Abstract
The limited symptom relief and side effects of current Alzheimer’s disease (AD) medications warrant urgent discovery and study of new anti-AD agents. The “cholinergic hypothesis” of AD prompts us to search for plant-derived acetylcholineesterase (AChE) inhibitors such as galanthamine that has been licensed in Europe for AD treatment. We used the unique amyloid β-expressing transgenic C. elegans CL4176, which exhibits paralysis when human Aβ1–42 is induced, to study two natural benzylphenethylamine alkaloids isolated from Lycoris radiata (L’ Her.) Herb, galanthamine and haemanthidine, and their synthetic derivatives 1,2-Di-O-acetyllycorine and 1-O-acetyllycorine for their anti-paralysis effects. Our data indicate that these Lycoris compounds effectively delay the paralysis of CL4176 worms upon temperature up-shift, and prolong the lives of these transgenic worms. Lycoris compounds were shown to significantly inhibit the gene expression of ace-1 and ace-2. Additionally, the Lycoris compounds may modulate inflammatory and stress-related gene expressions to combat the Aβ-toxicity in C. elegans.
Introduction
With more than 20 million cases worldwide, Alzheimer’s disease (AD) has been named the most common progressive neurodegenerative disease [1]. AD is characterized by cerebral degeneration, neuronal cell death, and the hallmark accumulation of 40–42 amino acid amyloid-β (Aβ) in plaques [2] and tau tangles in the affected brain nerve cells [1]. According to the “amyloid hypothesis” [3], [4], Aβ is produced by the cleavage of the ubiquitous amyloid precursor protein (APP) and the imbalance between the production and the clearance of Aβ in central nervous system, leads to neuronal degeneration. Up to now, there is no cure for this debilitating disease. The widely accepted “cholinergic hypothesis” of AD proposes that a serious loss of cholinergic function in the central nervous system is associated with the development of cognitive symptoms [5]. A number of acetylcholinesterase (AChE) inhibitor drugs, including tacrine, donepezil, and galantamine, have been developed and are used to restore the normal cholinergic function for synaptic transmission in the central nervous system of AD patients [6]. These drugs, which are moderately effective in alleviating the symptoms of AD, have a number of side effects, including gastrointestinal upsets [6], [7]. Other drugs used to treat AD patients, such as memantine, attempt to balance the cholinergic pathway by attenuating the function of N-methyl-D-aspartate (NMDA) receptors. Like AChE inhibitors, memantine offers only modest AD symptom relief along with gastrointestinal side effects [8]. As the world population continues to live longer and AD patient numbers steadily rise, the need for new, effective and safer pharmacological agents for AD therapy becomes increasingly pressing.
In recent years, we have been working on the isolation of active anti-AD compounds from traditional Chinese herbs. Lycoris Herb. is a member of Amaryllidaceae and a genus endemic to East Asia. Lycoris radiata (L’ Her.) Herb has been used in traditional Chinese medicine to treat sore throat, rheumatoid arthritis and snake bites. [9] Lycoris plants are rich in benzylphenethylamine alkaloids such as galanthamine, lycorine, lycoramine and lycorenine [10], [11]. We have previously reported the isolation of fifteen known benzylphenethylamine alkaloids in 2011 from the bulbs and flowers of Lycoris radiata [9]. Some alkaloids were first reported from Lycoris Herb. and some were first isolated from L. radiata, including haemanthidine. Both galantamine and lycoramine from L. radiata have been reported to have anti-AChE activities [12]. Being orally bio-available and stimulative to nicotinic acetylcholine receptors (nAchR) [13], galanthamine is licensed in Europe for AD treatment and is well tolerated by AD patients [12].
Besides the AChE inhibitory effects, galanthamine has been found to modulate inflammation by attenuating TNF-α (tumor necrosis factor-alpha) and NO (nitric oxide) release through the α7 nAChR [14] and p44/42 MAPK (mitogen-activated protein kinase) pathway in murine microglia [13]. Another AChE inhibitor, donepezil, has also been shown to decrease cytokine (oncostatin M, interleukin-1β and interleukin-6) levels in AD patient lymphocytes [15]. Tacrine, donepezil and huperzine, all AChE inhibitors, have been demonstrated to prevent hydrogen peroxide-induced cell death and Aβ peptide-induced oxidative cell death [16]–[18]. Since inflammatory process and oxidative damage have been implicated in neurodegenerative diseases, any AChE inhibitory agent with the additive anti-inflammatory and/or anti-oxidative effects would be expected to be superior for AD treatment [6], [7], [15].
Transgenic Caenorhabditis elegans (C. elegans) models have been established for Alzheimer’s disease since 1995 [19]. Nematode disease models have been used to study the mechanisms of AD toxicity [20] and to test the efficacies of drugs and nutri-supplements. By using transgenic CL4176 worms, which express the human Aβ1–42 in muscle tissues under a temperature-inducible system [20], it was reported that soy isoflavone glycitein could protect worms from Aβ-induced toxicity and this protection was credited to the anti-oxidative activity of glycitein [21]. Ginkgo biloba extract EGb761 and ginkgolides were shown to suppress the Aβ-induced pathological behaviors of several different Aβ-transgenic C. elegans, not by reducing oxidative stress but rather by modulating Aβ oligomeric species [22]. Arya et al. used transgenic C. elegans strains CL2006, which constitutively expresses Aβ in the body wall muscles, and CL2355, which has inducible neuronal Aβ expression, to show that reserpine (an FDA approved antihypertensive drug) could ameliorate Aβ toxicity [23].
In this study, we used transgenic C. elegans CL4176 to evaluate the Aβ toxicity- inhibitory effect of galanthamine and haemanthidine purified from Lycoris radiata (L’ Her.) Herb. and their derivatives 1,2-Di-O-acetyllycorine and 1-O-acetyllycorine. We demonstrate that these L. radiata compounds strongly inhibit Aβ toxicity and prolong the lifespan of CL4176 worms. Attenuation of Aβ toxicity in this model system mostly results from the inhibition of acetylcholineesterase gene expression. Modulation of inflammation and stress-related genes may also contribute to the anti-Aβ toxicity of Lycoris compounds. Our study indicates that Aβ transgenic CL4176 nematodes can be efficiently used to screen for AChE inhibitors.
