Abstract
Previous studies showed that cyclopenthenone-containing products resulting from oxidation of a natural phospholipid, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC) exhibit potent barrier-protective effects in the in vitro and in vivo models of lung endothelial cell (EC) barrier dysfunction, and these effects are associated with enhancement of peripheral actin cytoskeleton, cell-cell and cell-substrate contacts driven by activation of Rac and Cdc42 GTPases. Rap1 GTPase is another member of small GTPase family involved in control of cell-cell interactions; however its involvement in EC barrier-protective effects by OxPAPC remains unknown. This study examined a role of Rap1 in regulation of OxPAPC-induced interactions in adherens junctions (AJ) and tight junctions (TJ) as a novel mechanism of EC barrier preservation in vitro and in vivo. Immunofluorescence analysis, subcellular fractionation, and co-immunoprecipitation assays indicate that OxPAPC promoted accumulation of AJ proteins: VE-cadherin, p120-catenin, and β-catenin; and TJ proteins: ZO-1, occludin, and JAM-A in the cell membrane, and induced novel cross-interactions between AJ and TJ protein complexes, that were dependent on OxPAPC-induced Rap1 activation. Inhibition of Rap1 function suppressed OxPAPC-mediated pulmonary EC barrier enhancement and AJ and TJ interactions in vitro, as well as inhibited protective effects of OxPAPC against ventilator-induced lung injury in vivo. These results show for the first time a role of Rap1-mediated association between adherens junctions and tight junction complexes in the OxPAPC-induced pulmonary vascular EC barrier protection.
Keywords: OxPAPC, small GTPases, endothelium, permeability, cytoskeleton, cell contacts
INTRODUCTION
Cell membrane phospholipids and phospholipids present in circulating lipoproteins may undergo oxidation by lipoxygenases or reactive oxygen and nitrogen species as result of ventilator-induced lung injury, trauma, or septic inflammation (Fu and Birukov, 2009; Kalyanaraman, 2004; Morrow and Roberts, 2002; Pennathur et al., 2004; Waters, 2004). Under these conditions, lung vascular barrier function is largely compromised, and lysophospholipids and terminal products of lipid oxidation contribute to development of lung inflammation and vascular leak (Qiao et al., 2006; Quinlan et al., 1996; Usatyuk and Natarajan, 2004). However, specific products of phospholipid oxidation such as 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC) reveal potent anti-inflammatory (Bochkov et al., 2002) and barrier-protective (Birukov et al., 2004a; Birukova et al., 2007b) effects. OxPAPC-induced endothelial barrier enhancement plays essential role in prevention of lung vascular leak and pulmonary edema associated with LPS- and ventilator-induced lung injury (Birukova et al., 2007c; Ma et al., 2004; Nonas et al., 2008; Nonas et al., 2006). OxPAPC enhances endothelial cell (EC) barrier by stimulating the peripheral F-actin rim formation mediated by small GTPases Rac and Cdc42. In EC monolayers challenged with barrier disruptive agonists, OxPAPC attenuates agonist-induced Rho pathway of EC barrier dysfunction and accelerates Rac-dependent recovery of EC monolayer integrity (Birukov et al., 2004a; Birukova et al., 2007c; Nonas et al., 2008).
While some specific effects of OxPAPC can be partially inhibited by platelet activating factor (PAF) receptor antagonists (Kadl et al., 2002; Leitinger et al., 1997; Subbanagounder et al., 1999), PAF itself does not mimic barrier-protective OxPAPC effects (Birukova et al., 2007b), and instead is a well-recognized edemagenic agent (Goggel et al., 2004). Other OxPAPC effects on gene expression may be inhibited by prostaglandin receptor EP-2 antagonists (Li et al., 2006), while other study proposes G protein-coupled receptor-mediated activation of CS-1 fibronectin production by OxPL species (Cole et al., 2003). However, specific receptor(s) involved in activation of Rap1 or Rac signaling, cytoskeletal and cell junction enhancement, and barrier-protective effects of OxPAPC remain to be discovered.
