Abstract
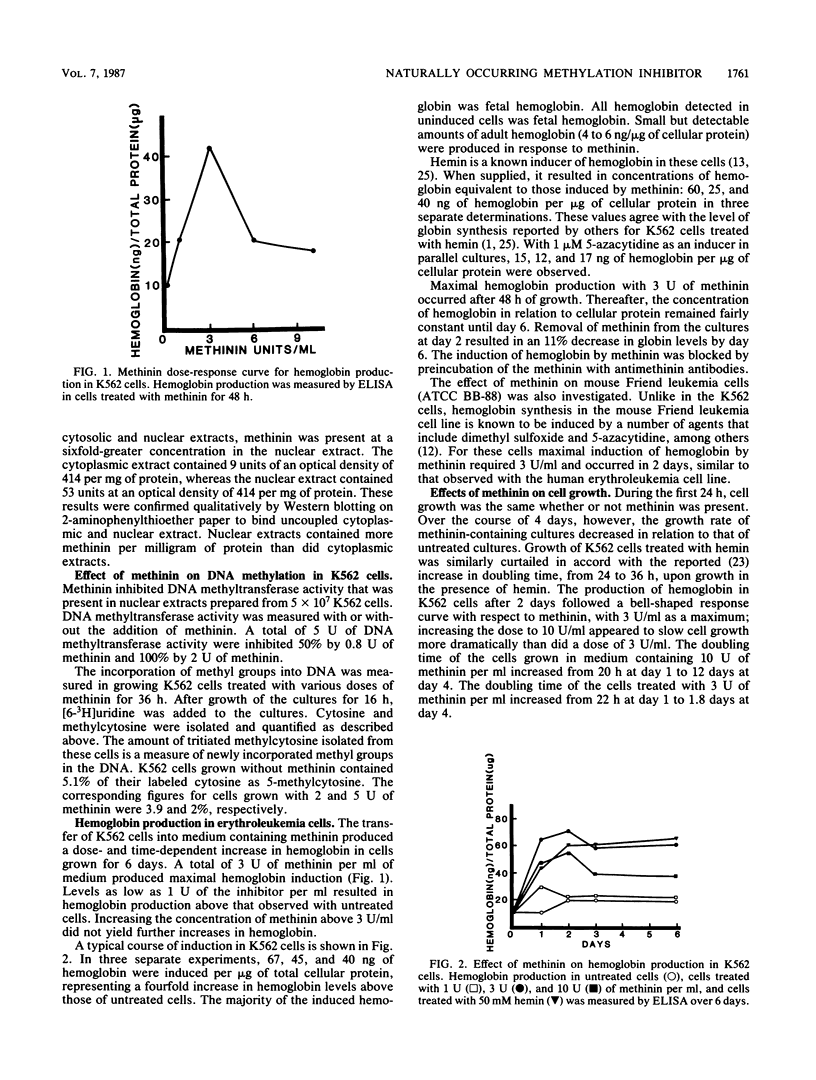
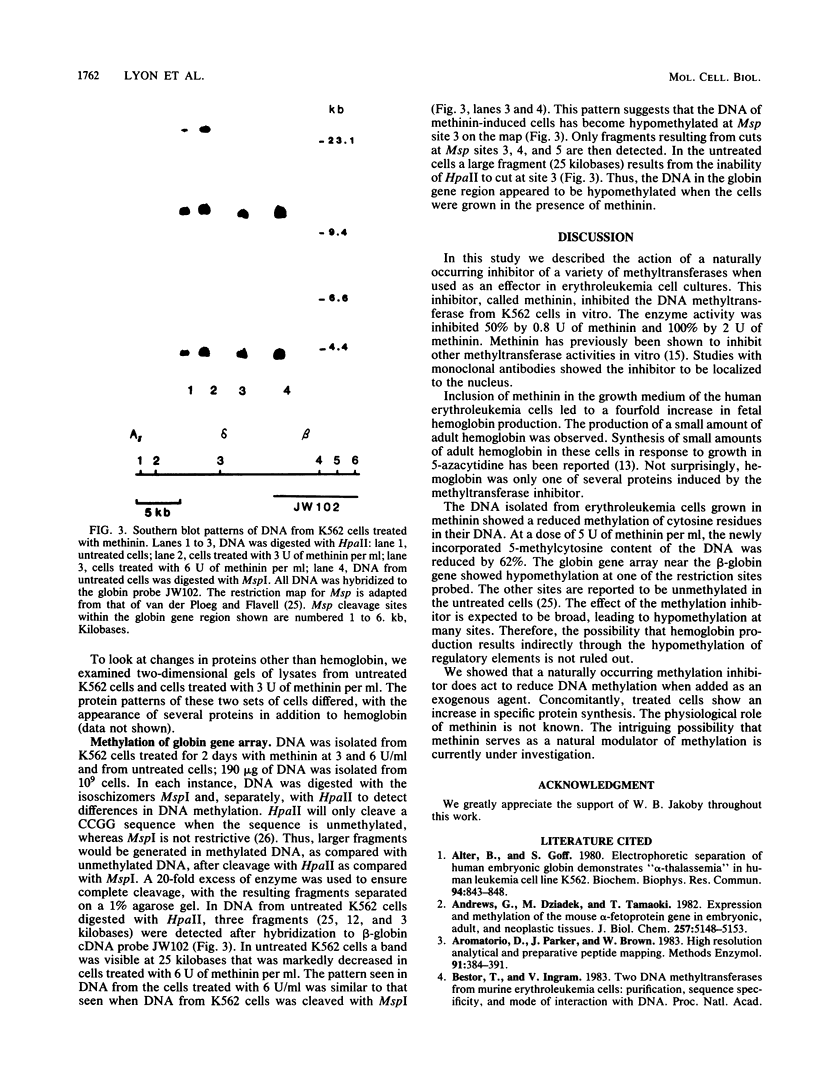
A naturally occurring methylation inhibitor isolated from rabbit liver and named methinin inhibits a number of methyltransferases. Methinin is a low-molecular-weight compound (1,400) that has an active amine group. This compound inhibits the DNA methyltransferase of human erythroleukemia cells (K562) in vitro. When the K562 cells were grown in medium containing methinin, fetal hemoglobin was produced. Small but detectable amounts of adult hemoglobin were also produced. Methinin was not toxic to these cells. The overall rate of genomic DNA methylation was reduced by 60% in cells grown in medium containing methinin. Southern blots of genomic DNA from methinin-treated cells and untreated cells hybridized to a 32P-labeled globin gene probe showed that one site in the globin gene region was hypomethylated. Methinin is a naturally occurring compound which inhibits DNA methylation both in vitro and in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Goff S. C. Electrophoretic separation of human embryonic globin demonstrates "alpha-thalassemia" in human leukemia cell line K562. Biochem Biophys Res Commun. 1980 Jun 16;94(3):843–848. doi: 10.1016/0006-291x(80)91311-x. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Dziadek M., Tamaoki T. Expression and methylation of the mouse alpha-fetoprotein gene in embryonic, adult, and neoplastic tissues. J Biol Chem. 1982 May 10;257(9):5148–5153. [PubMed] [Google Scholar]
- Aromatorio D. K., Parker J., Brown W. E. High-resolution analytical and preparative peptide mapping by a combination of ion-exchange chromatography and thin-layer chromatography. Methods Enzymol. 1983;91:384–391. doi: 10.1016/s0076-6879(83)91036-4. [DOI] [PubMed] [Google Scholar]
- Bestor T. H., Ingram V. M. Two DNA methyltransferases from murine erythroleukemia cells: purification, sequence specificity, and mode of interaction with DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5559–5563. doi: 10.1073/pnas.80.18.