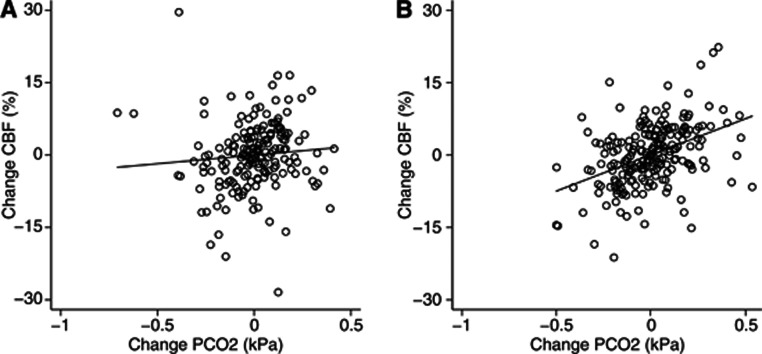
The overall effect of PETCO2 was not different between males and females. As shown in Figure 2, the within-subject effect of changes in PETCO2 was significantly higher in males than in females (15.2%/kPa vs. 3.7%/kPa, P=0.019), but when excluding all extreme changes in CBF (>15%) or PETCO2 (>0.4 kPa) from the mean values, the difference was no longer significant (15.5%/kPa vs. 13.6%/kPa, P=0.694).
Figure 2.
Within-subject effect of changes in end-tidal PCO2 on CBF by gender. Figures show CBF change vs. end-tidal PCO2 change in females (A) and males (B). Removing extreme measurements, the slopes of the regression lines are not different (see Results). CBF, cerebral blood flow; PETCO2, end-tidal expiratory PCO2.
No interactions between gender and Hct, scan day or measurement number, respectively, were observed.
Cerebral Blood Flow Variability
Relative between- and within-subject variability were 13.2% and 8.3%, respectively, in the subjects in whom Hct was measured when only relative background, study day, and measurement number were entered as fixed effects. In the same population including all COIs reduced relative between- and within-subject variability to 12.2% and 7.7% in model B. The effects of PETCO2, Hct, and gender on variance component estimates are summarized in Table 3.
Table 3. Relative influences of gender, hematocrit, and end-tidal PCO2 on variance components.
| Full model B |
Model B without |
||||
|---|---|---|---|---|---|
| PETCO2 | Hct | Gender | All COI | ||
| Between, σb2 | 0.01319 | 0.01332 (−0.97%) | 0.01365 (−3.38%) | 0.01361 (−3.11%) | 0.01534 (−14.01%) |
| Within, σw2 | 0.00555 | 0.00617 (−10.18%) | 0.00583 (−4.96%) | 0.00555 (−0.15%) | 0.00641 (−13.52%) |
| Total, σt2 | 0.01874 | 0.01949 (−3.89%) | 0.01949 (−3.85%) | 0.01917 (−2.25%) | 0.02175 (−13.86%) |
Abbreviations: Hct, hematocrit; PETCO2, end-tidal PCO2.
Values in parenthesis refer to change in variance estimate when the covariate of interest (COI) is included in the model. All models include scan day, measurement number, and relative background and are applied to the subsample with Hct measured.
Within-subject variability was mostly influenced by PETCO2 and between-subject variability mostly by Hct, whereas gender appears to be of little added value when Hct is included in the model.
Discussion
To our knowledge, this study presents the analysis of the largest number of repeated CBF measurements in healthy subjects published to date. The analysis confirms large between-subject variability in CBF measurements. Including gender, Hct, and PETCO2 in the model explains only ∼14% of the observed variability. Residual variance may thus be related to differences in brain metabolism, though other factors influencing CBF or methodological issues affecting CBF measurements in a subject-specific manner may also be of importance.
