Abstract
Hypertension is associated with cerebral small vessel disease (SVD) and with diffuse white matter hyperintensities (WMH) on T2-weighted magnetic resonance imaging (MRI). We tested whether stroke-prone spontaneously hypertensive rats (SHRSP), a model of chronic hypertension, exhibit WMH. Male SHRSP (age 10 months) without stroke symptoms were compared with age-matched male WKY rats. Stroke-prone spontaneously hypertensive rats exhibited no WMH on MRI scans (T2, T2*, diffusion tensor imaging) and no neuropathological lesions. While leptomeningeal arteries exhibited fibrohyaline wall thickening, with decreased smooth muscle actin relative to WKY, deep penetrating arterioles within the caudate nuclei had no vasculopathy. We conclude that WMH are not an obligate feature of stroke-free SHRSP aged up to 10 months.
Keywords: experimental, hypertension, immunohistochemistry, magnetic resonance, neuropathology, white matter disease
Introduction
Diffuse white matter hyperintensities (WMH) are a frequent finding on T2-weighted magnetic resonance imaging (MRI) scans, especially in elderly patients.1, 2 White matter hyperintensities are linked to cerebrovascular pathology such as cerebral small vessel disease (SVD), but the pathogenic mechanisms are not fully known.1, 3 Age and hypertension are risk factors for both WMH and SVD.2, 4
Stroke-prone spontaneously hypertensive rats (SHRSP) develop chronic, severe hypertension from 9 to 10 weeks of age5, 6, 7 with subsequent vascular pathology, reduction in cerebral perfusion, and a spectrum of spontaneous stroke lesions.5, 8, 9, 10, 11
Here, we tested the hypothesis that chronically hypertensive SHRSP develop WMH, using MRI and subsequent histopathology. We also examined leptomeningeal arteries and deep penetrating arterioles for SVD-like vasculopathy.8, 10 Most prior MRI studies have used young adult SHRSP, often fed a sodium-rich diet to precipitate stroke events.8 There are few studies of older, non-salt loaded SHRSP before stroke onset, with conflicting results with regard to white matter pathology.8 Here, we studied male SHRSP (to avoid confounding effects of female reproductive hormones) with normal diet, and without stroke symptoms. Rats were studied at age 10 months, as above this age stroke incidence and mortality increase markedly.6, 7, 12
Materials and methods
Male SHRSP aged 10 months (range 45 to 48 weeks; n=9) and male WKY rats (44 to 49 weeks; n=6) underwent MRI (T2, T2*, and DTI (diffusion tensor imaging)) on a Bruker Biospec 7 T/30 cm system (Wikingerstrasse, Karlsruhe, Germany) with gradient coil (121 mm ID, 400 mT/m) and 72 mm birdcage resonator. Diffusion tensor imaging was carried out with a four shot spin echo planar imaging sequence for assessment of white matter. Mean diffusivity (MD) and fractional anisotropy (FA) in corpus callosum and the caudate nuclei were computed, as described in the Supplementary File.
All animals included in this study had no stroke symptoms (defined here as: inactivity and lethargy; hunched appearance; piloerection; uncoordinated movements; loss of weight, and appetite). Rats were anesthetized with isoflurane in a mixture of N2O/O2 (70/30). All procedures were approved by the University of Glasgow Ethical Review Panel and complied with the Animals (Scientific Procedures) Act 1986. This report was prepared following consideration of the ARRIVE guidelines (http://www.nc3rs.org.uk/arrive).
While still anesthetized, rats were transcardially perfused with heparinized saline followed by 4% paraformaldehyde. Adjacent coronal sections (10 μm) underwent histological staining (n=9 SHRSP; n=6 WKY) with hematoxylin and eosin (H&E), or with Luxol fast blue counterstained with cresyl violet (LFB-CV), or elastic Van Gieson (EVG) stain, or immunohistochemical labeling for smooth muscle α-actin (SMA), myelin basic protein (MBP), or the oligodendrocyte marker Olig2. Immunolabeling was visualized using diaminobenzidine chromagen (Envision kit, Dako, Ely, UK), counterstained with hematoxylin.
