Abstract
Acute lung injury, sepsis, lung inflammation, and ventilator-induced lung injury are life-threatening conditions associated with lung vascular barrier dysfunction, which may lead to pulmonary edema. Increased levels of atrial natriuretic peptide (ANP) in lung circulation reported in these pathologies suggest its potential role in the modulation of lung injury. Besides well recognized physiological effects on vascular tone, plasma volume, and renal function, ANP may exhibit protective effects in models of lung vascular endothelial cell (EC) barrier dysfunction. However, the molecular mechanisms of ANP protective effects are not well understood. The recently described cAMP-dependent guanine nucleotide exchange factor (GEF) Epac activates small GTPase Rap1, which results in activation of small GTPase Rac-specific GEFs Tiam1 and Vav2 and Rac-mediated EC barrier protective responses. Our results show that ANP stimulated protein kinase A and the Epac/Rap1/Tiam/Vav/Rac cascade dramatically attenuated thrombin-induced pulmonary EC permeability and the disruption of EC monolayer integrity. Using pharmacological and molecular activation and inhibition of cAMP- and cGMP-dependent protein kinases (PKA and PKG), Epac, Rap1, Tiam1, Vav2, and Rac we linked ANP-mediated protective effects to the activation of Epac/Rap and PKA signaling cascades, which dramatically inhibited the Rho pathway of thrombin-induced EC hyper-permeability. These results suggest a novel mechanism of ANP protective effects against agonist-induced pulmonary EC barrier dysfunction via inhibition of Rho signaling by Epac/Rap1-Rac and PKA signaling cascades.
Keywords: small GTPases, PKA, guanine nucleotide exchange factors, pulmonary endothelium, permeability
INTRODUCTION
Atrial natriuretic peptide (ANP) regulates a variety of physiological functions including blood pressure, progesterone secretion, and release of rennin, vasopressin, and endothelin by interacting with plasma membrane-bound receptors and elevating intracellular cGMP concentrations, or affecting ion channels (see (Ahluwalia et al., 2004) for review). The role of ANP in the cardiovascular system has been well studied, and investigations have concentrated mainly on the diuretic, natriuretic and vasodilating properties of ANP (Baxter, 2004). However, it is increasingly recognized that ANP functions are not restricted to regulation of volume homeostasis. In vivo and in vitro models of lung injury show that ANP can protect endothelial barrier function apart from its vasodilatory and natriuretic action (Irwin et al., 2001; Irwin et al., 2005; Klinger et al., 2006; Mitaka et al., 1998; Tanabe et al., 1996). However, molecular mechanisms that could explain the protective effects of ANP remain unexplored.
Recent studies have described the involvement of small GTPases Rho and Rac in ANP-mediated regulation of EC permeability (Furst et al., 2005; Klinger et al., 2006). Rho and Rac play important roles in the regulation of cytoskeletal remodeling and EC barrier regulation by mechanical forces and bioactive molecules (Birukov et al., 2004; Birukova et al., 2005a; Birukova et al., 2006a; Birukova et al., 2004c; Essler et al., 1998; Majumdar et al., 1998; Sanders et al., 1999). Rho and Rho-associated kinase may directly catalyze myosin light chain (MLC) phosphorylation or act indirectly via inactivation of MLC phosphatase (van Nieuw Amerongen et al., 2000; Vouret-Craviari et al., 1998) and cause actomyosin-driven cell contraction and EC barrier dysfunction. In turn, EC barrier enhancement is associated with Rac-mediated formation of a peripheral F-actin rim, increased association of focal adhesion proteins, and enlargement of intercellular adherens junctions (Birukov et al., 2004; Garcia et al., 2001; Liu et al., 2002). Thus, a precise balance between Rho- and Rac-mediated signaling is essential for EC barrier regulation. Rac and Rho GTPases act as a molecular switch, cycling between the active GTP-bound and the inactive GDP-bound state which is regulated by guanine nucleotide exchange factors (GEFs) facilitating exchange of GDP for GTP, GTPase-activating proteins (GAPs), which increase the intrinsic rate of GTP hydrolysis by Rho GTPases, and by guanine nucleotide dissociation inhibitors (RhoGDI) which associate with inactivated Rho and Rac (Bishop and Hall, 2000; Boguski and McCormick, 1993; Zheng, 2001). Recent studies have suggested several mechanisms of Rac-mediated downregulation of Rho pathway (Rosenfeldt et al., 2006), including direct Rac interaction with RhoGDI (Wong et al., 2006), PAK1-dependent inhibition of Rho-specific GEF p115RhoGEF, and Rac stimulation of Rho-specific GTPase-activating protein p190-RhoGAP (Herbrand and Ahmadian, 2006). However, the mechanisms of Rac-Rho crosstalk, while critical for endothelial permeability responses (Birukov et al., 2004; Birukova et al., 2006b; Kouklis et al., 2004; Shikata et al., 2003), are still poorly understood.
The second messenger, cAMP, is intimately involved in barrier regulation of the pulmonary vascular endothelium (Birukova et al., 2004b; Cullere et al., 2005; Parker, 2000; Sayner et al., 2006). Several reports indicate the involvement of cAMP and cAMP-dependent protein kinase (PKA) in physiological responses elicited by ANP (Kulhanek-Heinze et al., 2004; Sanghi et al., 2005). Effects of cAMP-elevating agents on EC monolayers have been mostly associated with the activation of PKA-dependent mechanisms of endothelial barrier protection (Birukova et al., 2004b; Liu et al., 2001; Yuan et al., 1997). Recent studies have described a novel PKA-independent pathway of Rac activation via cyclic AMP-activated guanine nucleotide exchange factor (GEF) Epac1 and small GTPase Rap1, which activate Rac-specific GEFs Tiam1 and Vav2 (Bos, 2003; de Rooij et al., 1998).
In this study, we explored cGMP- and cAMP-mediated signaling pathways triggered by ANP and investigated the involvement of PKA and Epac-Rap1 cascades in the ANP-induced activation of Rac and its effector PAK1. Using the inhibitory approach and siRNA-based target protein depletion, we also examined the role of PKA- and Epac-Rap-dependent pathways of Rac activation induced by ANP as protective mechanisms against thrombin-induced Rho activation and EC barrier dysfunction.
MATERIALS AND METHODS
Cell culture and reagents
Human pulmonary artery endothelial cells (HPAEC) were obtained from Cambrex (Walkersville, MD). The cells were maintained in a complete culture medium according to the manufacturer's recommendations and used for experiments at passages 5-9. Texas Red-conjugated phalloidin and Alexa Flour 488 were purchased form Molecular Probes (Eugene, OR). Rho1, Rac1 and VE-cadherin antibodies were purchased from BD Transduction Laboratories (San Diego, CA); Tiam1, Epac1, Rap1, p115-RhoGEF, phospho-Vav2, and pan-Vav2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and HRP-linked anti-mouse and anti-rabbit IgG, di-phospho-MLC, phospho-PKA substrate, phospho-PAK1, and PAK1 antibodies were obtained from Cell Signaling Inc (Beverly, MA). Anti-GEF-H1 antibodies were kindly provided by our co-author Dr. G. Bokoch. NSC-23766, GGTI-298, Rp-8-Br-cGMPS, 8-CPT-2’-O-Me-cAMP, and 8-Br-PET-cGMPS were purchased from Calbiochem (La Jolla, CA). N6-Benzoyl-cAMP was purchased from Biolog (Hayward, CA). Cell permeable PKA inhibitory peptide (PKI) was purchased from Promega (Madison, WI). Unless otherwise specified, all biochemical reagents were obtained from Sigma (St. Louis, MO).