Spontaneous vs. Induced PCO2 Changes
Increasing arterial PCO2 by inhalation of CO2 produces an exponential increase in CBF of ∼30%/kPa increase in arterial PCO2.5, 22 The estimated effect of CO2 in the present analysis is much lower, ∼10% to 15%/kPa. It should be noted though, that no attempt was made to alter arterial PCO2 and that the effects of small, spontaneous variations in PETCO2 may differ from that of larger changes induced by inhalation of CO2. Interestingly, transcranial doppler studies investigating the effect of spontaneous variations in PETCO2 on middle cerebral artery flow velocities have reported an effect in the order of 15%/kPa, thus supporting a smaller effect of spontaneous vs. induced CO2 changes.23
In contrast, most studies have failed to show an effect of arterial PCO2 on CBF across subjects during resting conditions breathing room air which has been interpreted as each subject being adapted to its habitual PCO2 level.22, 24, 25 Our analysis shows a significant between-subject effect of CO2, although this effect tends to be smaller than the within-subject effect. Interestingly, one study applying 133Xe SPECT have reported a CO2 reactivity of 2% per mm Hg (or ∼16%/kPa) across subjects.26 Findings in 133Xe-SPECT studies may differ from those using positron emission tomography due to the use of a closed gas delivery system. Such may induce changes in breathing pattern and possibly also cause CO2 accumulation depending on the efficacy of the CO2 absorption system. It is thus possible that the gas administration systems may induce within-subject CO2 effects manifesting as between-subject effects due to subject- or time-specific effects on PETCO2.
Of note, according to variance component estimates, including PETCO2 has little, if any, effect on between-subject variability. This is probably explained by colinearity of the COI's due to gender-related differences in PETCO2 and Hct (both of which are lower in females than in males).
Nature of Gender Effects
Most studies of healthy subjects have shown that CBF is higher in female than in male subjects, whereas no gender differences have been observed as to cerebral metabolic rate of oxygen (CMRO2)3, 6, 25 or neuronal density.27 Our data show that higher CBF in females is largely explained by lower Hct levels. In agreement with our findings, a recent study found that CBF is inversely correlated with increasing hemoglobin levels and that the gender effect was not significant when hemoglobin was included in the model.25 In contrast, CMRO2 was not related to gender or hemoglobin, suggesting that CBF is regulated to maintain O2 delivery across different hemoglobin levels. However, others have observed that with increasing age, the CBF of females approaches that of males, but this is not the case for hemoglobin.6 The authors suggest that higher CBF in younger females may be related to higher levels of estrogen in premenopausal women, which is known to favor vasodilation by enhancing endothelial-derived nitric oxide and prostacyclin pathways.28 Though not significant at the 5% level, CBF tended to be higher in females than in males even when corrected for the effect of Hct, thus lending some support to an importance of sex hormones in CBF homeostasis.
Such mechanisms have also been suggested to underlie an enhanced CO2 reactivity as assessed by transcranial Doppler in premenopausal women compared with age-matched males and postmenopausal women.29, 30 These observations, however, have recently been contradicted by a functional magnetic resonance imaging study showing higher blood oxygen level-dependent response to CO2 inhalation in males than in females.31
Our data strongly contradict an enhanced effect of spontaneous CO2 variations in premenopausal women and when excluding extreme measurements the data does not support any gender difference. To our knowledge, no previous studies have investigated the effect of gender on CO2 reactivity using established perfusion methods. The conflicting findings may in part reflect differences in methodologies: the stimulus applied, population studied, and method used to assess CBF changes.