Further details on all Materials and methods are given in the Supplementary appendix.
Results
Magnetic Resonance Imaging Data
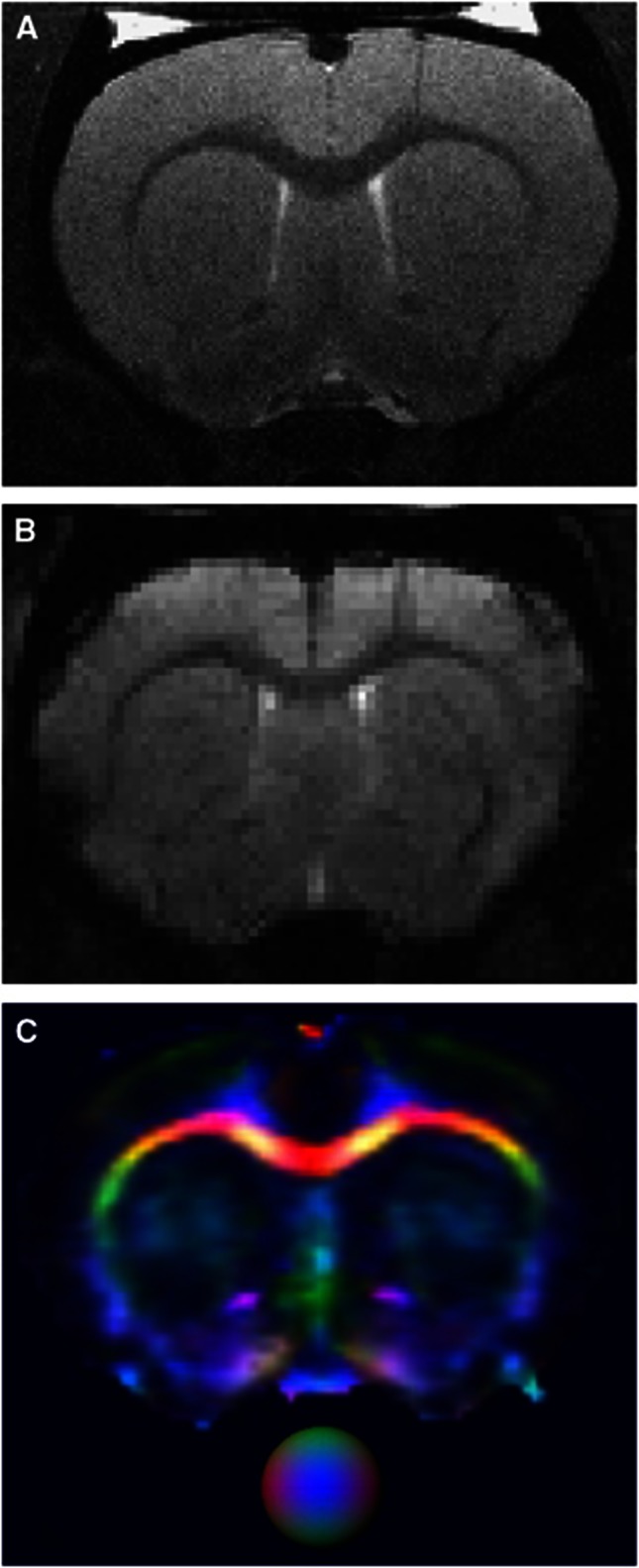
Magnetic resonance imaging scans confirmed the absence of stroke lesions in SHRSP (n=9). On T2-weighted scans, no WMH were seen in white matter areas (corpus callosum, internal capsule, and external capsule) in SHRSP (Figure 1; Supplementary Figure S1) or WKY (not shown). Analysis of FA and MD on DTI scans revealed no evidence for white matter damage in SHRSP (Table 1). Stroke-prone spontaneously hypertensive rats displayed modest reduction in MD within the corpus callosum (14%) and the caudate nuclei (4%) and increase in FA in the corpus callosum (6%) relative to WKY (Table 1). Stroke-prone spontaneously hypertensive rats (Figure 1; Supplementary Figure S1) and WKY (not shown) demonstrated no evidence of hemorrhage on gradient-echo T2* scans or T2-weighted images. For the T2* sequence the in-plane resolution is 0.260 mm, so the area of one voxel is 0.26 × 0.26=0.0676 mm2, with a slice thickness of 1.5 mm. The smallest detectable hemorrhage would be∼4 voxels, that is, 0.5 × 0.5 × 1.5 mm3.