Depletion of Rac1, Tiam1, Vav2, Epac1, Rap1, and GEF-H1 in EC
To reduce the content of target proteins HPAEC were treated with specific siRNA duplexes which guide sequence-specific degradation of homologous mRNA (Elbashir et al., 2001). To down-regulate endogenous expression of GEF-H1, Tiam1, Vav2, or Rac1 HPAEC were treated with gene-specific siRNA duplexes, as described elsewhere (Birukov et al., 2004; Birukova et al., 2006a; Gampel and Mellor, 2002; Malliri et al., 2004). To deplete endogenous Epac1 and Rap1, Stealth™ Select 3 RNAi (Homo sapiens) sets were used. Pre-designed Stealth siRNAs of standard purity were ordered from Invitrogen (Carlsbad, CA), and transfection of EC with siRNA was performed as previously described (Birukov et al., 2004; Birukova et al., 2006a). Non-specific, non-targeting siRNA (Dharmacon Research, Lafayette, CO) was used as a control treatment. Seventy-two hours after transfection, cells were harvested and used for experiments.
Rac, Rho, and Rap activation assays were performed using commercially available assay kits purchased from Upstate Biotechnology (Lake Placid, NY), as we have previously described (Birukov et al., 2004). In brief, after stimulation, cell lysates were collected, and GTP-bound Rac, Rho or Rap were captured using pull-down assays with immobilized PAK1-PBD, rhotekin-RBD, and RalGDS-RBD respectively, according to the manufacturer's protocols. The levels of activated small GTPases as well as total Rho, Rac or Rap content were evaluated by western blot analysis and quantified by scanning densitometry of autoradiography films. The levels of activated GTPases were normalized to Rac, Rho and Rap content in total cell lysates for densitometry evaluations.
Measurement of transendothelial electrical resistance
Measurements of transendothelial electrical resistance (TER) across confluent HPAEC monolayers were performed using the electrical cell-substrate impedance sensing system (ECIS) (Applied Biophysics, Troy, NY) as previously described (Birukov et al., 2004; Birukova et al., 2006a; Birukova et al., 2004a; Birukova et al., 2004c).
Measurements of intracellular cAMP and cGMP levels, and protein kinase A activities were performed using using non-radioactive HitHunter Chemiluminescence assays (Amersham Bioscience, Piscataway, NJ), and PepTag non-radioactive assays (Promega, Madison, WI) according to the manufacturer's instructions.
Immunofluorescence staining
Endothelial monolayers plated on glass cover slips were treated with the agonist of interest, fixed in 3.7% formaldehyde solution in PBS for 10 min at 4° C, washed three times with PBS, permeabilized with 0.1% triton X-100 in PBS-Tween (PBST) for 30 min at room temperature, and blocked with 2% BSA in PBST for 30 min. Incubation with VE-cadherin antibodies were performed in blocking solution (2% BSA in PBST) for 1 hr at room temperature followed by staining with Alexa 488-conjugated secondary antibodies. Actin filaments were stained with Texas Red- conjugated phalloidin. After immunostaining, slides were analyzed using a Nikon video imaging system (Nikon Instech Co., Tokyo, Japan) as described elsewhere (Birukov et al., 2004; Birukova et al., 2004c).
Immunoblotting
After stimulation, cells were lysed, and protein extracts were separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with specific antibodies as previously described (Birukova et al., 2005b). Intensities of immunoreactive protein bands were quantified using Image Quant software.
Statistical analysis
Results are expressed as means ± SD of three to five independent experiments. Stimulated samples were compared to controls by unpaired Student's t-tests. For multiple-group comparisons, a one-way variance analysis (ANOVA), followed by the post hoc Fisher's test, were used. P<0.05 was considered statistically significant.
RESULTS
Effects of ANP on cAMP and cGMP pathways
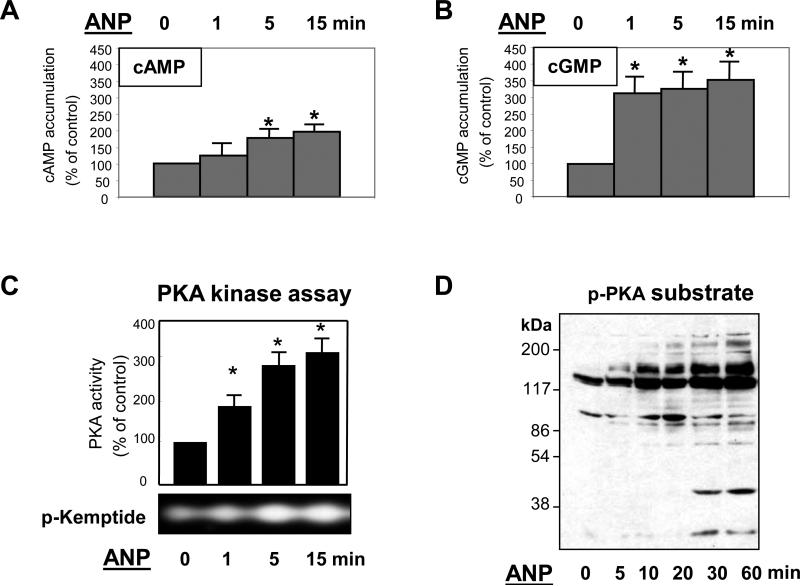
ANP ligation to the NPR-A receptor, which is a membrane-bound guanylate cyclase, stimulates the production of intracellular cGMP and activates cGMP-dependent protein kinase (PKG) (Ahluwalia et al., 2004; Koller and Goeddel, 1992). Our results show that, in addition to significant elevation of intracellular cGMP levels, ANP treatment of human pulmonary EC induced time-dependent increases in intracellular cAMP concentrations (Figure 1A,B) and activation of cAMP-dependent protein kinase (PKA) (Figure 1C). Consistently with these findings, ANP treatment stimulated phosphorylation of intracellular PKA protein substrates (Figure 1D).
Figure 1. Effect of ANP on cAMP, cGMP, and PKA activation.
A and B: EC were stimulated with 100nM of ANP for indicated periods of time, and intracellular cAMP (Panel A) and cGMP (Panel B) levels were determined using a non-radioactive immunoassay, as described in Materials and Methods. Results are mean ± SD of three independent experiments. *P<0.001. C: Cell lysates were analyzed for PKA activity by non-radioactive in vitro PKA assay. The inset represents fluorescent phosphorylated form of PKA substrate kemptide separated from non-phosphorylated form by 0.8% agarose gel electrophoresis. The fluorescence intensity was detected and quantified by a EagleEye Image System. Results are mean ± SD of three independent experiments. *P<0.001. D: PKA-mediated phosphorylation of endogenous substrates was monitored by immunoblotting with anti-phospho-PKA substrate antibody.
Activation of small GTPases and guanine nucleotide exchange factors by ANP
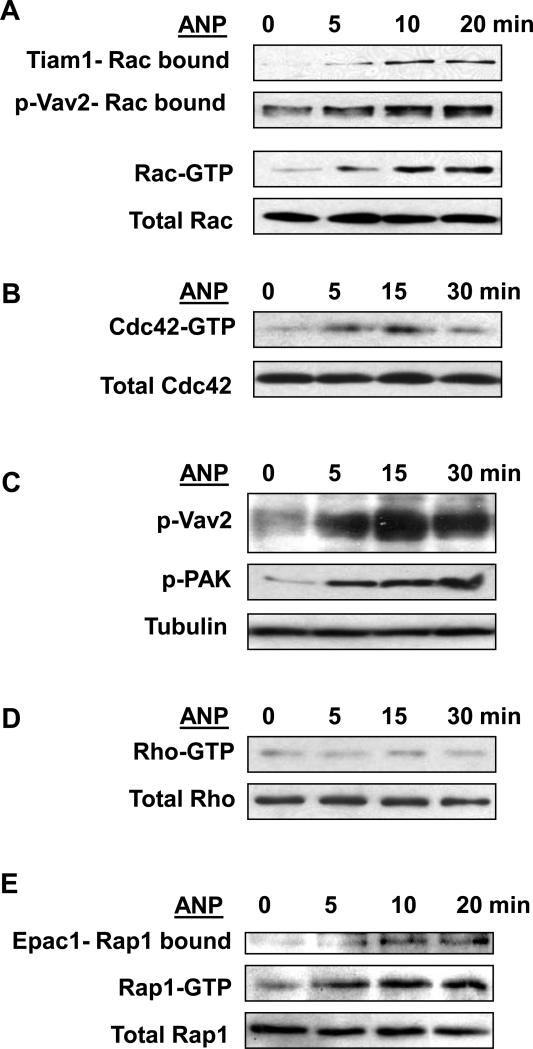
Direct measurements of small GTPase activity in human pulmonary EC showed activation of Rac and Cdc42 upon ANP stimulation (Figure 2A,B). Moreover, ANP stimulation increased association of Rac-specific GEFs Tiam1 and phosphorylated Vav2 with activated Rac (Rac-GTP) bound to PAK1-PBD agarose (Figure 2A, upper panels). Consistently with these data, ANP treatment induced time-dependent phosphorylation of Vav2 and Rac effector PAK1, which were detected in total cell lysates (Figure 2C). Vav2 phosphorylation observed in these experiments reflects the activation of Rac-specific Vav2 nucleotide exchange activity (Aghazadeh et al., 2000). In contrast, Rho activity was not affected by ANP (Figure 2D).
