Abstract
In adult mammals, the subventricular zone of the lateral ventricles (SVZ) and the subgranular zone of the dentate gyrus (DG) demonstrate ongoing neurogenesis, and multipotent neural stem/progenitor cells (NSCs) in these two regions exhibit different intrinsic properties. However, investigation of the mechanisms underlying such differences has been limited by a lack of efficient methods for isolating NSCs, particularly from the adult DG. Here we describe a protocol that enables us to isolate self-renewing and multipotent NSCs from the SVZ and the DG of the same adult mouse. The protocol involves the microdissection of the SVZ and DG from one adult mouse brain, isolation of NSCs from specific regions, and cultivation of NSCs in vitro. The entire procedure takes 2 to 3 hours. Since only one mouse is needed for each cell isolation procedure, this protocol will be particularly useful for studies with limited availability of mice, such as mice that contain multiple genetic modifications.
Introduction
Neural stem cells are characterized by their abilities to self-renew and to generate the different cell types found in the central nervous system. Isolation and in vitro analyses of neural stem/progenitor cells (NSCs) have proved to be an important method for deciphering the cellular and molecular mechanisms underlying neurogenesis, and for optimizing stem cell-based treatment of neurological disorders and injuries1. In the adult mammalian brain, NSCs exist mainly in two neurogenic regions: the subgranular zone of the dentate gyrus (DG) of the hippocampus, which generates excitatory glutamatergic granule neurons in the DG, and the subventricular zone (SVZ) of the lateral ventricles, which produces inhibitory GABAergic and dopaminergic interneurons of the olfactory bulb2. In addition to their destinies to become different types of neurons, NSCs in these two regions also respond differently to neurotrophic factors and physiological or pathological conditions; however, the molecular mechanisms governing the different properties of NSCs and neurogenesis in the SVZ and the DG remain largely unknown. It is therefore of interest to determine the intrinsic molecular signature of NSCs in these two regions, ideally in the same adult animals.
Over the past 20 years, multiple research groups have developed methodologies for isolating NSCs or progenitors from adult mouse brains. The first successful adult NSC cultures were neurospheres isolated from the SVZ and maintained under nonadherent conditions3,4. Later, some laboratories showed that SVZ NSCs can also be maintained in adherent monolayer culture5,6. However, spontaneous differentiation has been seen in mouse NSCs maintained as monolayer cells (based on our own observation). Thus although both neurosphere and adherent culture systems have pros and cons7, neurospheres, which provide a three-dimensional environment, may be better for maintenance of NSCs8. Therefore, neurosphere culture remains the most popular and reliable method for isolating and maintaining adult NSCs. Notably, until the current protocol was devised9, adult SVZ NSCs have been isolated from pooled tissues of at least two, and frequently four to six, adult mice.
Although a number of publications have demonstrated successful isolation of NSCs from the SVZ3–6, isolation of NSCs from the adult DG has been a challenge, largely due to the fact that the adult DG has significantly fewer NSCs than the SVZ. Early studies of adult hippocampal neurogenesis used progenitor cells isolated from whole adult forebrain or whole hippocampus of pooled tissues from five to six mice10. However, two groups isolated neurospheres from whole hippocampal tissue, but showed that these spheres do not exhibit self-renewal ability11,12. A breakthrough was made by Babu et al, who demonstrated that multipotent NSCs can be isolated from the DG. They microdissected the DG tissues from six adult mice and isolated NSCs using Percoll-based separation. The isolated DG NSCs could maintain their self-renewal and multipotency in monolayer culture13. Nevertheless, the need for six mice for each cell isolation posed a practical hurdle in studies with limited availability of animals. Since direct comparisons between littermates are necessary, for studies involving double and even triple transgenic animals, large numbers of mice had to be bred to complete just one cell preparation. Therefore, a much more efficient method was required for isolating NSCs from the adult DG. We developed such a method9 which in addition has the advantage that NSCs can be isolated both from the SVZ and the DG of the same animal.
