Abstract
Despite recent advances in our understanding of the molecular and cellular mechanisms behind vascular conducted responses (VCRs) in systemic arterioles, we still know very little about their potential physiological and pathophysiological role in brain penetrating arterioles controlling blood flow to the deeper areas of the brain. The scope of the present review is to present an overview of the conceptual, mechanistic, and physiological role of VCRs in resistance vessels, and to discuss in detail the recent advances in our knowledge of VCRs in brain arterioles controlling cerebral blood flow. We provide a schematic view of the ion channels and intercellular communication pathways necessary for conduction of an electrical and mechanical response in the arteriolar wall, and discuss the local signaling mechanisms and cellular pathway involved in the responses to different local stimuli and in different vascular beds. Physiological modulation of VCRs, which is a rather new finding in this field, is discussed in the light of changes in plasma membrane ion channel conductance as a function of health status or disease. Finally, we discuss the possible role of VCRs in cerebrovascular function and disease as well as suggest future directions for studying VCRs in the cerebral circulation.
Keywords: cerebral blood flow, endothelium, intercellular communication, neurovascular coupling, vascular conducted response, vascular smooth muscle
Introduction
The regulation of blood flow to the brain is under dynamic and precise control to ensure adequate oxygen and nutrient supply as well as to washout metabolic waste products. Regional blood flow control to peripheral tissues is accomplished by neurohormonal mechanisms, and is fine-tuned by local control mechanisms working either through the release of local vasoactive metabolites or mechanical forces such as shear stress or wall stress acting on the endothelium and smooth muscle cell layer of feeding arterioles and precapillary arterioles. The brain, however, encapsulated in a confined space in the skull and protected by the blood–brain barrier, relies almost entirely on local mechanisms to match the demand and supply of energy to the neurons of the brain, a mechanism known as neurovascular coupling.1 Theoretical work, however, suggests that in arteriolar networks nonlocal mechanisms are necessary for achieving optimal performance with regard to the delivery of oxygen and nutrients.2, 3 It has been speculated that vascular conducted responses (VCRs) could be such a nonlocal mechanism and thereby assist in coordinating and enhancing the effects of changes in local vascular resistances in brain arteriolar networks and thereby contribute to the effective and highly dynamic distribution of blood flow to areas of the brain undergoing large changes in neuronal activity.4, 5
Definition of vascular conducted response
A VCR is, by definition, initiated by a local stimulation causing vasodilation or constriction, which rapidly spreads bidirectionally along small blood vessels independent of blood flow or perivascular nerves.6, 7 Most studies have focused on VCRs elicited by local application of agonists to arterioles, but it is clear that local stimulation of either capillaries or small venules may likewise initiate a VCR that spreads upstream into the supplying arterioles.8, 9 Conduction velocity has been estimated to >1 to 3 mm/s in intracellular Ca2+ or diameter measurements with limited time-resolution10, 11, 12, 13 and >20 to ∼45 mm/s in electrophysiological recordings with higher time-resolution.14, 15 For comparison, the velocity of intercellular Ca2+ waves spreading along arterioles has been estimated to∼0.1 mm/s.16, 17 Thus, conducted vasomotor responses spread with velocities that are 1 to 3 orders of magnitude faster than the spread of intercellular Ca2+ waves. The ability of a local vasoactive stimulus to transform into a conducted vasomotor response is dependent on the type of agonist applied, as well as on the vascular cell type targeted by the agonist. For example, it has been shown that a local depolarization imposed on the vascular smooth muscle cell (VSMC) layer in rat renal and mesenteric arterioles conducts with typical length constants of a few hundred micrometers.12, 18, 19 Conversely, in skeletal muscle feed arteries and arterioles local application of acetylcholine (ACh), which activates muscarinic receptors on endothelial cells (ECs) and leads to hyperpolarization, conducts with limited decay for up to a couple of millimeters.15, 20, 21
Physiological Role of Vascular Conducted Responses
The functional role of conducted vasomotor signals within the microcirculation, especially in feed arteries and arterioles, is most likely to coordinate changes in resistance and flow in vascular networks, which would enable changes to be manifested more efficiently and rapidly throughout tissues in response to, for example, changes in metabolic need. For example, to meet the metabolic needs associated with an increased neuronal activity, it is necessary to dilate not only the blood vessels within the cortex, but also the larger penetrating and pial arterioles that supply the corresponding cortical area. This necessitates a mechanism that allows local vascular signals from neurons or glial cells to have an effect also on more remote parts of the microcirculation. One such possible mechanism is the VCR.4 Likewise, in exercising skeletal muscle, an increased metabolic demand leading to dilatation of arterioles deep within the tissue, would benefit from conduction of the dilatation in a retrograde manner to coordinate a simultaneous dilatation of feed arterioles and arteries, resulting in a more efficient and instant supply of oxygen and nutrients to the tissue.3, 22 In the kidney, the tubuloglomerular feedback mechanism, which constricts the afferent arteriole secondary to an increased glomerular filtration rate, is thought to rely in part on the upstream conduction of a depolarization along the glomerular microvasculature.12, 23 In disease states exhibiting an increased microvascular resistance, for example, diabetes or hypertension, it follows that impaired conducted vasodilatation or augmented conducted vasoconstriction could contribute to or even be responsible for the increased arteriolar resistance.
How Vascular Conducted Responses Are Typically Measured
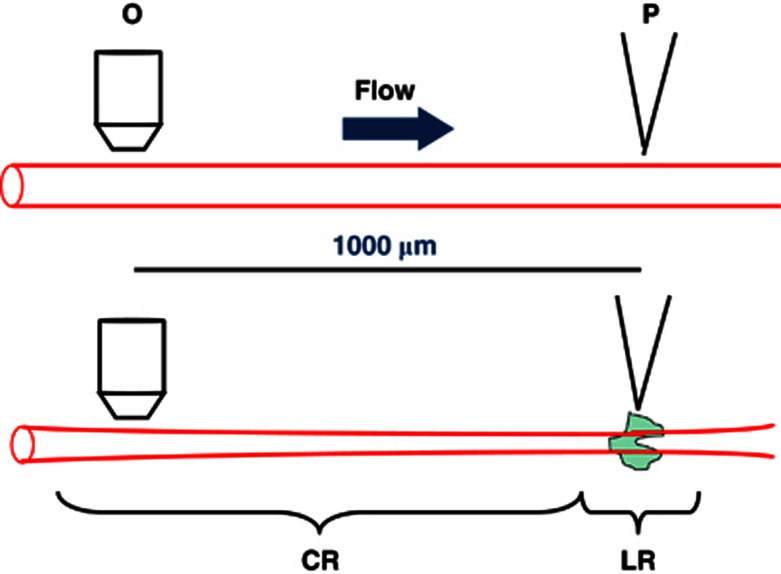
The methodological approach depends on whether measurements are performed with isolated arterioles (ex vivo) or in an anesthetized animal with some part of its microcirculation exposed for in vivo/intravital microscopy measurements. Isolated arterioles are usually mounted between glass pipettes for intraluminal perfusion at physiological pressure. However, very small arterioles may also be studied without pressure and flow, being positioned at the bottom of a recording chamber using suction micropipettes or bioadhesive proteins.24 Measurements of local and conducted diameters require a light microscope equipped with a digital or video camera for recording of the experiments. The local responses are elicited by placing the tip of a micropipette (tip 1 to 5 μm) adjacent to an arteriole for application of agonists such as norepinephrine (NE), phenylephrine (PE), ACh, or KCl, etc. Often a microiontophoresis device is used to deliver agonists, which ensures a tight control of agonist delivery. For delivery of sufficient quantities of KCl, it is necessary to use a pressure microejection device. Alternatively, a local response may be elicited by direct electrical stimulation via a micropipette whereby the vessel is exposed to a train of unipolar pulses causing perturbations of the membrane potential.25, 26 The experiments are usually performed by initially recording the local response a few times, then moving the objective to an upstream location for recording of the conducted (remote) response to repetitive local stimulation (see Figure 1). Alternatively, the stimulation site is moved to different downstream sites while the conducted responses are observed consecutively at a fixed upstream site. The distance to the upstream location is usually between 500 to 2,000 μm depending on the type of vessel and agonist applied.