Results
Isolation and Synthesis of L. radiata Compounds
From the bulbs and flowers of L. radiata, we obtained fifteen known alkaloids [9]. Galanthamine and haemanthidine were selected for further study. According to a previous report, 1-O-acetyllycorine possesses potent activity against electric eel acetylcholinesterase (eeAChE, IC50 = 0.96 µM) [24]. Therefore, we also synthesized this compound and its analogue 1,2-di-O-acetyllycorine and used both in the following studies.
Natural and Synthetic L. radiata Compounds Delayed Paralysis of Aβ-Transgenic C. elegans CL4176 Nematodes
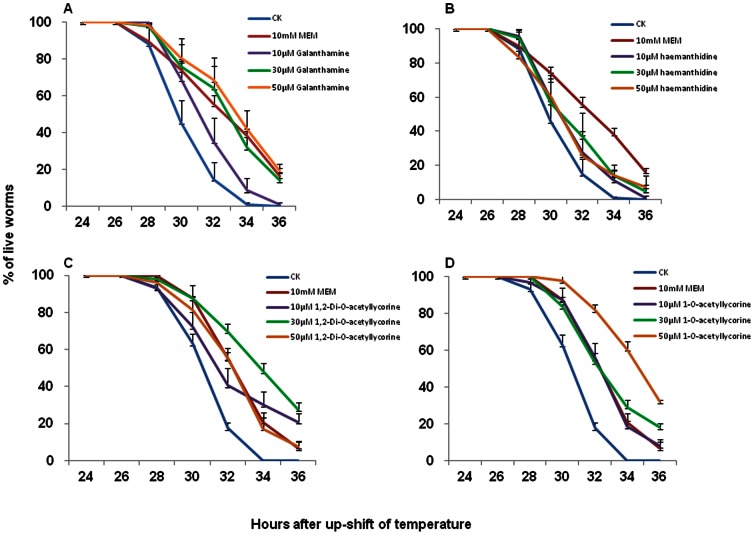
CL4176 worms were synchronously hatched and raised on NGM plates containing OP50 bacteria and compounds at 16°C. After two days, the temperature was shifted to the permissive 23°C to induce expression of the Aβ1–42 peptide. We consistently observed that 26 hours after the temperature up-shift, non-treated worms started to become paralyzed and die due to the expression of human Aβ1–42. In order to compare the efficacy of anti-Aβ activity of L. radiata compounds with memantine, the current medication used to lessen symptoms in AD patients, we included memantine as one of the experimental treatments in these studies. While memantine has been shown to be effective in delaying the paralysis of CL4176 upon temperature shift to 23°C, the concentration used in that study was not reported [25]. We tested different concentrations of memantine in the paralysis assay with CL4176 and found that it was effective only in the millimolar range (data not shown). Consequently, we used 10 mM of memantine as a reference throughout these studies. We showed that galanthamine could reduce the mortality of CL4176 compared to the control (CK) worms which were not treated with any compound (Fig. 1A). The control worms reached 100% mortality 34 hr post temperature up-shift. In contrast, 8% of worms exposed to 10 µM galanthamine were still alive at 36 hr. The worm survival rates were further increased to 32% and 42% by 30 µM and 50 µM of galanthamine 34 hr post temperature up-shift. Our data showed that the anti-paralysis effect of 50 µM galanthamine was greater than that of 10 mM memantine. We further demonstrated that 50 µM galanthamine could prolong the lifespan of CL4176 up to 40 hr.
Figure 1. Time course of the paralysis assay of CL4176 C. elegans.
Synchronized worms were hatched and fed on compound-containing NGM medium for two days at 16°C. The temperature was then shifted to 23°C. The survival rate (% of live worms) was recorded and plotted against the hours post temperature shift. The worms were treated with (A) galanthamine, (B) haemanthidine, (C) 1,2-Di-O-acetyllycorine and (D) 1-O-acetyllycorine at 10, 30 and 50 µM concentrations. In comparison, one group of worms were not treated (the non-treated control sample, CK) and the other group ware treated with 10 mM memantine. Three independent experiments were carried out to give the average rates for worm survival and to calculate standard deviations.
Our data also showed that haemanthidine, which we isolated for the first time from L. radiata, could reduce the mortality of CL4176, with 11%, 14% and 14% worms still alive 34 hr after the temperature up-shift at concentrations of 10 µM, 30 µM and 50 µM (Fig. 1B). At these same concentrations, haemanthidine appeared to be less effective at delaying the paralysis of CL4176 nematodes as compared to galanthamine.
The first synthetic alkaloid 1,2-Di-O-acetyllycorine at 50 µM was shown to be comparable to10 mM memantine in preventing paralysis of CL4176 worms expressing the Aβ1–42 peptide (Fig. 1C). At 30 µM, however, 1,2-Di-O-acetyllycorine increased the worm survival rate to 48% 34 hr post temperature up-shift, which was similar to the effect of 50 µM galanthamine (Fig. 1A).
The second synthetic alkaloid 1-O-acetyllycorine exhibited similar anti-paralysis effects in CL4176 worms as 10 mM memantine, and was comparable to 30 µM galanthamine at the same concentration (Fig. 1D). However, 50 µM 1-O-acetyllycorine increased the worm survival rate of CL4176 to the greatest level (61% alive), compared to all the compounds tested.
Our data indicate that the new alkaloid haemanthidine from L. radiata and our synthetic alkaloids 1,2-Di-O-acetyllycorine and 1-O-acetyllycorine could reduce Aβ-toxicity with similar potency to the known AChE inhibitor galanthamine. We have shown our Lycoris compounds were effective at the micromolar concentration range versus the millimolar concentration range required for the current AD medication memantine. Therefore, 50 µM for all Lycoris compounds and 10 mM for memantine were subsequently used in the following experiments.