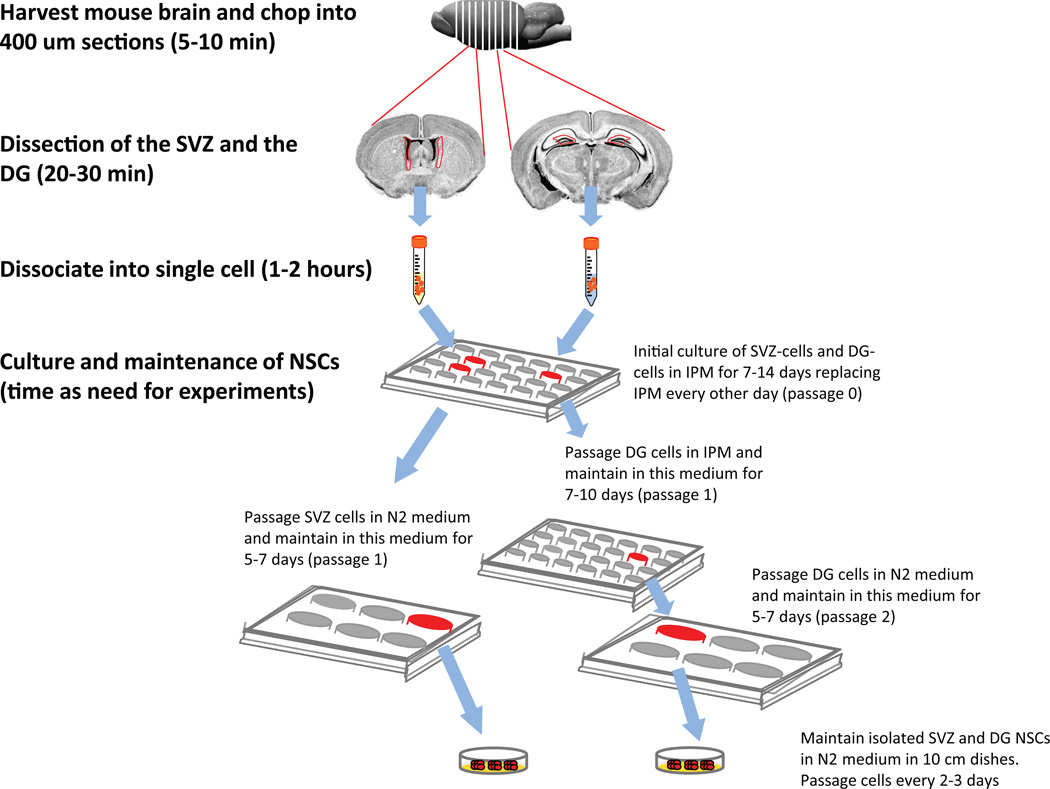
This protocol for isolating DG and SVZ NSCs as neurospheres (Figure 1) is based on that used in Babu et al13 and further optimized in our subsequent papers9,14–16. Whereas Babu et al. used pooled tissue from six adult mice13, our protocol requires tissue from only one mouse. In addition, our protocol allows for the isolation of multipotent NSCs from the DG and the SVZ of the same mouse. Although two earlier studies have presented isolation of neurospheres from both the SVZ and the DG tissue at the same time by pooling two to four animals, with their methods only the SVZ neurospheres exhibited self-renewal ability11,12. As demonstrated in our publications9,14–16 and described below, our method represents two breakthroughs: first, we only need a single adult mouse for each cell isolation, and second, we can reliably isolate multipotent NSCs from the SVZ and the DG of the same adult mouse.
Figure 1.
A schematic overview of the major steps in this protocol. These procedures have been approved the Institutional Animal Care and Use Committee at University of Wisconsin-Madison.
Materials
Reagents
8- to 12-week-old male mice. We have used Nestin-GFP mice17.
Hank’s Balanced Salt Solution (HBSS; Invitrogen/Gibco, Cat. No. 14025-126)
Neurobasal Medium (Invitrogen/Gibco, Cat. No. 21103-049)
Dulbecco's Modified Eagle’s Medium and Ham's F-12 Medium in a 1:1 ratio (DMEM/F-12; Omega Scientific, Cat. No. DM-25)
B27 serum-free supplement (B27; Invitrogen/Gibco, Cat. No. 17504-044)
N2 supplement (N2; Invitrogen/Gibco, Cat. No. 17502-408)
GlutaMAX (Invitrogen/Gibco, Cat. No. 35050-038)
L-Glutamine (Invitrogen/Gibco, Cat. No. 2503-081)
Antibiotic-Antimycotic (Anti-Anti; Invitrogen/Gibco, Cat. No. 15240-062)
Basic fibroblast growth factor (FGF-2; PeproTech: Cat. No. 100-18B-B)
Epidermal growth factor (EGF; PeproTech: Cat. No. 100-15)
MACS® Neural Tissue Dissociation kit (Miltenyi Biotec, Cat. No. 130-092-628)
Beta-mercaptoethanol (EM Sciences, MX031OMB-1); Caution: Hazardous material. Handle with care. Open it in a biological safety cabinet (BSC) while wearing gloves. In case of skin contact, immediately wash with plenty of water and seek medical attention. If inhaled, move to fresh air and seek medical attention.
Percoll (Amersham Biosciences, Cat. No.17-0891-01)
Dulbecco’s Phosphate-Buffered Saline 7.4 (10X) (DPBS; Invitrogen/Gibco, Cat. No.14200-059)
Dulbecco’s Phosphate-Buffered Saline 7.4 (1X) (DPBS; Invitrogen/Gibco, Cat. No.14190-136)
Trypsin-EDTA (10X) (Invitrogen/Gibco, Cat. No. 15400-054)
Trypsin inhibitor (Sigma-Aldrich, Cat. No. T6522)
Poly-L-ornithine (Sigma-Aldrich, Cat. No. P-3655)
Laminin (BD Biosciences, Cat. No. 354232)
Retinoic acid (RA, Sigma-Aldrich, Cat. No. R2625)
Forskolin (FSK, Sigma-Aldrich, Cat. No. F6886)
Dimethyl Sulfoxide (DMSO, Sigma-Aldrich, Cat. No. D2650)
Equipment
Cell culture disposables: Petri dishes (BD Falcon, Cat. No. 353003), centrifuge tubes (Corning, Cat. No. 430828 and Cat. No. 430055), pipettes (Eppendorf, Cat. No. 022470302), pipette tips (TipOne, Cat. No. 1111-2830)
Pasteur pipettes (Fisher Scientific, Cat. No. 22-209-361)
24-well tissue culture plates (BD Falcon, Cat. No. 353047)
Razor blades (Personna, Cat. No. BP9020)
Dissecting tools: dissecting scissors (Roboz, Cat. No. RS-5840, Cat. No. RS-6942), dissecting forceps (Roboz, Cat. No. RS-5237, Cat. No. RS-5095)
Dissecting microscope (Nikon, Cat. No. SMZ645)
Tissue culture hood (SteriGuard, Cat. No. SG603aHE)
CO2 incubator set to 5% CO2 and 37 °C (Thermo Scientific, Cat. No. 50116048)
Sterile swabs (Fisher Scientific, Cat. No. 14-959-92B)
Low-speed cell culture centrifuge (Eppendorf 5702, Cat. No. 022626001)
McIIwain Tissue Chopper (The Mickle Laboratory Engineering Co. LTD, Cat. No. 10180)
MACSmix Tube Rotator (Miltenyi Biotec, Cat. No. 130-090-753)
Inverted microscope (Olympus, CK41) with an Infinity digital camera
Reagent Setup
FGF-2 and EGF: Store as 10-µl aliquots at 1 mg/ml concentration in −80 °C. For working solutions, add 90 ul of Neurobasal Medium to each stock to give a final concentration of 100 µg/ml; these can be stored at 4 °C for up to 2 weeks.