Figure 1.
Experimental approach for measurement of conducted vasomotor responses in arterioles (here depicted as local and conducted vasoconstriction). A micropipette (P) tip for delivery of agonist (green shade) is placed adjacent to an arteriole. It is important to make sure that the agonist is carried away from the vessel with the superfusate flow. At 1,000 μm in the upstream direction, the arteriole diameter is recorded through a microscope objective. The magnitude of the conducted response (CR) is seen to decay with distance from the local response (LR). See text for further details.
The change in EC and VSMC intracellular Ca2+ concentration ([Ca2+]i) have also been measured during a VCR. Here, local and conducted measurements were performed using either ratiometric (Fura-2, Fura-PE3) or single-wavelength excitation (Fluo-3) of Ca2+ indicators using epifluorescence microscopy.11, 13, 27, 28, 29 For simultaneous recording of Ca2+ signals at multiple sites along an arteriole during a VCR, an objective with low magnification ( × 20) and high numerical aperture and a sensitive CCD camera for capturing a sufficient amount of fluorescence has been used.13, 18 Conducted vasoconstriction is associated with an increase in [Ca2+]i in both the VSMC and the EC, and the change in [Ca2+]i showed an attenuation with distance from the local stimulation site that paralleled the degree of vasoconstriction.13, 18, 27, 29 The wide-field recordings of [Ca2+]i can be used as a measure of the conducted signal, and therefore provides detailed information on the strength of the conducted signal along the vessel. Such data have enabled a detailed mathematical description of the conduction process by nonlinear curve-fitting and computer simulations.18
The electrical intercellular communication underlying the conduction of vasomotor responses along the vascular wall has been measured using sharp microelectrodes inserted in either VSMC or EC at various locations along the arteriole.27, 30, 31, 32, 33 Usually investigators impale only one cell at the time and stimulate at various locations along the vessel successively, ending up with a number of Vm measurements along the conduction pathway obtained at different time points. A few studies have also obtained Vm recordings in arterioles at dual sites, making it possible to record conducted responses in VSMCs and ECs simultaneously, or to inject current at the local site and record Vm deflections at the conducted site.15, 30 This technique has provided crucial insight into which cell type(s) is involved in initiation and rapid spreading of the VCRs along the vessel wall.
Intravital microscopy for recording of VCRs in arterioles from intact, anesthetized animals has been performed using the hamster cheek pouch preparation, the rat mesenteric preparation and the mouse cremaster muscle preparation. These studies have mainly reported diameter measurements, but importantly Ca2+ dynamics in ECs in vivo were recently reported using a transgenic mouse expressing a GCamP2 Ca2+ sensor under the control of a Cx40 promotor found only in ECs of the vasculature and Purkinje fibers of the heart.16 Finally, sharp microelectrode measurements of Vm in VSMCs and ECs during VCRs in the above-mentioned in vivo models are routinely performed in only a few laboratories.10, 32, 34, 35
Molecular and Cellular Mechanisms Involved in Conduction of Vasomotor Signals
There is consensus in the literature that conducted vasodilatation is preceded by spreading of a hyperpolarization between the cells in the vascular wall, and that conducted vasoconstriction is initiated by a local depolarization conducted intercellularly to distant sites. However, the cell type(s) involved in these processes is still under debate, and seems to depend on the nature of the local stimulus and the cell type stimulated. As an example, local application of ACh onto an arteriole activates muscarinic receptors on the endothelium at the local site, which leads to Gα/q activation, diacylglycerol, and inositol triphosphate (IP3) production causing Ca2+ store release and capacitative Ca2+ entry through receptor- and storage-operated cation channels in the ECs.36, 37 This activates endothelial small and intermediate conductance Ca2+-activated K+ channels (KCa2.3 and KCa3.1), which leads to a hyperpolarization of ECs. The ECs are interconnected by gap junctions and the local endothelial hyperpolarization therefore spreads longitudinally from EC to EC along the length of the vessel.20, 38, 39, 40, 41 Subsequently, the hyperpolarization spreads from the ECs into the VSMCs through gap junctions in the myoendothelial projections.42, 43, 44, 45 As ECs are 30 to 140 μm long and oriented along the longitudinal axis of the vessel, each EC may establish contact with as many as 20 VSMCs.46, 47 The hyperpolarizing current from ECs to VSMCs results in a reduction in the number of open voltage-gated (L-type) Ca2+ channels in the VSMCs, a fall in VSMC [Ca2+]i and vasodilatation. As the hyperpolarization spreads along the endothelium, more and more VSMCs are hyperpolarized and eventually relaxed.
