Abstract
We have previously shown that orphanin FQ (also known as nociceptin; OFQ/N) attenuates the motor stimulatory effect of cocaine and blocks locomotor sensitization induced by cocaine. Furthermore, we have shown that cocaine treatment altered the level of endogenous OFQ/N, raising the possibility that endogenous OFQ/N and its receptor (NOP) may be crucial in these actions of cocaine. Accordingly, in the present study, we sought to determine the role of NOP receptors in psychomotor stimulation and locomotor sensitization induced by cocaine or amphetamine. Mice lacking the NOP receptor and their wild-type littermates were habituated to motor activity chambers for 1 h, injected with cocaine (0, 15 or 30 mg/kg) or amphetamine (0, 1 or 3 mg/kg), and motor activity was recorded for 1 h. For sensitization induced by these drugs, mice were treated with saline or the highest dose of each drug once daily for three consecutive days and tested on day 8. On this day, mice were habituated to the chambers for 1 h, then received a challenge dose of cocaine (15 mg/kg) or amphetamine (1 mg/kg), and motor activity was recorded for 1 h. Cocaine and amphetamine each induced hyperlocomotion but the extent of this response was not different between NOP receptor null mice and their controls. Sensitization developed to the motor stimulatory action of each drug, but the magnitude of cocaine-induced sensitization was only higher in null mice compared to their controls. Together, the present results suggest that the endogenous OFQ/N/NOP receptor system may modulate the development of cocaine-induced locomotor sensitization.
Keywords: Cocaine, Amphetamine, Locomotor sensitization, Orphanin FQ/Nociceptin (OFQ/N), NOP receptor, opioid receptor-like (ORL1) receptor, Knockout mouse
1. Introduction
Cocaine addiction is a serious public health issue which places a significant burden on society and economy. It is recognized as a chronic relapsing brain disorder characterized by compulsive drug seeking and drug taking behaviors with loss of control and associated negative consequences. The transition from occasional drug use to compulsive drug use involves instrumental, and classical conditioning processes [for reviews, see (Hyman et al., 2006; Kelley, 2004; Koob, 2000; Nestler and Landsman, 2001; Wolf, 2002)]. Thus, when administration of cocaine or another drug of abuse is often associated with a distinct environment, the context serves as a cue and this cue-drug association can induce subjective feelings even in the absence of the drug. Once this conditioned response develops and is consolidated, it can last for months and even years, due to its chronic nature (Hyman and Malenka, 2001). Importantly, subsequent exposure to the same surroundings, even in the absence of drugs, recalls old memories in abstinent addicts, thereby leading to craving and relapse (Foltin and Haney, 2000; Grant et al., 1996; Hyman et al., 2006; Kelley, 2004; Nestler and Landsman, 2001).
Locomotor sensitization is a long-lasting increase in locomotor activity that develops following repeated intermittent administration of cocaine and other psychoactive drugs (Kalivas and Weber, 1988; Post and Rose, 1976; Robinson and Becker, 1986; Stripling and Ellinwood, 1977a; b). This phenomenon which involves conditioned learning may play a role in the development and maintenance of drug addiction via enhanced motivational valence of cues associated with drug administration. Although there is little evidence for the development of this phenomenon in humans and that the disparity between animal and human data questions the clinical relevance of sensitization in rodents [for review, see (Narendran and Martinez, 2008)], behavioral sensitization is thought to mimic at least some aspects of addiction, particularly craving (Robinson and Berridge, 1993; 2000). Despite decades of research in this area, the underpinning mechanism of this phenomenon is not fully characterized. Thus, further research is needed to assess the role of neurochemicals found in brain regions implicated in this process.
The opioid receptor-like (ORL1) receptor (also known as NOP receptor) and its endogenous ligand, orphanin FQ (Reinscheid et al., 1995) or nociceptin (Meunier et al., 1995), are expressed in the CNS and especially in brain regions implicated in motivated behaviors (Neal et al., 1999a; Neal et al., 1999b). Considering that neuronal adaptive changes that occur along the mesolimbic dopaminergic and corticolimbic glutamatergic neurons are the hallmarks of locomotor sensitization [for review, see (Thomas et al., 2008)], and that OFQ/N negatively regulates dopaminergic and glutamatergic neurotransmission [for review, see (Murphy, 2010)], drugs targeting NOP receptors could regulate the development of sensitization. Indeed, we have previously shown that intracerebroventricular OFQ/N administration reduced the motor stimulatory effect of cocaine and blocked the development of locomotor sensitization in rodents (Bebawy et al., 2010; Lutfy et al., 2001; Lutfy et al., 2002). Notably, we have previously demonstrated in rats that repeated intermittent cocaine treatment that led to locomotor sensitization increased the level of endogenous OFQ/N in the hippocampus (Lutfy et al., 2008). However, the role of the endogenous OFQ/N/NOP receptor system in cocaine sensitization largely remains unexplored. Thus, using mice lacking the NOP receptor and their wild-type littermates, we sought to assess the role of OFQ/N/NOP receptor system in cocaine-induced motor stimulation and locomotor sensitization. For comparison, we examined the role of this system in motor stimulation and locomotor sensitization induced by amphetamine.
