Abstract
Their unique patterns of size, numbers, and stratification indicate that amacrine cells have diverse functions. These are mostly unknown, as studies using imaging and electrophysiological methods have only recently begun. However, some of the events that occur within the amacrine cell population—and some important unresolved puzzles—can be stated purely from structural reasoning.
Keywords: Retina, Neuron, Stratification, Ganglion, Types, Review
Introduction
When the extreme diversity of amacrine cells was first learned, the often asked question was: why is it necessary to have so many types of amacrine cells? During the subsequent decade, answers for a few types of amacrines have been revealed. Among other things, the coding properties of retinal ganglion cells turn out to be subtler than had been imagined, and in some cases, this can be traced to particular types of amacrine cells (reviews: Gollisch & Meister, 2010; Masland, 2011; Werblin, 2011).
The more general answer, though, is that there are lots of types of amacrine cell because there are lots of types of ganglion cell. In round numbers, if there were 15 different functional types of ganglion cell and 30 types of amacrine cell, this would be a ratio of 2 amacrine cells per ganglion cell type—not a surprising number for a dedicated microcircuit of the central nervous system. We will return to these numbers at the end. I will start by discussing some generalizations about amacrine function, focusing in particular on functional principles that can be derived from the shapes, numbers, and sizes of the various amacrine cell populations. The aim here was to consider amacrine cells as a total population, and I will rely on the surveys by MacNeil and her colleagues (MacNeil & Masland, 1998; MacNeil et al., 1999), which combined photofilling of amacrine cells, as a sampling method, with three-dimensional imaging of cells previously Golgi stained by Elio Raviola and John Heussy. This was done for the retina of the rabbit and has limits that have been discussed elsewhere (Masland, 2011), but until a next generation survey is available (Briggman & Denk, 2006; Anderson et al., 2011; Helmstaedter et al., 2011), it remains a useful starting point.
Some unsolved questions of amacrine cell function
In this discussion, I will focus on the contributions of amacrine cells to the receptive fields of ganglion cells, because the relative dimensions of the amacrine and ganglion cells define inescapable features of their interactions. For communications between amacrine cells and bipolar cells or other amacrine cells, such guidance is not available and fewer generalizations are possible. Some individual cases are discussed in detail elsewhere (Demb & Singer, 2012; Taylor, 2012).
For convenience, amacrine cells will be divided into wide field, medium field, and narrow field. In the rabbit retina, there is a tight cluster of cells with dendritic fields at 172 ± 5 μm diameter (Fig. 1) and a distinctly different group with processes in excess of 1 mm. It would not be surprising if these turn out to be no more than easily noticed clusters on a continuum of dendritic field diameter, but the division by size is useful because dendritic field spread is an important determinant of a cell’s function in visual processing.
Fig. 1.
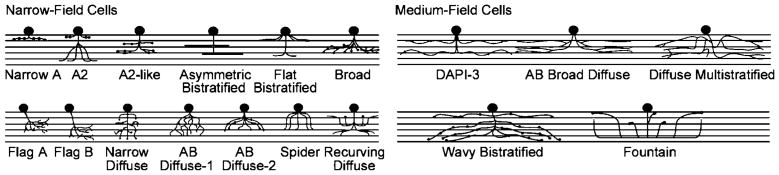
Narrow- and medium-field amacrine cells. The icons represent the levels of stratification of the different amacrine cell types within the IPL and roughly show their spread. Details of the dendrites are represented only when they are a particularly striking feature of the type. For a sense of scale, note that the dendritic field diameters of all of the medium-field cells are near 175 μm. Adapted from Masland (2001).
Almost all amacrine cells communicate among several strata of the inner plexiform layer
The starburst cell, one of the most famous amacrines, is narrowly stratified, but this is not the case for the majority of amacrine cells. Communication among levels is most striking for narrow-field amacrines, of which only 2 of the 13 narrow-field types found by MacNeil et al. (1999) were restricted to branching in narrow strata. It has been termed “vertical integration,” to contrast with lateral integration, as in center–surround antagonism. More specific formulations have recently been proposed. For example, crossover inhibition has been proposed as a linearizing mechanism for ganglion cell responses (Molnar et al., 2009; Werblin, 2010).
An interesting twist on the multistratification of narrow-field amacrine cells is that gap junctions in principle allow them to be excitatory in one place and inhibitory in another. Most if not all amacrine cells release GABA or glycine, inhibitory neurotransmitters. However, many amacrine cells are coupled by gap junctions to retinal ganglion cells or other amacrines. If these gap junctions conduct enough charge to affect neighboring neurons, they would allow the amacrine cell to be excitatory at some contacts and inhibitory at others, since a gap junction is a sign-conserving synapse. This clearly happens for amacrine AII, which has large conventional gap junctions. The role of the small contacts that allow neurobiotin flow(down an infinite concentration gradient) but may not allow much current flow remains to be learned. It must be remembered that the receptive fields of most ganglion cells have about the same spatial extent as their dendritic field, (Peichl & Wässle, 1983; Yang & Masland, 1994) despite the cell being dye coupled to a wide spatial extent of other retinal neurons; this suggests that the effect of the gap junctions is a subtle one, as in the creation of cross correlations between ganglion cell firings. Since some of these junctions are between cells of differing functional types, however, this is clearly not the whole story (see Vaney, 2002).