Solution A: 1XHBSS containing 30 mM glucose, 2 mM Hepes, 26 mM NaHCO3. Store at 4 °C for up to 1 month.
Initial Proliferation Medium (IPM): To 96 ml Neurobasal medium, add 2 ml of B27, 1 ml of GlutaMAX, 1 ml of Anti-Anti, 20 ng/ml of FGF-2, and 20 ng/ml EGF. Store at 4 °C for up to 2 weeks.
N2 Medium: To 500 ml DMEM/F-12 medium, add 5 ml of N2, 5 ml of glutamine, and 5 ml of Anti-Anti. Before passaging NSCs, add FGF-2 and EGF to N2 medium to make them 20 ng/ml working medium. Store at 4 °C up to 1 month.
Diluted Beta-Mercaptoethanol: add 34.7 µl of Beta-Mercaptoethanol into 10 ml of sterile H2O. Make fresh dilution for each cell isolation.
Enzyme Mix #1: For each isolation, mix 2 ml “MACS® Solution 2” and 50 µl “MACS® Solution 1” from MACS® Neural Tissue Dissociation kit with 2.5 µl diluted BME. Double this amount for isolations from two mice.
Enzyme Mix #2: For each isolation, mix 20 µl “MACS® Solution 3” and 10 µl “MACS® Solution 4” from MACS® Neural Tissue Dissociation kit. Double this amount for isolation from two mice.
Percoll solution (only needed for isolation from more than one animal; see below): To 22.5 ml Percoll, add 2.5 ml 10XDPBS. Store at 4 °C for up to 1 month.
Trypsin inhibitor solution: store at −20 °C as a 5-ml aliquot of 2.5 mg/ml in DPBS. For working solution, dilute stock solution to 0.25 mg/ml concentration in DPBS. Store at 4 °C up to 6 months.
Retinoic acid: Dissolve in DMSO and store as 0.5-ml aliquot at 1 mM concentration at −20 °C.
Forskolin: Dissolve in DMSO and store as 0.5-ml aliquot at 1 mM concentration at −20 °C.
Differentiation medium: N2 medium with a final concentration of 1 µM retinoic acid and 1 µM forskolin. CRITICAL Make fresh every time.
Cryoprotectant medium: N2 medium with 10% of DMSO. CRITICAL Make fresh every time.
Poly-L-ornithine solution: Dissolve in sterile Milli-Q H2O and store as 0.5-ml aliquot at 10 mg/ml at −20 °C. For working solutions, dilute the store solution into sterile Milli-Q H2O to make a final concentration of 10 µg/ml. CRITICAL Make working solution fresh every time.
Laminin solution: Dilute laminin in sterile PBS to make a final concentration of 5 µg/ml.CRITICAL Make fresh every time.
Procedure
Microdissection of dentate gyrus and subventricular zone from mouse brain (Timing: 40–60 minutes)
Euthanize a mouse (8–12 weeks old) using a protocol approved by your Institutional Animal Care and Use Committee. Mice used here were deeply anesthetized by an intraperitoneal injection of either 230 mg/kg Sodium Pentobarbital or 400mg/kg Avertin, followed by euthanasia through decapitation..….
Using large scissors cut off the head just above the cervical spinal cord region. Then, using small pointed scissors, make a medial caudal-rostral cut and remove the skin of the head.
Using small scissors make a longitudinal incision at the base of the skull and continue cutting along the sagittal suture. Then, using pointed forceps grasp the skull of one hemisphere at the bottom of the incision and peel it outward to expose the brain. Repeat with the other hemisphere.
-
Turn the head of the animal upside-down and, while cutting the optic nerves, allow the brain to slip into a 50-ml Falcon tube containing 20 ml of cold HBSS.
Critical step: Keep brain in cold Solution A on ice during subsequent dissection.