Adherens junctions (AJ) provide mechanical adhesion between neighboring cells via transmembrane proteins cadherins bound together in a homotypic and Ca2+-dependent fashion. Through their cytoplasmic tail, cadherins interact with the catenin family of intracellular proteins (α-, β-, γ-catenins, p120-catenin), which provide anchorage of cell-cell junction membrane complexes to the actin cytoskeleton (Aberle et al., 1996). In addition, p120-catenin has been recently identified as a novel modulator of Rac and Rho activity at the cell-cell contacts (Anastasiadis and Reynolds, 2001; Grosheva et al., 2001; Wildenberg et al., 2006).
Similar to adherens junctions, tight junctions (TJ) mediate adhesion and communication between adjacent and contacting cells. TJ are composed of integral membrane proteins: occludin, claudins, Junctional Adhesion Molecule-A (JAM-A), and intracellular proteins, including ZO-1 and cingulin (Bazzoni et al., 2000; Dejana, 2004; Miyoshi and Takai, 2005). Although AJ and TJ are separate structural entities, under certain conditions their components may interact with each other. For example, interactions of Rap1 effector afadin with ZO-1 and α-catenin have been found in epithelial cell lines (Takai and Nakanishi, 2003). Whether AJ-TJ associations can be formed in pulmonary endothelium, what is their role in regulation of EC permeability, and how they may be regulated by Rap1 GTPase remains completely unknown.
Small GTPase Rap1 is activated by extracellular signals through several regulatory proteins and may function in diverse processes, ranging from modulation of growth and differentiation to enhancement of cell adhesions and lung endothelial permeability (Birukova et al., 2009; Bos et al., 2001; Fukuhara et al., 2005; Kooistra et al., 2005). Rap1 activity is negatively regulated by Rap-specific GTPase activator protein RapGAP. Ectopic expression of Rap-GAP blocks Rap1 activation and abolishes Rap1-mediated adhesion and enhancement of adherens junctions in epithelial cells (Fukuyama et al., 2006; Rangarajan et al., 2003). Via activation of Rac-specific guanine nucleotide exchange factors Tiam1 and Vav2, Rap1 may activate Rac GTPase and trigger additional Rac-mediated mechanisms of EC barrier enhancement (Birukova et al., 2007f). However, the involvement of Rap1 in the mechanisms of OxPAPC-induced barrier enhancement and cell junction rearrangements remains unknown.
In this study we examined effects of OxPAPC on the adherens junction and tight junction assembly and interactions, investigated a role of small GTPase Rap1 in the regulation of adherens junction and tight junction association by OxPAPC, and tested a novel Rap1-dependent mechanism of lung vascular barrier preservation by oxidized phospholipids in pulmonary endothelial monolayers and in the in vivo model of ventilator induced lung injury.
MATERIALS AND METHODS
Cell culture and reagents
Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza (Allendale, NJ). Cells were maintained in a complete culture medium according to the manufacturer's recommendations and used for experiments at passages 5-7. Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO). Rap1, VE-cadherin, β- and p120-catenin, JAM-A and occludin antibodies were purchased from BD Transduction Laboratories (San Diego, CA); p115RhoGEF and cMyc antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); ZO-1 antibody was purchased from Invitrogen (Carlsbad, CA). All reagents for immunofluorescence were purchased from Molecular Probes (Eugene, OR).
Lipid oxidation and analysis
Non oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC) was obtained from Avanti Polar Lipids (Alabaster, AL). PAPC was oxidized by exposure to air for 72 hours. The extent of oxidation was measured by positive ion electrospray mass spectrometry (ESIMS) as previously described (Watson et al., 1997). After completion of oxidation, the phospholipids were stored at –70°C dissolved in chloroform and used within 2 weeks after mass spectrometry testing. All oxidized and non-oxidized phospholipid preparations were analyzed by the limulus amebocyte assay (BioWhittaker, Frederick, MD) and shown negative for endotoxin.