Less widely appreciated than for narrow-field amacrine cells, another source of vertical communication is for medium-field cells—in the rabbit, the group of five amacrine cell types with dendritic spread around 175 μm(Fig. 1). An example is the fountain cell (MacNeil et al., 1999; Wright & Vaney, 2000). The medium-field cells are problematic in other ways as well (see below), and it is hard to imagine what a cell of this degree of spread is doing in its vertical communication. Even A17, a wide-field cell that plainly does most of its work at feedback synapses with the rod bipolar terminal, receives noticeable amacrine cell input where its dendrites slope across the inner plexiform layer (IPL) to reach the layer 4/5 boundary. The feedback synapse between A17 and the rod bipolar terminal appears to be functionally isolated from the rest of the cell; its geometry would be well suited to such isolation, as the fat swelling where the synapses reside is separated from the main trunk of the amacrine cell by a hyper-thin (0.2 μm) stalk, and there is now direct physiological evidence that this is the case (Ellias & Stevens, 1980; Sandell et al., 1989; Grimes et al., 2010). If so, what is the purpose of the inputs that occur not on the layer 5 swellings but directly upon the more proximal dendrites, which stretch vertically across all strata of the IPL? One possibility is that these control the gain of the whole cell, allowing for simultaneous modulation of all the local feedback synapses at once.
Finally, and unexpectedly, some of the other wide-field amacrine cells communicate between layers (Fig. 2). At one time, we and others believed that wide-field cells were invariably monostratified. However, more recent studies in the mouse, where the IPL is thick and genetically marked cells are available, show that this not always the case. Good examples exist among the polyaxonal cells, initially described in the rabbit and monkey (Vaney et al., 1988; Dacey, 1989; Famiglietti, 1992a,b,c) and present in abundance in the mouse (Badea & Nathans, 2004; Lin & Masland, 2006). The polyaxonal cells have separate axonal and dendritic arbors. Often the dendritic arbor is narrower than the axonal one, so that they receive input in a relatively narrow central zone but radiate output for wider distances across the retina (Völgyi et al., 2001). In general, the dendritic arbor is narrowly stratified and the axonal arbor is also narrowly stratified. What this means is that the cells receive input from a very specific functional type (at most a few types) of bipolar cell and radiate their output to a very specific type (at most a few types) of retinal ganglion or amacrine cell (Fig. 2B). In those cases where both arbors lie within the same stratum, the interactions mediated by the wide-field cell should occur between functionally related neurons since functionally similar neurons participate in each IPL stratum. However, in those cases where the cells are multistratified, more complex connectivity is indicated (Fig. 3). A hypothetical example: a polyaxonal cell might receive synaptic input only from an OFF-transient bipolar cell and send its outputs specifically to the local edge detecting type of ganglion cell. Since the amacrine cell is believed to be GABAergic, this would create a very wide OFF-transient inhibitory field, but only for that specific type of ganglion cell.
Fig. 2.
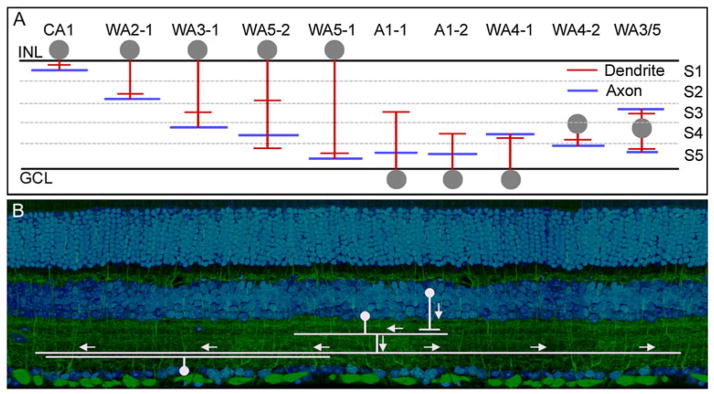
(A) Polyaxonal amacrine cells of the mouse retina. Dendrites are represented in red and axons in blue. Note that these cells are present in most layers of the IPL, and that some have axons and dendrites in different layers. It is not known whether this is an exhaustive list or whether more polyaxonal cells remain to be discovered. These cells were first well described in the retina of the rabbit (Vaney et al., 1988; Famiglietti, 1992a,b,c). Lin and Masland (2006) surveyed GFP-M retinas (Feng et al., 2000) for the presence of long-range amacrine cells. Badea and Nathans (2004) and Gustincich et al. (1997) used different genetically based strategies and their results are included here. No standard nomenclature exists; for details, see the original publications. The lateral spread of the processes is not represented, except to indicate that the spread of the dendritic arbor in these cells is narrower than the spread of the axonal arbor. (B) Polyaxonal cells transmit information from one or a few types of bipolar and amacrine cells to specific other groups of cells, here illustrated for one type of bipolar cell and one type of ganglion cell. This is shown by both the laminar structure of the polyaxonal cells, and the fact that their physiological effects are restricted to subsets of ganglion cells (Olveczky et al., 2003; Roska & Werblin, 2003).