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Weich N., Schoenbrun B., Schneiderman N., Acs G. Hypomethylation of DNA during differentiation of Friend erythroleukemia cells. J Cell Biol. 1980 Aug;86(2):366–370. doi: 10.1083/jcb.86.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B., Scangos G. Expression of transferred thymidine kinase genes is controlled by methylation. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6299–6303. doi: 10.1073/pnas.79.20.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Gomes U. C., Shanley B. C. An endogenous inhibitor of indoleamine-N-methyltransferase in cerebrospinal fluid. Life Sci. 1978 Aug 21;23(7):697–704. doi: 10.1016/0024-3205(78)90069-3. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y., Cedar H., Razin A. Substrate and sequence specificity of a eukaryotic DNA methylase. Nature. 1982 Feb 18;295(5850):620–622. doi: 10.1038/295620a0. [DOI] [PubMed] [Google Scholar]
- Karlsson S., Nienhuis A. W. Developmental regulation of human globin genes. Annu Rev Biochem. 1985;54:1071–1108. doi: 10.1146/annurev.bi.54.070185.005231. [DOI] [PubMed] [Google Scholar]
- Lyon E. S., Jakoby W. B. Arylamine N-methyltransferase. Methylation of the indole ring. J Biol Chem. 1982 Jul 10;257(13):7531–7535. [PubMed] [Google Scholar]
- Lyon E. S., McPhie P., Jakoby W. B. Methinin: a peptide inhibitor of methylation. Biochem Biophys Res Commun. 1982 Sep 30;108(2):846–850. doi: 10.1016/0006-291x(82)90907-x. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Marzullo G., Rosengarten H., Friedhoff A. J. A peptide-like inhibitor of N-methyltransferase in rabbit brain. Life Sci. 1977 Mar 1;20(5):775–783. doi: 10.1016/0024-3205(77)90026-1. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Powers D. A., Fishbein J. C., Place A. R. Thin-layer peptide mapping with sequencing at the nanomole level. Methods Enzymol. 1983;91:466–486. doi: 10.1016/s0076-6879(83)91044-3. [DOI] [PubMed] [Google Scholar]
- Rutherford T., Clegg J. B., Higgs D. R., Jones R. W., Thompson J., Weatherall D. J. Embryonic erythroid differentiation in the human leukemic cell line K562. Proc Natl Acad Sci U S A. 1981 Jan;78(1):348–352. doi: 10.1073/pnas.78.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]