Protein depletion using siRNA approach
To deplete endogenous Rap1, p120-catenin, or ZO-1 Stealth™ Select 3 RNAi sets were used. Pre-designed siRNAs of standard purity were ordered from Invitrogen (Carlsbad, CA), and transfection of EC with siRNA was performed as previously described (Singleton et al., 2009). Nonspecific, non-targeting siRNA was used as a control treatment. After 48 hrs of transfection cells were used for experiments or harvested for western blot verification of specific protein depletion. Our preliminary testing demonstrated that nearly complete depletion of Rap1, p120-catenin, or ZO-1 significantly decreased basal levels of electrical resistance, led to disruption of EC barrier and even caused cell detachment in select experiments. Therefore, the experiments were performed after 48 hrs of siRNA transfection. These conditions provided significant target protein depletion verified by western blot analysis but caused only modest reduction in basal transmonolayer electrical resistance in TER studies in comparison to non-specific RNA controls (data not shown). For in vivo depletion, polymer-based administration of non-specific or Rap1-specific siRNA conjugated with polycation polyethilenimine (PEI-22) shown to promote lung-specific DNA and siRNA delivery (Thomas et al., 2005a; Thomas et al., 2005b) was used as described elsewhere (Singleton et al., 2009). PEI-22 was kindly provided by Dr. A. Klibanov. SiRNA at 4 mg/kg showed the most significant inhibition of the target gene after 72 hrs of transfection, as determined by western blot analysis. Treated mice showed no signs of non-specific siRNA-induced inflammation. Nonspecific, non-targeting siRNA (Dharmacon, Lafayette, CO) was used as a control treatment for both in vitro and in vivo experiments.
Expression plasmids and transfection
Plasmid encoding Rap-GAP bearing cMyc tag was kindly provided by Dr. L. Quilliam. EC were used for transient transfections according to protocol described previously (Birukov et al., 2004a; Birukova et al., 2004). Control transfections were performed with empty vectors. For permeability measurements, more effective introduction of cDNA into the cell, nucleofection of HPAEC was performed using a kit from Amaxa Biosystems (Gaithersburg, MD). Optimized protocol of nucleofection is provided by manufacturer and used with minor modifications described previously (Birukova et al., 2006; Birukova et al., 2008a).
Rap1 activation assay
Activation of Rap1 GTPase in pulmonary EC culture was analyzed using Rap in vitro pulldown assay kit available from Millipore (Billerica, MA) according to the manufacturer's protocols, as previously described (Birukova et al., 2007f).
Measurement of transendothelial electrical resistance
The cellular barrier properties were analyzed by measurements of transendothelial electrical resistance (TER) across confluent human pulmonary artery endothelial monolayers using an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) as previously described (Birukova et al., 2004).
Immunofluorescence
Endothelial monolayers plated on glass cover slips were subjected to immunofluorescence staining, as described previously (Birukova et al., 2007e). After immunostaining, slides were analyzed using a Nikon video imaging system (Nikon Instech Co., Tokyo, Japan). Confocal microscopy was performed using Leica SP2A OBS Laser Scanning Confocal microscope, and images were processed with Image J software (National Institute of Health, Washington, USA) and Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) software.
Differential protein fractionation
Confluent HPAEC were stimulated with the agonist of interest, and after rapid wash with ice cold PBS, cytosolic fraction was isolated by centrifugation using extraction buffer containing 50 mM Tris-HCl pH 7.4, 100 mM sodium chloride, 0.01% digitonin, and protease/phosphatase inhibitor cocktail. Next, pellets were resuspended in extraction buffer containing 0.05 mol/L Tris-HCl pH 7.4, 2% Triton X-100, 100 mM sodium chloride and protease/phosphatase inhibitor cocktail and incubated on ice for 30 min. The membrane fraction was isolated by centrifugation (5 min, 16,000 g). Pellets containing cytoskeletal fraction were dissolved in 1xSDS sample buffer.
Co-immunoprecipitation and immunoblotting
Co-immunoprecipitation studies and western blot analysis were performed using confluent HPAEC monolayers treated with vehicle or stimulated with OxPAPC, as described previously (Birukova et al., 2007e).
Mechanical ventilation protocol
All animal care and treatment procedures were approved by the University of Chicago Institutional Animal Care and Use Committee and were handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male C57BL/6J mice, 8-10 week old, with average weight 20-25 grams (Jackson Laboratories, Bar Harbor, ME) were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg). Tracheotomy was performed and the trachea was cannulated with a 20-gauge-one inch long catheter (Johnson and Johnson), which was tied into place to prevent air leak. The animals have been placed on mechanical ventilator (Harvard Apparatus, Boston, MA). Mice were randomized to concurrently receive sterile saline solution or OxPAPC (1.5 mg/kg, intravenous injection in the external jugular vein) followed by 4 hours of mechanical ventilation with high tidal volume (30 ml/kg, HTV) ventilation. At the end of experiment, measurements of cell count and protein concentration in bronchoalveolar lavage fluid (BAL) were performed as previously described (Birukova et al., 2007c; Fu et al., 2009).