L. radiata Compounds Slightly Reduced the Levels of Aβ in Transgenic C. elegans CL4176
Since the paralysis and the subsequent death of CL4176 are caused by the Aβ toxicity, naturally we wanted to examine if our Lycoris compounds would have any direct effect on the expression of Aβ ˜Transgenic C. elegans CL4176 expresses Aβ in the worm muscle cells [19]. It has been shown that CL4176 worms actually do not form Aβ plaques in the worm bodies. Rather, Aβ is expressed mostly in a soluble form [25], [26]. We used the fluorescent thioflavin T stain, which detects non-aggregated proteins more specifically than thioflavin S [27], to quantify the levels of Aβ in CL4176 worms treated with L. radiata compounds. Pure synthetic Aβ obtained from Sigma (cat. # A9810) was initially used to optimize the assay condition by preparing 0, 1, 5, 10 to 100 µg Aβin 100 µl volume each with 20 µM thioflavin T. It was determined that the Aβ concentration effect could be assessed by our Synergy HT Plate Reader using 440 nm excitation and 482 nm emission. At least 50 worms were collected from each treatment and sonicated. Equal amount of total soluble protein was used for each sample and assayed in triplicates and the experiment was repeated three times. Our results demonstrated that there was no reduction in the level of Aβ by any of the Lycoris compounds or by memantine 26 hr after the temperature shift to 23°C. As mentioned above, at 26 hr post temperature up-shift, the worms became paralyzed and started to die. We then repeated the treatments and collected worms 32 hr post temperature up-shift. The thioflavin T assay results showed that the Aβ levels in the galanthamine, haemanthidine, 1,2-Di-O-acetyllycorine, 1-O-acetyllycorine and memantine treated worms were 91.5% ±4.86 (p = 0.019), 91.37% ±7.72 (p = 0.062), 91.23% ±9.26 (p = 0.088), 93.61% ±1.41 (p = 0.001) and 98.27% ±1.83 (p = 0.085), compared to the non-treated worms. These data indicate that galanthamine significantly reduced the Aβ level by approximately 8.5% and the synthetic 1-O-acetyllycorine significantly reduced the Aβ level in worms by approximately 6.4% compared to the non-treated worms. In comparison, the data also indicate that memantine did not seem to reduce the Aβ level much.
Quantitative real-time RT-PCR (qRT-PCR) was carried out to assess the capability of Lycoris compounds to inhibit the Aβ gene at the transcript level. By using the primers designed for the Aβ transgene and the F23B2.13 gene encoding an RNA polymerase small subunit as a non-variable endogenous control [28] (Table 1), the relative gene expression of Aβ was assessed by the 2−ΔΔCt method. Our qRT-PCR results from three independent experiments showed that galanthamine significantly reduced the Aβ transgene expression by an average of 2.72-fold (p<0.01) compared to the non-treated sample. The results also showed that 1,2-Di-O-acetyllycorine reduced the Aβ gene expression by 1.32-fold (p<0.05) and 1-O-acetyllycorine by 1.3-fold (p<0.01). Since it is generally accepted that a 2-fold change in gene expression is considered significant, we can assume the effect of 1,2-Di-O-acetyllycorine and 1-O-acetyllycorine on Aβ gene expression was negligible. Haemanthidine and memantine, however, did not seem to have any inhibitory effect on the Aβ transgene expression at the transcript level.
Table 1. Oligonucleotide primers used in qRT-PCR studies.
| Gene | Forward primer | Reverse primer |
| Aβ | CCGACATGACTCAGGATATGAAGT | CACCATGAGTCCAATGATTGCA |
| ace-1 | AGTGGGCTCCTGTTCGAGAA | CCAATAGAAAATCACCATCGACAA |
| ace-2 | CAATAATCAACTCATGGGCATCA | TTTTCGCGAGACGAAACGA |
| F22E5.6 | TCCCCATACGAAACAACACA | CTCCTCCCAGCTTTTCCACAA |
| ZC239.12 | CCAGAAGAATCCCCATACGA | TCCTCCTCCAACTTTTCCAAA |
| Y46H3A.D | GGTGCAGTTGCTTCGAATCTT | TCTTCCTTGAACCGCTTCTTTC |
| Y46H3A.E | AAACAAAATCGGAACATGGATACTT | TGGAGCCTCAATTTGGAGTTTTC |
| F23B2.13 | CGCCGAAAATGAAATCAAAC | GGGCGTCGTACACCATCA |
L. radiata Compounds Inhibited Acetylcholine Esterase Gene Expression in Aβ-Transgenic C. elegans CL4176 Nematodes
To delineate the anti-paralysis mechanisms of natural and synthetic L. radiata compounds in Aβ-transgenic C. elegans CL4176 nematodes, we investigated the ability of the natural and synthetic L. radiata compounds to inhibit the AChE gene expression in Aβ-transgenic C. elegans CL4176 nematodes. Total worm RNA was isolated from CL4176 nematodes 26 hr after the temperature up-shift. At this point, the worms had fed for a total of 48 hr on medium containing both the compounds and OP50 bacteria and just started to become paralyzed. Unlike vertebrates with only one AChE gene [29], C. elegans and other nematodes have multiple ace genes. C. elegans ace-1 gene is expressed in all body-wall and vulval muscle cells [30] while ace-2 is expressed almost exclusively in neurons [31]. ace-3 is expressed in pharyngeal muscle cells and neurons [32]. ACE-1 and ACE-2 account for 95% of the total enzymatic activity, ACE-3 for the remainder 5% and ACE-4′s activity is normally undetectable. Consequently, we focused on the effects of L. radiata compounds on gene expression of ace-1 and ace-2.