2. Materials and Methods
2.1. Subjects
Male mice lacking the NOP receptor (2–3 months old at the onset of experiments) bred in-house were the offspring of heterozygous breeding pairs crossed for 10–12 generations on C57BL/6J mouse strain. Pups were weaned between the ages of 21–24 days and genotyped. Only knockout mice and their wild-type littermates were used for the experiments; whereas, heterozygous mice were used for future breeding pairs. Mice were housed 2–4 per cage with free access to water and food in temperature- and humidity-controlled room. All the experimental procedures were conducted according to the National Institute of Health (NIH) guideline for the proper use of animals in research and were approved by the Institutional Animal Care and Use Committee (IACUC) at Western University of Health Sciences (Pomona, California, USA).
2.2. Experimental Protocols
2.2.1. The role of NOP receptors in motor stimulation and locomotor sensitization induced by cocaine
To determine the role of NOP in the acute motor stimulatory action of cocaine, male mice lacking the NOP receptor and their wild-type littermates/aged- and sex-matched wild-type controls were habituated to motor activity chambers (14 cm length × 14 cm width × 22 cm height) for 1 h, then injected with saline or cocaine (15 or 30 mg/kg, i.p.) and motor activity was recorded for an additional 1 h using the Videomex-V system (Columbus Instruments, Columbus, OH, USA). To determine the role of NOP receptors in sensitization developed to cocaine-induced motor stimulation, mice were treated with saline or cocaine (30 mg/kg, i.p.) for three consecutive days and mice were tested for locomotor sensitization on day 8. On this day, mice were habituated to the test chambers for 1 h, injected with cocaine (15 mg/kg) and motor activity was recorded for 1 h.
2.2.2. The role of NOP receptors in motor stimulation and locomotor sensitization induced by amphetamine
To assess the role of NOP receptors in amphetamine-induced motor stimulation, male mice lacking the NOP receptor and their wild-type littermates/controls were habituated to the test chambers for 1 h, then injected with saline or amphetamine (1 or 3 mg/kg), and motor activity was recorded for 1 h. To elucidate the role of NOP receptors in locomotor sensitization induced by amphetamine, mice were injected with saline or amphetamine (3 mg/kg, i.p.) once daily for three consecutive days and then tested for locomotor sensitization on day 8 following a challenge dose of amphetamine (1 mg/kg, i.p.).
2.3. Drugs
Cocaine hydrochloride and d-amphetamine sulfate were purchased from Sigma-Aldrich (St. Louis, MO, USA), and dissolved in normal saline on each test day. Drugs were injected intraperitoneally (i.p.) in a volume of 0.1 mL per 10 g of body weight.
2.4. Data Analysis
Data represent mean (±S.E.M.) of distance traveled during 15-min epochs or the entire 1-h test period. Repeated measures analysis of variance (ANOVA) or two-factor ANOVA was used to analyze the data. The Newman-Keuls post-hoc test was used to reveal significant difference between groups. P<0.05 was considered statistically significant.