-
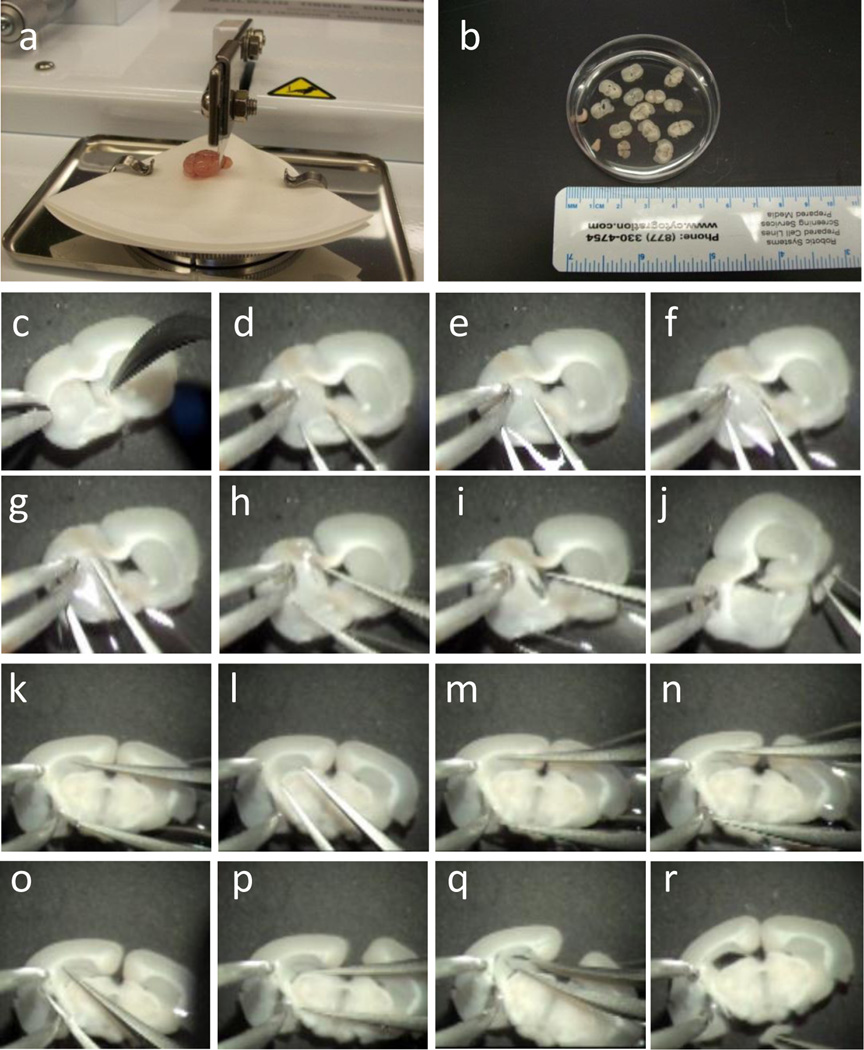
Transfer the brain from the 50-ml tube to a 10-cm petri dish containing 20 ml of cold Solution A. Place a small piece of filter paper onto Tissue Chopper, slightly wet the filter paper using a wet sterile swab, and then set the brain onto the wet filter paper using curved-pointed forceps (Figure 2a). Chop brain into 400-um coronal sections using the McIIwain Tissue Chopper and use wet sterile swab to collect the sections containing SVZ (~6 sections) and hippocampus (~5 sections) into a 6-cm petri dish filled with 5 ml of Solution A (Figure 2b).
Critical step: Keep the petri dish containing brain sections and solution A on ice during the chopping and dissection period.
? Troubleshooting
-
Dissect out the SVZ (Figure 2c–j; Figure 3a–c; Supplementary Video1) and the DG (Figure 2k–r; Figure 3d–f; Supplementary Video 2) under a dissecting microscope and keep the dissected tissue in separate 15-ml Falcon tubes, each containing 10 ml of cold Solution A.
Critical step: This and subsequent steps are optimized for isolating NSCs from the SVZ and DG preparation of one mouse. If you plan to isolate cells from pooled tissues of more than one mouse, we recommend that you add the step of Percoll purification to remove excess myelin and other cell types (see step 12).
? Troubleshooting
Figure 2.
Microdissection of the SVZ and the DG of a single adult mouse. (a) Photograph of one adult mouse brain placed on the platform on the tissue chopper. Note that the orientation of the brain and the position of the blade are specific for generating coronal brain slices. (b) A photograph of 400-µm thick brain slices in Solution A. (c–j) Images demonstrating the dissection of the left SVZ from one of the thick brain sections (also see Supplementary Video 1). The experimenter held the brain section containing the SVZ still using the left forceps (c), and then used the right forceps to make a ventral-to-dorsal cut between the left striatum and the left ventricle (d–g). (h) Another cut was then made at the dorsal SVZ along the corpus callosum. (i) The left SVZ tissue is now separated from the rest of the brain section. (j) The isolated SVZ tissue held by the right forceps and the brain section with the left SVZ tissue removed. (k–r) Images showing the dissection of the left DG tissue from one of the thick brain sections (also see Supplementary Video 2). The experimenter held the brain section containing the DG with the left forceps (k), and then used the right forceps to cut between the DG and the CA1 region (l–n), followed by a vertical cut between the DG and the CA3 region (o). (q) A third cut was then made to separate the DG from striatum, avoiding the inclusion of any ventricular zone tissue (p). (r) The view of the isolated left DG held by the right forceps and the brain section with the left DG tissue removed. Same procedures were carried out for isolating the right SVZ and the right DG tissues from the same animal. The above procedures have been approved the Institutional Animal Care and Use Committee at University of Wisconsin-Madison
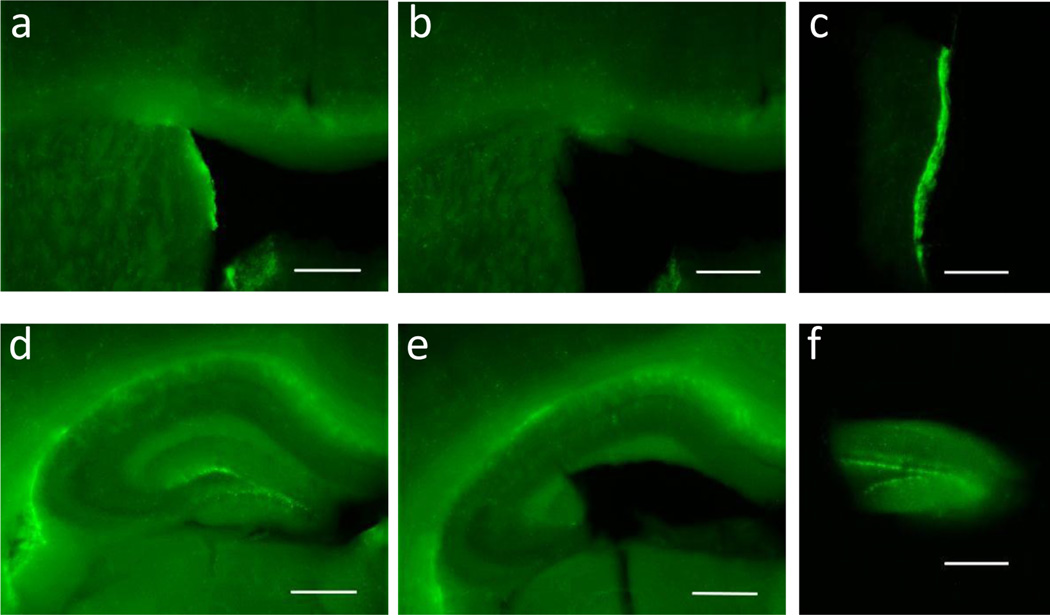
Figure 3.