Statistical Analysis
Results are expressed as means ± SD of three to six independent experiments. Stimulated samples were compared to the controls using the unpaired Student's t-test. P<0.05 was considered statistically significant.
RESULTS
OxPAPC induces enhancement of adherens junctions and tight junctions
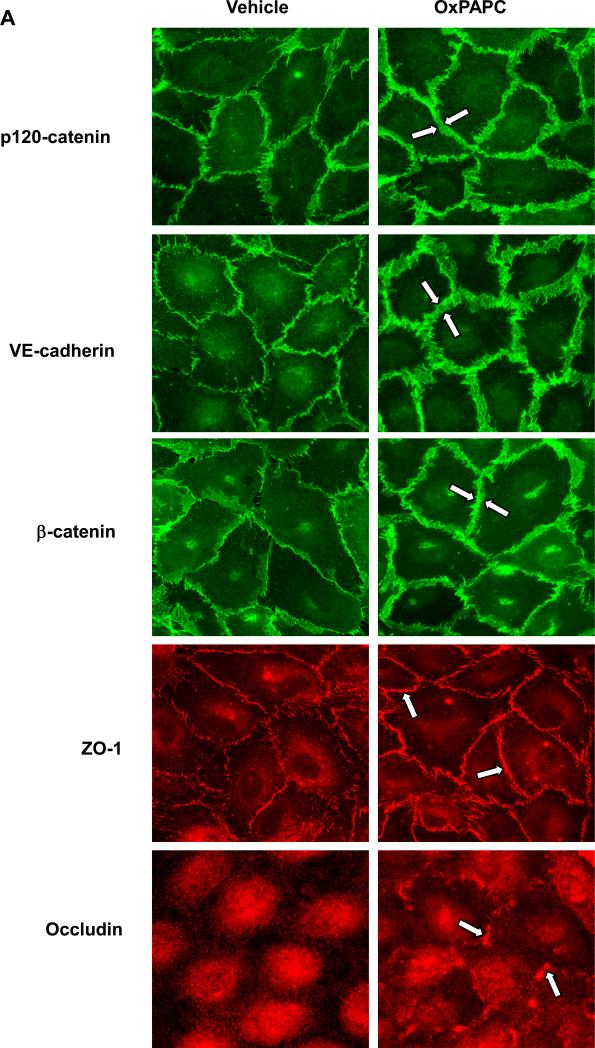
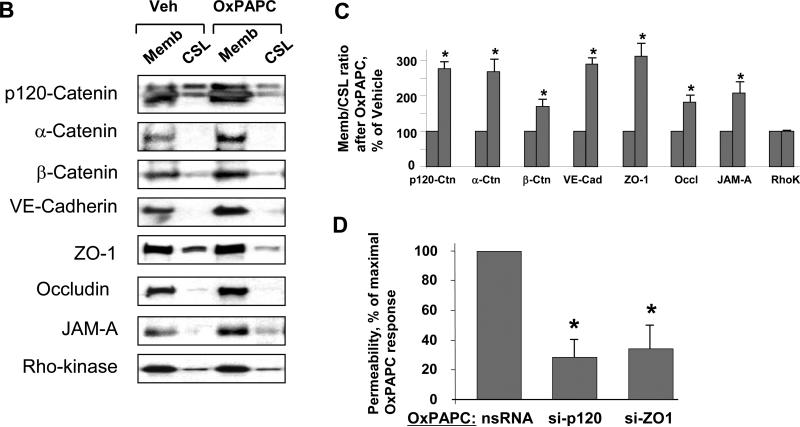
Barrier protective responses by endothelial cells to chemical and mechanical stimuli are associated with enhancement of peripheral F-actin rim, enlargement of VE-cadherin-positive cell junction areas, and increased interactions within junctional protein complexes (Birukov et al., 2002; Birukova et al., 2007a; Birukova et al., 2007e; Birukova et al., 2007f; Garcia et al., 2001). In agreement with barrier-protective effects on human pulmonary EC monolayers, OxPAPC treatment increased peripheral EC areas covered by adherens junctions (AJ), as detected by immunofluorescence staining for VE-cadherin, p120- and β-catenin (Figure 1A). Also, OxPAPC induced peripheral accumulation of ZO-1 and occludin suggesting enhancement of tight junction (TJ) complexes. Morphological changes of AJ and TJ protein localization were conformed by subcellular fractionation assays, which showed OxPAPC-induced translocation of VE-cadherin, p120-, α-, and β-catenin, ZO-1, occluding, and JAM-A to the membrane fraction (Figure 1B and C). Control experiments showed that OxPAPC did not change subcellular distribution of Rho effector Rho kinase, which is not involved in the mediation of barrier-protective effects by OxPAPC.