3. Results
3.1. The acute motor stimulatory action of cocaine was not altered in mice lacking the NOP receptor compared to their wild-type littermates/controls
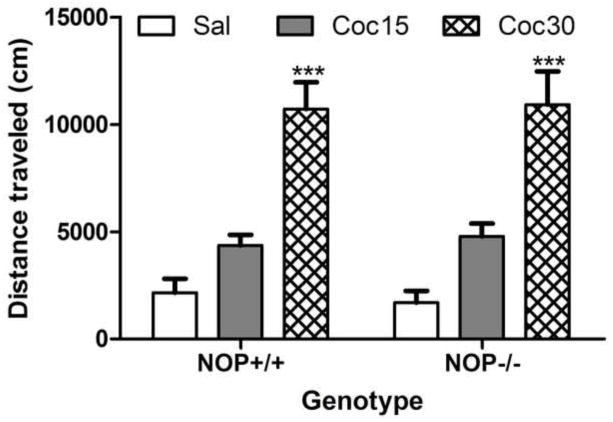
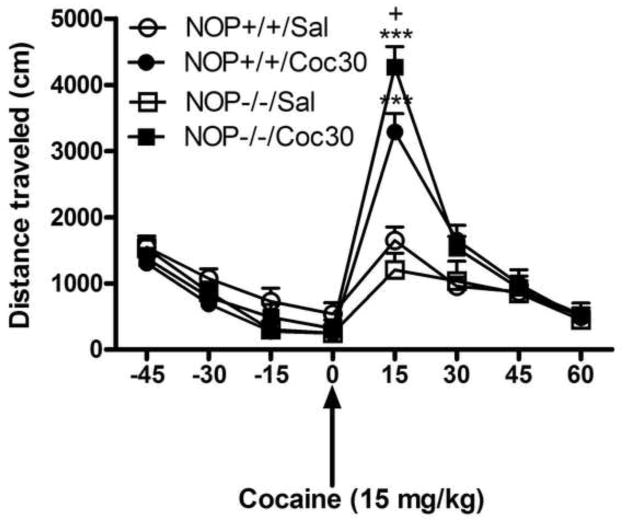
Figure 1 depicts the motor stimulatory action of cocaine (0, 15 or 30 mg/kg) in mice lacking the NOP receptor and their wild-type littermates on day 1. A two-factor ANOVA revealed a significant effect of cocaine’s dose (F2,45 = 36.96; P<0.0001), but no significant effect of genotype (Fl,45 = 0.01; P>0.05) and no significant interaction between the two factors (F2,45 = 0.08; P>0.05), showing that cocaine dose-dependently increased locomotor activity but the magnitude of this response was not altered in mice lacking the NOP receptor compared to their wild-type littermates/controls (P>0.05). Figure 2 illustrates the motor stimulatory action of a challenge dose of cocaine (15 mg/kg) given on day 8 in mice lacking the NOP receptor and their wild-type littermates with prior drug or no drug experience, i.e., treated with cocaine (30 mg/kg) or saline on days 1–3, respectively. As can be observed, the motor stimulatory action of cocaine was significantly greater in mice with prior cocaine experience compared to their respective saline-treated controls (Fig. 2). Repeated measures ANOVA revealed a significant interaction between pretreatment, i.e., saline vs. cocaine given on days 1–3, genotype and time in response to cocaine administration on the test day (F7,210 = 2.67; P<0.01). The post-hoc analysis of the data showed that the magnitude of this sensitized response was enhanced in mice lacking the NOP receptor compared to their wild-type littermates with prior cocaine exposure (Fig. 2; P<0.05).
Fig. 1.
Basal or cocaine-stimulated locomotor activity was not altered in mice lacking the NOP receptor compared to their wild-type controls. Mice were habituated to the motor activity chambers for 1 h, injected with saline or cocaine (15 or 30 mg/kg) and motor activity was recorded for 1 h. Data represent mean (±S.E.M.) of total distance traveled during the 1-h test period (n = 7–11 mice per dose/genotype). ***P<0.001 vs. saline- or cocaine (15mg/kg)-treated mice for each genotype
Fig. 2.