Fig. 3.
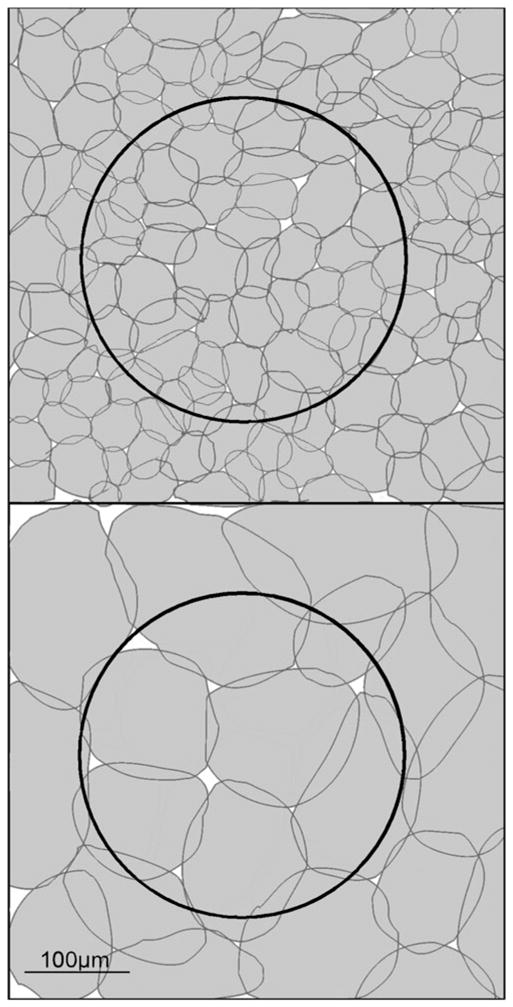
The relative sizes of amacrine cells and ganglion cells. This is a whole-mount view, with the boundaries of the dendritic fields outlined. A ganglion cell of roughly average dendritic field diameter (250 μm) for the rabbit is shown by the large dark outline. A mosaic of narrow-field (left) or wide-field (right) amacrine cells is shown in white. Many narrow-field amacrine cells are present within the dendritic field of this ganglion cell; they must create some sort of a functional subunit within the receptive field, but this functionality has been little explored. In contrast, medium-field amacrine cells have a confusing overlap with the dendritic field of the ganglion cell; it is not obvious how the activities of these two cells can be spatially integrated.
Amacrine cells create functional subunits within the receptive fields of many ganglion cells
The term “local subunit” was coined by Barlow and Levick (1965) to capture the experimental finding that the ON–OFF directionally selective (DS) ganglion cell of the rabbit retains its ability to detect the direction of movement for stimuli much smaller than the whole extent of the receptive field. In their description, the DS cell can detect a 50-μm movement of a 50-μm diameter spot, no matter where the spot lies within the much wider receptive field. This is now known to be mediated by the overlapping sectors of the starburst amacrine cell and is the reason for the apparent redundancy of that cell’s coverage of the retina (Masland et al., 1984; Vaney, 1991; Euler et al., 2002; Fried et al., 2005).
But local subunits exist within the receptive fields of other types of ganglion cell, the best studied being the Y cell of the cat and the parasol cell of the monkey. For these cells, the classic evidence is (a) their sensitivity to small stimuli moved within the receptive field and (b) the fact that they can be driven by counterphased gratings finer than the overall size of the receptive field (Soodak et al., 1991). The physiology of many other types of medium- and large-field ganglion cells remains unexplored, but physical geometry alone shows that such subunits must exist for many of them as well. This is illustrated, for a generic medium-sized ganglion cell of the rabbit, in Fig. 3. What these subunits contribute to visual signaling is entirely unknown.
New contextual effects remain to be discovered
A variety of contextual effects have been described—contextual here defined as effects on the behavior of a ganglion cell from outside the boundaries of its classical receptive field. Simple long-range interactions were described long ago (Levick et al., 1965), and modern studies describe functions of increasing subtlety. A prototype is the segregation of relative image movement from movement of the whole visual input, apparently a way to distinguish real movement of an object from the whole image movement caused by the eye’s resting tremor (Chiao & Masland, 2003; Olveczky et al., 2003). A similar mechanism appears to cause saccadic suppression—it depresses the firing of some ganglion cells during rapid shifts of the whole visual image (Roska & Werblin, 2003).