Study Limitations
Compared with positron emission tomography, CBF measurements using 133Xe SPECT have poorer spatial and temporal resolution. However, by using a freely diffusible tracer the ability of the method to quantify CBF in absolute terms is superior to many current methods. The CBF values obtained in early studies based on measurement of the wash out of arterially injected 133Xe are still considered as the reference values for gray- and white-matter perfusion.32, 33
Measurement of CBF with dynamic SPECT of inhaled 133Xe as implemented in the Kanno-Lassen algorithm is associated with a number of errors and assumptions influencing accuracy of the measurements including (1) Compton scatter due to the low energy of 133Xe causing overestimation of CBF in low flow areas, (2) use of a fixed partition coefficient for all brain voxels, (3) partial volume problems leading to underestimation of CBF in mixed brain tissue areas, and (4) use of a standard delay of the lung curve used as input function. It has been concluded that these factors to some extent will cancel out each other leading to a total overestimation of CBF by ∼10%.26
However, these and other unavoidable errors associated with the method may not only induce random errors, lowering method precision, but also cause overestimation of between subjects variability if these causes subject-specific effects on CBF quantitation. Between-subject variation in scatter due to structural differences, variation in lung curve delay due to differences in cardiac function, variation in partition coefficient due to differences in Hct, and different breathing pattern are examples of subject-specific factors that may cause overestimation of between-subject variability. The quantitative significance is difficult to assess analytically. In the present analysis, some of these factors were accounted for in the regression model. Correcting the partition coefficient for Hct reduced the effects of Hct but did not change the estimates of variability. Breathing rate did not influence CBF and was excluded from the model. The poor spatial resolution due to scattered photons induces significant partial volume effects. However, including only young subjects free of atrophy and using only whole-brain CBF values we expect structural variability to be of minor influence.
Due to the retrospective design, only physiologic measures obtained by the primary investigators were available for analysis. Also, variations in camera performance, gas inhalation system, and interobserver variability of the various manual postprocessing steps cannot be avoided. To minimize these effects, we normalized CBF and PETCO2 in five periods. This procedure may potentially limit the ability to correctly assess between-subject effects. Still, the number of observations and subjects in each period are equal to or higher than in most previous studies of CBF in healthy subjects. Alternatively, these time effects could have been corrected for by entering each project as fixed or random effects. Doing so yielded very similar estimates of between-subject variability. Since all measurements in each subject were confined to one project (and consequently to one study period), the analysis at the subject level should not be affected by such time or project-related effects.
In the analysis, baseline measurements and subsequent placebo measurements were pooled. As shown in Table 2, small systematic changes related to measurement number and study day were observed. Since these effects are corrected for changes in PETCO2, the within-day changes most likely reflect a (biphasic) placebo effect whereas between-day changes could be interpreted as habituation.
Also, the study design does not allow us to investigate high frequency variations in CBF induced by spontaneous fluctuations related to in PETCO2 or low frequency variations related to menstrual cycle in female subjects.23, 34
Metabolic Basis of Between-Subject Variability
Previous studies show a very similar between-subject variability in healthy young subjects with between-subject coefficient of variation of 16% using the Kety Schmidt technique1 and of 15% to 20% using various magnetic resonance imaging and positron emission tomography methods.4 Relative between-subject variability in the present study was ∼13%. These figures are very similar to the coefficient of variation of 13% of both CMRO2 and neuron density reported in brains of healthy subjects.1, 27
These observations offer an attractive explanation linking variability in CBF to differences in metabolic demands related to neuron density. Such a conclusion may be an oversimplification. Variability in CMRO2 may depend both to neuronal density (trait factors) and to neuronal activity (state-dependent factors). It has been estimated that the two fractions account for 40% and 60% of the total energy expenditures, respectively.35 From the present data, the size or variability of these two fractions cannot be estimated. Although a close coupling of CBF, CMRO2, glucose utilization and neuronal activity is assumed, the mechanisms coupling CBF to neuronal activity are complex and are not yet fully understood. The coupling of CBF to CMRO2 may be nonlinear and such nonlinearity could contribute to the finding of larger between-subject variability for CBF than for CMRO2 (17% vs. 13%) in an analysis of Kety-Schmidt measurements.1 Furthermore, most previous studies have not addressed the within-subject relationship of CBF and CMRO2. Examining the early studies using the Kety-Schmidt technique shows that the degree of correlation between whole-brain CMRO2 and CBF varies substantially between studies and one study even failed to show such a correlation.36
Exogenous factors, such as vasoactive substances, ingestion of caffeine, and changes in arterial PCO2, may disrupt the coupling of CBF to CMRO2. Endogenous factors, such as variability of mitochondrial uncoupling, in which glucose consumption is uncoupled from ATP formation in the mitochondria, have also been suggested to account for the twofold variability in CMRO2 observed in healthy subjects.37 Mitochondrial dysfunction has been implicated in brain aging and neurodegenerative diseases. Mitochondrial uncoupling, in particular through the action of uncoupling protein 2, is believed to exert neuroprotective effects by reducing production of reactive oxygen species and consequently oxidative stress.38 Although purely speculative, mitochondrial uncoupling could serve as an alternative explanation for the variability of CBF and its association with brain health.