In the case of a vasoconstrictor, such as NE or PE applied locally onto arterioles and activating Gα/q-coupled receptors in VSMCs, both monophasic conducted vasoconstriction as well as biphasic local vasoconstriction followed by a conducted vasodilatation has been observed depending on the vascular bed under study.25, 48, 49 In the first case, a local depolarization caused by activation of receptor-operated cation channels and/or PKC-mediated inhibition of Ca2+- and/or voltage-activated K+ channels in VSMC, is conducted along the vessel through gap junctions coupling VSMCs with VSMCs. In addition, the depolarization may spread into the underlying endothelium, and can be conducted through this cellular pathway along the vessel wall. As the depolarization spreads into distant VSMCs, L-type channels are activated and the concomitant Ca2+ entry and rise in [Ca2+]i leads to conducted vasoconstriction. In the latter case where a transient local vasoconstriction is followed by a secondary conducted vasodilatation, evidence has shown that a local Gα/q-mediated IP3 release and Ca2+ increase in VSMC may spread via myoendothelial gap junctions into adjacent ECs to increase their local [IP3] and [Ca2+]i to activate endothelial Ca2+-activated K+ channels thereby causing a secondary conducted hyperpolarization and vasodilatation.48, 49, 50, 51 Application of a local high KCl concentration has also been widely used as a tool to induce conducted depolarization and vasoconstriction. This leads to a conducted vasoconstriction of rather limited amplitude, which is thought to rely primarily on intercellular communication via the VSMC layer. Interestingly, the tendency of local vasoconstrictor application acting on VSMCs to induce conducted vasoconstriction with limited amplitude compared with agonists acting on ECs, have been explained by a higher dissipation of current through gap junctions and ion channels for VSMC-initiated responses. Thus, with a sufficiently strong local depolarization of VSMCs to overcome current dissipation, the depolarization can spread into adjacent ECs and be conducted with limited decay along this pathway as well.52
As noted, the KCl-induced conducted signal is smaller in amplitude compared with the vasomotor signals induced by local vasodilator or vasoconstrictor hormones, which are often conducted without significant decay within the distances of 1 to 2 mm usually investigated in this type of study. This has led to the hypothesis that a regenerative mechanism exist in the vascular wall, which would account for propagation of a hyper- or depolarization over long distances in the microcirculation.15, 26, 35, 53 In hamster retractor muscle feed arteries, it was shown that inward-rectifier (KIR) K+ channels possess the inherent biophysical properties necessary to facilitate the conducted hyperpolarization and vasodilatation to local ACh application. Using a range of in vitro methods and computational modeling, it was shown that the negative-slope conductance of KIR channels during hyperpolarization of VSMCs would augment the initial hyperpolarization as it conducts through VSMCs along the vascular wall.54 Thus, this was the first concrete molecular evidence of a regenerative mechanism.
Previous studies suggested that voltage-gated Na+ channels may be expressed in the vascular wall, either in ECs53 or in sensory nerve terminals adjacent to arteriolar VSMCs55 and that activation of these channels might contribute to the regenerative conduction process. This topic is still not completely resolved, but recent studies did not find a role for TTX-sensitive13, 25, 29 or TTX-insensitive Na+ channels18 in conducted depolarization in rat renal or mesenteric arterioles.
Recently, a new hypothesis argues against the requirement of a regenerative mechanism for nondecaying conducted vasodilatation. This model, which gained support from experimental evidence in mouse cremaster arterioles in vivo, proposes that the conducted hyperpolarization is more negative than the range of membrane potentials at which L-type channel window currents occur, thus causing the vasodilatation to be maximal over a long segment of the arteriole, while the conducted hyperpolarization in fact decays electrotonically.34 Thus, only at large distances from the local site is the hyperpolarization small enough to allow a limited Ca2+ entry through L-type channels, which would tend to decay the conducted vasodilatation. It will be interesting to see this model investigated in more vascular beds and using virtual arteriolar models incorporating the crucial ion channels and intercellular resistances known to affect conduction.
Physiological Modulation of Vascular Conducted Responses
An interesting question is whether VCRs are subject to physiological or pathophysiological modulation in the sense that the extent of the conduction can be modified by physiological factors, for example, hormones or nervous activity. The extent or spread of a VCR can be expressed by its length constant λ. The length constant is the distance from the site of stimulation where the response has decayed to ∼63% of the initial value.7, 56 The intercellular electrical circuit of an arteriole consists of an inner conduction pathway with resistance Rj determined by the combined gap junctional and cytoplasmic resistances, and an outer semipermeable leak pathway consisting of the plasma membrane with the resistance Rm. In analogy with electrotonic conduction in axons, the length constant λ in arterioles depends on the ratio between the resistance of the plasma membrane and the resistance of the intercellular compartment: λ=√(Rm/Rj). This implies that the conducted responses can be regulated by modulating either the gap junctional resistance or the resistance of the plasma membrane. The latter is primarily determined by the activity of the K+ channels present in the cell membrane.57, 58
The gap junctional resistance depends on the number of gap junctional channels, the single channel conductance and the open probability.59 Several factors, like changes in gene activity, intracellular trafficking of connexins, [Ca2+]i, intracellular pH, and posttranslational modifications of connexins, for example, phosphorylation, are able to modify one or more of these three parameters, and, thus, modulate gap junctional resistance.59 The connexins found in the vascular wall are primarily Cx37, Cx40, Cx43, and Cx45.59 Cx40 appears to be the dominant connexin, and it is found primarily in the ECs. In accordance with the central role of gap junctions, Cx40 knockout mice has impaired conducted vasodilation,20 and nonspecific inhibitors of gap junctions like carbenoxolone or palmitoleic acid completely abolish the VCR.13, 60 Despite the central role of gap junctions in VCR, there are at present no experimental data to show that modifications of gap junctional resistance plays a role in physiological or pathophysiological modulations of VCRs.
In a recent study, the hypothesis was tested that an increase of Rm caused by inhibition of plasma membrane K+ channels would lead to augmented conducted vasomotor responses in rat mesenteric terminal arterioles. Using an experimental and computational approach, it was demonstrated that BKCa and KV channels limit conducted vasoconstriction in intact rat mesenteric arterioles by effectively dissipating current out of the VSMCs and limiting the transfer of current to other cells in the wall via gap junctions.18 As both BKCa and KV channels are depolarization-activated, it is an interesting question whether conducted hyperpolarization could be modified by plasma membrane K+ channel activity. In rat mesenteric small artery, simultaneous inhibition of BKCa and KV channels using a cocktail of pharmacological drugs, lead to augmentation of conducted hyperpolarization and vasodilatation to local ACh and isoproterenol application.61 Presumably, these blockers eliminate the dissipation of hyperpolarizing current along the arteriole; however, it is somewhat unexpected to see an effect of inhibiting these voltage-gated channels as hyperpolarization could be expected to completely close BKCa and KV channels. Recently, however, nonvoltage-gated endothelial SKCa and IKCa channels were also shown to limit the conducted hyperpolarization to local ACh in an isolated endothelial tube preparation from mouse epigastric arteries,62 demonstrating that the major K+ conductances in both ECs and VSMCs can play a role in modifying the conducted vasomotor responses by constituting a regulated leak pathway in the vascular wall as the conducted signal is passing through the cells. This dissipation of current effectively limits the conduction and would therefore serve as a means of physiological regulation of conduction and perhaps explain the difference in λ between responses obtained in different animal models with altered ion channel expression as a consequence of a treatment or disease.
Aging is associated with a reduction in BKCa channel expression and function in rat coronary and skeletal muscle arteries,63, 64 and this might alter conducted vasomotor responses in arterioles from aged individuals. In hypertensive animals, cerebral artery BKCa channels are upregulated,65 whereas in diabetic mice and rats the β1-subunit of the BKCa channel is downregulated, leading to impaired function of the channel.66, 67 Thus, it can be expected that the changes in BKCa channel expression and function modifies the length constant of VCRs in these diseases. Having defined a key role of K+ channels for modulation of VCRs, it can be predicted that intracellular regulators of K+ channel activity, such as PKC, PKA, 20-HETE, epoxyeicosatrienoic acid (EETs), prostaglandins, and PIP2 would be capable of regulating VCRs.