Saccadic suppression was seen for some types of ganglion cells and not others, but for most of the contextual effects the definition of which types of ganglion cells participate is incomplete at best. It was noted, for example, that selectivity for object motion may be a special feature of a few of the parallel pathways that convey retinal output to the brain, but the identities of these pathways were not learned (Olveczky et al., 2003). The primary reason is that there is no agreed upon classification of the physiological types of retinal ganglion cell nor is it possible to distinguish many of the types using checkerboard stimulation. The principles of IPL organization indicate that very specific types of cell are probably involved—this is inevitable given the specificity of lamination of wide-field amacrines described above and the plethora of wide-field amacrine cells. It seems quite possible that all ganglion cells participate in long-range, contextual, interactions—even that individual ganglion cells participate in more than one such interaction. The long-range interactions discovered thus far were found in response to test conditions that are intuitively easy to grasp—saccadic suppression has been known for a long time from perceptual experiments. The plethora of wide-field amacrines suggests that other, less obvious and more cell-type specific, interactions may exist.
Medium-field amacrine cells pose a particular conceptual difficulty
The puzzle derives from comparing the size of their dendritic fields, on average ~175 μm in the rabbit, with the size of the receptive fields of the retinal ganglion cells (Rockhill et al., 2002). What function could these amacrine cells serve for ganglion cells, which average roughly 250 μm in dendritic field diameter and thus have similarly sized receptive fields? The problem is that these medium-field amacrine cells are too large to create local subunits within the dendritic field but too narrow to carry out the contextual functions mediated by the wide-field amacrine cells. Because the medium-field cells must overlap with the dendritic field boundaries of the ganglion cells, they would seem inevitably to degrade the sharpness of the receptive fields of the ganglion cells. It does not matter whether their action is in aggregate excitatory or inhibitory nor whether they influence only ganglion cells or bipolar and amacrine as well: one imagines them creating a “scalloped” edge to the receptive field of the ganglion cell (Fig. 3).
An easy way out is to postulate that sectors of the dendritic arbor are electronically isolated. That is evidently the case for some wide-field cells—the starburst amacrine cells and A17. But the medium-field cells are much smaller than those two and have less finely branched dendritic arbors, both features that would make electrotonic isolation harder. In contrast to the starburst cells, also, is that the medium-field cells busily communicate among the retinal layers, again suggesting some sort of a different function. Perhaps electrotonic isolation is in fact the case—the cells could carry out some sort of up and down function in local patches, but their geometry suggests a more specialized function, as yet unimagined.
Amacrine cells probably carry out undiscovered paracrine functions
The famous example is of course the dopaminergic cells, studied in precise detail by Raviola and others (Gustincich et al., 1999; Contini & Raviola, 2003; Dorenbos et al., 2007; Hirasawa et al., 2009). These widely spreading amacrines: (1) carry out diffuse release of dopamine, (2) simultaneously carry out traditional synaptic release, (3) are dual neurotransmitter neurons, releasing both dopamine and GABA, and (4) modulate the activity of many retinal neurons during light and dark adaptation. The spatial extent of the paracrine effects is remarkably broad, extending even to triggering the migration of melanin in the cells of the retinal pigment epithelium (Burnside, 2001).
There are strong hints of other such functions by other amacrine cells. A possible one is the still mysterious function of acetylcholine in the starburst amacrine cells. These are GABAergic neurons and GABA does the work of creating null direction inhibition in the DS ganglion cells. Although it was the presence of acetylcholine that first pointed out the existence of the starburst cells and allowed their identification (Masland & Mills, 1979), the function of acetylcholine is not understood. It potentiates the response to movement in all directions, suggesting that it is released by a mechanism distinct from that of GABA (Chiao & Masland, 2003). And there have always been odd features of acetylcholine release. The first is that it is only partly Ca++ dependent (Masland & Cassidy, 1987). Eliminating Ca++ blocks light-evoked acetylcholine release but reduces the resting release by only one third. This is reminiscent of the “acetylcholine leak” demonstrated in classic studies of the superior cervical ganglion—a release that is actually greater than the traditional conventionally mediated release (Phyllis, 2005). Another odd feature is that both acetylcholinesterase and acetylcholine receptors are more widely distributed across the IPL than the synapses of the starburst cells (Keyser et al., 2000). Both these things suggest that there is some level of diffuse release of acetylcholine.