To study the steady state expression levels of the ace-1 and ace-2 genes, we performed qRT-PCR assays using specific forward and reverse primers for these genes (Table 1) with total nematode RNA isolated from Aβ-transgenic C. elegans CL4176. The relative levels of ace-1 and ace-2 gene expression were assessed by the 2−ΔΔCt method using the F23B2.13 gene as a non-variable endogenous control, averaged from three independent experiments. Galanthamine reduced the gene expression of ace-1 by 7.61-fold, the synthetic 1,2-Di-O-acetyllycorine decreased ace-1 expression by 4.62-fold and haemanthidine decreased ace-1 expression by 2.73-fold (Fig. 2A). Our results also demonstrated that the synthetic 1-O-acetyllycorine reduced the expression of ace-1 in CL4176 worms by only 1.6-fold and that memantine had no inhibitory effect on ace-1. Additionally, we demonstrated that the synthetic 1,2-Di-O-acetyllycorine reduced ace-2 expression by 2.97-fold (p<0.05), galanthamine reduced ace-2 expression by 2.94-fold (p<0.05), synthetic 1-O-acetyllycorine decreased ace-2 expression by 2.67-fold (p<0.01) and haemanthidine decreased ace-2 expression by 2.04-fold (p<0.01). Memantine had a slightly inhibitory effect, reducing ace-2 levels by 1.39-fold which was negligible.
Figure 2. Real-time qRT-PCR analysis of the steady state acetylcholine esterase gene expression in transgenic C. elegans.
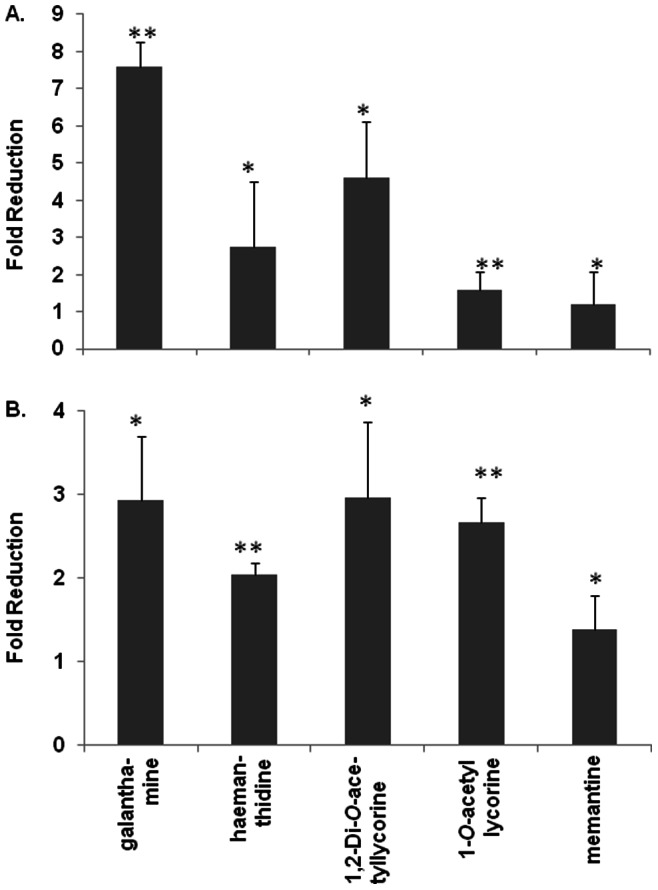
Total RNA was isolated from worms 26 hr post temperature up-shift and subjected to DNase digestion. cDNA was produced using the High Capacity cDNA Synthesis Kit (Life Technologies) with random primers. qPCR was performed with Applied Biosystem SDS7300 instruments and primers specific for ace-1 (A) and ace-2 (B) genes. The gene expression levels were assessed by the 2−ΔΔCt method using F23B2.13 gene as a non-variable endogenous control. The fold-reductions and standard deviations for gene expression were calculated from three independent experiments by comparison with non-treated samples. The student t-test was conducted to assess the statistical significance (*p<0.05; **p<0.01).
L. radiata Compounds Inhibited Inflammation and Stress-Associated Gene Expression Which May Contribute to the Anti-Aβ Paralysis Effect in C. elegans CL4176
As inflammation is implicated in Aβ-toxicity and AChE inhibitors have been shown to modulate inflammation to improve the cholinergic health [7], [15], [28], we decided to evaluate the ability of L. radiata compounds to reduce the expression of inflammation and stress-associated genes in CL4176 C. elegans. Pro-inflammatory genes and cytokines such as TNFα, IL-1 and IL-6 are associated with human neurodegenerative diseases. While C. elegans contains homologues of TNFA1P1 (TNFα-induced protein 1), this gene has not been associated with Aβ toxicity in human [28]. However, it has been reported that the treatment of TNFα in human cell cultures exposed to Aβ can protect hippocampal neurons from Aβ toxicity [33]. Link et al. have shown that the gene expression levels of two C. elegans homologs of human TNFA1P1, F22E5.6 and ZC239.12, were up-regulated in transgenic CL4176 upon temperature up-shift [28]. They also showed that TNFA1P1 expression was increased in AD patient brain tissues [28]. Additionally, Link et al. reported that the expression of human αB-crystallin (CRYAB), a well-studied stress-inducible chaperone protein, was enhanced in AD patient brain tissues. Equivalently, the C. elegans CRYAB-homologous HSP16 (heat shock protein 16)-2 (Y46H3A.D) and HSP16-4 (Y46H3A.E) gene expressions were up-regulated in transgenic CL4176 worms following the temperature up-shift [28].
We used the same primers in Link et al. [28] (Table 1) to perform qRT-PCR on total RNA isolated from CL4176 26 hr after the temperature shift. We found that galanthamine, haemanthidine and 1,2-Di-O-acetyllycorine at 50 µM significantly reduced the gene expression of TNFA1P-homolog F22E5.6 (p<0.01∼0.05), with haemanthidine being the most effective compound with an average of 4.8-fold reduction compared to the non-treated sample (Table 2). 1,2-Di-O-acetyllycorine at 50 µM was also shown to affect another TNFA1P1-homolog, ZC239.12, whose gene expression was reduced by 5.5-fold (a significance level of p = 0.006). Additionally, our data indicate that 50 µM haemanthidine reduced the gene expression of two stress-related HSP-16 genes by 1.99- and 2.07-fold, corresponding to a statistical significance values of p = 0.007, and 0.025, respectively. These results show us that galanthamine, haemanthidine and 1,2-Di-O-acetyllycorine may be involved in limiting inflammation and stress-related cellular damage caused by Aβ toxicity in transgenic CL4176 worms. Interestingly, we found that 10 mM memantine could reduce the expression of these four genes related to inflammation and stress in CL4176 C. elegans (Table 2).