Figure 2. Effect of ANP on activation of small GTPases Rac and Rac-dependent signaling, Cdc42, Rho, and Rap1.
EC were stimulated with 100nM of ANP for indicated periods of time. A: Results of Rac activation assay. Upper panels depict Tiam1 and p-Vav2 bound to activate Rac (Rac-GTP). Middle panel depicts the levels of activated Rac, and the lower panel shows total Rac content in EC lysates. B: Results of Cdc42 activation assay. Upper panel depicts the levels of activated Cdc42, and the lower panel shows total Cdc42 content in EC lysates. C: Phosphorylation of Vav2 and PAK1 in control and ANP-stimulated EC was determined in the total lysates using specific antibodies. Equal loading was confirmed by probing of membranes for β-tubulin. D: Results of Rho activation assay. Upper panel depicts the levels of activated Rho, and the lower panel shows total Rho content in EC lysates. E: Rap1 activation pull-down assay. Upper panels depict Epac1 bound to activate Rap1 (Rap-GTP). Lower panel shows total Rap1 content in EC lysates. Results are representative of three independent experiments.
Recent reports have described a novel mechanism of cAMP-dependent regulation of the Rac pathway via cAMP-binding nucleotide exchange factor Epac, which activates small GTPase Rap1 (Arthur et al., 2004; Fukuhara et al., 2005; Price et al., 2004). Our results show an ANP-induced transient activation of Rap1 detected by in vitro Rap1 activation assay (Figure 2E). Similarly to results depicted in Figure 2A, ANP stimulation caused increased association of Rap1-specific exchange factor Epac1 with activated Rap1 (Rap1-GTP) bound to Ral GDS-RBD agarose.
Involvement of PKA, Epac/Rap1, and Tiam1-Vav2 cascade in the ANP-induced Rac activation
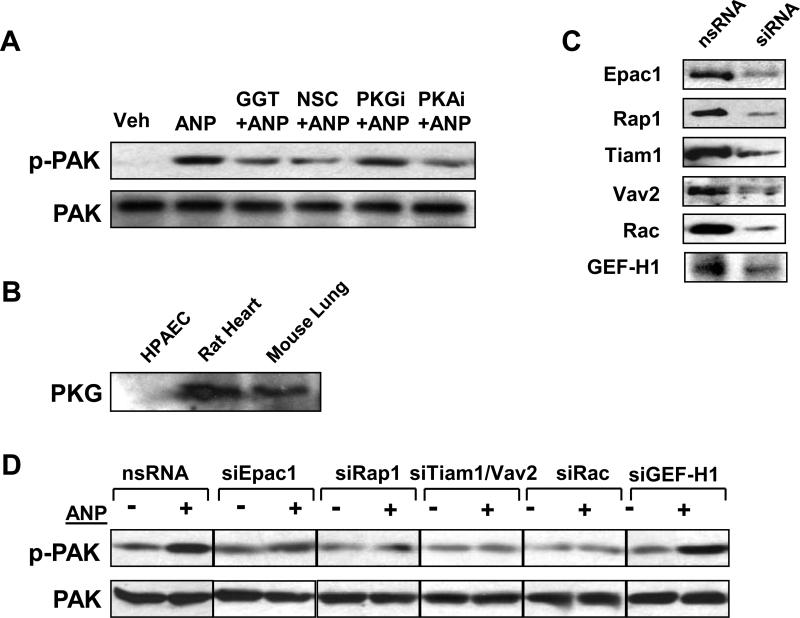
In the following experiments, we examined a potential involvement of PKA-, PKG-, and Epac/Rap-dependent mechanisms in the ANP-mediated activation of Rac-dependent signaling. Pharmacological inhibition of Rap1 processing by a cell permeable inhibitor (GGTI-298, 10μM, 30 min) abolished ANP-induced PAK autophosphorylation associated with Rac activation (Bokoch, 2003), whereas PKG inhibitor Rp-8-Br-cGMPS (10μM, 30 min) was ineffective (Figure 3A). In agreement with previous observations, PKG expression was not detected in human pulmonary artery endothelial cells used in this study (Figure 3B and (Moldobaeva et al., 2005) ). Therefore, in our model inhibitor of PKG was used as a negative control. Treatment of EC with Rac inhibitor NSC-23766 (200 μM, 30 min) served as a positive control and markedly inhibited ANP-induced PAK1 autophosphorylation. Importantly, EC pretreatment with the cell permeable PKA inhibitory peptide PKI (10μM, 30 min) also dramatically attenuated ANP-induced PAK autophosphorylation, suggesting involvement of the PKA-dependent mechanism in Rac activation by ANP (Figure 3A).
Figure 3. Involvement of Epac/Rap1-, Tiam1-Vav2- and PKA-dependent mechanisms in the regulation ANP-mediated Rac activation.
A: EC were pretreated with NSC-23766 (200μM), PKI (10μM), GGTI-298 (10μM), or Rp-8-Br-cGMPS (10μM) for 30 min followed by stimulation with ANP (100 nM, 15 min). Phosphorylation of PAK1 was determined in the total lysates using phospho-PAK1 specific antibody. Results are representative of three independent experiments. B: Expression of PKG has been analyzed by western blot in samples obtained from HPAEC, rat heart, and mouse lung. C: Pulmonary EC were transfected with siRNA specific to Epac1, Rap1, Tiam1, Vav2, Rac, or GEF-H1. Depletion of target proteins induced by specific siRNA duplexes was confirmed by immunoblotting with appropriate antibody, as compared to treatment with non-specific RNA. Results are representative of three to five independent experiments. D: Pulmonary EC were transfected with specific siRNAs followed by ANP stimulation and detection of PAK1 phosphorylation using specific antibody. Control cells were treated with non-specific RNA. Results are representative of three independent experiments.
To further substantiate the involvement of the Epac/Rap1 pathway in ANP-induced Rac activation, we performed sequential protein depletion of Epac, Rap1, Vav2, Tiam1 and Rac using transfection with specific siRNAs (Figure 3C). The expression levels of housekeeping proteins actin and β-tubulin were analyzed in all siRNA experiments and showed no changes in control and siRNA-transfected EC (data not shown). Inhibition of Epac, Rap1, Vav2, Tiam1, or Rac markedly attenuated PAK1 phosphorylation after 15 min of ANP treatment, in comparison with EC transfected with nonspecific RNA duplexes (Figure 3D). Depletion of each member of the Epac cascade tested in these experiments had no effect on the levels of other studied proteins (data not shown). Additional control experiments showed that a siRNA-induced downregulation of Rho-specific exchange factor GEF-H1 was without effect on ANP-induced phosphorylation of PAK1.