There is also much evidence for the release of nitric oxide by some retinal neurons, again a substance that would diffuse throughout the retina with widespread modulatory effects (Vaney, 2004; Hoffpauir et al., 2006; Wilson et al., 2011). New diffusible neuroactive substances continue to be discovered, and it would not be surprising if other amacrine cells participate in this type of signaling. The control of sensitivity (dark adaptation) is unlikely to be the sole function of these cells.
The synaptic complexes of the IPL remain a daunting challenge
In their pioneering study of the IPL, Dowling and Boycott (1966) described an array of complex synaptic arrangements, including chain (serial) synapses among amacrine cells. The implications are hard to grasp and these descriptions have been largely ignored for the last 40 years. However, the facts remain as described. A recent example is the circuitry of the ON–OFF DS cell (Dacheux et al., 2003). The starburst cells synapse upon the DS cells within the starburst fascicles, which contain the dendrites of several starbursts and at least one ON–OFF DS cell. There, the starburst amacrines synapse upon the dendrites of the DS cells, but they also synapse on each other, forming chains of synapses. A more general study of these local arrangements revealed complexity that is, if anything, even greater than seen by Dowling and Boycott (1966) and offered a conceptual framework for their classification (Marc & Liu, 2000). One of these arrangements is shown in Fig. 4. Perhaps the next step, at least for the narrow-field cells, is reconstruction by connectomic strategies, which would allow linking of the local synaptic complexes to their parent cell types (Briggman & Denk, 2006; Anderson et al., 2011; Helmstaedter et al., 2011).
Fig. 4.
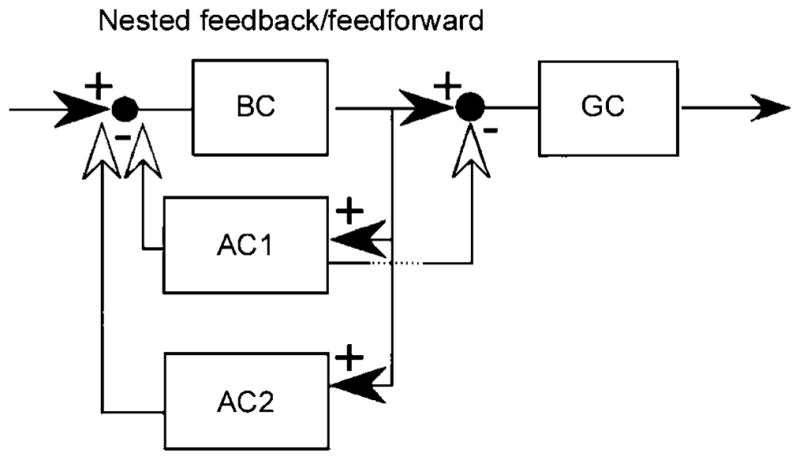
One of the many possible consequences of synapses among bipolar, amacrine, and ganglion cells. The one shown here was termed “nested feedforward inhibition” by Marc and Liu (2000), who pointed out that several such logics are possible. Reproduced by permission from the Journal of Comparative Neurology.
Amacrine cell diversity is related to ganglion cell diversity
How many types of amacrine cells will ultimately be found? The issue depends substantially on the number of ganglion cells, and counting both cell types suffers from some of the same difficulties. Wässle and colleagues defined two anatomical types of retinal ganglion cell, termed α and β, in the retina of the cat. These were shown to represent the physiological X and Y cells, the pioneering demonstration of morphological–physiological correspondence (Cleland et al., 1975; Peichl & Wässle, 1981). But these experimenters also noted the existence of a large number of other cell types—approximately 40% of all retinal ganglion cells in the mid-peripheral retina.
Later investigators have struggled to define the remaining ganglion cell types (O’Brien et al., 2002; Dacey, 2004). The first explicit attempt at a complete catalogue using modern cell-filling methods was Rockhill et al. (2002) in the rabbit retina. They identified 11 cell types but noted a group of medium-field ganglion cells that they were unable to classify. Five groups have attempted exhaustive ganglion cell classification in the mouse retina—more difficult because retinal ganglion cells in the mouse are less distinctive morphologically than those of the rabbit, cat, or monkey. Sun et al. (2002) used biolistic filling of the cells and traditional subjective classification methods. Badea and Nathans (2004), Kong et al. (2005), and Coombs et al. (2006) used a variety of cell-filling techniques followed by cluster analysis. Völgyi et al. (2009) defined cell types on the basis of tracer coupling. There were two encouraging results: (1) Many cell types—certainly the majority of all ganglion cells—were identified in most studies. For example, all groups identified a very small bushy ganglion cell usually termed “cluster 1.” (2) There was rough agreement on certain features of the population as a whole, specifically, that the number of cells exceeds a dozen. Unfortunately, an unequivocal division into types did not occur. The number of putative cell types was 19, 11, 12, 14, and 22 in the respective studies.