Microdissection of the SVZ and the DG from the brain of an adult Nestin-GFP transgenic mouse. (a) Coronal 400-µm section of the brain containing the SVZ before dissection. Note the bright GFP+ cells in the SVZ. (b, c) The same brain section as in (a) but after dissection of SVZ. Note the absence of the bright GFP+ cells in the SVZ and the intact morphology of surrounding regions, including the corpus callosum. (d) Coronal 400-µm section of the brain containing the hippocampus and the DG. (e, f) The DG was isolated from the rest of the tissue by microdissection, and the tissues containing the DG have bright GFP+ cells. Scale bar = 500 µm. The above procedure has been approved the Institutional Animal Care and Use Committee at University of Wisconsin-Madison.
Isolation of neural progenitor cells (Timing: 1–2 hours)
-
7.
After finishing the dissection of all the brain sections, spin down the tissue chunks in a low-speed centrifuge at 200 g for 1 min at room temperature (20–25 °C).
-
8.
In the TC hood remove the supernatant and add 2 ml of Enzyme Mix 1 to each 15-ml conical tubes containing tissue chunks. Place the conical tubes onto the MACSmix Tube Rotator and rotate at room temperature for 20 min.
Caution: Do not incubate longer than 30 min, as this will decrease the viability of the cells.
-
9.
Add 30 µl of Enzyme Mix 2 into each tube and rotate for another 20 min.
Caution: Do not incubate longer than 30 min, as this will reduce the viability of the cells.
? Troubleshooting
-
10.
Pre-wet a fire-polished glass pipette to triturate tissue by pipetting up and down 10–20 times until there is no tissue clump, and then add 8 ml of N2 medium to each tube to dilute the Enzyme Mix. Pellet the cells at 200 g for 5 min at room temperature.
Caution: Avoid generating air bubbles when triturating the tissue, as this will reduce the viability of the cells.
Critical step: It is important to dissociate the SVZ and the DG to single cells, as any remaining aggregates can result in reduced yield.
? Troubleshooting
-
11.
Wash cells twice with 10 ml of N2 Medium and spin down at 200 g for 5 min at room temperature each time.
-
12.If you are isolating cells from pooled tissues of 2 or more mice, re-suspend each cell pellet with 5.5 ml of N2 medium, add 5.5 ml of Percoll/PBS solution, and mix by inverting the tubes. Pellet the cells at 400 g for 15 min at room temperature. Then wash the cells 3 times with 10 ml N2 medium and spin down at 200 g for 5 min at room temperature each time to collect cells.Critical step: Gently remove the supernatant, as the pellet may not be firmly attached to the bottom of the tube.Critical step: It is critical to remove Percoll by washing thoroughly, as residual Percoll will affect cell viability.
-
13.
Wash one more time with 8 ml of Initial Proliferation Medium (IPM).
-
14.
Re-suspend each cell pellet with 1 ml of IPM for DG, 2 ml of IPM for SVZ, and then plate cells into one well of a 24-well tissue culture plate for DG, and 2 wells for SVZ cells. Incubate cells in the CO2 incubator for 48 h.
? Troubleshooting
-
15.
Change half the IPM, avoiding removing any cells. Continue to incubate cells for 7–14 days, changing half the IPM every other day and monitoring the cells for the formation of neural spheres. Neural spheres should form in both cultures in 1–2 weeks (Figure 4).
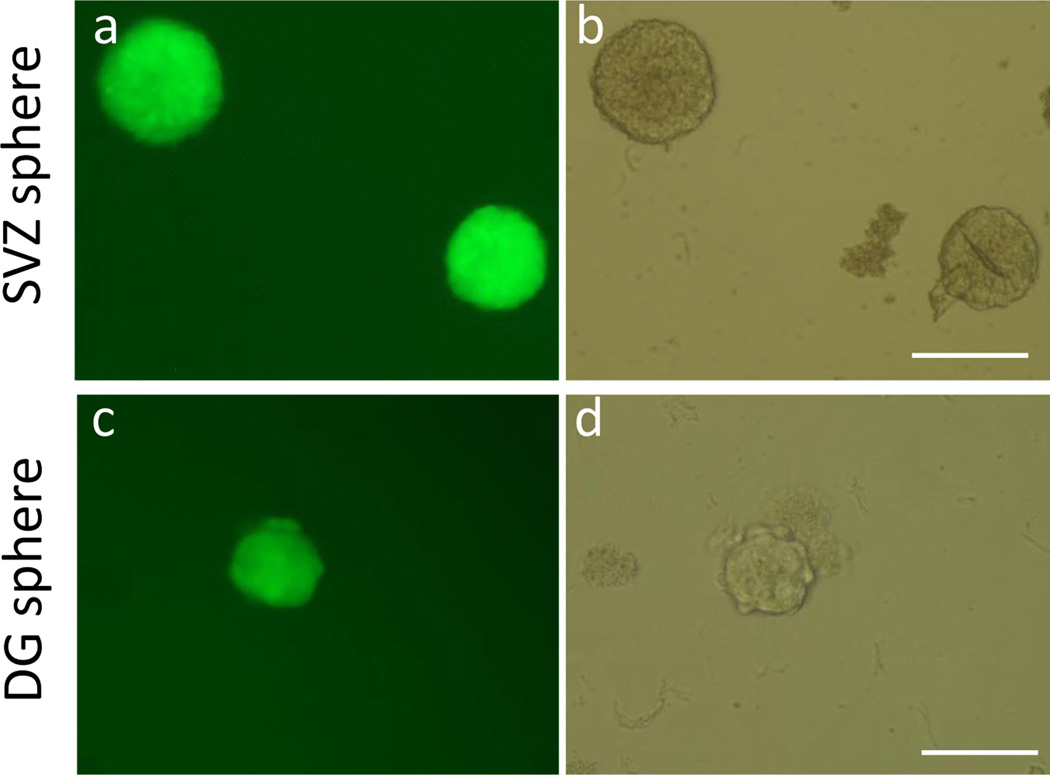
Figure 4.