ANP attenuates Rho-mediated EC barrier dysfunction
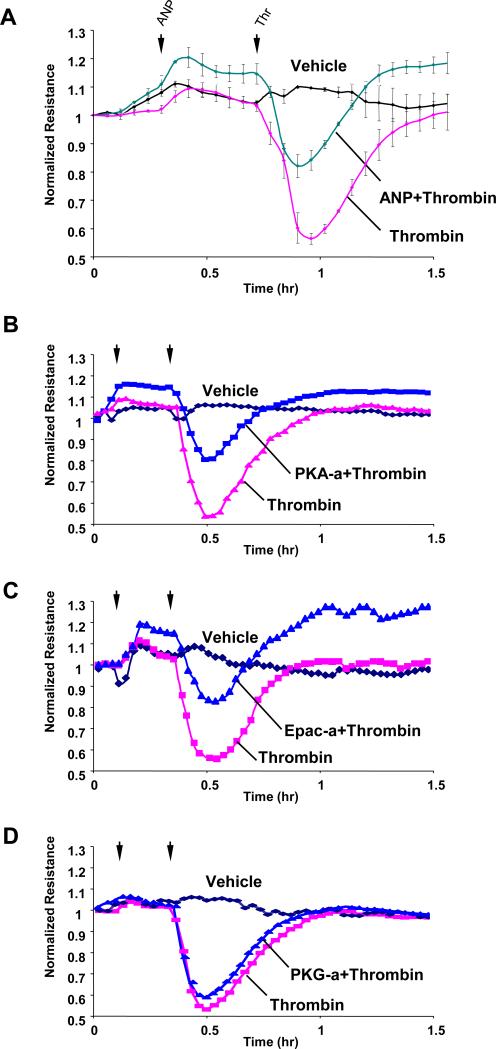
In the following experiments, the potential protective effects of ANP were tested in the model of thrombin-induced pulmonary EC barrier dysfunction. ANP dramatically attenuated thrombin-induced EC hyper-permeability, which was monitored by changes in transendothelial electrical resistance (TER) (Figure 4A). Because our data showed ANP-induced stimulation of cGMP, cAMP, PKA and Epac, we next tested the involvement of these mechanisms in ANP-mediated protective effects against thrombin-induced EC hyper-permeability. PKA activator (N6-Benzoyl-cAMP, 100μM) and Epac activator (8-CPT-2’-O-Me-cAMP, 100μM) markedly attenuated thrombin-induced TER decline, with TER levels elevated above the baseline in EC treated with Epac activator, whereas PKG activator (SP-8-Br-PET-cGMPS, 100μM) exhibited marginal effects (Figure 4B-D). Because cultured human pulmonary artery endothelial cells exhibit residual levels of PKG expression (Moldobaeva et al., 2005), in these experiments PKG activator served as a negative control. Taken together, these data suggest the involvement of PKA and Epac signaling pathways in ANP-mediated barrier protection in pulmonary EC.
Figure 4. Effect of ANP, PKA, Epac, and PKG activators on thrombin-induced permeability.
Human pulmonary EC were treated with ANP (100 nM, marked by first arrow). At the time point indicated by second arrow, cells were stimulated with thrombin (0.5 U/ml), and TER was monitored over the time (upper panel) (A). In complementary experiments N6-Benzoyl-cAMP (100μM) (B), 8-CPT-2’-O-Me-cAMP (100μM) (C), or SP-8-Br-PET-cGMPS (100μM) (D) were used instead of ANP.
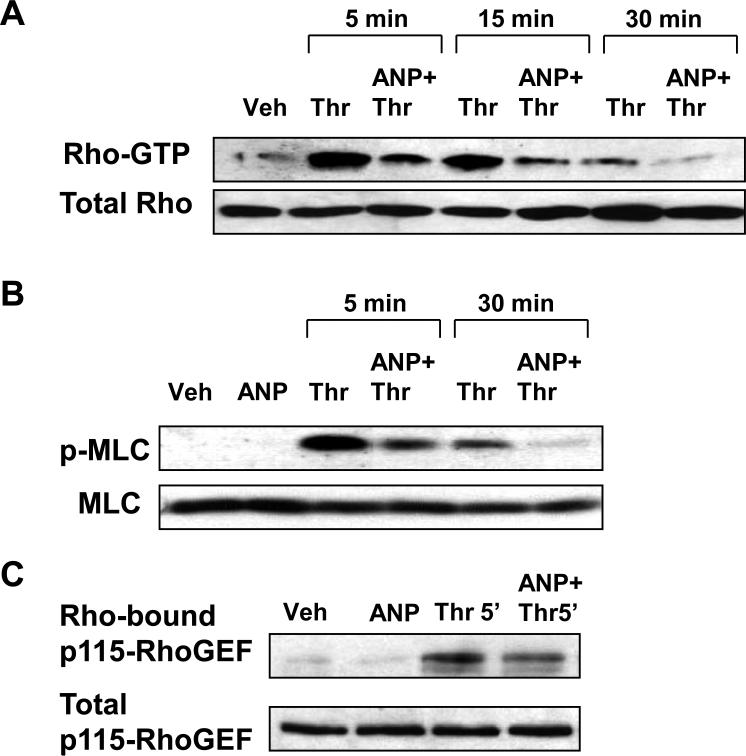
Because thrombin-induced EC barrier dysfunction is mediated by the Rho pathway (Birukova et al., 2004c), we next examined the effects of ANP on thrombin-induced Rho signaling. ANP pretreatment dramatically attenuated thrombin-induced Rho activation (Figure 5A) and MLC phosphorylation (Figure 5B) during the acute phase (5 - 15 min) of thrombin-induced barrier dysfunction and completely inhibited Rho activation and MLC phosphorylation after 30 min of thrombin challenge, the time point corresponding to the onset of EC monolayer recovery (Figure 4A). Remarkably, ANP pretreatment, which suppressed thrombin-induced Rho activation, also significantly attenuated Rho association with its activator, p115-RhoGEF (Figure 5C).
Figure 5. Effect of ANP on thrombin-induced modulation of Rho- and Rac-dependent pathways.
Pulmonary EC were pre-incubated with ANP (100nM, 15 min), followed by treatment with thrombin (0.5 U/ml) for 5 min, 15 min or 30 min.A: Rho activation pull-down assay. Upper panel depicts the levels of activated Rho, and the lower panel shows total Rho content in EC lysates. B: Phosphorylation of MLC in EC pretreated with ANP followed by thrombin challenge was detected by western blot with diphospho-MLC specific antibodies. The lower panel represents the membrane re-probed with pan-MLC antibody. C: The upper panel depicts p115Rho-GEF associated with activated Rho. The lower panel represents p115Rho-GEF content in the total lysates detected by western blot. Results in each group are representative of three independent experiments.
Effects of ANP on thrombin-induced actin stress fiber formation and disruption of pulmonary EC monolayer integrity
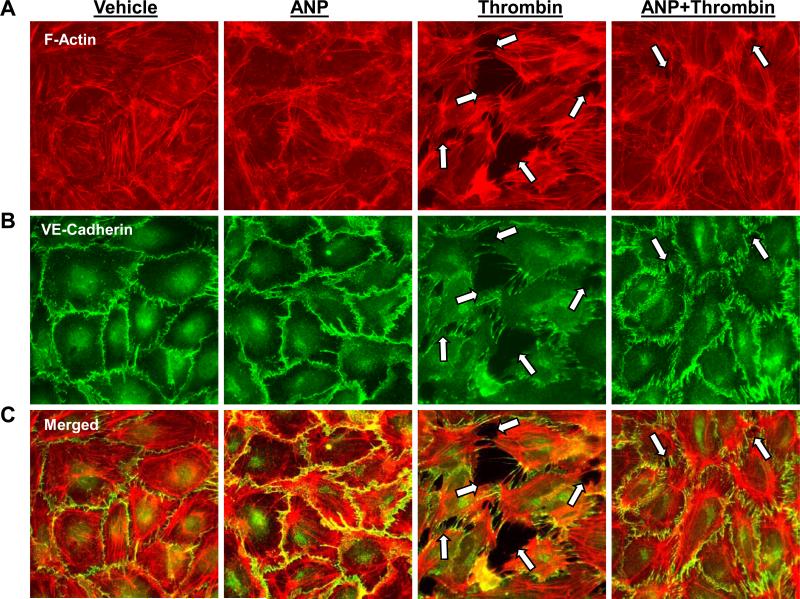
Treatment of pulmonary EC with ANP alone caused slight decrease in central stress fibers and increased VE-cadherin immunoreactivity at the areas of intercellular junctions (Figure 6). Consistently with the protective effects of ANP against thrombin-induced hyper-permeability, thrombin-induced stress fiber formation (Figure 6A), disruption of VE-cadherin-positive adherens junctions (Figure 6B), and paracellular gap formation (Figure 6A-C) was dramatically attenuated by 15-min pretreatment of EC with ANP prior to thrombin challenge.