For both ganglion and amacrine cells, one should remember that wide-field cell types are hard to discover because of their small numbers. It does not take many wide-field cells to cover the retina, so that a small absolute number of ganglion cells can create a large number of true—that is, functionally distinct—types. In addition, the estimated number of types, of both ganglion and amacrine cells, is affected by unspoken criteria. How should one count similar cells that carry out variants of a single function? Should the ON–OFF DS cell be treated as one type or four, since each responds best to one of four cardinal directions? The same ambiguity exists for ON DS cells (three directions) and the melanopsin cells, which are all intrinsically sensitive to light but came in five varieties with slight structural variations. In aggregate, then, these cell groupings can be treated as 3 or 12 cell types. A similar confusion exists for the polyaxonal amacrine cells. As noted earlier, these have at least 10 different stratification patterns in the mouse (Fig. 2). They could be considered to be doing a single task and thus be treated as a single amacrine cell type: collecting input from a central zone and sending a wave of inhibition outward to the other cells of the inner nuclear layer. At a finer granularity, however, which particular groups of cells communicate in this way—which set of bipolar inputs initiates inhibition of which other retinal neurons—begins to matter. This is reflected in the obviously different stratification of different wide-field amacrine cells. If this form of specialized connectivity is taken to define a type, then each of the >10 polyaxonal cells in the mouse would be counted as a separate cell “type.”
Thus, it is possible that the number of ganglion cell types is near 12, and it is also possible that the number is near 30, depending on the number of as-yet-undiscovered cells and how the counting is done. At a hypothetical ratio of two amacrine cells per ganglion cell, the number of amacrine cell types would then range from 24 to 60. The encouraging news is that one can now see the way to a definitive answer, which would be one in which the total populations of ganglion and amacrine cells could be accounted for quantitatively by the sum of the individual cells of that class, as now appears to have been accomplished for bipolar cells (Jeon et al., 1998; Wässle et al., 2009). At that point the list of cells would be specific, and the question of “what is a type” could be returned, without regret, to the philosophers.
Acknowledgments
Supported by NIH grant RO1 017169. R.H.M. is a Senior Investigator of Research to Prevent Blindness. I thank Michael Becker for help with the figures and the members of the Jakobs–Masland lab for reading the manuscript.
References
- Anderson JR, Jones BW, Watt CB, Shaw MV, Yang JH, Demill D, Lauritzen JS, Lin Y, Rapp KD, Mastronarde D, Koshevoy P, Grimm B, Tasdizen T, Whitaker R, Marc RE. Exploring the retinal connectome. Molecular Vision. 2011;17:355–379. [PMC free article] [PubMed] [Google Scholar]
- Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. The Journal of Comparative Neurology. 2004;480:331–351. doi: 10.1002/cne.20304. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit’s retina. The Journal of Physiology. 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Denk W. Towards neural circuit reconstruction with volume electron microscopy techniques. Current Opinion in Neurobiology. 2006;16:562–570. doi: 10.1016/j.conb.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Burnside B. Light and circadian regulation of retinomotor movement. Progress in Brain Research. 2001;131:477–485. doi: 10.1016/s0079-6123(01)31038-5. [DOI] [PubMed] [Google Scholar]
- Chiao CC, Masland RH. Contextual tuning of direction-selective retinal ganglion cells. Nature Neuroscience. 2003;6:1251–1252. doi: 10.1038/nn1147. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Levick WR, Wässle H. Physiological identification of a morphological class of ganglion cell in the cat. The Journal of Physiology. 1975;248:151–171. doi: 10.1113/jphysiol.1975.sp010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs J, van der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140:123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Contini M, Raviola E. GABAergic synapses made by a retinal dopaminergic neuron. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1358–1363. doi: 10.1073/pnas.0337681100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. Axon-bearing amacrine cells of the macaque monkey retina. The Journal of Comparative Neurology. 1989;284:275–293. doi: 10.1002/cne.902840210. [DOI] [PubMed] [Google Scholar]
- Dacey D. Origins of perception: Retinal ganglion cell diversity and the creation of parallel visual pathways. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- Dacheux RF, Chimento MF, Amthor FR. Synaptic input to the on-off directionally selective ganglion cell in the rabbit retina. The Journal of Comparative Neurology. 2003;456:267–278. doi: 10.1002/cne.10521. [DOI] [PubMed] [Google Scholar]