Interestingly, such effects may explain the modulation of VCRs that have been observed under certain conditions. Conducted vasoconstriction to local depolarization or NE application in mesenteric terminal arterioles was augmented by systemic Ang II infusion and abolished by the Ang II receptor antagonist losartan in anesthetized rats.68 In isolated rat mesenteric terminal arterioles preconstricted with NE and neuropeptide Y, the λ for conducted vasoconstriction to local depolarization was increased in a similar manner.18 We suggest that the action of systemic or topical administration of Gα/q-coupled receptor agonists to modulate the VCRs may be explained by PIP2 depletion or PKC-mediated inhibition of vascular BKCa and KV channels. In contrast to the above effects of agonists, sympathetic nerve activation in skeletal muscle arterioles was previously noted to inhibit conducted vasodilatation to local ACh application. This effect was mediated via α1- and α2-adrenergic receptors, and it was hypothesized that NE released from arteriolar varicosities caused a decrease in Rm by opening depolarizing ion channels in VSMCs, which would increase the dissipation of conducted hyperpolarizing current along the vessel.69
The length and branching of the vessel(s) under study will also influence the VCRs. Short arteriolar segments with electrically sealed ends tend to have larger remote responses, and thus larger λ values, because of the smaller total plasma membrane area available for dissipative currents. On the other hand, increasing vessel length can be expected to cause increased dissipation of current into the intercellular and extracellular compartments. When estimating λ, it is therefore important to utilize an equation that incorporates the method of reflection that takes into account the variable degree of dissipation as a function of vessel length.56, 70 Branching of the vessels, as in an intact microcirculatory network, would also effectively dissipate the conducted vasomotor signals due to significant current dissipation into the vascular cells along the side branches.31
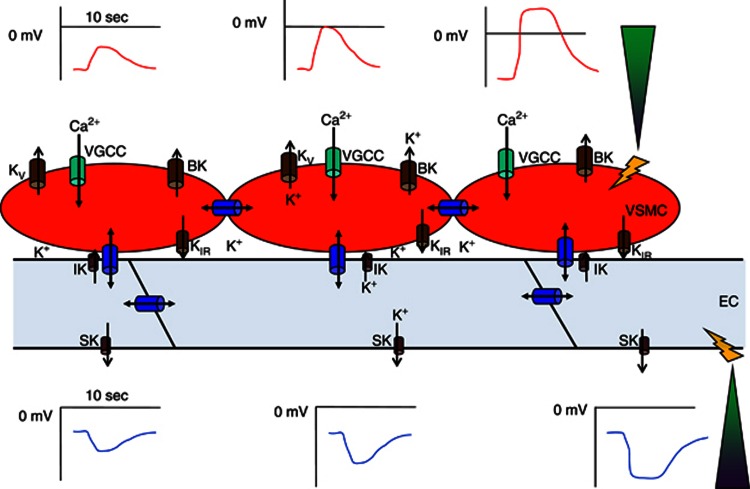
Figure 2 summarizes the molecular and cellular mechanisms involved in conduction and modulation of VCRs in arterioles. Homocellular coupling of ECs and VSMCs is achieved through gap junctions between neighboring cells, and heterocellular coupling occurs by gap junctions in myoendothelial projections passing through the internal elastic lamina. Voltage-dependent Ca2+ channels are the effectors linking the conducted electrical signals with the appropriate relaxation or contraction of VSMCs. Small and intermediate conductance Ca2+-activated K+ channels are important for initialization of the conducted hyperpolarization to a local rise in EC [Ca2+]i. In some vascular beds, the negative-slope conductance of inward-rectifier KIR channels augment hyperpolarizations as they conduct through the VSMC pathway. Finally, several types of K+ channels in EC (SKCa, IKCa) and VSMC (BKCa, KV) limit intercellular conduction of electrical signals due to charge dissipation across the cell membrane.
Figure 2.
Conduction mechanism involved in vascular conducted responses (VCRs). The schematic shows coupled endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) in the arteriolar wall, equipped with ion channels and gap junctions necessary to sustain conduction through the intercellular compartment(s) along the vessel. On the right, two pipette tips (green color) show delivery of a depolarizing (upper panel, VSMC) and hyperpolarizing (lower panel, EC) current to initiate conduction along the vessel. Both the depolarization (red curve) and hyperpolarization (blue curve) is seen to decay with distance from local stimulus. Blue barrels depict gap junctions coupling EC–EC, and VSMC–VSMC, as well as myoendothelial coupling (EC–VSMC). Green barrels depict voltage-gated Ca2+ channels (VGCC) whose activity convert the de- or hyperpolarizations into an increased or decreased Ca2+ influx into VSMC. Brown barrels depict various K+ channels in EC and VSMC, whose function is to modify the electrical responses as they are conducted along the EC and VSMC pathways. See text for further explanations. BK, big conductance Ca2+-activated K+ channels; IK, intermediate conductance Ca2+-activated K+ channels; SK, small conductance Ca2+-activated K+ channels; KV, voltage-gated K+ channels; KIR, inward-rectifier K+ channels. The duration bar is arbitrarily set to 10 seconds in the plot of Vm versus distance. The ECs and VSMCs are not drawn to scale.
Vascular Conducted Responses in the Cerebral Microcirculation
The most intensively studied conducted vasomotor responses in the cerebral circulation are those initiated by local ATP, ADP, or adenosine application onto rat cerebral penetrating arterioles (passive diameter <100 μm) isolated from middle cerebral arteries. Here, local adenosine application elicited both local and conducted vasodilatations,71, 72 which conducted rapidly in a decaying manner.71 ATP and ADP caused initial local vasoconstriction followed by a secondary local vasodilatation, which was conducted rapidly and in a decaying manner to remote sites.71, 72, 73 The local constriction to ATP was inhibited by low concentration of pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (3 μM) and α,β-methylene ATP (1 μM), showing that P2X-receptors are involved.73 The local secondary dilatation was reduced by impairment of endothelial function using air emboli, while the local constriction was enhanced by this procedure.73 The local dilatation was also sensitive to blockers of NOS (nitric oxide synthase) and Cytochrome P450 (CYP450), as well as to inhibition of endothelial IKCa by luminal application of TRAM-34, and to abluminal application of iberioxin to inhibit smooth muscle BKCa channels. Upon local ATP application a transient depolarization followed by a hyperpolarization preceded the local vasomotor responses.73 Taken together, these data indicate that local ATP activates smooth muscle P2X receptors causing local depolarization and vasoconstriction. At the same time, local ATP activates endothelial P2Y receptors, leading to a local rise in EC [Ca2+]i, which activates NOS, PLA2, and CYP450 enzymes. The local EC Ca2+ increase will cause hyperpolarization and secondary local dilatation by activating endothelial IKCa channels directly,36, 58 and indirectly activating VSMC BKCa channels through a PLA2/CYP450/EET-dependent mechanism.74, 75 The conducted dilatations to local ATP were dependent on an intact endothelium and were preceded by conducted hyperpolarization,73 consistent with electrotonic conduction and electromechanical coupling of the conducted vasomotor responses as observed in other systemic arterioles.15, 27, 32 Nitric oxide synthase or cyclooxygenase inhibition did not affect the conducted vasodilatation. CYP450 inhibition, on the other hand, strongly attenuated both local and conducted dilatations to ATP.73 However, the CYP450 inhibitor was applied to the bath and not via pipette to the local or remote sites, so it is difficult to conclude whether EETs play a role in the local response only, or in the conduction process per se. As EETs are known to activate Ca2+-activated K+ channels, it cannot be excluded that EET activation along arterioles could theoretically contribute to conducted hyperpolarization. However, EETs are usually released in response to receptor activation and/or local Ca2+ increases and it is therefore difficult to imagine how EETs could be activated on a time scale fast enough to account for the spreading electrotonic hyperpolarization underlying the conducted vasodilatation in arterioles. The data presented by Dietrich et al73 suggest that the conducted vasodilatations to ATP are initiated by local hyperpolarizations and conducted electrotonically in a decaying manner via intercellular coupling of ECs and spread via myoendothelial junctions to the VSMC layer to cause remote vasodilatations.