Representative images of neurospheres. (a) A fluorescent image and (b) a phase contrast image of SVZ neurospheres appeared at 7 days after initial plating (passage 0). (c) A fluorescent image and (d) a phase contrast image of a DG neurosphere appeared at 14 days after first plating (passage 0). Scale bar = 200 µm.
Passage of neural progenitor cells (20–30 min)
-
16.
After the 7–14 days of culture, collect all primary spheres without disturbing the attached cells; spin at 200 g for 5 min at room temperature.
-
17.
Carefully remove the medium and add 1 ml of 0.025% trypsin/EDTA to each tube. Dissociate the spheres using a pre-wet 1-ml blue tip by pipetting up and down 20 times to digest the spheres within 2 min. Add 1 ml of trypsin inhibitor to each tube inactivate trypsin and pipette up and down 10 more times. Add 5 ml of IPM to each tube, mix by inverting the tubes a few times, and pellet the cells at 200 g for 5 min at room temperature.
-
18.
Re-suspend the DG cell pellet in 1 ml of IPM and the SVZ cell pellet in 2 ml of N2 medium. Dilute a 10-µl aliquot from each sample in 10 µl of 0.5% Trypan blue and count viable cells using a hemocytometer. Adjust cell density until cells are countable on a hemocytometer.
-
19.
Plate the DG cells into one well of a 24-well plate, and the SVZ cells into one well of 6-well plate and incubate cells.
-
20.
Replace half the medium with fresh IPM for the DG cells and fresh N2 medium for the SVZ cells every other day for 1 week. Avoid taking any cells out when changing out half the medium. After the first passage, maintain the DG and the SVZ NSCs in N2 medium and passage NSCs by seeding at 3*104-1*105 cells/ml every 2–3 days. In our lab, we have passaged NSCs derived from both the DG and the SVZ for extended periods (>20 passages). However, to avoid potential effects of prolonged in vitro culture, we use early passage (<p10) cells for NSC proliferation and differentiation analysis9,14–16.
-
21.
Proliferating NSCs can be differentiated down to neuronal and glial lineages to determine their multipotency (option A) or cyopreserved for future analysis (option B).
Option A) Differentiation of neural progenitor cells (6 days)
-
Add poly-L-ornithine solution to the plates you plan to differentiate the cells on 2 days prior to starting differentiation, and then rock and shake the plates to be sure the entire surface is covered by liquid. Incubate the plates overnight at room temperature.
Critical step: From this point on, the plates/slides must not be allowed to dry out.
-
Remove the majority of the poly-L-ornithine solution from the plates and wash the plates 3 times with sterile Milli-Q H2O.
? Troubleshooting
-
Add laminin solution to the plates and incubate the plates overnight in the 37 °C tissue culture incubator.
? Troubleshooting
Collect NSCs from step 20 in a 15-ml tube and spin at 200 g for 5 min at room temperature.
Carefully take out the medium and add 1 ml of 0.025% trypsin/EDTA. Dissociate the neurospheres using a pre-wet 1-ml tip by pipetting up and down about 20 times within 1 min to digest the spheres. Add 1 ml of trypsin inhibitor to inactivate trypsin and pipette up and down 10 more times. Add 5 ml of N2 medium, mix by inverting the tubes a few times, and pellet the cells at 200 g for 5 min at room temperature.
Re-suspend cell pellet in 1 ml of N2 medium. Count viable cells by Trypan blue exclusion and seed the cells in the coated plates at a cell density of 1*105 cells/ml. Incubate the plates overnight in a 37 °C tissue culture incubator.
Replace half the N2 medium with differentiation medium. Continue to incubate cells, replacing the medium every day for 4 days. Differentiated cells can then be collected by either direct cell lysis for molecular biochemical analysis or by fixation using 4% paraformaldehyde for histological analysis using cell lineage-specific antibodies as published9,14–16.
Option B) Cryopreservation of neurospheres
Collect spheres in a 15-ml tube from a 10-cm dish and spin at 200 g for 5 min at room temperature.
Carefully take out the medium. Re-suspend the pellet in 2 ml of freezing medium using a 1-ml pipette to gently disaggregate the pellet, and then add 1 ml to a 2-ml cryovial.
-
Transfer vials into a −80 °C freezer overnight and then transfer vials into a liquid nitrogen tank for long-term storage.
PAUSEPOINT Cells can be stored in liquid nitrogen for more than one year. XXX.
Thawing of neurospheres
-
iv)
Carefully take the frozen vial from a liquid nitrogen tank and put into a 37 °C water bath with gentle shaking until the vial is completely thawed.
? Troubleshooting
-
v)
Add the thawed cells into a 15-ml tube with pre-warm N2 medium and spin at 200 g for 5 min at room temperature.