Figure 1.
Magnetic resonance imaging (MRI) scans of stroke-free stroke-prone spontaneously hypertensive rats (SHRSP) brain. MRI scans (coronal section) from a representative male SHRSP aged 48 weeks. T2-weighted (A), gradient-echo T2*-weighted (B) and color-coded fractional anisotropy map (C). The color ball in (C) indicates the three directional vectors (red–blue–green). Scans show no white matter hyperintensities (WMH) (A), no significant hemorrhage (B) and no loss of white matter anisotropy (C).
Table 1. Diffusion tensor imaging and morphometric data for SHRSP and WKY.
| WKYa | SHRSPa | P valueb | |
|---|---|---|---|
| DTI data | |||
| FA, caudate nuclei | 0.277 (0.025) | 0.263 (0.020) | 0.345 |
| MD, caudate nuclei (mm2/s × 10−3) | 0.760 (0.011) | 0.732 (0.011) | 0.0019** |
| FA, corpus callosum | 0.610 (0.024) | 0.649 (0.032) | 0.035* |
| MD, corpus callosum (mm2/s × 10−3) | 0.985 (0.084) | 0.846 (0.041) | 0.016* |
| Histological data | |||
| MBP-positive area fraction (%): corpus callosum | 47.3 (5.6) | 67.7 (6.8) | <0.001*** |
| MBP-positive bundle cross section area: caudate nuclei (μm2) | 1710 (630) | 1020 (270) | 0.043* |
| MBP-positive bundle density: caudate nuclei (bundles/mm2) | 146 (40) | 207 (50) | 0.019* |
| Olig2-positive cells per mm2 | 1480 (120) | 1760 (470) | 0.084 |
| Fraction of cells positive for Olig2 (%) | 46.8 (9.9) | 48.1 (8.0) | 0.81 |
| Sclerotic index: large leptomeningeal arteries | 0.26 (0.03) | 0.31 (0.04) | 0.032* |
| SMA-positive area fraction (%): large leptomeningeal arteries | 63.9 (15.4) | 45.9 (12.7) | 0.038* |
| Sclerotic index: deep penetrating arterioles, caudate nuclei | 0.48 (0.09) | 0.44 (0.11) | 0.44 |
DTI, diffusion tensor imaging; FA, fractional anisotropy; MBP, myelin basic protein; MD, mean diffusivity; SHRSP, stroke-prone spontaneously hypertensive rats; SMA, smooth muscle actin.
*P<0.05, **P< 0.01, ***P<0.001.
Data are expressed as mean (s.d.).
Student's unpaired t-test.
Histopathology and Morphometry
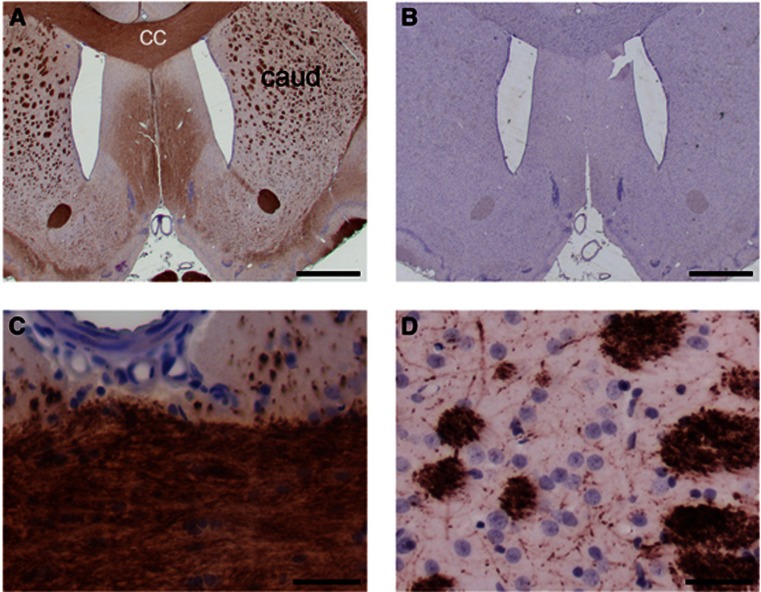
Blinded examination of H-E and LFB-CV-stained histological sections by a registered neuropathologist (LRB) revealed no evidence of the following in SHRSP (n=9): vacuolation in corpus callosum or caudate white matter bundles; astrocytosis; microinfarcts; fibrinoid necrosis of vessel walls; microhemorrhage; macrophage invasion. Robust, uniform MBP immunolabeling was seen in corpus callosum and in caudate white matter bundles of SHRSP (Figure 2).