Cocaine sensitization was enhanced in mice lacking the NOP receptor compared to their wild-type littermates/controls. Mice were habituated to the motor activity chambers for 1 h, injected with saline or cocaine (30 mg/kg) and motor activity was recorded for 1 h. Mice received their respective treatment once daily for three consecutive days and then tested on day 8. On this day, mice were habituated to the testing chambers for 1 h, injected with cocaine (15 mg/kg) and motor activity was recorded at 15 min session for a total of 1 h. The data are expressed as mean (±S.E.M.) of distance traveled at 15-min epochs (n = 7–11 mice per treatment per genotype). ***P<0.001 vs. their respective saline-treated controls; +P<0.05 vs. cocaine-treated wild-type mice
3.2. The role of NOP receptors in amphetamine-induced motor stimulation and locomotor sensitization
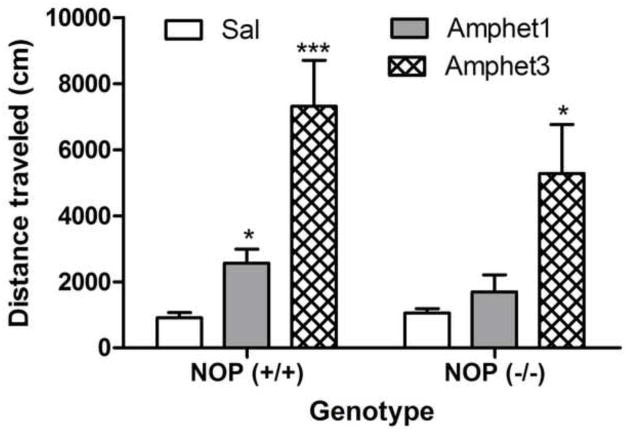
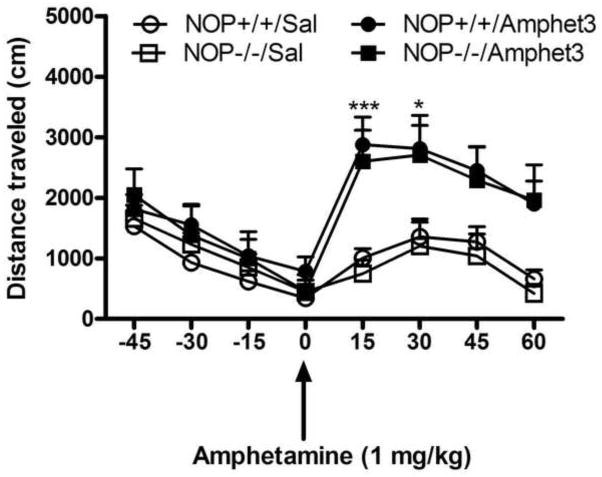
The motor stimulatory action of amphetamine (1 or 3 mg/kg) in mice lacking the NOP receptor and their wild-type littermates/controls is shown in figure 3. A repeated-measure ANOVA revealed a significant effect of amphetamine’s dose (F2,38 = 12.49; P<0.0001) but no significant effect of genotype (F1,38 = 0.87; P>0.05) and no significant interaction between the two factors (F2,38 = 0.45; P>0.05), showing that amphetamine dose-dependently increased locomotor activity but the magnitude of this response was not altered in mice lacking the NOP receptor compared to their wild-type littermates (Fig. 3). Figure 4 illustrates the motor stimulatory effect of a challenge dose of amphetamine (1 mg/kg) given on day 8 in mice lacking the NOP receptor and their wild-type littermates with prior amphetamine experience compared to their saline-treated controls. As observed with cocaine, mice treated with amphetamine on days 1–3 exhibited greater locomotor activity compared to their saline-treated controls in response to the challenge dose of amphetamine given on day 8 (Fig. 4). Repeated measures ANOVA revealed no significant interaction between pretreatment, i.e., saline vs. amphetamine given on days 1–3, genotype and time in response to amphetamine injection on the test day (F7,168 = 0.38; P>0.05). However, there was a significant effect of pretreatment, suggesting that amphetamine induced locomotor sensitization but this response was not different between mice lacking the NOP receptor and their wild-type littermates.
Fig. 3.
The motor stimulatory action of amphetamine was not altered in mice lacking the NOP receptor compared to their wild-type littermates/controls. Mice were habituated to the motor activity chambers for 1 h, injected with saline or amphetamine (1 or 3 mg/kg) and motor activity was recorded for 1 h. The data represent mean (±S.E.M.) of total distance traveled during the entire 1-h test period (n = 6–10 mice per dose per genotype). *P<0.05, ***P<0.001 compared to their respective saline-treated controls
Fig. 4.
Locomotor sensitization induced by amphetamine was not altered in mice lacking the NOP receptor compared to their wild-type littermates/controls. Mice were habituated to the motor activity chambers for 1 h, injected with saline or amphetamine (3 mg/kg) and motor activity was recorded for 1 h. Mice received their respective treatment once daily for three consecutive days and then tested on day 8. On this day, mice were habituated to the testing chambers for 1 h, injected with amphetamine (1 mg/kg) and motor activity was recorded for 1 h. The data represent mean (±S.E.M.) of distance traveled during the 15-min epochs (n = 6–8 mice per genotype per treatment). *P<0.05, ***P<0.001 compared to their respective saline-treated controls
4. Discussion
In the present study, we found that amphetamine or cocaine each dose-dependently increased locomotor activity but this response was not altered in mice lacking the NOP receptor compared to their wild-type littermates. In addition, locomotor sensitization induced by amphetamine was not different between mice lacking the NOP receptor and their wild-type controls. However, a greater sensitized response was detected in NOP receptor null mice compared to their wild-type littermates. Together, the present results suggest that the OFQ/N/NOP receptor system may be involved in cocaine-induce locomotor sensitization.