Figure 6. Effect of ANP on thrombin-induced EC remodeling of actin cytoskeleton, adherens junctions and monolayer disruption.
A and B: EC grown on glass coverslips were preincubated with ANP (100nM 15 min), followed by thrombin treatment (0.5 U/ml) for 15 min and double immunofluorescence staining with Texas Red phalloidin to detect actin filaments (A) and with VE-cadherin antibodies to visualize adherens junctions (B). C: Merged images depict ANP-induced accumulation of peripheral F-actin at adherens junctions’ areas and ANP-mediated preservation of EC monolayer integrity against thrombin-induced disruption. Paracellular gaps and disrupted intercellular contacts are marked by arrows. Results are representative of three independent experiments.
Role of PKA, Rac, and Rap1 in ANP protective effects against thrombin-induced EC barrier dysfunction
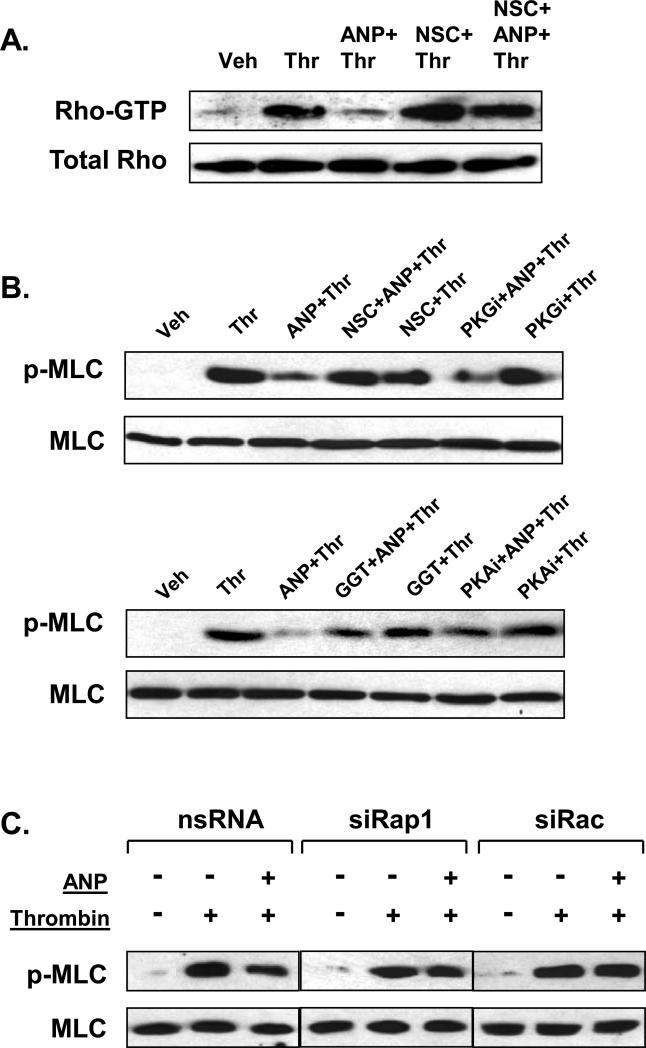
PKA, Epac-Rap and Rac are intimately involved in the mechanisms of endothelial barrier protection (Birukova et al., 2006b; Cullere et al., 2005; Garcia et al., 2001; Kooistra et al., 2005; Qiao et al., 2003). As noted above, an inhibitor of PKG was used as a negative control. In the following experiments we examined the involvement of PKA-, PKG-, Epac/Rap-, and Rac-dependent signaling in the ANP-mediated EC protection against thrombin-induced barrier dysfunction. Pharmacological Rac inhibition by NSC-23766 suppressed the inhibitory effect of ANP on thrombin-induced Rho activation (Figure 7A). In agreement with these observations, downregulation of Rac suppressed inhibitory effects of ANP on thrombin-induced MLC phosphorylation (Figure 7B). Importantly, inhibition of Rap1 processing (GGTI-298, 10μM, 30 min) or PKA activity (PKI, 10μM, 30 min) dramatically decreased inhibitory effect of ANP on thrombin-induced MLC phosphorylation, whereas pretreatment with PKG inhibitor (Rp-8-Br-cGMPS, 10μM, 30 min) did not influence effects of ANP (Figure 7B). Taken together, these results suggest the role of PKA- and Rap-Rac-dependent mechanisms in the inhibition of Rho pathway of EC barrier dysfunction by ANP.
Figure 7. Effects of Rac, Rap1, and PKA inhibition on modulation of thrombin-induced Rho signaling by ANP.
A: EC preincubated with vehicle or NSC-23766 (200 μM, 30min), were treated with ANP (100nM, 15 min) and stimulated with thrombin (0.5 U/ml, 15 min). Control cells were treated with thrombin alone. Rho activation pull-down assays were performed as described in Methods. B: HPAEC were pretreated with NSC-23766 (200μM), Rp-8-Br-cGMPS (10μM) (upper panels), GGTI-298 (10μM), or PKI (10μM) (lower panels) for 30 min followed by ANP stimulation (100nM, 15 min) and thrombin challenge (0.5 U/ml, 15 min). Phosphorylation of MLC was determined in the total lysates using phospho-specific antibodies. Results are representative of three independent experiments. C: Pulmonary EC transfected with Rap1- or Rac-specific siRNA or non-specific RNA duplexes were stimulated with vehicle or ANP (100 nM, 15 min), followed by thrombin addition (0.5 U/ml, 15 min). Phosphorylated MLC was determined by immunoblotting using MLC phospho-specific antibodies. The lower panel represents the membrane re-probed with pan-MLC antibody.
To further delineate the role of the Rap1/Rac-dependent mechanisms in barrier protective effects by ANP, we depleted endogenous Rap1 protein pool using siRNA-based approach. Down-regulation of Rap1 expression significantly reduced inhibitory effects of ANP on thrombin-induced MLC phosphorylation associated with thrombin-induced EC hyper-permeability (Figure 7C). Furthermore, siRNA-based depletion of endogenous Rac dramatically abolished the inhibitory effects of ANP on thrombin-induced MLC phosphorylation.
Our results show that PKG inhibitor neither affected ANP-induced, Rac-dependent PAK autophosphorylation (Figure 3), nor influenced thrombin-induced MLC phosphorylation, nor it affected attenuation of thrombin-induced MLC phosphorylation by ANP (Figure 7). These data clearly show that the PKG mechanism is not involved in the barrier protective effects elicited by ANP in our cell model of acute endothelial barrier dysfunction, most likely due to residual levels of PKG expression in this model (Moldobaeva et al., 2005). Consistently with these results, PKG activator failed to attenuate thrombin-induced permeability in human pulmonary artery EC (Figure 4). However, we do not exclude a possibility for PKG involvement in the barrier regulation in other EC models with considerable PKG expression levels. Indeed, gene transfer of recombinant PKG significantly modified permeability responses of cultured HPAEC to hydrogen peroxide treatment (Moldobaeva et al., 2005).
DISCUSSION
Clinical observations and animal studies strongly suggest beneficiary effects of ANP in pathological settings such as ischemia/reperfusion, acute lung injury, vascular wound repair and pulmonary hypertension. Moreover, recent studies have suggested the role of PKA mechanisms in protective effects of ANP against ischemia-reperfusion-induced spinal cord injury (Nakayama et al., 2007) and ischemia-induced apoptosis in perfused livers (Kulhanek-Heinze et al., 2004).