Figure 2.
Histological examination of white matter. Immunohistochemical labeling of myelin basic protein (MBP) in brain sections from a representative stroke-prone spontaneously hypertensive rat (SHRSP), aged 47 weeks. (A) MBP immunolabeling (brown) displays histological white matter areas in low magnification view of a coronal section. The corpus callosum (cc) and caudate (caud) are marked. (B) A neighboring section treated identically but without primary antibody shows no immunoreactivity. (C, D) Higher magnification images confirm integrity of myelin in corpus callosal white matter (C) and in caudate white matter bundles (D). The central azimuthal artery serves as an anatomical landmark in (C). Nuclear chromatin is counterstained with hematoxylin (blue). Scale bars: 1,000 μm (A, B) and 20 μm (C, D).
Morphometric analysis of MBP and Olig2 labeling showed no white matter damage in corpus callosum or in caudate white matter bundles of SHRSP (n=9, Figure 2; Supplementary Figure S2) or WKY (n=6; Table 1). Stroke-prone spontaneously hypertensive rats had somewhat greater MBP-positive area density (1.4-fold) in the corpus callosum relative to WKY (Table 1). In the caudate nuclei, SHRSP had greater density of MBP-positive bundles (1.4-fold) and lower bundle density (0.59-fold; Table 1). Within corpus callosum, the area density and cell fraction of Olig2-positive cells did not differ between SHRSP and WKY (Table 1; Supplementary Figure S2).
Leptomeningeal arteries in SHRSP exhibited thickened, hyalinized walls with abnormal SMA labeling in myocytes (Supplementary Figure S3), while WKY rats had uniform medial SMA labeling in equivalent vessels, without hyalinosis (Supplementary Figure S3). Leptomeningeal arteries in SHRSP exhibited elevated sclerotic index (SI) relative to WKY (1.2-fold; Table 1) and reduced SMA-positive area fraction (0.72-fold; Table 1) relative to WKY. The SI of penetrating SMA-labeled vessels (assumed to be deep penetrating arterioles) in the caudate nuclei did not differ between groups (Table 1; Supplementary Figure S3).