In another study76 on isolated cerebral penetrating arterioles (passive diameter ∼55 μm), local elevation of [KCl] from 3 to 5 mM produced a minor local constriction followed by robust dilatation, and the dilatation conducted rapidly >1 mm with minimal decay. The local initial constriction was most likely caused by an initial Nernstian depolarization induced by the change in EK in VSMCs. The local secondary dilatation was blocked by ouabain but not by BaCl2 applied luminally or abluminally, indicating that activation of the Na+/K+-ATPase but not KIR channels mediate the local hyperpolarization and dilatation to a local [K+] increase from 3 to 5 mM.76 The local hyperpolarization seems to be conducted through the endothelial pathway, since the conducted dilatation to local KCl was significantly reduced after passing air emboli through arterioles. The conducted dilatations were reduced, but not abolished, by abluminal BaCl2, whereas there were no effects of blocking KV, KCa, or KATP channels using a combination of 4-aminopyridine, tetraethylammonium, and glibenclamide.76 These data suggest that KIR channels function to amplify the conducted hyperpolarizations, most likely due to their inherent negative-slope conductance as suggested for skeletal muscle arterioles.54 Bath application of ouabain blocked the conducted dilatations to the same extent as the local dilatations, but ouabain was not applied locally, so it cannot be determined whether the Na+/K+-ATPase contributes to the conduction process per se. It is interesting that the conducted dilatations only decayed slightly with distance. This suggests that cerebral penetrating arterioles may have: (1) a very well-coupled endothelial layer, (2) expression of KIR channels that may act as amplifiers of conducted hyperpolarizations, (3) a hyperpolarized voltage threshold beyond which dilatations are maximal, and (4) no major dissipative ion currents in EC or VSMC during conducted hyperpolarizations. If these requirements are met, the conducted dilatations could theoretically spread almost without attenuation over large distances in unbranched arterioles.
In rat penetrating arterioles (passive diameter 55 to 70 μm) isolated from pial arteries, local ATP application elicited local vasoconstriction followed by vasodilatation, as well as a conducted vasodilatation.5 In pial arterioles, prostaglandin F (PGF)2α elicited local vasoconstriction and simultaneous conducted vasodilatation. Conducted dilations to ATP and PGF2α were interpreted to be mediated via an endothelium-dependent mechanism.5 In pial arterioles, local adenosine caused local vasodilatation, but did not consistently produce conducted dilatation.
In an in vivo study of pial arterioles (15 to 40 μm) in halothane anesthetized rats equipped with an open cranial window, stimulation of parallel fibers in the cerebellar cortex produced both strong local vasodilatation, presumably caused by a local production of glutamate leading to local NO and adenosine release, as well as an upstream remote vasodilatation in larger feeding arterioles outside of the activated cortex area.4 However, in this latter study, it could not be excluded that part of the upstream vasodilation was due to flow-mediated vasodilation.
Taken together, pial and cerebral penetrating arterioles do possess powerful conducted vasomotor responses that are conducted electrotonically via an endothelial pathway. The latter study4 demonstrates the important concept that local increases in neuronal activity are coupled with upstream dilatation of arterioles at sites outside the stimulated area, indicating an important role for conducted vasomotor responses in neurovascular coupling.
Perspectives for Studying Cerebral Vascular Conducted Responses
The ultimate goal in any physiological and pathophysiological study of the microcirculation in the brain is to gain knowledge of the in vivo function of the brain. Therefore, in addition to studying isolated cerebral arterioles, the study of VCRs in vivo would be highly desirable. However, the anatomy of the brain and its blood supply offers considerable obstacles in pursuing in vivo studies of the important penetrating arterioles. These are not readily visible on the brain surface, but branch downwards into the brain. It seems probable that such studies would be advanced by employment of sophisticated imaging techniques enabling researchers to record responses deep within brain tissues, coupled with spatially well-defined focal stimulation of brain arterioles. Such methods are already available, for example, multiphoton microscopy of brain arterioles77, 78 and localized uncaging of Ca2+ or IP3 in a single or few cells.79, 80, 81 As gap junctions, voltage-gated Ca2+ channels and endothelial-dependent responses are known to be sensitive to anesthetic concentrations of propofol, thiopental, halothane, isoflurane, sevoflurane, and other general anesthetic agents82, 83, 84, 85, 86, 87 it is of paramount importance to validate the method of anesthesia carefully in such in vivo studies.
Potential Role of Vascular Conducted Responses in Cerebrovascular Disease
Subarachnoid hemorrhage is associated with regional cerebral hypoperfusion that is treatment-resistant and may cause prolonged episodes of regional brain ischemia. It has been speculated that the associated hypoperfusion to subarachnoid hemorrhage could be caused by either augmentation of conducted vasoconstriction or impairment of conducted vasodilatation in penetrating cerebral arterioles. By mimicking the effects of locally released oxyhemoglobin during subarachnoid hemorrhage, Kajita et al72 showed that the conducted vasodilatations to local ATP, ADP, and adenosine application were markedly reduced in isolated cerebral penetrating arterioles exposed to oxyhemoglobin, and in parallel the conducted vasoconstriction to PGF2α was increased.