Figure 1. Effect of OxPAPC on cell contact remodeling.
A: Endothelial cells grown on glass coverslips were stimulated with OxPAPC (10 μg/ml, 30 min). Redistribution of AJ proteins VE-cadherin, p120-catenin, and β-catenin and TJ proteins ZO-1 and occludin was examined by immunofluorescence staining with corresponding antibodies. Enhancement of AJ and TJ is shown by arrows. B and C: HPAEC were stimulated with OxPAPC (10 μg/ml, 30 min) followed by isolation of cytosolic and membrane fractions. Translocation of AJ and TJ proteins to the membrane fraction was detected with specific antibodies (B). Result of densitometry shown as mean ± SD, * p<0.05 (C). D: Pulmonary endothelial cells grown on gold microelectrodes for TER measurements were transfected with siRNA specific to p120-catenin or ZO-1. Control cells were transfected with non-specific RNA. After 48 hrs of transfection, cells were stimulated with OxPAPC (10 μg/ml) or vehicle, and TER changes were measured after 30 min of OxPAPC stimulation; n=4-6 per condition; *p<0.05.
A role of AJ and TJ in the mediation of OxPAPC barrier-protective effects was further tested in knockdown experiments. Protein depletion of AJ protein p120-catenin or TJ protein ZO-1 in the pulmonary EC was performed using siRNA approach, as described in the Methods section. EC transfected with non-specific RNA duplexes were used as controls. After 48 hours of transfection, OxPAPC-induced TER changes were monitored over the time. Down-regulation of protein expression by 50-60% confirmed by western blot analysis did not significantly affect basal TER readings (data not shown). Depletion of p120-catenin or ZO-1 significantly attenuated barrier-protective effects of OxPAPC on pulmonary EC (Figure 1D).
OxPAPC promotes assembly of adherens junction and tight junction protein complexes
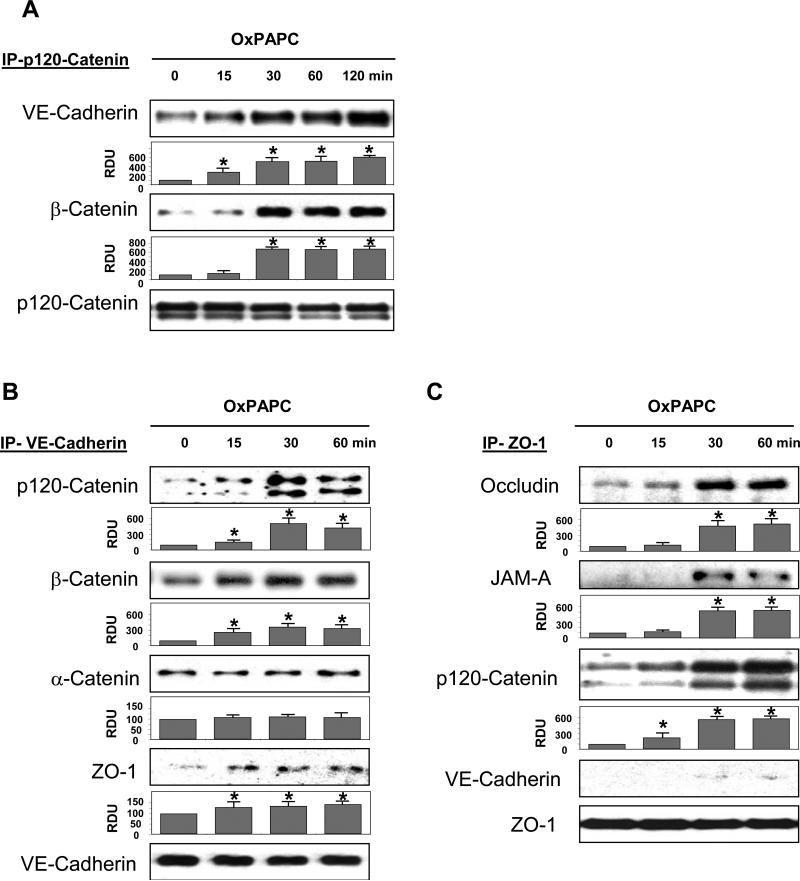
To test potential interaction between AJ and TJ complexes, we performed co-immunoprecipitation studies. OxPAPC increased association of VE-cadherin and β-catenin with p120-catenin in a time-dependent manner, which was observed after 30 min and remained elevated at least during 60 min after OxPAPC stimulation (Figure 2A).