Discussion
White matter hyperintensities are commonly seen on clinical T2-weighted MRI scans and their prevalence and extent increase with advancing age and cardiovascular risk factors, particularly hypertension.2, 4 More confluent WMH, especially those in deep white matter sites, are associated with SVD.2 In DTI scans of human subjects, WMH exhibit elevated MD and reduced FA, interpreted as loss of axons and myelin structure.2, 13, 14 Neuropathological features of WMH are demyelination, cell and axonal atrophy, and gliosis, accompanied by fibrinohyalinotic changes and vessel wall thickening in deep penetrating arteries.1, 2, 3
Our MRI data suggest that WMH do not accompany chronic hypertension in stroke-free SHRSP at age 10 months. Our histological data supported these findings, with no depletion of MBP or loss of oligodendrocytes. Previous MRI studies of SHRSP have used high salt diet to augment hypertension and stroke likelihood.8, 15, 16 In agreement with our findings, other groups found no changes on T2-weighted scans of stroke-free SHRSP on normal diet at 5–8 months of age.11, 16 Some prior MRI studies included stroke-free SHRSP above this age and, in agreement with our data, these showed no WMH.9, 15 Some previous histological studies reported white matter lesions in non-salt loaded SHRSP17 while others, including ourselves, found no white matter damage.16, 18
Contrary to our hypothesis, SHRSP exhibited reduced MD in the corpus callosum and caudate nuclei relative to WKY, and the corpus callosum displayed elevated FA (Table 1), indicative of increased white matter structure. This was corroborated by increased MBP in the corpus callosum (Table 1). These paradoxical findings may be a response to chronic hypoperfusion in the white matter of SHRSP.9 Changes in myelination occur more rapidly in rodent brain than in humans.19, 20, 21
Leptomeningeal arteries of SHRSP exhibited fibrohyaline thickening and loss of cellularity, confirmed by increased SI and decreased SMA-positive area (Table 1), in accord with the chronic hypertensive phenotype of SHRSP.6, 8 As with our findings, earlier morphometric analyses observed arterial thickening in large leptomeningeal vessels of SHRSP relative to WKY.22, 23 We saw no wall thickening in deep penetrating arterioles, and no erythrocyte accumulation in deep brain vessels.11
There are several limitations of our study. First, the SI parameter does not encompass all aspects of SVD arteriopathy. It is an approximate measure of arterial or arteriolar stenosis at single-vessel level, for neuropathological reporting of SVD.24, 25 Second, the Glasgow SHRSP colony used here may have undergone some genotypic shift from the Japanese founder colony.7 However, they exhibited characteristic coat coloration associated with SHRSP, and leptomeningeal arterial thickening indicative of chronic hypertension. Third, our data do not exclude WMH in SHRSP that survive beyond 10 months. Notch3R169C transgenic mice accumulate arterial collagen from age 5 months, but white matter lesions only at age 18–20 months.26 These Notch3R169C mice are not hypertensive or stroke-prone.26
It should be emphasized that the SHRSP studied here are a selected population, free from stroke symptoms. Incidence of stroke in the Glasgow SHRSP colony is∼70% at 9 months of age, which accords with incidence in other groups.6, 9, 11, 12 In detailed studies of lifespan by another group, survival of untreated male SHRSP was 100% at 9 months of age, 50% at 13 months, with mortality complete at 15 months.6, 12 Stroke and mortality were seen considerably earlier in original reports from the Kyoto founder colony, with average lifespan of 276 days (∼9 months) and 89% stroke incidence (n=60/67).5, 7 It seems likely that there are some differences in pathology and stroke incidence between the founder colony and the other colonies that have been established. There may also be an effect of different husbandry regimes and different criteria for selection of breeding pairs.
In conclusion, SHRSP which were stroke-free at age 10 months exhibited fibrohyaline changes in leptomeningeal arteries, but no vasculopathy in deep penetrating arterioles, and no WMH. Our data do not support the hypothesis that stroke-free SHRSP develop WMH, and suggest that WMH do not necessarily accompany chronic hypertension over the timescale studied.
Acknowledgments
The authors thank their colleagues Yvette Bland, Celia Cope, Lindsay Gallagher, Raymond Moss, and James Mullin for their expert assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
AHH gratefully acknowledges financial support from the St George's Hospital Charity, the Neuroscience Research Foundation and Action Medical Research (UK).