Practical Implications for Future Studies
Our findings underline the importance of including measures of arterial PCO2, oxygen transport capacity, and gender in the analysis of CBF measurements.
Knowledge of the normal between- and within-subject variability of CBF is also of importance for planning studies. Using these estimates it can be calculated that for detection of a 10% change in CBF a total of 76 subjects (38 in each group) are required when performing one measurement in each subject in a group comparison study design but only 11 subjects are needed in a within-subject effect study design. Including Hct, gender and PETCO2 as covariates reduces the number of subject required to 65 and 10 in the two study designs, respectively.
Normal between- and within-subject variations in CBF are important in other areas of quantitative brain imaging, such as receptor binding studies in which the delivery of the receptor ligand is proportional to the local CBF. Establishing normal variability of CBF is also essential for interpreting studies relating brain perfusion to brain health.
Conclusion
The present study confirms large between-subject variability in CBF measurements and that gender, Hct, and PETCO2 explain only a small part of this variability. This implies that a large fraction of CBF variability may be due to unknown exogenous or endogenous factors such as differences in neuron density or metabolic efficacy that could be subject for further studies.
Acknowledgments
The authors would like to thank laboratory assistants Lene Elkjær, Danish Headache Center, and Annette Foldager, Section of Clinical Physiology and Nuclear Medicine, Department of Diagnostics, Glostrup Hospital, for their assistance in retrieving data, Søren Holm, Department of Clinical physiology, PET and Nuclear Medicine, Rigshospitalet for advice on technical aspects of 133Xe SPECT measurements, Klaus Holst, Department of Biostatistics, University of Copenhagen for advice on the statistical analysis and previous and current researchers at Danish Headache Center, Glostrup Hospital (J Sitarz, K Petersen, HW Schytz) for granting us access to data and study records.
The authors declare no conflict of interest.
Footnotes
This work was supported by the Danish Council for Independent Research (Medical Sciences).
References
- Madsen PL, Holm S, Herning M, Lassen NA. Average blood flow and oxygen uptake in the human brain during resting wakefulness: a critical appraisal of the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1993;13:646–655. doi: 10.1038/jcbfm.1993.83. [DOI] [PubMed] [Google Scholar]
- Ito H, Kanno I, Kato C, Sasaki T, Ishii K, Ouchi Y, et al. Database of normal human cerebral blood flow, cerebral blood volume, cerebral oxygen extraction fraction and cerebral metabolic rate of oxygen measured by positron emission tomography with 15O-labelled carbon dioxide or water, carbon monoxide and oxygen: a multicentre study in Japan. Eur J Nucl Med Mol Imaging. 2004;31:635–643. doi: 10.1007/s00259-003-1430-8. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Henriksen OM, Larsson HB, Hansen AE, Gruner JM, Law I, Rostrup E. Estimation of intersubject variability of cerebral blood flow measurements using MRI and positron emission tomography. J Magn Reson Imaging. 2012;35:1290–1299. doi: 10.1002/jmri.23579. [DOI] [PubMed] [Google Scholar]
- Olesen J, Paulson OB, Lassen NA. Regional cerebral blood flow in man determined by the initial slope of the clearance of intra-arterially injected 133Xe. Stroke. 1971;2:519–540. doi: 10.1161/01.str.2.6.519. [DOI] [PubMed] [Google Scholar]
- Aanerud JFA. Brain Energy Metabolism and Blood Flow: Effects of Gender and Aging. Århus: Århus Universitet; 2011. [Google Scholar]