The endogenous OFQ/N/NOP receptor system is expressed throughout the CNS and in particular in brain regions involved in motivational and emotional behaviors (Neal et al., 1999a; Neal et al., 1999b). The mRNA for NOP receptor has been found on mesolimbic dopaminergic neurons (Maidment et al., 2002; Norton et al., 2002). Activation of NOP receptor is coupled to opening of potassium channels (Connor et al., 1996a; Vaughan and Christie, 1996), closure of calcium channels (Connor and Christie, 1999; Connor et al., 1996b; Knoflach et al., 1996) and inhibition of adenylyl cyclase (Meunier et al., 1995; Reinscheid et al., 1995), positioning the OFQ/N/NOP receptor system to regulate the activity of these neurons (Murphy et al., 1996; Murphy and Maidment, 1999; Murphy et al., 2004; Zheng et al., 2002) as well as the ability of addictive drugs to elevate dopamine levels in the nucleus accumbens (Di Giannuario et al., 1999; Koizumi et al., 2004; Lutfy et al., 2001) or elicit reward (Murphy et al., 1999; Sakoori and Murphy, 2004; 2008).
Furthermore, we have shown that OFQ/N attenuates basal and cocaine-stimulated motor activity (Bebawy et al., 2010; Lutfy et al., 2001). Additionally, we have demonstrated that single cocaine administration reduces the level of OFQ/N in the VTA and to a lesser extent in the striatum, thalamus and cortical regions (Lutfy et al., 2008), raising the possibility that the endogenous OFQ/N/NOP receptor system may be involved in the acute action of cocaine. Consistent with this notion, earlier we discovered that the conditioned place preference (CPP) induced by a single conditioning with cocaine was enhanced in mice lacking the NOP receptor (Marquez et al., 2008). Given that local administration of OFQ/N in the VTA reduces the motor stimulatory effect of cocaine (Lutfy et al., 2002) and that acute cocaine may cause the release of OFQ/N in the VTA (Lutfy et al., 2008), raising the possibility that the endogenous OFQ/N/NOP receptor system may play a functional role in acute motor stimulatory action of cocaine. However, our results revealed that cocaine dose-dependently increased locomotor activity in mice lacking the NOP receptor and their wild-type littermates (Fig. 2) but the magnitude of this response was not altered in mutant mice, suggesting that endogenous OFQ/N may not play a functional role in the acute motor stimulatory effect of cocaine in mice. Given that exogenous OFQ/N reduces cocaine-induced hyperlocomotion (Bebawy et al., 2010; Lutfy et al., 2001; Lutfy et al., 2002), the lack of an effect of endogenous OFQ/N on cocaine-induced motor stimulation could be due to the fact that the level of endogenous OFQ/N, released in response to cocaine administration (Lutfy et al., 2008), may not be high enough to regulate the motor stimulatory action of cocaine but this amount is sufficient to regulate cocaine reward (Marquez et al., 2008). Indeed, higher doses of OFQ/N are required to alter cocaine-induced motor stimulation than cocaine-induced CPP (Bebawy et al., 2010; Sakoori and Murphy, 2004).