The VCRs are most likely also important for reperfusion following brain ischemia. A recent study in rats investigated the conducted vasodilatation to local ATP or adenosine application after experimental middle cerebral artery occlusion at 1 and 24 hours reperfusion, respectively. It was demonstrated that conducted vasodilatation to ATP and adenosine was augmented after prolonged reperfusion, only. Thus, it is possible that regional hypoperfusion and ischemia in the brain is compensated for by augmentation of conducted dilatation without concurrent changes in conducted vasoconstriction.71
Cortical spreading depression (CSD) is associated with changes in the diameter of arterioles on the cortical surface, as well as changes in blood flow distribution in the cortex. A recent study reported that a wave of vascular dilatation is running ahead of the leading edge of CSDs induced by local depolarization of the cortex in anesthetized rats and mice equipped with cranial windows.88 The spreading dilatation was independent of underlying cortical or parenchymal activity and consistently followed the pattern of arteriolar networks on the cortical surface and was never observed to traverse cortical areas independent of arterioles. Furthermore, the rate of spreading dilatations was about twice the rate observed for CSDs (4 mm/min versus 2 mm/min) and this lead the authors to speculate that changes in the local parenchymal chemical environment, such as astrocytic Ca2+ waves, could trigger an electrical event that might initiate conducted vasodilatation along parenchymal arterioles.88 However, it must be pointed out that the conduction velocity of conducted vasomotor responses is several orders of magnitude faster than the spreading dilatations observed in the latter study. Although this could be influenced by the choice of model or anestethic agent, we would argue that conducted hyperpolarizations along the endothelial pathway leading to remote dilatations, cannot explain the phenomenon of CSDs. Much slower intercellular Ca2+ waves have been described in ECs in culture,89 in isolated vessels,17 and in vivo.16 The intercellular Ca2+ waves are dependent on the PLC pathway, IP3 diffusion, Ca2+-induced Ca2+ release, and gap junctions.90 It remains to be investigated whether intercellular Ca2+ waves in brain blood vessels are involved in CSD.
Conclusions and perspectives
The conduction of vasomotor responses along the arteriolar wall depends on intercellular communication of an initial local de- or hyperpolarization along the electrically coupled cells of the vascular wall, and on the degree of current dissipation across the plasma membrane through several types of ion channels. Although the local stimuli to initiate VCRs in cerebral arterioles might not be the same as in other systemic arterioles, the mechanisms of conduction do not seem to be different. The role of VCRs in regulating cerebral perfusion in health and disease deserves more attention due to their potential involvement in neurovascular coupling and in conditions under which brain ischemia might occur. The development of methods for studying VCRs in cerebral arterioles in vivo is needed to advance our understanding of their role in brain blood flow control in health and disease.
The authors declare no conflict of interest.
Footnotes
This study was financially supported by The Danish Medical Research Council, The Danish Heart Foundation, The Novo Nordisk Foundation, The Lundbeck Foundation, and The AP Møller Foundation for the Advancement of Medical Science.
References
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy TK, Pries AR, Secomb TW. Theoretical comparison of wall-derived and erythrocyte-derived mechanisms for metabolic flow regulation in heterogeneous microvascular networks. Am J Physiol Heart Circ Physiol. 2012;302:H1945–H1952. doi: 10.1152/ajpheart.01176.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarelius I, Pohl U. Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol (Oxf) 2010;199:349–365. doi: 10.1111/j.1748-1716.2010.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–659. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- Dietrich HH, Kajita Y, Dacey RG. Local and conducted vasomotor responses in isolated rat cerebral arterioles. Am J Physiol. 1996;271:H1109–H1116. doi: 10.1152/ajpheart.1996.271.3.H1109. [DOI] [PubMed] [Google Scholar]
- Gustafsson F, Holstein-Rathlou NH. Conducted vasomotor responses in arterioles: characteristics, mechanisms and physiological significance. Acta Physiol Scand. 1999;167:11–21. doi: 10.1046/j.1365-201x.1999.00603.x. [DOI] [PubMed] [Google Scholar]
- Segal SS, Damon DN, Duling BR. Propagation of vasomotor responses coordinates arteriolar resistances. Am J Physiol. 1989;256:H832–H837. doi: 10.1152/ajpheart.1989.256.3.H832. [DOI] [PubMed] [Google Scholar]
- Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res. 1998;56:43–53. doi: 10.1006/mvre.1998.2076. [DOI] [PubMed] [Google Scholar]
- Dietrich HH. Effect of locally applied epinephrine and norepinephrine on blood flow and diameter in capillaries of rat mesentery. Microvasc Res. 1989;38:125–135. doi: 10.1016/0026-2862(89)90021-6. [DOI] [PubMed] [Google Scholar]
- Wölfle SE, Schmidt VJ, Hoyer J, Kohler R, de Wit C. Prominent role of KCa3.1 in endothelium-derived hyperpolarizing factor-type dilations and conducted responses in the microcirculation in vivo. Cardiovasc Res. 2009;82:476–483. doi: 10.1093/cvr/cvp060. [DOI] [PubMed] [Google Scholar]
- Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol. 2007;579:175–186. doi: 10.1113/jphysiol.2006.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AJ, Holstein-Rathlou NH, Marsh DJ. Internephron coupling by conducted vasomotor responses in normotensive and spontaneously hypertensive rats. Am J Physiol. 1997;272:F372–F379. doi: 10.1152/ajprenal.1997.272.3.F372. [DOI] [PubMed] [Google Scholar]
- Braunstein TH, Inoue R, Cribbs L, Oike M, Ito Y, Holstein-Rathlou NH, et al. The role of L- and T-type channels in local and remote calcium responses in rat mesenteric terminal arterioles. J Vasc Res. 2009;46:138–151. doi: 10.1159/000151767. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Neild TO, Segal SS. Conduction of hyperpolarization along hamster feed arteries: augmentation by acetylcholine. Am J Physiol Heart Circ Physiol. 2002;283:H102–H109. doi: 10.1152/ajpheart.00038.2002. [DOI] [PubMed] [Google Scholar]
- Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, et al. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res. 2007;101:1300–1309. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- Uhrenholt TR, Domeier TL, Segal SS. Propagation of calcium waves along endothelium of hamster feed arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1634–H1640. doi: 10.1152/ajpheart.00605.2006. [DOI] [PubMed] [Google Scholar]
- Hald BO, Jacobsen JC, Braunstein TH, Inoue R, Ito Y, Sorensen PG, et al. BKCa and KV channels limit conducted vasomotor responses in rat mesenteric terminal arterioles. Pflügers Arch. 2012;463:279–295. doi: 10.1007/s00424-011-1049-8. [DOI] [PubMed] [Google Scholar]