- Birk S, Sitarz JT, Petersen KA, Oturai PS, Kruuse C, Fahrenkrug J, et al. The effect of intravenous PACAP38 on cerebral hemodynamics in healthy volunteers. Regul Pept. 2007;140:185–191. doi: 10.1016/j.regpep.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Birk S, Petersen KA, Kruuse C, Guieu R, Jonassen O, Eisert W, et al. The effect of circulating adenosine on cerebral haemodynamics and headache generation in healthy subjects. Cephalalgia. 2005;25:369–377. doi: 10.1111/j.1468-2982.2005.00867.x. [DOI] [PubMed] [Google Scholar]
- Birk S, Kruuse C, Petersen KA, Jonassen O, Tfelt-Hansen P, Olesen J. The phosphodiesterase 3 inhibitor cilostazol dilates large cerebral arteries in humans without affecting regional cerebral blood flow. J Cereb Blood Flow Metab. 2004;24:1352–1358. doi: 10.1097/01.WCB.0000143536.22131.D7. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Sitarz J, Birk S, Rahmann AM, Oturai PS, Fahrenkrug J, et al. Vasoactive intestinal polypeptide evokes only a minimal headache in healthy volunteers. Cephalalgia. 2006;26:992–1003. doi: 10.1111/j.1468-2982.2006.01149.x. [DOI] [PubMed] [Google Scholar]
- Petersen KA, Birk S, Lassen LH, Kruuse C, Jonassen O, Lesko L, et al. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia. 2005;25:139–147. doi: 10.1111/j.1468-2982.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther. 2005;77:202–213. doi: 10.1016/j.clpt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kruuse C, Thomsen LL, Jacobsen TB, Olesen J. The phosphodiesterase 5 inhibitor sildenafil has no effect on cerebral blood flow or blood velocity, but nevertheless induces headache in healthy subjects. J Cereb Blood Flow Metab. 2002;22:1124–1131. doi: 10.1097/00004647-200209000-00010. [DOI] [PubMed] [Google Scholar]
- Kruuse C, Jacobsen TB, Thomsen LL, Hasselbalch SG, Frandsen EK, Dige-Petersen H, et al. Effects of the non-selective phosphodiesterase inhibitor pentoxifylline on regional cerebral blood flow and large arteries in healthy subjects. Eur J Neurol. 2000;7:629–638. doi: 10.1046/j.1468-1331.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- Kruuse C, Jacobsen TB, Lassen LH, Thomsen LL, Hasselbalch SG, Dige-Petersen H, et al. Dipyridamole dilates large cerebral arteries concomitant to headache induction in healthy subjects. J Cereb Blood Flow Metab. 2000;20:1372–1379. doi: 10.1097/00004647-200009000-00012. [DOI] [PubMed] [Google Scholar]
- Schytz HW, Wienecke T, Jensen LT, Selb J, Boas DA, Ashina M. Changes in cerebral blood flow after acetazolamide: an experimental study comparing near-infrared spectroscopy and SPECT. Eur J Neurol. 2009;16:461–467. doi: 10.1111/j.1468-1331.2008.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schytz HW, Wienecke T, Oturai PS, Olesen J, Ashina M. The cholinomimetic agent carbachol induces headache in healthy subjects. Cephalalgia. 2009;29:258–268. doi: 10.1111/j.1468-2982.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- Wienecke T, Olesen J, Ashina M. Prostaglandin I2 (epoprostenol) triggers migraine-like attacks in migraineurs. Cephalalgia. 2010;30:179–190. doi: 10.1111/j.1468-2982.2009.01923.x. [DOI] [PubMed] [Google Scholar]
- Wienecke T, Olesen J, Oturai PS, Ashina M. Prostaglandin E2(PGE2) induces headache in healthy subjects. Cephalalgia. 2009;29:509–519. doi: 10.1111/j.1468-2982.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- Celsis P, Goldman T, Henriksen L, Lassen NA. A method for calculating regional cerebral blood flow from emission computed tomography of inert gas concentrations. J Comput Assist Tomogr. 1981;5:641–645. doi: 10.1097/00004728-198110000-00006. [DOI] [PubMed] [Google Scholar]