- Demb J, Singer J. Intrinsic properties and functional circuitry of the AII amacrine cell. Visual Neuroscience. 2012:29. doi: 10.1017/S0952523811000368. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E. Expression of circadian clock genes in retinal dopaminergic cells. Visual Neuroscience. 2007;24:573–580. doi: 10.1017/S0952523807070538. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: Electron microscopy. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Ellias SA, Stevens JK. The dendritic varicosity: A mechanism for electrically isolating the dendrites of cat retinal amacrine cells? Brain Research. 1980;196:365–372. doi: 10.1016/0006-8993(80)90401-1. [DOI] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Polyaxonal amacrine cells of rabbit retina: Morphology and stratification of PA1 cells. The Journal of Comparative Neurology. 1992a;316:391–405. doi: 10.1002/cne.903160402. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Polyaxonal amacrine cells of rabbit retina: Size and distribution of PA1 cells. The Journal of Comparative Neurology. 1992b;316:406–421. doi: 10.1002/cne.903160403. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Polyaxonal amacrine cells of rabbit retina: PA2, PA3, and PA4 cells: Light and electron microscopic studies with a functional interpretation. The Journal of Comparative Neurology. 1992c;316:422–446. doi: 10.1002/cne.903160404. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:45–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Directional selectivity is formed at multiple levels by laterally offset inhibition in the rabbit retina. Neuron. 2005;46:117–127. doi: 10.1016/j.neuron.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Grimes WN, Zhang J, Graydon CW, Kachar B, Diamond JS. Retinal parallel processors: More than 100 independent microcircuits operate within a single interneuron. Neuron. 2010;65:873–885. doi: 10.1016/j.neuron.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollisch T, Meister M. Eye smarter than scientists believed: Neural computations in circuits of the retina. Neuron. 2010;65:150–164. doi: 10.1016/j.neuron.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustincich S, Feigenspan A, Sieghart W, Raviola E. Composition of the GABA receptors of retinal dopaminergic neurons. The Journal of Neuroscience. 1999;19:7812–7822. doi: 10.1523/JNEUROSCI.19-18-07812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustincich S, Feigenspan A, Wu DK, Koopman LJ, Raviola E. Control of dopamine release in the retina: A transgenic approach to neural networks. Neuron. 1997;18:723–736. doi: 10.1016/s0896-6273(00)80313-x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Denk W. High-accuracy neurite reconstruction for high-throughput neuroanatomy. Nature Neuroscience. 2011;14:1801–1808. doi: 10.1038/nn.2868. [DOI] [PubMed] [Google Scholar]
- Hirasawa H, Puopolo M, Raviola E. Extrasynaptic release of GABA by retinal dopaminergic neurons. Journal of Neurophysiology. 2009;102:146–158. doi: 10.1152/jn.00130.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffpauir B, Mcmains E, Gleason E. Nitric oxide transiently converts synaptic inhibition to excitation in retinal amacrine cells. Journal of Neurophysiology. 2006;95:2866–2877. doi: 10.1152/jn.01317.2005. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. The Journal of Neuroscience. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser KT, Macneil MA, Dmitrieva N, Wang F, Masland RH, Lindstrom JM. Amacrine, ganglion, and displaced amacrine cells in the rabbit retina express nicotinic acetylcholine receptors. Visual Neuroscience. 2000;17:743–752. doi: 10.1017/s095252380017508x. [DOI] [PubMed] [Google Scholar]
- Kong JH, Fish DR, Rockhill RL, Masland RH. Diversity of ganglion cells in the mouse retina: Unsupervised morphological classification and its limits. The Journal of Comparative Neurology. 2005;489:293–310. doi: 10.1002/cne.20631. [DOI] [PubMed] [Google Scholar]
- Levick WR, Oyster CW, Davis DL. Evidence that McIlwain’s periphery effect is not a stray light artifact. Journal of Neurophysiology. 1965;28:555–559. doi: 10.1152/jn.1965.28.3.555. [DOI] [PubMed] [Google Scholar]
- Lin B, Masland RH. Populations of wide-field amacrine cells in the mouse retina. The Journal of Comparative Neurology. 2006;499:797–809. doi: 10.1002/cne.21126. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme diversity among amacrine cells: Implications for function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: Matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. The Journal of Comparative Neurology. 1999;413:305–326. [PubMed] [Google Scholar]
- Marc RE, Liu W. Fundamental GABAergic amacrine cell circuitries in the retina: Nested feedback, concatenated inhibition, and axosomatic synapses. The Journal of Comparative Neurology. 2000;425:560–582. doi: 10.1002/1096-9861(20001002)425:4<560::aid-cne7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nature Neuroscience. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Masland RH. Cell populations of the retina: The Proctor lecture. Investigative Ophthalmology and Visual Science. 2011;52:4581–4591. doi: 10.1167/iovs.10-7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH, Cassidy C. The resting release of acetylcholine by a retinal neuron. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1987;232:227–238. doi: 10.1098/rspb.1987.0071. [DOI] [PubMed] [Google Scholar]