Supplementary Material
References
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Haybaeck J, Loitfelder M, Weis S, Cavalieri M, et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011;122:171–185. doi: 10.1007/s00401-011-0851-x. [DOI] [PubMed] [Google Scholar]
- Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Chalmers J, Coskun O, Besancon V, Bousser MG, Guillon P, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- Yamori Y, Horie R, Handa H, Sato M, Fukase M. Pathogenetic similarity of strokes in stroke-prone spontaneously hypertensive rats and humans. Stroke. 1976;7:46–53. doi: 10.1161/01.str.7.1.46. [DOI] [PubMed] [Google Scholar]
- Linz W, Heitsch H, Scholkens BA, Wiemer G. Long-term angiotensin II type 1 receptor blockade with fonsartan doubles lifespan of hypertensive rats. Hypertension. 2000;35:908–913. doi: 10.1161/01.hyp.35.4.908. [DOI] [PubMed] [Google Scholar]
- Yamori Y, Horie R. Developmental course of hypertension and regional cerebral blood flow in stroke-prone spontaneously hypertensive rats. Stroke. 1977;8:456–461. doi: 10.1161/01.str.8.4.456. [DOI] [PubMed] [Google Scholar]
- Hainsworth AH, Markus HS. Do in vivo experimental models reflect human cerebral small vessel disease? A systematic review. J Cereb Blood Flow Metab. 2008;28:1877–1891. doi: 10.1038/jcbfm.2008.91. [DOI] [PubMed] [Google Scholar]
- Henning EC, Warach S, Spatz M. Hypertension-induced vascular remodeling contributes to reduced cerebral perfusion and the development of spontaneous stroke in aged SHRSP rats. J Cereb Blood Flow Metab. 2010;30:827–836. doi: 10.1038/jcbfm.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EL, Smith C, Sudlow CL, Wardlaw JM. Is the spontaneously hypertensive stroke prone rat a pertinent model of sub cortical ischemic stroke? A systematic review. Int J Stroke. 2011;6:434–444. doi: 10.1111/j.1747-4949.2011.00659.x. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Bueche CZ, Garz C, Kropf S, Angenstein F, Goldschmidt J, et al. The pathologic cascade of cerebrovascular lesions in SHRSP: is erythrocyte accumulation an early phase. J Cereb Blood Flow Metab. 2012;32:278–290. doi: 10.1038/jcbfm.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz W, Jessen T, Becker RH, Scholkens BA, Wiemer G. Long-term ACE inhibition doubles lifespan of hypertensive rats. Circulation. 1997;96:3164–3172. doi: 10.1161/01.cir.96.9.3164. [DOI] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, et al. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009;73:1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology. 2001;57:2307–2310. doi: 10.1212/wnl.57.12.2307. [DOI] [PubMed] [Google Scholar]
- Guerrini U, Sironi L, Tremoli E, Cimino M, Pollo B, Calvio AM, et al. New insights into brain damage in stroke-prone rats: a nuclear magnetic imaging study. Stroke. 2002;33:825–830. doi: 10.1161/hs0302.104111. [DOI] [PubMed] [Google Scholar]
- Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke. 2012;43:1115–1122. doi: 10.1161/STROKEAHA.111.643080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JX, Tomimoto H, Akiguchi I, Wakita H, Shibasaki H, Horie R. White matter lesions and alteration of vascular cell composition in the brain of spontaneously hypertensive rats. Neuroreport. 2001;12:1835–1839. doi: 10.1097/00001756-200107030-00015. [DOI] [PubMed] [Google Scholar]
- Dong YF, Kataoka K, Tokutomi Y, Nako H, Nakamura T, Toyama K, et al. Beneficial effects of combination of valsartan and amlodipine on salt-induced brain injury in hypertensive rats. J Pharmacol Exp Ther. 2011;339:358–366. doi: 10.1124/jpet.111.182576. [DOI] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Kuhlmann T, Antel JP. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta. 2011;1812:184–193. doi: 10.1016/j.bbadis.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Tanaka R, Shimura H, Watanabe T, Mori H, Onodera M, et al. Phosphodiesterase III inhibition promotes differentiation and survival of oligodendrocyte progenitors and enhances regeneration of ischemic white matter lesions in the adult mammalian brain. J Cereb Blood Flow Metab. 2010;30:299–310. doi: 10.1038/jcbfm.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach GL, Walmsley JG, Hart MN. Composition and mechanics of cerebral arterioles in hypertensive rats. Am J Pathol. 1988;133:464–471. [PMC free article] [PubMed] [Google Scholar]
- Nordborg C, Fredriksson K, Johansson BB. The morphometry of consecutive segments in cerebral arteries of normotensive and spontaneously hypertensive rats. Stroke. 1985;16:313–320. doi: 10.1161/01.str.16.2.313. [DOI] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Lammie GA, Brannan F, Slattery J, Warlow C. Nonhypertensive cerebral small-vessel disease. An autopsy study. Stroke. 1997;28:2222–2229. doi: 10.1161/01.str.28.11.2222. [DOI] [PubMed] [Google Scholar]
- Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120:433–445. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.