- Steinhausen M, Endlich K, Nobiling R, Parekh N, Schutt F. Electrically induced vasomotor responses and their propagation in rat renal vessels in vivo. J Physiol. 1997;505 (Pt 2:493–501. doi: 10.1111/j.1469-7793.1997.493bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, et al. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- Segal SS, Welsh DG, Kurjiaka DT. Spread of vasodilatation and vasoconstriction along feed arteries and arterioles of hamster skeletal muscle. J Physiol. 1999;516 (Pt 1:283–291. doi: 10.1111/j.1469-7793.1999.283aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol. 2001;536:937–946. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Toma I, Sosnovtseva OV, Peti-Peterdi J, Holstein-Rathlou NH. Electrotonic vascular signal conduction and nephron synchronization. Am J Physiol Renal Physiol. 2009;296:F751–F761. doi: 10.1152/ajprenal.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DS, Sim SB, Cha HJ. Cell adhesion biomaterial based on mussel adhesive protein fused with RGD peptide. Biomaterials. 2007;28:4039–4046. doi: 10.1016/j.biomaterials.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Gustafsson F, Andreasen D, Salomonsson M, Jensen BL, Holstein-Rathlou NH. Conducted vasoconstriction in rat mesenteric arterioles: role for dihydropyridine-insensitive Ca(2+) channels. Am J Physiol Heart Circ Physiol. 2001;280:H582–H590. doi: 10.1152/ajpheart.2001.280.2.H582. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: electrotonic versus regenerative conduction. Am J Physiol Heart Circ Physiol. 2008;295:H2001–H2007. doi: 10.1152/ajpheart.00063.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Xia J, Duling BR. Endothelial cell signaling during conducted vasomotor responses. Am J Physiol Heart Circ Physiol. 2003;285:H119–H126. doi: 10.1152/ajpheart.00643.2002. [DOI] [PubMed] [Google Scholar]
- Duza T, Sarelius IH. Conducted dilations initiated by purines in arterioles are endothelium dependent and require endothelial Ca2+ Am J Physiol Heart Circ Physiol. 2003;285:H26–H37. doi: 10.1152/ajpheart.00788.2002. [DOI] [PubMed] [Google Scholar]
- Salomonsson M, Gustafsson F, Andreasen D, Jensen BL, Holstein-Rathlou NH. Local electric stimulation causes conducted calcium response in rat interlobular arteries. Am J Physiol Renal Physiol. 2002;283:F473–F480. doi: 10.1152/ajprenal.00247.2001. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- Segal SS, Neild TO. Conducted depolarization in arteriole networks of the guinea-pig small intestine: effect of branching of signal dissipation. J Physiol. 1996;496 (Pt 1:229–244. doi: 10.1113/jphysiol.1996.sp021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol. 1998;274:H178–H186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- Xia J, Little TL, Duling BR. Cellular pathways of the conducted electrical response in arterioles of hamster cheek pouch in vitro. Am J Physiol. 1995;269:H2031–H2038. doi: 10.1152/ajpheart.1995.269.6.H2031. [DOI] [PubMed] [Google Scholar]
- Wölfle SE, Chaston DJ, Goto K, Sandow SL, Edwards FR, Hill CE. Non-linear relationship between hyperpolarisation and relaxation enables long distance propagation of vasodilatation. J Physiol. 2011;589:2607–2623. doi: 10.1113/jphysiol.2010.202580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GJ, Neild TO, Segal SS. Contribution of active membrane processes to conducted hyperpolarization in arterioles of hamster cheek pouch. Microcirculation. 2004;11:425–433. doi: 10.1080/10739680490457836. [DOI] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2006;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res. 2004;62:345–356. doi: 10.1016/j.cardiores.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res. 2003;60:643–653. doi: 10.1016/j.cardiores.2003.09.017. [DOI] [PubMed] [Google Scholar]
- de Wit C. Connexins pave the way for vascular communication. News Physiol Sci. 2004;19:148–153. doi: 10.1152/nips.01520.2004. [DOI] [PubMed] [Google Scholar]
- Gustafsson F, Mikkelsen HB, Arensbak B, Thuneberg L, Neve S, Jensen LJ, et al. Expression of connexin 37, 40 and 43 in rat mesenteric arterioles and resistance arteries. Histochem Cell Biol. 2003;119:139–148. doi: 10.1007/s00418-002-0493-0. [DOI] [PubMed] [Google Scholar]
- Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium- derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Goto K, Rummery NM, Hill CE. Developmental changes in myoendothelial gap junction mediated vasodilator activity in the rat saphenous artery. J Physiol. 2004;556:875–886. doi: 10.1113/jphysiol.2003.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function. J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TL, Duling BR. Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc Res. 1997;53:113–120. doi: 10.1006/mvre.1996.1999. [DOI] [PubMed] [Google Scholar]
- Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Yashiro Y, Duling BR. Integrated Ca(2+) signaling between smooth muscle and endothelium of resistance vessels. Circ Res. 2000;87:1048–1054. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]
- Yashiro Y, Duling BR. Participation of intracellular Ca2+ stores in arteriolar conducted responses. Am J Physiol Heart Circ Physiol. 2003;285:H65–H73. doi: 10.1152/ajpheart.00662.2002. [DOI] [PubMed] [Google Scholar]
- Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, et al. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol. 2012;302:C1226–C1242. doi: 10.1152/ajpcell.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- Tran CH, Vigmond EJ, Plane F, Welsh DG. Mechanistic basis of differential conduction in skeletal muscle arteries. J Physiol. 2009;587:1301–1318. doi: 10.1113/jphysiol.2008.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Chen CC, Campbell KP, Damon DN, Day KH, Ramos S, et al. Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone. Am J Physiol Heart Circ Physiol. 2007;293:H1371–H1383. doi: 10.1152/ajpheart.01368.2006. [DOI] [PubMed] [Google Scholar]
- Jantzi MC, Brett SE, Jackson WF, Corteling R, Vigmond EJ, Welsh DG. Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2006;291:H1319–H1328. doi: 10.1152/ajpheart.00217.2006. [DOI] [PubMed] [Google Scholar]
- Thengchaisri N, Rivers RJ. Remote arteriolar dilations caused by methacholine: a role for CGRP sensory nerves. Am J Physiol Heart Circ Physiol. 2005;289:H608–H613. doi: 10.1152/ajpheart.01290.2004. [DOI] [PubMed] [Google Scholar]
- Hald BO, Jensen LJ, Sorensen PG, Holstein-Rathlou NH, Jacobsen JC. Applicability of cable theory to vascular conducted responses. Biophys J. 2012;102:1352–1362. doi: 10.1016/j.bpj.2012.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH. Gap Junctions. Comprehen Physiol. 2012;2:1981–2035. doi: 10.1002/cphy.c110051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen CM, Salomonsson M, Braunstein TH, Nielsen MS, Holstein-Rathlou NH. Connexin mimetic peptides fail to inhibit vascular conducted calcium responses in renal arterioles. Am J Physiol Regul Integr Comp Physiol. 2008;295:R840–R847. doi: 10.1152/ajpregu.00491.2007. [DOI] [PubMed] [Google Scholar]