Figure 2. Effect of OxPAPC on interaction between adherens junctions and tight junctions.
A: Cells were stimulated with OxPAPC (10 μg/ml) for various time periods or left untreated followed by immunoprecipitation of p120-catenin. VE-cadherin and β-catenin content in the immunoprecipitates was detected with specific antibodies. Equal protein loading was confirmed by re-probing of membranes with p120-catenin antibody. B and C: HPAEC were stimulated with OxPAPC (10 μg/ml, 30 min). Co-immunoprecipitation using antibody to VE-caherin (B) or ZO-1 (C) was performed, and associated proteins were detected by western blot with corresponding antibodies. Result of densitometry shown as mean ± SD, * p<0.05 D: EC monolayers grown on glass coverslips were stimulated with OxPAPC (10 μg/ml, 30 min) followed by double immunofluorescence staining for p120-catein (red) and ZO-1 (green). Merged images depict areas of protein co-localization which appear in yellow and marked by arrows. Shown are representative results of three independent experiments.
The results of immunofluorescence staining and subcellular fractionation (Figure 1) show coordinated OxPAPC-induced peripheral localization of AJ and TJ. In the next set of experiments, we directly examined potential interactions between AJ and TJ protein complexes using co-immunoprecipitation approach. Besides activation of VE-cadherin interactions with p120-catenin and β-catenin, OxPAPC also stimulated interaction of VE-cadherin with TJ protein ZO-1 (Figure 2B).
Reverse immunoprecipitation experiments using antibodies to ZO-1 showed enhanced interaction of ZO-1 with other TJ proteins occludin and JAM-A upon OxPAPC challenge, as well as with p120 catenin, but not with VE-cadherin (Figure 2C). These findings suggest indirect interactions of ZO-1 with VE-cadherin containing protein complex and additional scaffolding proteins associated with p120-catenin, which mediate AJ-TJ association in response to OxPAPC.
To further substantiate association between AJ and TJ complexes, we used double immunofluorescence staining and confocal microscopy to analyze co-localization of p120-catenin (red) and ZO-1 (green) in the pulmonary EC after OxPAPC stimulation (Figure 2D). Merged images showed a weak co-localization between p120-catenin and ZO-1 in quiescent EC (Figure 2D, left panel). OxPAPC treatment induced co-localization of p120-catenin and ZO-1 at the cell-cell junctions (Figure 2D, right panel).