Table 2. Inhibition of inflammation and stress-associated gene expression in CL4176 nematodes.
| Gene | Description | Compounds | Average Fold reduction | P value | |
| F22E5.6 | TNFA1P1 homolog | galanthamine | 2.17 | 0.039* | |
| haemanthidine | 4.8 | 0.033* | |||
| 1,2-Di-O-acetyllycorine | 1.39 | 0.012* | |||
| 1-O-acetyllycorine | 1.49 | 0.152 | |||
| memantine | 2.78 | 0.011* | |||
| ZC239.12 | TNFA1P1 homolog | galanthamine | 2.11 | 0.054 | |
| haemanthidine | 0.98 | 0.079 | |||
| 1,2-Di-O-acetyllycorine | 5.55 | 0.0066** | |||
| 1-O-acetyllycorine | 1.41 | 0.15 | |||
| memantine | 7.3 | 0.002** | |||
| Y46H3A.D | HSP16-2 | galanthamine | 1.17 | 0.0048* | |
| haemanthidine | 1.99 | 0.007** | |||
| 1,2-Di-O-acetyllycorine | 0.99 | 0.002** | |||
| 1-O-acetyllycorine | 2.06 | 0.098 | |||
| memantine | 2.29 | 0.007** | |||
| Y46H3A | HSP16-41 | galanthamine | 0.94 | 0.009** | |
| haemanthidine | 2.07 | 0.025* | |||
| 1,2-Di-O-acetyllycorine | 0.77 | 0.008** | |||
| 1-O-acetyllycorine | 1.81 | 0.071 | |||
| memantine | 3.12 | 0.019* | |||
Average fold-reduction of gene expression was calculated from three independent qRT-PCR analyses by comparing samples from treated and non-treated CL4176 worms collected 26 hr after temperature shift. Student t-test was used to calculate p values for statistical significance,
p<0.05,
p<0.01.
Antioxidant Activity did not Contribute to the Anti-Aβ Paralysis of CL4176 Nematodes by L. radiata Compounds
Besides Aβ toxicity, oxidative stress has been widely postulated to contribute to the etiology of Alzheimer’s disease [7], [26]. Aβ-transgenic C. elegans CL4176 have been shown to be under increased oxidative stress upon the temperature shift to 23°C [21]. We used the dye DCF-DA to measure intracellular levels of H2O2-related reactive oxidative species (ROS) in C. elegans CL4176 following exposure to compounds and temperature shift for 26 hr. Our data showed that exposure to the Lycoris compounds had no effect on ROS levels in treated worms compared to non-treated worms. This result indicates that antioxidant activity was not one of the factors contributing to Lycoris compounds’ anti-paralysis activity in CL4176 worms.
Cytotoxicity of L. radiata Compounds in Mammalian Cells
Ultimately, we would like to screen for natural and synthetic AChE inhibitors that have potent anti-AD effects but do not induce undesirable side effects for patients. The crinine and lycorine alkaloids have been reported to have noteworthy cytotoxicity [9], [34], [35]. We tested the cytotoxicity of our Lycoris compounds at the highest concentration of 50 µM that was used in the C. elegans in FHs 74 Int cells (human fetal small intestine normal cells, ATCC # CCL-241). With the MTS proliferation test, we demonstrated that the natural haemanthidine, a crinine type of alkaloid, was the most toxic and exhibited an average (from two independent experiments) of 28% ±2.62 inhibition rate of cell proliferation compared to non-treated cells which coincided with the previous report. Our results showed that the natural galanthamine and the synthetic 1,2-Di-O-acetyllycorine were not toxic at all to FHs 74 Int cells at 50 µM concentration. The synthetic 1-O-acetyllycorine was also shown to inhibit the proliferation of FHs 74 Int cells by 12.8% ±3.98.
Discussion
As the world population is growing older, the number of patients with Alzheimer disease (AD) is steadily increasing worldwide. With the limitations of improving the AD symptoms and the unfavorable side effects of current AD medications, it is imperative that we actively search and screen for new AD-controlling agents. We describe here the utilization of Aβ-transgenic C. elegans to study the inhibitory effects of two natural and two synthetic compounds from Lycoris radiata on paralysis of the worms caused by the Aβ toxicity. Our results showed that the natural galanthamine and haemanthidine and the synthetic 1,2-Di-O-acetyllycorine and 1-O-acetyllycorine could effectively attenuate the toxicity of Aβ expressed in transgenic CL4176 worms after the temperature shift from 16°C to 23°C (Fig. 1). Although it has been reported that the popular AD medication memantine could delay the paralysis of CL4176 nematodes [25], its effective concentration was not reported. We showed in this study that the Aβ toxicity attenuation by the Lycoris compounds was achieved at 10–50 µM concentrations. In comparison, 10 mM memantine had to be used to achieve similar anti-paralysis effect in CL4176 worms. Memantine, a known attenuator of N-methyl-D-aspartate (NMDA) receptors, is used to keep the degeneration of cholinergic cells in check [8]. One of our Lycoris compounds, galanthamine, is a known acetylcholine esterase (AChE) inhibitor that has been licensed in Europe for AD treatment. Both galanthamine and haemanthidine isolated naturally from Lycoris are alkaloids. Both the NMDA attenuator and AChE inhibitors are presumed to function by maintaining the healthy cholinergic status of cells. Therefore, the nearly 1000-fold lower concentration needed for Lycoris compounds to produce similar anti-paralysis effects in CL4176 indicates that they are much more effective than memantine in this system.