- Beleznai TZ, Yarova PL, Yuill KH, Dora KA. Smooth muscle Ca(2+)-activated and voltage-gated K(+) channels modulate conducted dilation in rat isolated small mesenteric arteries. Microcirculation. 2011;18:487–500. doi: 10.1111/j.1549-8719.2011.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer EJ, Segal SS. Tuning electrical conduction along endothelial tubes of resistance arteries through Ca(2+)-activated K(+) channels. Circ Res. 2012;110:1311–1321. doi: 10.1161/CIRCRESAHA.111.262592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang LS, Kim S, Dominguez JM, Sindler AL, Dick GM, Muller-Delp JM. Aging and muscle fiber type alter K+ channel contributions to the myogenic response in skeletal muscle arterioles. J Appl Physiol. 2009;107:389–398. doi: 10.1152/japplphysiol.91245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca(2+)-activated K(+) channels in coronary smooth muscle during aging. Circ Res. 2001;88:210–216. doi: 10.1161/01.res.88.2.210. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hudetz AG, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats: evidence for their protection against cerebral vasospasm. Circ Res. 1998;82:729–737. doi: 10.1161/01.res.82.6.729. [DOI] [PubMed] [Google Scholar]
- McGahon MK, Dash DP, Arora A, Wall N, Dawicki J, Simpson DA, et al. Diabetes downregulates large-conductance Ca2+-activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100:703–711. doi: 10.1161/01.RES.0000260182.36481.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Zheng YM, Van RD, Rathore R, Liu QH, Singer HA, et al. Functional and molecular evidence for impairment of calcium-activated potassium channels in type-1 diabetic cerebral artery smooth muscle cells. J Cereb Blood Flow Metab. 2008;28:377–386. doi: 10.1038/sj.jcbfm.9600536. [DOI] [PubMed] [Google Scholar]
- Gustafsson F, Holstein-Rathlou NH. Angiotensin II modulates conducted vasoconstriction to norepinephrine and local electrical stimulation in rat mesenteric arterioles. Cardiovasc Res. 1999;44:176–184. doi: 10.1016/s0008-6363(99)00174-1. [DOI] [PubMed] [Google Scholar]
- Haug SJ, Segal SS. Sympathetic neural inhibition of conducted vasodilatation along hamster feed arteries: complementary effects of alpha1- and alpha2-adrenoreceptor activation. J Physiol. 2005;563:541–555. doi: 10.1113/jphysiol.2004.072900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GJ, Neild TO. An equation describing spread of membrane potential changes in a short segment of blood vessel. Phys Med Biol. 1999;44:N217–N221. doi: 10.1088/0031-9155/44/10/402. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Nguyen TS, Meno JR, Britz GW. Postischemic augmentation of conducted dilation in cerebral arterioles. Stroke. 2007;38:124–130. doi: 10.1161/01.STR.0000252157.93998.47. [DOI] [PubMed] [Google Scholar]
- Kajita Y, Dietrich HH, Dacey RG. Effects of oxyhemoglobin on local and propagated vasodilatory responses induced by adenosine, adenosine diphosphate, and adenosine triphosphate in rat cerebral arterioles. J Neurosurg. 1996;85:908–916. doi: 10.3171/jns.1996.85.5.0908. [DOI] [PubMed] [Google Scholar]
- Dietrich HH, Horiuchi T, Xiang C, Hongo K, Falck JR, Dacey RG. Mechanism of ATP-induced local and conducted vasomotor responses in isolated rat cerebral penetrating arterioles. J Vasc Res. 2009;46:253–264. doi: 10.1159/000167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Dietrich HH, Hongo K, Dacey RG. Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke. 2002;33:2692–2699. doi: 10.1161/01.str.0000034791.52151.6b. [DOI] [PubMed] [Google Scholar]
- Lecoq J, Parpaleix A, Roussakis E, Ducros M, Houssen YG, Vinogradov SA, et al. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat Med. 2011;17:893–898. doi: 10.1038/nm.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Bonev AD, Nelson MT, Wellman GC. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc Natl Acad Sci USA. 2012;109:E1387–E1395. doi: 10.1073/pnas.1121359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisov DV, Gelber SE, Walker JW, Wang SS. Synapse specificity of calcium release probed by chemical two-photon uncaging of inositol 1,4,5-trisphosphate. J Biol Chem. 2007;282:25517–25526. doi: 10.1074/jbc.M609672200. [DOI] [PubMed] [Google Scholar]
- Wang SS, Augustine GJ. Confocal imaging and local photolysis of caged compounds: dual probes of synaptic function. Neuron. 1995;15:755–760. doi: 10.1016/0896-6273(95)90167-1. [DOI] [PubMed] [Google Scholar]
- Yoshino J, Akata T, Shirozu K, Izumi K, Hoka S. Diabetes-associated alterations in volatile anesthetic actions on contractile response to norepinephrine in isolated mesenteric resistance arteries. Anesthesiology. 2010;112:595–606. doi: 10.1097/ALN.0b013e3181ce9e80. [DOI] [PubMed] [Google Scholar]
- Wentlandt K, Samoilova M, Carlen PL, El BH. General anesthetics inhibit gap junction communication in cultured organotypic hippocampal slices. Anesth Analg. 2006;102:1692–1698. doi: 10.1213/01.ane.0000202472.41103.78. [DOI] [PubMed] [Google Scholar]
- Yamakage M, Chen X, Tsujiguchi N, Kamada Y, Namiki A. Different inhibitory effects of volatile anesthetics on T- and L-type voltage-dependent Ca2+ channels in porcine tracheal and bronchial smooth muscles. Anesthesiology. 2001;94:683–693. doi: 10.1097/00000542-200104000-00024. [DOI] [PubMed] [Google Scholar]
- Xuan YT, Glass PS. Propofol regulation of calcium entry pathways in cultured A10 and rat aortic smooth muscle cells. Br J Pharmacol. 1996;117:5–12. doi: 10.1111/j.1476-5381.1996.tb15147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantz J, Cordier J, Giaume C. Effects of general anesthetics on intercellular communications mediated by gap junctions between astrocytes in primary culture. Anesthesiology. 1993;78:892–901. doi: 10.1097/00000542-199305000-00014. [DOI] [PubMed] [Google Scholar]
- Buljubasic N, Rusch NJ, Marijic J, Kampine JP, Bosnjak ZJ. Effects of halothane and isoflurane on calcium and potassium channel currents in canine coronary arterial cells. Anesthesiology. 1992;76:990–998. doi: 10.1097/00000542-199206000-00020. [DOI] [PubMed] [Google Scholar]
- Brennan KC, Beltran-Parrazal L, Lopez-Valdes HE, Theriot J, Toga AW, Charles AC. Distinct vascular conduction with cortical spreading depression. J Neurophysiol. 2007;97:4143–4151. doi: 10.1152/jn.00028.2007. [DOI] [PubMed] [Google Scholar]
- Domenighetti AA, Beny JL, Chabaud F, Frieden M. An intercellular regenerative calcium wave in porcine coronary artery endothelial cells in primary culture. J Physiol. 1998;513 (Pt 1:103–116. doi: 10.1111/j.1469-7793.1998.103by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leybaert L, Sanderson MJ. Intercellular Ca2+ waves: mechanisms and function. Physiol Rev. 2012;92:1359–1392. doi: 10.1152/physrev.00029.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]