However, the signaling mechanisms involved in ANP protective effects on the pulmonary EC barrier dysfunction associated with acute lung injury remain virtually unexplored. Inhibitory effects of ANP on Rho-dependent signaling have been implicated in the ANP protective effects against thrombin-induced pulmonary EC permeability (Klinger et al., 2006), and ANP-induced activation of Rac GTPase has been observed (Furst et al., 2005). However, upstream mechanisms of ANP-dependent small GTPase regulation remained unclear. This study described a novel mechanism underlying ANP protective effect against thrombin-induced EC barrier compromise via inhibition of Rho activities by PKA and Epac-Rap1-Rac signaling cascades.
Although activation of membrane-bound guanylate cyclase activity and increased cGMP production is considered to be a major signaling response to ANP, several observations suggest involvement of cAMP- and PKA-mediated signaling in physiologic responses to ANP (Nakayama et al., 2007; Surapisitchat et al., 2007). On the other hand, some authors were unable to detect cAMP increase in EC upon ANP stimulation (Kishi et al., 1994; Yonemaru et al., 1992). A role of endothelial heterogeneity in responses to humoral and mechanical environment is well recognized (Gebb and Stevens, 2004), and apparently conflicting results obtained by different groups clearly suggest that cAMP elevation in response to ANP appears to be dependent on the endothelial cell source (bovine, porcine or human EC) and origin (systemic vs pulmonary circulation; macrovascular versus microvascular beds).
ANP may elevate cAMP concentration via cGMP-mediated inhibition of cAMP phosphodiesterase (Surapisitchat et al., 2007). In addition, the dual regulation of PKA and Epac-Rap1 by cAMP and cGMP has been also described. Under certain conditions, PKA can act as a target molecule for cGMP (Cornwell et al., 1994; Schumacher et al., 1992). The other report demonstrates PKA-independent activation of Rap1 by both cAMP and cGMP analogs and suggests activation of Rap1 through a cAMP/cGMP-regulated guanine nucleotide exchange factor (von Lintig et al., 2000). These two possibilities may account for ANP-induced elevation of cAMP and activation of PKA and Epac/Rap1 signaling reported in this study.
Because cultured pulmonary EC used in this study did not express detectable levels of PKG (Figure 3B and (Mackie et al., 1986; Moldobaeva et al., 2005) ), this model allowed a more detailed analysis of PKG-independent pathways involved in ANP-mediated EC barrier regulation. In vivo, PKG expression levels also depend on multiple factors and may be affected by various pathological conditions. The decreased PKG levels in vascular smooth muscle cells have been found in some models of hypertension and vascular injury. For example in vasculature, PKG expression can be suppressed by hypoxia or high glucose exposure (Liu et al., 2007; Zhou et al., 2007). Pro-inflammatory cytokines decreased PKG expression in vascular smooth muscle cells (Browner et al., 2004a; Browner et al., 2004b). Chronic hypoxia via Rho-dependent mechanisms inhibited PKG in pulmonary vessels (Gao et al., 2007; Zeng et al., 2006). Thus, the role of PKA and other signaling mechanisms may become predominant even in PKG-expressing cells and tissues under conditions when PKG activity or expression is suppressed by pathological factors.
Our data show that ANP induced the activation of Rac and Cdc42, but not Rho in human pulmonary EC. Furthermore, we demonstrate for the first time the critical involvement of the PKA- and Epac/Rap1-dependent mechanisms in ANP-induced activation of Rac pathway. Activation of cAMP-dependent GEF Epac stimulates small GTPase Rap1, which in turn induces membrane localization of Rac-specific GEFs Tiam1 and Vav2 (Arthur et al., 2004), local activation of Rac, and Rac-dependent remodeling of cytoskeleton and adherens junctions associated with EC barrier-protective response (Cullere et al., 2005; Fukuhara et al., 2005; Kooistra et al., 2005; Rangarajan et al., 2003).
In addition to the Epac-Rap1 mechanism, cAMP-induced activation of Tiam1 nucleotide exchange activity may be achieved by PKA-mediated phosphorylation (O'Connor and Mercurio, 2001). In turn, phosphorylation of Vav2 induces conformational change and makes Vav2 DH domain available for interaction with Rac (Aghazadeh et al., 2000; Bustelo, 2000). Consistently with these findings, our data show that ANP causes elevation of cAMP and cGMP levels and stimulation of PKA and Epac/Rap1 signaling leading to Tiam1/Vav2-dependent Rac activation.
Our data show the inhibitory effects of ANP on Rho activation and EC barrier dysfunction caused by thrombin. These effects may be due to ANP-induced activation of cAMP/PKA and direct phosphorylation of RhoA, which has an inhibitory effect on RhoA activity (Dong et al., 1998; Lang et al., 1996). Another reported mechanism of PKA-mediated Rho inhibition is PKA-mediated phosphorylation of Rho-GDP dissociation inhibitor, a negative regulator of small GTPase Rho, which results in Rho inactivation and blocks Rho-dependent mechanism of EC hyperpermeability (Qiao et al., 2003).
Accumulating evidence suggests that small GTPases may regulate the activity of each other. However, precise mechanisms of crosstalk between Rac and Rho remain to be determined. Our results show that ANP treatment decreased thrombin-induced association of p115-RhoGEF with Rho (Figure 5C). On the other hand, ANP stimulated the Rac-PAK1 pathway (Figure 2). Recent report indicates that Rac effector PAK1 may associate with and inhibit p115-RhoGEF, which plays a major role in signaling from thrombin receptors to Rho (Rosenfeldt et al., 2006). ANP may also inactivate Rho directly by PKA-dependent phosphorylation or indirectly, via PKA-dependent phosphorylation of Rho-GDI (Dong et al., 1998; Lang et al., 1996; Qiao et al., 2003). Alternatively, activated Rac may regulate RhoA activity via direct interaction with RhoGDI (Wong et al., 2006). Activation of Rac downstream target PAK1 may further increase the Rac activity and enhance Rac inhibitory effects on Rho pathway (Herbrand and Ahmadian, 2006). Collectively, these results emphasize complexity of the mechanisms regulating the crosstalk between Rac and Rho. Therefore, we believe that PAK1-dependent inhibition of p115RhoGEF is not a sole mechanism of ANP-mediated Rho inactivation. Further investigation of the mechanisms of Rac/Rho cross-regulation is the focus of our ongoing studies.
Agonist-induced elevation of cAMP has been previously associated with barrier-protective effects on endothelium via PKA-dependent phosphorylation of several targets including myosin light chain, Rho and Rho-GDI leading to decreased myosin light chain phosphorylation and inhibition of cell contraction and gap formation (Mehta and Malik, 2006). Our recent study (Birukova et al., 2007) and report by Cullere et al. (Cullere et al., 2005) describes signaling pathways activated by prostaglandin E2 (PGE2) and prostacyclin and demonstrates that barrier-protective effects of these prostaglandins on the thrombin-induced permeability are mediated by the PKA and Epac/Rap pathways, which converge on Rac activation and lead to the enhancement of peripheral actin cytoskeleton and adherens junctions. We suggest that cAMP-dependent activation of the PKA and Epac/Rap pathways may be a common mechanism of EC barrier protection by physiologic cAMP-elevating agonists including ANP in the settings of acute lung injury caused by high tidal volume mechanical ventilation or bacterial infection.