In order to evaluate the effects of Lycoris compounds on AChE genes, real-time quantitative RT-PCR analysis was conducted with total RNA isolated from worms that had been treated and incubated at higher temperature (23°C) for 26 hr. This time point was chosen since this is when the first symptoms of paralysis were observed and it allowed us to examine early changes in gene expression associated with the results of the treatment itself, rather than the downstream consequence of paralysis [28]. Our data demonstrated that the natural compound galanthamine was the most inhibitory to the gene expression of ace-1 followed by the synthetic 1,2-Di-O-acetyllycorine and the natural compound haemanthidine (Fig. 2A). We have also shown that all of our Lycoris compounds could inhibit the gene expression of ace-2 by around 3-fold. Interestingly, we did not find memantine inhibitory to the expression of ace-1 and ace-2. To our knowledge, there has been no previous report of using Aβ-transgenic C. elegans CL4176 nematodes to study the anti-AChE activity of any AChE inhibitors. It is understood that CL4176 expresses Aβ in muscle cells of worms upon temperature up-shift and the paralysis phenotype is a result of the Aβ toxicity. However, as the creators of CL4176 pointed out that 67 genes were up-regulated and 240 genes were down-regulated in CL4176 upon temperature up-shift [28]. Since there are many mechanisms proposed for Aβ toxicity, so far there is no strong indication of which gene changes are responsible for the Aβ toxicity in CL4176 worms [28]. It is likely that the AChE inhibiting activities of our Lycoris compounds provide some protection to CL4176 worms against Aβ toxicity, just as memantine, an attenuator of N-methyl-D-aspartate (NMDA) receptors for cholinergic health, was found to be able to delay the paralysis of CL4176 [25]. At least, our data suggested that CL4176 C. elegans can be used effectively to screen compounds that are inhibitory to AChE gene expression.
Since inflammation and oxidative-induced stress are associated with Aβ-toxicity and some AChE inhibitors including galanthamine have been shown to modulate inflammation process [13], [14], we evaluated our Lycoris compounds for their inhibition of two C. elegans human homologs of TNFA1P1 genes, F22E5.6 and ZC239.12 and two homologs of human αB-crystallin (CRYAB), HSP16-2 (Y46H3A.D) and HSP16-4 (Y46H3A.E). These four genes have been shown to be up-regulated in CL4176 worms following the temperature up-shift [28]. Our results indicated that galanthamine and haemanthidine significantly reduced the gene expression of F22E5.6 and 1,2-Di-O-acetylcorine highly significantly reduced the expression of ZC239.12. Haemanthidine was also found to be noticeably inhibitory to expression of the two HSP16 genes (Table 2). These data suggest that Lycoris compounds can both suppress AChE gene expression and are able to modulate expression of inflammation-related and stress-related genes in transgenic C. elegans CL4176. Since C. elegans really do not have the inflammation system per se, the benefits of Lycoris compounds’ anti-inflammation activity will need to be further evaluated in animal models.
Our data suggested that only galanthamine could reduce the transgene Aβ expression by 2.7-fold in CL4176 worms and the inhibitory effect on Aβ transcript level from haemanthidine, 1-O-acetyllycorine and 1,2-Di-O-acetyllycorine was negligible. However, all four Lycoris compounds could slightly reduce the Aβ peptide level in CL4176 worms by 6.4% to 8.5%. Since all four Lycoris compounds were shown to inhibit paralysis of CL4176 after temperature up-shift (Fig. 1), the slight reduction in Aβ transgene expression at both the transcript and peptide levels by these compounds may play a small role in extending the lifespan of CL4176 worms. Whether this slight Aβ reduction results directly from the AChE inhibition/cholinergic health-promoting activity of our Lycoris compounds needs to be further elucidated.
Additionally, we did not find anti-oxidant activity contribute to the anti-paralysis effect of the four Lycoris compounds. Therefore, our findings in this study strongly indicate that the anti-paralysis effects of our Lycoris compounds mainly result from their AChE gene inhibition and inflammation/stress-related gene modulation. However, it will be interesting to find out the inter-relationship amongst the Aβ toxicity and the cholinergic health and the lifespan extension promoted by the Lycoris compounds in CL4176 worms in future studies.
The present study demonstrates that human Aβ-expressing transgenic C. elegans CL4176 can be used effectively to screen for compounds that are inhibitory to AChE gene expression. Although we have shown that human fetal intestinal epithelial cells provide an easy system to test the cytotoxicity of AChE inhibitors, further efficacy studies need to be conducted in mouse and monkey models of Alzheimer’s disease before any new compound can be studied in human clinical trials.
Materials and Methods
Isolation and Synthesis of L. radiata Compounds
The isolation of galanthamine and haemanthidine from L. radiata has been reported in our previously published paper [23]. 1-O-Acetyllycorine [36] and 1,2-di-O-acetyllycorine [37] were prepared using established procedures.
Paralysis Assay of Aβ-Transgenic C. elegans CL4176 Nematodes
Aβ-transgenic C. elegans CL4176 nematodes [genotype: smg-1(cc546ts); dvIs27] were obtained from CGC (Caenorhabditis Genetics Center) and maintained on NGM (Nematode Growth Medium) plates (60 mm petri dishes) at 16°C. Paralysis assays were performed according to the method of Dr. C. Link [25]. In brief, one week before initiating the paralysis assay, gravid adult worms were picked onto NGM plate spread with OP50 bacteria to lay eggs for 2–4 hr at 16°C. The gravid adults were picked off the plates and the progeny were allowed to grow for 7 days into “second day” gravid adults. One day before the initiation of the paralysis assay, NGM plates were prepared with 50 µl of fresh OP50 bacterium mixed with Lycoris compounds at different concentrations or 10 mM memantine and incubated at 37°C overnight. At day 1 of the initiation of the paralysis assay, the second day gravid adults were allowed to lay eggs on NGM plates containing OP50 and compounds. Approximately 50 progeny were maintained on each plate for each treatment and incubated at 16°C for two days. The worms were then incubated at 23°C and the survival rates were recorded. Worms without movement after prodding were recorded as dead.