-
vi)
Carefully take out the medium. Re-suspend the pellet with 10 ml of pre-warm N2 medium containing FGF-2 and EGF, and then seed the cells in a 10-cm dish. Incubate the cells in a 37 °C tissue culture incubator and continue to passage as described in step 20.
TROUBLESHOOTING
See Table 1 for troubleshooting guidance.
TABLE 1.
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 5–6 | Too few cells are recovered | Loss of brain tissue during chopping of brain | Slightly wet filter paper and wet the blade in each chopping |
| Insufficient mechanical dissociation | Enhance dissection skills by speeding up the dissection procedure and obtaining dissected tissue that only contains SVZ and DG | ||
| 9–10 | Low cell yield | Digestion may be incomplete | Digest longer in Enzyme Mix 1 and 2 up to 30 min |
| Pasteur pipette is not fire-polished well | Improve fire-polishing of the Pasteur pipette | ||
| Cells stick to the Pasteur pipette | Pre-wet the Pasteur pipette before trituration | ||
| 13 | Excess of debris in the culture | Too much striatal tissue containing dead cells and myelin | Enhance dissection accuracy and try to cut the SVZ and DG tissue thinly; look at an aliquot under the microscope for the absence of aggregates after trituration |
| 20 | Poor cell adherence | Non-optimal coating | Prepare fresh coating solution |
| 21-B) | Too few cells are recovered | Insufficient thawing | Try to freeze cells in higher concentration. Thaw cells quickly to avoid damage from DMSO |
TIMING
Steps 1–6, Dissection of the SVZ and the DG: 40 min to 1 h
Steps 7–13, Isolation of NSCs: 1–2 h
Step 14, Culturing NSCs: 7–14 d
Steps 15–20, Maintenance of NSCs: as needed for experiments
Steps 21-A), Differentiation of NSCs: 6 days
Steps 21-B), Cryopreservation of neurospheres: 20 min
Steps 21-B), Thawing of NSCs: 15 min
ANTICIPATED RESULTS
This protocol is optimized for the isolation of self-renewing and multipotent NSCs from two adult neurogenic regions, the SVZ and the DG, of a single adult mouse. If the protocol is carried out properly, the SVZ neurospheres should appear in about one week and the DG neurospheres in about two weeks post-isolation. If no sphere appears at the expected time, then the isolation may have failed, and a longer culture time will not help based on our experience. Please refer to Troubleshooting for methods of improvement. Figure 4 shows images of typical SVZ neurospheres (498 ± 15.2 µm diameter) at seven days post-initial plating and DG neurospheres (202 ± 10.7 µm) at 14 days post-initial plating. SVZ neurospheres are normally bigger and also need to be passaged sooner than DG neurospheres.
The NSCs isolated from both regions are positive for several well-characterized NSC markers, such as Nestin and Sox2, and can proliferate, as demonstrated using a BrdU incorporation assay (Figure S1a–d). Quantitative analyses published from our lab have shown that 99.8% of DG and SVZ NSCs isolated using this protocol are Nestin-positive, 88.2% are Sox2-positive, and all Sox2-positive cells are Nestin-positive9. In addition, during 16 hours of BrdU pulse labeling, 61.6 % of SVZ NSCs and 48.5% of DG NSCs incorporate BrdU9.
NSCs isolated from both regions are multipotent and can differentiate into both neurons and glia under differentiation conditions (i.e., growth factor withdrawal and the addition of retinoic acid and/or forskolin). As shown in the examples for DG (Figure S1e–f), the lineages of differentiated cells were identified using immunocytochemistry, with neurons detected by Tuj1 and astrocytes by GFAP. Quantitative analyses published from our lab have shown that, in a typical differentiation experiment, SVZ NSCs yield 10.5 % Tuj1-positive neurons and 12.0% GFAP-positive astrocytes, while DG NSCs yielded 12.0 % Tuj1-positive neurons and 19.0% GFAP-positive astrocytes9. Although the percentage of neuronal and astrocyte differentiation is limited, it has proven to be a faithful model for studying molecular mechanisms regulating neurogenesis in the adult brain, as shown in our publications9,14–16.
If maintained as suggested, NSCs derived from both the DG and the SVZ can be passaged for extended periods (>20 passages) without a loss of NSC characteristics; however, extended passaging would increase chromosome aberration and reduce the capacity to differentiate into neurons. Adult NSCs isolated using this method can be used for self-renewal analysis starting at passage 0 and cell fate analysis starting at passage 1. They can also be expanded for gene expression and biochemical analyses.
Since this protocol is simple and reproducible, we expect that many laboratories will be able to easily adapt it. Because it uses only one adult mouse for each cell preparation it significantly reduces the number of animals needed per assay and is therefore extremely useful to neuroscientists using complex transgenic lines. One of the most interesting applications of this protocol is the ability to conduct a direct comparison between DG and SVZ NSCs, which should help understand the differential regulation of DG and SVZ neurogenesis in the same animals, a largely unexplored research area. We anticipate that our easy-to-adapt protocol will promote this type of study and move this important field forward.
Despite this, we should note that in vitro culture will modify the properties of isolated cells. While EGF and FGF-2 have been used for long-term culture of NSCs without changing the self-renewal and multipotent properties of NSCs, a recent study shows that treatment of NSCs with EGF and FGF-2 leads to alterations in cell cycle length and mode of cell division18. Slow division is an important hallmark of NSCs in vivo. NSCs in the adult DG are found to reenter the cell cycle after two to three weeks of first cell division, while immediate progenitors are fast-dividing cells19. The culture of NSCs as neurospheres with EGF and FGF-2 accelerates cell division, but comes at the cost of the above-mentioned alteration of NSC biology in vivo. Even though nearly all isolated NSCs express Nestin, these isolated cells are likely a mixture of different types of fast-dividing progenitors, with very few, if any, slow-dividing NSCs. Furthermore, many types of immediate progenitors exist in the DG, and isolated cells are a mixture of different subtypes; one should be cautious using these cells for NSC analysis at the single-cell level.