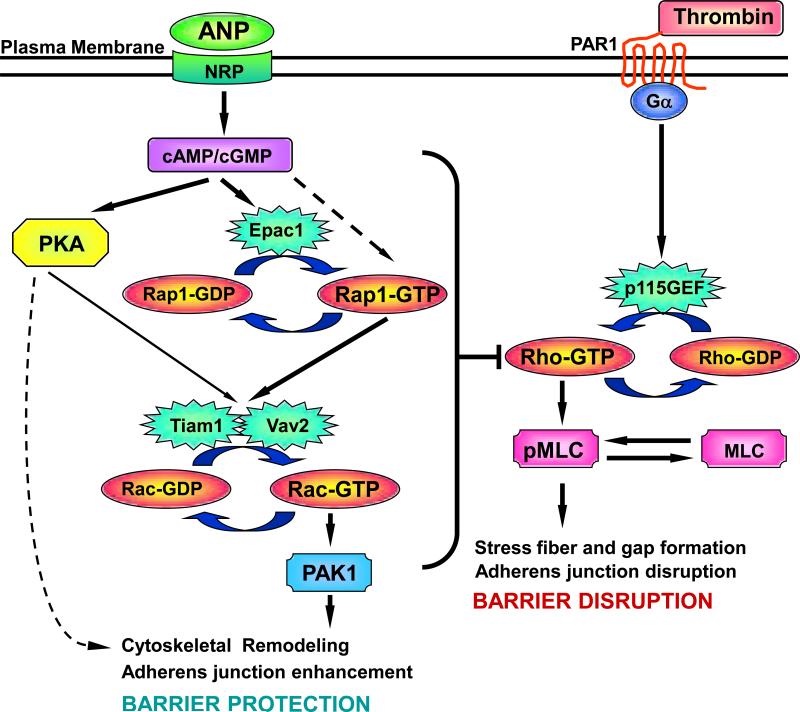
In summary, the results of this study demonstrate that ANP markedly attenuates barrier dysfunction induced by edemagenic mediator thrombin and promotes EC barrier recovery following pathological stimulation. Importantly, our data suggest a novel mechanism of ANP-mediated protective effects against agonist-induced pulmonary EC barrier dysfunction, which involves the regulation of small GTPases Rac and Rho by Rap- and PKA-dependent mechanisms. Based on previous reports and the results of this study, we propose a hypothetical scheme of ANP-mediated regulation of small GTPases and EC permeability by cAMP and cGMP (Figure 8). Activation of Rap1 by cGMP via putative cGMP-activated GEF has been reported previously (von Lintig et al., 2000). In addition, Rap1 activity is regulated by cAMP-dependent guanine nucleotide exchange factor Epac1 (Bos, 2003), which also appears to be activated by high concentrations of cGMP (Christensen et al., 2003). Activation of Epac/Rap1- and PKA-dependent signaling then leads to Tiam1/Vav2-dependent activation of Rac. Activated Rac stimulates PAK1 and cytoskeletal Rac effectors. Activation of Rac signaling also inhibits Rho activity, possibly via PAK1-dependent inhibition of p115RhoGEF activity, attenuates Rho-mediated barrier disruption and may contribute to the maintenance of EC monolayer integrity in injured lungs. These findings may represent a fundamental mechanism of endothelial cellular response to a spectrum of barrier-protective agonists. Thus, molecular mechanisms of the protective effects of ANP in the model of pulmonary EC barrier dysfunction described in this study may be utilized for identification of novel protein targets and development of new therapies for prevention of pulmonary vascular leak associated with acute lung inflammation and injury.
Figure 8. Upstream mechanisms of ANP-induced Rac activation and modulation of Rho pathway of EC barrier dysfunction.
Stimulation of EC with ANP elevates intracellular cAMP/cGMP levels and stimulates cAMP-dependent protein kinase (PKA) and cAMP-activated guanine nucleotide exchange factor Epac1, which activates its effector small GTPase Rap1. Epac1 may be also activated by high local cGMP concentrations (Christensen et al., 2003). In addition, Rap1 may be activated by putative cGMP-specific GEF (von Lintig et al., 2000). Activated PKA and Rap1 promote Rac activation via stimulation of Rac specific GEFs Tiam1 and Vav2. Activated Rac attenuates the Rho pathway of endothelial barrier dysfunction via reduction of Rho activity, which leads to decreased myosin light chain phosphorylation, EC contraction, and less severe endothelial barrier dysfunction. In addition, PKA may directly affect EC cytoskeletal organization and monolayer barrier properties via modulation of myosin light chain kinase activity or VASP-dependent relaxation of actin cytoskeleton.
AKNOWLEDGEMENTS
This work was supported by grants from National Heart, Lung, and Blood Institutes (HL076259 and HL075349), AHA Scientist Development grant and ALA Biomedical Research grant for AAB. The authors also wish to thank Nurgul Moldobaeva for superb laboratory assistance.
REFERENCES
- Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102(5):625–633. doi: 10.1016/s0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Ahluwalia A, MacAllister RJ, Hobbs AJ. Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res Cardiol. 2004;99(2):83–89. doi: 10.1007/s00395-004-0459-6. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167(1):111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter GF. The natriuretic peptides. Basic Res Cardiol. 2004;99(2):71–75. doi: 10.1007/s00395-004-0457-8. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95(9):892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Adyshev D, Gorshkov B, Birukov KG, Verin AD. ALK5 and Smad4 are involved in TGF-beta1-induced pulmonary endothelial permeability. FEBS Lett. 2005a;579(18):4031–4037. doi: 10.1016/j.febslet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AA. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006a;290(3):L540–548. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Gorshkov B, Liu F, Garcia JG, Verin AD. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol. 2005b;289(1):L75–84. doi: 10.1152/ajplung.00447.2004. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Smurova K, Adyshev DM, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 2004a;18(15):1879–1890. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol. 2006b;168(5):1749–1761. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Liu F, Garcia JG, Verin AD. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol. 2004b;287(1):L86–93. doi: 10.1152/ajplung.00441.2003. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004c;67(1):64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Alekseeva E, Fu P, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313(11):2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-Activated Kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4(9):733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Browner NC, Dey NB, Bloch KD, Lincoln TM. Regulation of cGMP-dependent protein kinase expression by soluble guanylyl cyclase in vascular smooth muscle cells. J Biol Chem. 2004a;279(45):46631–46636. doi: 10.1074/jbc.M408518200. [DOI] [PubMed] [Google Scholar]
- Browner NC, Sellak H, Lincoln TM. Downregulation of cGMP-dependent protein kinase expression by inflammatory cytokines in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004b;287(1):C88–96. doi: 10.1152/ajpcell.00039.2004. [DOI] [PubMed] [Google Scholar]
- Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20(5):1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Doskeland SO. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278(37):35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1994;267(5 Pt 1):C1405–1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105(5):1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396(6710):474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Dong JM, Leung T, Manser E, Lim L. cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKalpha. J Biol Chem. 1998;273(35):22554–22562. doi: 10.1074/jbc.273.35.22554. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273(34):21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25(1):136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst R, Brueckl C, Kuebler WM, Zahler S, Krotz F, Gorlach A, Vollmar AM, Kiemer AK. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res. 2005;96(1):43–53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- Gampel A, Mellor H. Small interfering RNAs as a tool to assign Rho GTPase exchange-factor function in vivo. Biochem J. 2002;366(Pt 2):393–398. doi: 10.1042/BJ20020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Portugal AD, Negash S, Zhou W, Longo LD, Usha Raj J. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L678–684. doi: 10.1152/ajplung.00178.2006. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108(5):689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res. 2004;68(1):1–12. doi: 10.1016/j.mvr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Herbrand U, Ahmadian MR. p190-RhoGAP as an integral component of the Tiam1/Rac1-induced downregulation of Rho. Biol Chem. 2006;387(3):311–317. doi: 10.1515/BC.2006.041. [DOI] [PubMed] [Google Scholar]
- Irwin DC, Rhodes J, Baker DC, Nelson SE, Tucker A. Atrial natriuretic peptide blockade exacerbates high altitude pulmonary edema in endotoxin-primed rats. High Alt Med Biol. 2001;2(3):349–360. doi: 10.1089/15270290152608525. [DOI] [PubMed] [Google Scholar]
- Irwin DC, Tissot van Patot MC, Tucker A, Bowen R. Direct ANP inhibition of hypoxia-induced inflammatory pathways in pulmonary microvascular and macrovascular endothelial monolayers. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L849–859. doi: 10.1152/ajplung.00294.2004. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Ashikaga T, Watanabe R, Numano F. Atrial natriuretic peptide reduces cyclic AMP by activating cyclic GMP-stimulated phosphodiesterase in vascular endothelial cells. J Cardiovasc Pharmacol. 1994;24(3):351–357. doi: 10.1097/00005344-199409000-00001. [DOI] [PubMed] [Google Scholar]