- Holm S. Dynamic single photon emission computerized tomography of the human brain for measurement of regional cerebral blood flow. A critical appraisal. University of Copenhagen: Copenhagen; 1985. [Google Scholar]
- Rostrup E, Knudsen GM, Law I, Holm S, Larsson HB, Paulson OB. The relationship between cerebral blood flow and volume in humans. Neuroimage. 2005;24:1–11. doi: 10.1016/j.neuroimage.2004.09.043. [DOI] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Ito H, Kanno I, Ibaraki M, Suhara T, Miura S. Relationship between baseline cerebral blood flow and vascular responses to changes in PaCO2 measured by positron emission tomography in humans: implication of inter-individual variations of cerebral vascular tone. Acta Physiol (Oxf) 2008;193:325–330. doi: 10.1111/j.1748-1716.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- Ibaraki M, Shinohara Y, Nakamura K, Muira S, Kinoshita F, Kinoshita T. Interindividual variations of cerebral blood flow, oxygen delivery, and metabolism in relation to hemoglobin concentration measured by positron emission tomography in humans. J Cereb Blood Flow Metab. 2010;30:1296–1305. doi: 10.1038/jcbfm.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata N, Henriksen L, Vorstrup S, Holm S, Lauritzen M, Paulson OB, et al. Regional cerebral blood flow assessed by 133Xe inhalation and emission tomography: normal values. J Comput Assist Tomogr. 1985;9:861–866. doi: 10.1097/00004728-198509000-00004. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006;101:1252–1261. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Happe V, Hartmann C, Schabet M. Gender-related effects of indomethacin on cerebrovascular CO2 reactivity. J Neurol Sci. 1999;162:127–132. doi: 10.1016/s0022-510x(98)00288-3. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Dichgans J, Niemeier M, Schabet M. Changes of cerebrovascular CO2 reactivity during normal aging. Stroke. 1998;29:1311–1314. doi: 10.1161/01.str.29.7.1311. [DOI] [PubMed] [Google Scholar]
- Kassner A, Winter JD, Poublanc J, Mikulis DJ, Crawley AP. Blood-oxygen level dependent MRI measures of cerebrovascular reactivity using a controlled respiratory challenge: reproducibility and gender differences. J Magn Reson Imaging. 2010;31:298–304. doi: 10.1002/jmri.22044. [DOI] [PubMed] [Google Scholar]
- Law I, Iida H, Holm S, Nour S, Rostrup E, Svarer C, et al. Quantitation of regional cerebral blood flow corrected for partial volume effect using O-15 water and PET: II. Normal values and gray matter blood flow response to visual activation. J Cereb Blood Flow Metab. 2000;20:1252–1263. doi: 10.1097/00004647-200008000-00010. [DOI] [PubMed] [Google Scholar]
- Hoedt-Rasmussen K. Regional cerebral blood flow. The intra-arterial injection method. Acta Neurol Scand. 1967;43 (Suppl 27:1–81. [PubMed] [Google Scholar]
- Krejza J, Mariak Z, Huba M, Wolczynski S, Lewko J. Effect of endogenous estrogen on blood flow through carotid arteries. Stroke. 2001;32:30–36. doi: 10.1161/01.str.32.1.30. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Feinberg I, Lane MH. Bilateral studies of cerebral oxygen uptake in young and aged normal subjects and in patients with organic dementia. J Clin Invest. 1960;39:491–500. doi: 10.1172/JCI104063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjedde A, Aanerud J, Peterson E, Ashkanian M, Iversen P, Vafaee M, et al. Variable ATP yields and uncoupling of oxygen consumption in human brain. Adv Exp Med Biol. 2011;701:243–248. doi: 10.1007/978-1-4419-7756-4_32. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005;6:829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]