- Masland RH, Mills JW. Autoradiographic identification of acetylcholine in the rabbit retina. The Journal of Cell Biology. 1979;83:159–178. doi: 10.1083/jcb.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH, Mills JW, Cassidy C. The functions of acetylcholine in the rabbit retina. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1984;223:121–139. doi: 10.1098/rspb.1984.0086. [DOI] [PubMed] [Google Scholar]
- Molnar A, Hsueh HA, Roska B, Werblin FS. Crossover inhibition in the retina: Circuitry that compensates for nonlinear rectifying synaptic transmission. Journal of Computational Neuroscience. 2009;27:569–590. doi: 10.1007/s10827-009-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien BJ, Isayama T, Richardson R, Berson DM. Intrinsic physiological properties of cat retinal ganglion cells. The Journal of Physiology. 2002;538:787–802. doi: 10.1113/jphysiol.2001.013009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olveczky BP, Baccus SA, Meister M. Segregation of object and background motion in the retina. Nature. 2003;423:401–408. doi: 10.1038/nature01652. [DOI] [PubMed] [Google Scholar]
- Peichl L, Wässle H. Morphological identification of on- and off-centre brisk transient (Y) cells in the cat retina. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1981;212:139–153. doi: 10.1098/rspb.1981.0030. [DOI] [PubMed] [Google Scholar]
- Peichl L, Wässle H. The structural correlate of the receptive field centre of α ganglion cells in the cat retina. The Journal of Physiology. 1983;34:309–324. doi: 10.1113/jphysiol.1983.sp014807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phyllis JW. Acetylcholine release from the central nervous system: A 50-year retrospective. Critical Reviews in Neurobiology. 2005;17:161–217. doi: 10.1615/critrevneurobiol.v17.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, Macneil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. The Journal of Neuroscience. 2002;22:3831–3843. doi: 10.1523/JNEUROSCI.22-09-03831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Werblin F. Rapid global shifts in natural scenes block spiking in specific ganglion cell types. Nature Neuroscience. 2003;6:600–608. doi: 10.1038/nn1061. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Masland RH, Raviola E, Dacheux RF. Connections of indoleamine-accumulating cells in the rabbit retina. The Journal of Comparative Neurology. 1989;283:303–313. doi: 10.1002/cne.902830210. [DOI] [PubMed] [Google Scholar]
- Soodak RE, Shapley RM, Kaplan E. Fine structure of receptive-field centers of X and Y cells of the cat. Visual Neuroscience. 1991;6:621–628. doi: 10.1017/s0952523800002613. [DOI] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of rat retinal ganglion cells. Visual Neuroscience. 2002;19:483–493. doi: 10.1017/s0952523802194107. [DOI] [PubMed] [Google Scholar]
- Taylor R, Smith WR. The role of starburst amacrine cells in visual signal processing. Visual Neuroscience. 2012:29. doi: 10.1017/S0952523811000393. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI. The mosaic of amacrine cells in the mammalian retina. Progress in Retinal Research. 1991;9:49–100. [Google Scholar]
- Vaney DI. Retinal neurons: Cell types and coupled networks. Progress in Brain Research. 2002;136:239–254. doi: 10.1016/s0079-6123(02)36020-5. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Type 1 nitrergic (ND1) cells of the rabbit retina: Comparison with other axon-bearing amacrine cells. The Journal of Comparative Neurology. 2004;474:149–171. doi: 10.1002/cne.20110. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Peichl L, Boycott BB. Neurofibrillar long-range amacrine cells in mammalian retinae. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1988;235:203–219. doi: 10.1098/rspb.1988.0072. [DOI] [PubMed] [Google Scholar]
- Völgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of ganglion cell subtypes in the mouse retina. The Journal of Comparative Neurology. 2009;512:664–687. doi: 10.1002/cne.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi B, Xin D, Amarillo Y, Bloomfield SA. Morphology and physiology of the polyaxonal amacrine cells in the rabbit retina. The Journal of Comparative Neurology. 2001;440:109– 125. doi: 10.1002/cne.1373. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. The Journal of Neuroscience. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina. Correcting the nonlinearities in synaptic transmission. Visual Neuroscience. 2010;27:1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. The retinal hypercircuit: A repeating synaptic interactive motif underlying visual function. The Journal of Physiology. 2011;589:3691–3702. doi: 10.1113/jphysiol.2011.210617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Nacsa N, Hart NS, Weller C, Vaney DI. Regional distribution of nitrergic neurons in the inner retina of the chicken. Visual Neuroscience. 2011;28:205–220. doi: 10.1017/S0952523811000083. [DOI] [PubMed] [Google Scholar]
- Wright LL, Vaney DI. The fountain amacrine cells of the rabbit retina. Visual Neuroscience. 2000;17:1145R–1156R. doi: 10.1017/s0952523800171160. [DOI] [PubMed] [Google Scholar]
- Yang G, Masland RH. Receptive fields and dendritic structure of directionally selective retinal ganglion cells. The Journal of Neuroscience. 1994;14:5267–5280. doi: 10.1523/JNEUROSCI.14-09-05267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