Quantitative Staining of Aβ with Thioflavin-T
Twenty-six or 32 hours post temperature shift from 16°C to 23°C, all the CL1476 worms for each treatment plate were collected by washing the plate with 1 ml 1× PBS (diluted from 10× PBS, Fisher) and transferred into a microfuge tube. The worms were pelleted by centrifugation at14k rpm for 2 min and sonicated with Branson Sonifier 150 at setting 2 for 15 sec ×4 times. The sonicated worms were centrifuged at 14 krpm for 2 min and the supernatant was transferred into a new tube. The concentration of total soluble protein in each sample was quantified by the Bradford method (BioRad). Equal amount of total protein from every sample was used in each independent experiment, which was divided into 3 replicates. Each replicate was mixed with 10 µl 10× PBS (Fisher) and 2 µl 1 mM thioflavin-T (Sigma) (final concentration of 20 µM) in a final volume of 100 µl. Fluorescence resulting from Aβ stained by thioflavin-T was measured by the Synergy HT Plate Reader using excitation at 440 nm and emission at 482 nm and averaged from at least three independent experiments.
Measurement of H2O2 Levels in CL4176 C. elegans
Twenty-six hours after the temperature up-shift, exactly 50 CL4176 worms from each treatment plate were collected by a platinum worm-picker into 100 µl 1× PBS (diluted from 10× PBS, Fisher) containing 1% Tween-20 in a microfuge tube and sonicated with Branson Sonifier 150 at setting 2 for 15 sec ×4 times. The intracellular levels of H2O2-related reactive oxidative species (ROS) in CL4176 worms were measured as described using 2,7-dichlorofluorescein diacetate (DCF-DA, Sigma Aldrich) [21]. Briefly, sonicated worms were pipetted into the wells of a 96-well plate containing 100 µl of 1× PBS plus 100 µM DCF-DA. The final concentration of DCF-DA in the assay is 50 µM. The fluorescence was recorded by Synergy HT Plate Reader at 485 nm excitation and 545 nm emission and averaged from three independent experiments.
Quantitative Real-Time RT-PCR (qRT-PCR) Analysis
Twenty-six hours post temperature shift from 16°C to 23°C, treated CL4176 worms were collected by washing the plate with 1 ml 1× PBS and transferred to microfuge tubes. The worms were pelleted by centrifugation at 14 krpm for 2 min. The total nematode RNA was isolated by the freeze-cracking method as described in the WormBook (http: //www.wormbook.org). Briefly, 500 µl Trizol reagent (Life Technologies) was added to each sample. The freeze-thaw was repeated three times by freezing in dry ice/ethanol bath and 5 min 37°C -incubation. After 100 µl chloroform was added, the worm suspension was then vortexed vigorously and centrifuged at 14 krpm for 3 min. Total nematode RNA in the supernatant was precipitated by ethanol, sodium acetate and centrifugation. Precipitated RNA was further purified by in gel-DNase digestion following the suggestion of Qiagen using the RNeasy column. RNA concentration and integrity was measured by a Nanodrop spectrophotometer.
cDNA was produced from total nematode RNA by reverse transcription using the High Capacity cDNA Synthesis Kit and random primers (Applied Biosystems/Life Technologies). Quantitative, real-time PCR (qPCR) was performed by Applied Biosystems SDS 7300 instrument using gene-specific forward and reverse primers (Table 1) with the following parameters: 1 cycle of 50°C for 2 min, 1 cycle of 95°C 10 min and 40 cycles of 95°C for 15 sec followed by 60°C for 1 min. The levels of relative gene expression were assayed by the 2−ΔΔCt method using the F23B2.13 gene encoding an RNA polymerase small subunit as a non-variable endogenous control.
Cytotoxicity Assay of Lycoris Compounds
Human fetal intestinal epithelial (FHs 74 Int) cells were purchased from ATCC and grown in complete Hybri-care medium (ATCC) supplemented with 30 ng/ml epidermal growth factor, 10% fetal bovine serum, 20 units/ml penicillin and 20 µg/ml streptomycin at 37°C with 5% CO2. 1×104 FHs 74 Int cells in 100 µl medium were seeded in each well of the 96-well tissue culture plate. After overnight growth, cells were washed with 1× PBS and incubated with 100 µl complete medium containing the Lycoris compounds at the final concentration of 50 µM. After 24 hr incubation, wells were washed with 1× PBS, 100 µl complete medium was added to each well. MTS (Promega) reagent (20 µl) was added into each well and the plate was incubated at 37°C with 5% CO2 for 2 hr. The cell viability was measured at 490 nm using the Synergy HT Plate Reader. The cytotoxicity of Lycoris compounds was assessed as the percentage reduction of cell viability compared to the cells-only controls. All samples were tested in triplicate and the assays were repeated twice.
Acknowledgments
The authors are greatly thankful to the critical review of the manuscript by Dr. Monica Driscoll (Rutgers University, New Jersey, USA).