Supplementary Material
Acknowledgments
We thank C.T. Strauss for editing the manuscript, and Y. Xing, E. Polich, W. Gilbert, and I. J. Bhuiyan for technical support. This work was supported by grants from the NIH to X.Z. (RO1MH080434 and RO1MH078972), from the International Rett Syndrome Foundation (IRSF) to X.Z., a center grant from the NIH to the Waisman Center (P30HD03352). W.G. is funded by a postdoctoral fellowship from University of Wisconsin Center for Stem Cells and Regenerative medicine. E.M.J. is funded by a NIH Molecular Biosciences Training Grant (MBTG: T32 GM07215), and N.E.P. is funded by a NIH Molecular and Cellular Pharmacology Training Grant (T32 GM008688).
Footnotes
Author Contributions
W.G. and X.Z developed the methodology. W.G., N.P., and E.J. followed the protocol to isolate cells from Nestin-GFP mouse. W.G and X.Z. wrote the protocol.
Competing Financial Interests
The authors declare that they have no competing financial interests
Supplementary Information
Video S1: Dissection of the SVZ
Video S2: Dissection of the DG
Figure S1 DG NSCs isolated using this protocol exhibit basic neural stem cell properties in vitro.
(a–d) Passage 8 DG neurospheres were dissociated to single cells and placed onto a poly-L-ornithine- and laminin-coated coverslip in N2 medium containing FGF2 and EGF (see protocol. At 18 hours post-plating, bromodeoxyuridine (BrdU) was added to the cells for 16 hours, followed by fixation using 4% parafaldehyde and subjected to histological analysis. The majority of DG NSCs expressed both neural stem cell markers Nestin (a, green) and Sox2 (b, red) and incorporated BrdU (c, white) and nuclear staining (d, blue). e, The merged view of a, b, c, and d.
(f, g) Passage 8 DG neurospheres were dissociated to single cells and placed onto a poly-L-ornithine- and laminin-coated coverslip in cell proliferation medium (see protocol. At 18 hours post-plating, medium was changed into differentiation medium (see protocol) and differentiated for 4 days. Cells were then fixed using 4% paraldehyde and subjected to histological analysis. DG NSCs differentiated into neurons and astrocytes as demonstrated by immunostaining cells using the astrocytic marker GFAP (f, green) and the neuronal marker Tuj1 (g, red). Scale bars = 50 µm. The detailed methods can be found in our published papers9,14–16.
Primary antibodies used here are:
Rat anti BrdU (1:1000, Abcam, ab6326)
Chicken anti Nestin (1:1000, Aves Lab, #mNES)
Mouse anti Sox2 (MAB4343, 1:1000, Millipore)
Rabbit anti GFAP (1:1000, Dako, Z0334)
Mouse anti Tuj1 (1:1000, Progega, G712A)
Secondary antibodies used here are:
Goat anti Chicken 488 (1:500, Invitrogen, A11039)
Goat anti Rat 568 (1:500, Invitrogen, A11077)
Goat anti Mouse 647 (1:500, Invitrogen, A21235)
Goat anti Rabbit 488 (1:500, Invitrogen, A11008)
Goat anti Mouse 568 (1:500, Invitrogen, A11004)
References
- 1.Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta Stone. Neuron. 2011;70:597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 4.Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods in enzymology. 2006;419:3–23. doi: 10.1016/S0076-6879(06)19001-1. [DOI] [PubMed] [Google Scholar]
- 5.Theus MH, Ricard J, Liebl DJ. Reproducible expansion and characterization of mouse neural stem/progenitor cells in adherent cultures derived from the adult subventricular zone. Chapter 2, Unit 2D 8. Current protocols in stem cell biology. 2012 doi: 10.1002/9780470151808.sc02d08s20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortega F, et al. Using an adherent cell culture of the mouse subependymal zone to study the behavior of adult neural stem cells on a single-cell level. Nature protocols. 2011;6:1847–1859. doi: 10.1038/nprot.2011.404. [DOI] [PubMed] [Google Scholar]
- 7.Sun T, et al. A comparison of proliferative capacity and passaging potential between neural stem and progenitor cells in adherent and neurosphere cultures. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2011;29:723–731. doi: 10.1016/j.ijdevneu.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Giachino C, Basak O, Taylor V. Isolation and manipulation of mammalian neural stem cells in vitro. Methods Mol Biol. 2009;482:143–158. doi: 10.1007/978-1-59745-060-7_9. [DOI] [PubMed] [Google Scholar]
- 9.Guo W, et al. RNA-binding protein FXR2 regulates adult hippocampal neurogenesis by reducing Noggin expression. Neuron. 2011;70:924–938. doi: 10.1016/j.neuron.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Molecular and cellular neurosciences. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 11.Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babu H, Cheung G, Kettenmann H, Palmer TD, Kempermann G. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS One. 2007;2:e388. doi: 10.1371/journal.pone.0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo W, et al. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nature medicine. 2011;17:559–565. doi: 10.1038/nm.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, et al. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y, et al. Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6:e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- 18.Costa MR, et al. Continuous live imaging of adult neural stem cell division and lineage progression in vitro. Development. 2011;138:1057–1068. doi: 10.1242/dev.061663. [DOI] [PubMed] [Google Scholar]
- 19.Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Developmental neuroscience. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.