We have previously showed that repeated intermittent cocaine treatment induced locomotor sensitization and this phenomenon was associated with an increase in the level of OFQ/N in the rat hippocampus (Lutfy et al., 2008). Considering that the hippocampus is involved in neuronal plasticity and that exogenous OFQ/N blocked the development of locomotor sensitization (Lutfy et al., 2002), we next examined whether the magnitude of locomotor sensitization would be altered in mice lacking the NOP receptor compared to their wild-type littermates. We observed that repeated intermittent cocaine treatment induced locomotor sensitization in mice of both genotypes. Interestingly, we discovered that the sensitized response was greater in NOP receptor null mice compared to their wild-type littermates. These results suggest that the OFQ/N/NOP receptor system may be involved in the development of sensitized response. Alternatively, the present results suggest that this system plays a functional role in the expression of conditioned locomotor activity observed in animals sensitized to cocaine. Interestingly, OFQ/N inhibits long-term potentiation in the hippocampus (Yu et al., 1997; Yu and Xie, 1998) and impairs spatial learning in rats (Sandin et al., 1997; Sandin et al., 2004). Moreover, enhanced LTP and spatial attention has been reported in mice lacking the NOP receptor (Mamiya et al., 2003; Manabe et al., 1998), raising the possibility that the enhanced sensitized response in mutant mice could have been due to enhanced learning, or altered memory retention. However, our results demonstrating that amphetamine-induced locomotor sensitization was not altered in mice lacking the NOP receptor compared to their wild-type littermates argues against the latter possibility. As stated above, exogenous OFQ/N attenuates the development of cocaine sensitization in mice (Bebawy et al., 2010) and that the level of endogenous OFQ/N is increased in the hippocampus in rats sensitized to cocaine (Lutfy et al., 2008), it is tempting to propose that the level of endogenous OFQ/N may be increased to reduce the magnitude of the sensitized response that develops in response to repeated cocaine administration. However, further studies are needed to confirm this assumption.
The differential role of the endogenous OFQ/N/NOP receptor system in the underlying mechanism of sensitization induced cocaine vs. amphetamine is somewhat intriguing because both drugs increase extracellular dopamine in the nucleus accumbens. However, cocaine competitively blocks the dopamine transporter and its action is dependent on ongoing impulse-dependent release of dopamine, whereas amphetamine inhibits the transporter only at low doses and has additional effects such as blockade of the vesicular monoamine transporter and the reversal of dopamine transporter at moderate to high doses, leading to efflux of dopamine [(Ramsson et al., 2011); for review, see (Schmitt and Reith, 2010)]. Thus, it seems possible that endogenous OFQ/N/NOP receptor system modulates the action of drugs, such as cocaine, which require ongoing cell firing and transmitter release.
5. Conclusion
We observed that the motor stimulatory effect of cocaine or amphetamine was not altered in mice lacking the NOP receptor compared to their wild-type mice. Likewise, locomotor sensitization induced by amphetamine was not different between mice of the two genotypes. On the other hand, the ability of cocaine to induce locomotor sensitization was enhanced in mice lacking the NOP receptor although the enhancement was not very robust. However, these results suggest that the endogenous OFQ/N/NOP receptor system may be involved in cocaine-induced locomotor sensitization. Considering that exogenous OFQ/N also blocks and even reverses cocaine sensitization (Bebawy et al., 2010; Lutfy et al., 2002), drugs that activate the NOP receptor may be of value in treating the behavioral and neuronal adaptive changes that occur following repeated cocaine treatment.
Acknowledgments
The authors thank Dr. Nazarian for his comments. This study was supported in part by the NIDA funded Grant # R01 DA016682 and MIDARP Grant # R24 DA017298.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bebawy D, Marquez P, Samboul S, Parikh D, Hamid A, Lutfy K. Orphanin FQ/nociceptin not only blocks but also reverses behavioral adaptive changes induced by repeated cocaine in mice. Biol Psychiatry. 2010;68:223–230. doi: 10.1016/j.biopsych.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Christie MD. Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol. 1999;26:493–499. doi: 10.1046/j.1440-1681.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Chieng B, Christie MJ. Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol. 1996a;119:1614–1618. doi: 10.1111/j.1476-5381.1996.tb16080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Yeo A, Henderson G. The effect of nociceptin on Ca2+ channel current and intracellular Ca2+ in the SH-SY5Y human neuroblastoma cell line. Br J Pharmacol. 1996b;118:205–207. doi: 10.1111/j.1476-5381.1996.tb15387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S, Catalani A, Loizzo A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett. 1999;272:183–186. doi: 10.1016/s0304-3940(99)00579-0. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Reinscheid RK, Civelli O, Kemp JA. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:6657–6664. doi: 10.1523/JNEUROSCI.16-21-06657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M, Midorikawa N, Takeshima H, Murphy NP. Exogenous, but not endogenous nociceptin modulates mesolimbic dopamine release in mice. Journal of neurochemistry. 2004;89:257–263. doi: 10.1111/j.1471-4159.2003.02322.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Annals of the New York Academy of Sciences. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Khaliq I, Carroll FI, Maidment NT. Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2002;164:168–176. doi: 10.1007/s00213-002-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Lam H, Narayanan S. Alterations in the level of OFQ/N-IR in rat brain regions by cocaine. Neuropharmacology. 2008;55:198–203. doi: 10.1016/j.neuropharm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidment NT, Chen Y, Tan AM, Murphy NP, Leslie FM. Rat ventral midbrain dopamine neurons express the orphanin FQ/nociceptin receptor ORL-1. Neuroreport. 2002;13:1137–1140. doi: 10.1097/00001756-200207020-00013. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Yamada K, Miyamoto Y, Konig N, Watanabe Y, Noda Y, Nabeshima T. Neuronal mechanism of nociceptin-induced modulation of learning and memory: involvement of N-methyl-D-aspartate receptors. Mol Psychiatry. 2003;8:752–765. doi: 10.1038/sj.mp.4001313. [DOI] [PubMed] [Google Scholar]
- Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, Nishi M, Noda T, Takahashi T, Sugimoto T, Nabeshima T, Takeshima H. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- Marquez P, Nguyen AT, Hamid A, Lutfy K. The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology. 2008;54:564–568. doi: 10.1016/j.neuropharm.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Murphy NP. The nociceptin/orphanin FQ system as a target for treating alcoholism. CNS Neurol Disord Drug Targets. 2010;9:87–93. doi: 10.2174/187152710790966713. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Ly HT, Maidment NT. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. doi: 10.1016/0306-4522(96)00322-3. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Maidment NT. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. Journal of neurochemistry. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Tan AM, Lam HA, Maidment NT. Nociceptin/orphanin FQ modulation of rat midbrain dopamine neurons in primary culture. Neuroscience. 2004;127:929–940. doi: 10.1016/j.neuroscience.2004.05.055. [DOI] [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999a;412:563–605. [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999b;406:503–547. [PubMed] [Google Scholar]
- Nestler EJ, Landsman D. Learning about addiction from the genome. Nature. 2001;409:834–835. doi: 10.1038/35057015. [DOI] [PubMed] [Google Scholar]
- Norton CS, Neal CR, Kumar S, Akil H, Watson SJ. Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol. 2002;444:358–368. doi: 10.1002/cne.10154. [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Ramsson ES, Howard CD, Covey DP, Garris PA. High doses of amphetamine augment, rather than disrupt, exocytotic dopamine release in the dorsal and ventral striatum of the anesthetized rat. Journal of neurochemistry. 2011;119:1162–1172. doi: 10.1111/j.1471-4159.2011.07407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology (Berl) 2004;172:129–136. doi: 10.1007/s00213-003-1643-3. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacology. 2008;33:877–891. doi: 10.1038/sj.npp.1301459. [DOI] [PubMed] [Google Scholar]
- Sandin J, Georgieva J, Schott PA, Ogren SO, Terenius L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur J Neurosci. 1997;9:194–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Sandin J, Ogren SO, Terenius L. Nociceptin/orphanin FQ modulates spatial learning via ORL-1 receptors in the dorsal hippocampus of the rat. Brain Res. 2004;997:222–233. doi: 10.1016/j.brainres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Annals of the New York Academy of Sciences. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Stripling JS, Ellinwood EH., Jr Augmentation of the behavioral and electrophysiologic response to cocaine by chronic administration in the rat. Exp Neurol. 1977a;54:546–564. doi: 10.1016/0014-4886(77)90256-4. [DOI] [PubMed] [Google Scholar]
- Stripling JS, Ellinwood EH., Jr Potentiation of the behavioral and convulsant effects of cocaine by chronic administration in the rat. Pharmacol Biochem Behav. 1977b;6:571–579. doi: 10.1016/0091-3057(77)90119-8. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br J Pharmacol. 1996;117:1609–1611. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Yu TP, Fein J, Phan T, Evans CJ, Xie CW. Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus. 1997;7:88–94. doi: 10.1002/(SICI)1098-1063(1997)7:1<88::AID-HIPO9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Yu TP, Xie CW. Orphanin FQ/nociceptin inhibits synaptic transmission and long-term potentiation in rat dentate gyrus through postsynaptic mechanisms. J Neurophysiol. 1998;80:1277–1284. doi: 10.1152/jn.1998.80.3.1277. [DOI] [PubMed] [Google Scholar]
- Zheng F, Grandy DK, Johnson SW. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br J Pharmacol. 2002;136:1065–1071. doi: 10.1038/sj.bjp.0704806. [DOI] [PMC free article] [PubMed] [Google Scholar]