- Klinger JR, Warburton R, Carino GP, Murray J, Murphy C, Napier M, Harrington EO. Natriuretic peptides differentially attenuate thrombin-induced barrier dysfunction in pulmonary microvascular endothelial cells. Exp Cell Res. 2006;312(4):401–410. doi: 10.1016/j.yexcr.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Koller KJ, Goeddel DV. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992;86(4):1081–1088. doi: 10.1161/01.cir.86.4.1081. [DOI] [PubMed] [Google Scholar]
- Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579(22):4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- Kouklis P, Konstantoulaki M, Vogel S, Broman M, Malik AB. Cdc42 regulates the restoration of endothelial barrier function. Circ Res. 2004;94(2):159–166. doi: 10.1161/01.RES.0000110418.38500.31. [DOI] [PubMed] [Google Scholar]
- Kulhanek-Heinze S, Gerbes AL, Gerwig T, Vollmar AM, Kiemer AK. Protein kinase A dependent signalling mediates anti-apoptotic effects of the atrial natriuretic peptide in ischemic livers. J Hepatol. 2004;41(3):414–420. doi: 10.1016/j.jhep.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. Embo J. 1996;15(3):510–519. [PMC free article] [PubMed] [Google Scholar]
- Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J. 2002;16(9):950–962. doi: 10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- Liu F, Verin AD, Borbiev T, Garcia JG. Role of cAMP-dependent protein kinase A activity in endothelial cell cytoskeleton rearrangement. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1309–1317. doi: 10.1152/ajplung.2001.280.6.L1309. [DOI] [PubMed] [Google Scholar]
- Liu S, Ma X, Gong M, Shi L, Lincoln T, Wang S. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic Biol Med. 2007;42(6):852–863. doi: 10.1016/j.freeradbiomed.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Nairn AC, Greengard P, Pitt BR, Lazo JS. Protein phosphorylation in cultured endothelial cells. J Cell Physiol. 1986;128(3):367–374. doi: 10.1002/jcp.1041280304. [DOI] [PubMed] [Google Scholar]
- Majumdar M, Seasholtz TM, Goldstein D, de Lanerolle P, Brown JH. Requirement for Rho-mediated myosin light chain phosphorylation in thrombin-stimulated cell rounding and its dissociation from mitogenesis. J Biol Chem. 1998;273(17):10099–10106. doi: 10.1074/jbc.273.17.10099. [DOI] [PubMed] [Google Scholar]
- Malliri A, van Es S, Huveneers S, Collard JG. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem. 2004;279(29):30092–30098. doi: 10.1074/jbc.M401192200. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Mitaka C, Hirata Y, Nagura T, Tsunoda Y, Amaha K. Beneficial effect of atrial natriuretic peptide on pulmonary gas exchange in patients with acute lung injury. Chest. 1998;114(1):223–228. doi: 10.1378/chest.114.1.223. [DOI] [PubMed] [Google Scholar]
- Moldobaeva A, Welsh-Servinsky LE, Shimoda LA, Stephens RS, Verin AD, Tuder RM, Pearse DB. Role of Protein Kinase G in Barrier Protective Effects of cGMP in Human Pulmonary Artery Endothelial Cells. Am J Physiol Lung Cell Mol Physiol. 2005 doi: 10.1152/ajplung.00434.2005. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Harada N, Asano M, Nomura N, Saito T, Mishima A, Okajima K. Atrial natriuretic peptide reduces ischemia/reperfusion-induced spinal cord injury in rats by enhancing sensory neuron activation. J Pharmacol Exp Ther. 2007;322(2):582–590. doi: 10.1124/jpet.107.120725. [DOI] [PubMed] [Google Scholar]
- O'Connor KL, Mercurio AM. Protein kinase A regulates Rac and is required for the growth factor-stimulated migration of carcinoma cells. J Biol Chem. 2001;276(51):47895–47900. doi: 10.1074/jbc.M107235200. [DOI] [PubMed] [Google Scholar]
- Parker JC. Inhibitors of myosin light chain kinase and phosphodiesterase reduce ventilator-induced lung injury. J Appl Physiol. 2000;89(6):2241–2248. doi: 10.1152/jappl.2000.89.6.2241. [DOI] [PubMed] [Google Scholar]
- Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279(34):35127–35132. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L972–980. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol. 2003;160(4):487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeldt H, Castellone MD, Randazzo PA, Gutkind JS. Rac inhibits thrombin-induced Rho activation: evidence of a Pak-dependent GTPase crosstalk. J Mol Signal. 2006;1:8. doi: 10.1186/1750-2187-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase [see comments]. Science. 1999;283(5410):2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Sanghi S, Kumar R, Smith M, Baker KM, Dostal DE. Activation of protein kinase A by atrial natriuretic peptide in neonatal rat cardiac fibroblasts: role in regulation of the local renin-angiotensin system. Regul Pept. 2005;132(1-3):1–8. doi: 10.1016/j.regpep.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Sayner SL, Alexeyev M, Dessauer CW, Stevens T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res. 2006;98(5):675–681. doi: 10.1161/01.RES.0000209516.84815.3e. [DOI] [PubMed] [Google Scholar]
- Schumacher H, Muller D, Mukhopadhyay AK. Stimulation of testosterone production by atrial natriuretic peptide in isolated mouse Leydig cells results from a promiscuous activation of cyclic AMP-dependent protein kinase by cyclic GMP. Mol Cell Endocrinol. 1992;90(1):47–52. doi: 10.1016/0303-7207(92)90100-k. [DOI] [PubMed] [Google Scholar]
- Shikata Y, Birukov KG, Birukova AA, Verin AD, Garcia JG. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J. 2003;17(15):2240–2249. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- Surapisitchat J, Jeon KI, Yan C, Beavo JA. Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ Res. 2007;101(8):811–818. doi: 10.1161/CIRCRESAHA.107.154229. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Ueda M, Endo M, Kitajima M. The effect of atrial natriuretic peptide on pulmonary acid injury in a pig model. Am J Respir Crit Care Med. 1996;154(5):1351–1356. doi: 10.1164/ajrccm.154.5.8912747. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87(4):335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- von Lintig FC, Pilz RB, Boss GR. Quantitative determination of Rap 1 activation in cyclic nucleotide-treated HL-60 leukemic cells: lack of Rap 1 activation in variant cells. Oncogene. 2000;19(35):4029–4034. doi: 10.1038/sj.onc.1203741. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V, Boquet P, Pouyssegur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell. 1998;9(9):2639–2653. doi: 10.1091/mbc.9.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KW, Mohammadi S, Isberg RR. Disruption of RhoGDI and RhoA regulation by a Rac1 specificity switch mutant. J Biol Chem. 2006;281(52):40379–40388. doi: 10.1074/jbc.M605387200. [DOI] [PubMed] [Google Scholar]
- Yonemaru M, Ishii K, Murad F, Raffin TA. Atriopeptin-induced increases in endothelial cell permeability are associated with elevated cGMP levels. Am J Physiol. 1992;263(3 Pt 1):L363–369. doi: 10.1152/ajplung.1992.263.3.L363. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Huang Q, Wu HM. Myosin light chain phosphorylation: modulation of basal and agonist-stimulated venular permeability. Am J Physiol. 1997;272(3 Pt 2):H1437–1443. doi: 10.1152/ajpheart.1997.272.3.H1437. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Zhuang S, Gloddek J, Tseng CC, Boss GR, Pilz RB. Regulation of cGMP-dependent protein kinase expression by Rho and Kruppel-like transcription factor-4. J Biol Chem. 2006;281(25):16951–16961. doi: 10.1074/jbc.M602099200. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26(12):724–732. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- Zhou W, Dasgupta C, Negash S, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol. 2007;292(6):L1459–1466. doi: 10.1152/ajplung.00143.2006. [DOI] [PubMed] [Google Scholar]