Funding Statement
This work was partially funded by the National Natural Science Foundation of China (31161140345, 31070288, 20972166), and Ministry of Education of China through its 111 and 985 programs (B08044, MUC985-9, MUC98506-01000101). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Science 314: 777–781. [DOI] [PubMed] [Google Scholar]
- 2. Monsonego A, Maron R, Zota V, Selkoe DJ, Weiner HL (2001) Immune hyporesponsiveness to amyloid beta-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer’s disease. Proc Natl Acad Sci U S A 98: 10273–10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297: 353–356. [DOI] [PubMed] [Google Scholar]
- 4. Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, et al. (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330: 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartus RT, Dean RL 3rd, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408–414. [DOI] [PubMed] [Google Scholar]
- 6. McGleenon BM, Dynan KB, Passmore AP (1999) Acetylcholinesterase inhibitors in Alzheimer’s disease. Br J Clin Pharmacol 48: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tabet N (2006) Acetylcholinesterase inhibitors for Alzheimer’s disease: anti-inflammatories in acetylcholine clothing! Age Ageing. 35: 336–338. [DOI] [PubMed] [Google Scholar]
- 8. Wenk GL, Quack G, Moebius HJ, Danysz W (2000) No interaction of memantine with acetylcholinesterase inhibitors approved for clinical use. Life Sci 66: 1079–1083. [DOI] [PubMed] [Google Scholar]
- 9. Wang H, Wang YH, Zhao FW, Huang QQ, Xu JJ, et al. (2011) Benzylphenethylamine alkaloids from the bulbs and flowers of Lycoris radiata . Chin Herbal Mediciens 3: 60–63. [Google Scholar]
- 10. Geng ZF, Lv HS, Zhang MH (2008) Supercritical CO2 extraction technology of lycorenine in L. radiata . Chin Tradit Herb Drugs 39: 543–546. [Google Scholar]
- 11. Wang L, Zhang XQ, Yin ZQ, Wang Y, Ye WC (2009) Two new amaryllidaceae alkaloids from the bulbs of Lycoris radiata. Chem Pharm Bull (Tokyo) 57: 610–611. [DOI] [PubMed] [Google Scholar]
- 12. Howes MJ, Houghton PJ (2003) Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav 75: 513–527. [DOI] [PubMed] [Google Scholar]
- 13. Giunta B, Ehrhart J, Townsend K, Sun N, Vendrame M, et al. (2004) Galantamine and nicotine have a synergistic effect on inhibition of microglial activation induced by HIV-1 gp120. Brain Res Bull 64: 165–170. [DOI] [PubMed] [Google Scholar]
- 14. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, et al. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388. [DOI] [PubMed] [Google Scholar]
- 15. Reale M, Iarlori C, Gambi F, Lucci I, Salvatore M, et al. (2005) Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer’s disease patients. Exp Gerontol 40: 165–171. [DOI] [PubMed] [Google Scholar]
- 16. Zhang HY, Tang XC (2000) Huperzine B, a novel acetylcholinesterase inhibitor, attenuates hydrogen peroxide induced injury in PC12 cells. Neurosci Lett 292: 41–44. [DOI] [PubMed] [Google Scholar]
- 17. Wang R, Zhou J, Tang XC (2002) Tacrine attenuates hydrogen peroxide-induced apoptosis by regulating expression of apoptosis-related genes in rat PC12 cells. Brain Res Mol Brain Res 107: 1–8. [DOI] [PubMed] [Google Scholar]
- 18. Xiao XQ, Wang R, Tang XC (2000) Huperzine A and tacrine attenuate beta-amyloid peptide-induced oxidative injury. J Neurosci Res 61: 564–569. [DOI] [PubMed] [Google Scholar]
- 19. Link CD (1995) Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci U S A 92: 9368–9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Link CD (2006) C. elegans models of age-associated neurodegenerative diseases: lessons from transgenic worm models of Alzheimer’s disease. Exp Gerontol 41: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 21. Gutierrez-Zepeda A, Santell R, Wu Z, Brown M, Wu Y, et al. (2005) Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC Neurosci 6: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, et al. (2006) Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci 26: 13102–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arya U, Dwivedi H, Subramaniam JR (2009) Reserpine ameliorates Abeta toxicity in the Alzheimer’s disease model in Caenorhabditis elegans. Exp Gerontol 44: 462–466. [DOI] [PubMed] [Google Scholar]
- 24. Elgorashi EE, Stafford GI, Van Staden J (2004) Acetylcholinesterase enzyme inhibitory effects of amaryllidaceae alkaloids. Planta Med 70: 260–262. [DOI] [PubMed] [Google Scholar]
- 25.Dostal V, Link CD (2010) Assaying beta-amyloid toxicity using a transgenic C. elegans model. JoVe. doi: 10.3791/2252. [DOI] [PMC free article] [PubMed]
- 26. Drake J, Link CD, Butterfield DA (2003) Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging 24: 415–420. [DOI] [PubMed] [Google Scholar]
- 27.Hatters DM, Griffin MDW (2011) Diagnostics for amyloid fibril formation: where to begin? Secaucus, NJ, Springer Science+Business Media, LLC. [DOI] [PubMed]
- 28. Link CD, Taft A, Kapulkin V, Duke K, Kim S, et al. (2003) Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiol Aging 24: 397–413. [DOI] [PubMed] [Google Scholar]
- 29. Combes D, Fedon Y, Toutant JP, Arpagaus M (2001) Acetylcholinesterase genes in the nematode Caenorhabditis elegans. Int Rev Cytol 209: 207–239. [DOI] [PubMed] [Google Scholar]
- 30. Culetto E, Combes D, Fedon Y, Roig A, Toutant JP, et al. (1999) Structure and promoter activity of the 5′ flanking region of ace-1, the gene encoding acetylcholinesterase of class A in Caenorhabditis elegans. J Mol Biol 290: 951–966. [DOI] [PubMed] [Google Scholar]
- 31. Combes D, Fedon Y, Toutant JP, Arpagaus M (2003) Multiple ace genes encoding acetylcholinesterases of Caenorhabditis elegans have distinct tissue expression. Eur J Neurosci 18: 497–512. [DOI] [PubMed] [Google Scholar]
- 32. Selkirk ME, Lazari O, Hussein AS, Matthews JB (2005) Nematode acetylcholinesterases are encoded by multiple genes and perform non-overlapping functions. Chem Biol Interact 157–158: 263–268. [DOI] [PubMed] [Google Scholar]
- 33. Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, et al. (1995) Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci U S A 92: 9328–9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Evidente A, Kireev AS, Jenkins AR, Romero AE, Steelant WF, et al. (2009) Biological evaluation of structurally diverse amaryllidaceae alkaloids and their synthetic derivatives: discovery of novel leads for anticancer drug design. Planta Med 75: 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Evidente A, Komienko A (2009) Anticancer evaluation of structurally diverse Amaryllidaceae alkaloids and their synthetic derivatives. Phytochem Rev 8: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakagawa Y, Uyeo S, Yajima H (1956) The double bond in lycorine. Chemistry and Industry 16: 1238–1239. [Google Scholar]
- 37. Lee SS, Venkatesham U, Rao CP, Lam SH, Lin JH (2007) Preparation of secolycorines against acetylcholinesterase. Bioorg Med Chem 15: 1034–1043. [DOI] [PubMed] [Google Scholar]