OxPAPC activates Rap1 GTPase
Small GTPase Rap1 plays a role in the maintenance and repair of E-cadherin junctions in epithelial monolayers (Asuri et al., 2008), mediates endothelial barrier protective response to elevation of intracellular cAMP concentrations (Birukova et al., 2007f; Cullere et al., 2005), and prevents lung endothelial barrier dysfunction caused by inflammatory agents and pathologic mechanical ventilation (Birukova et al., 2010; Birukova et al., 2009). Rap1 activation may be achieved via a novel cAMP-binding nucleotide exchange factor Epac (Arthur et al., 2004; Fukuhara et al., 2005), or via not yet well understood mechanisms associated with integrity of cell junctions (Asuri et al., 2008). Because OxPAPC clearly affects cell-cell junctions and may elevate intracellular cAMP levels (Birukov et al., 2004b; Birukova et al., 2007b), in the following experiments we examined Rap1 activation in response to OxPAPC. Direct measurements of small GTPase activity using pull-down assay showed time-dependent activation of Rap1 upon OxPAPC stimulation (Figure 3A). Rap1 involvement in the mediation of barrier-protective effect by OxPAPC was further evaluated by expression of Rap negative regulator, Rap-GAP, in EC monolayers. Rap-GAP construct bearing cMyc tag was introduced into pulmonary EC by nucleofection technique (Birukova et al., 2006), which allows high transfection efficiency. Control transfections were performed with empty vector. Expression of recombinant Rap-GAP was confirmed by western blotting with cMyc antibodies (data not shown). Expression of Rap-GAP decreased OxPAPC-induced elevation of EC resistance, while EC transfected with empty vector responded to OxPAPC stimulation by pronounced TER increase (Figure 3B).
Figure 3. Involvement of Rap1 in the OxPAPC-induced EC barrier enhancement.
A: EC were stimulated with OxPAPC (10 μg/ml) for the indicated periods of time. Effect of OxPAPC on Rap1 activation was evaluated by Rap1-GTP pulldown assay. Upper panel depicts the levels of activated, GTP-bound Rap1, and the lower panel shows total Rap1 content in EC lysates. B: EC monolayers were subjected to nucleofection with the Rap-GAP construct bearing cMyc-tag. Control transfection was performed with empty vector. After 24 hrs of transfection, cells were stimulated with OxPAPC. Changes in endothelial permeability were monitored by measurements of transendothelial electrical resistance (left panel). Right panel represents pooled TER measurements after 30 min of OxPAPC stimulation; n=4; *p<0.05. C: Sub-confluent EC were transiently transfected with cMyc-Rap-GAP followed by treatment with OxPAPC (10 μg/ml, 30 min). Cells were fixed and subjected to immunofluorescence staining with Texas-Red phalloidin to detect F-actin (upper panels) and cMyc antibody to detect transfected cells (lower panels). Insets depict closure of paracellular gaps in non-transfected cells and remaining paracellular gaps surrounding cMyc-Rap-GAP expressing cells after OxPAPC stimulation. Results are representative of three independent experiments.
Involvement of Rap1 mechanism in the OxPAPC-induced enhancement of EC monolayer integrity was further tested in the immunofluorescence studies. Expression of Rap-GAP did not affect the cell morphology of unstimulated sub-confluent EC culture, but impaired EC ability of sealing the gaps (shown by arrows) upon OxPAPC stimulation (Figure 3C).
Knockdown of Rap1 suppresses OxPAPC protective effects
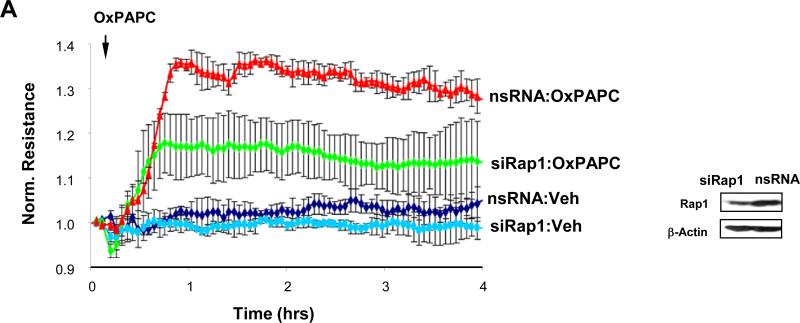
Human pulmonary EC grown on the gold microelectrodes for TER measurements were transfected with Rap1-specific siRNA duplexes. Control cells were transfected with non-specific RNA duplexes. The levels of Rap1 protein depletion that did not significantly affect the basal TER readings of EC cultures were used in these experiments. Downregulation of Rap1 expression diminished OxPAPC-induced TER increase (Figure 4A), which reflects an important role of Rap1 in the mechanisms of EC barrier enhancement by OxPAPC.
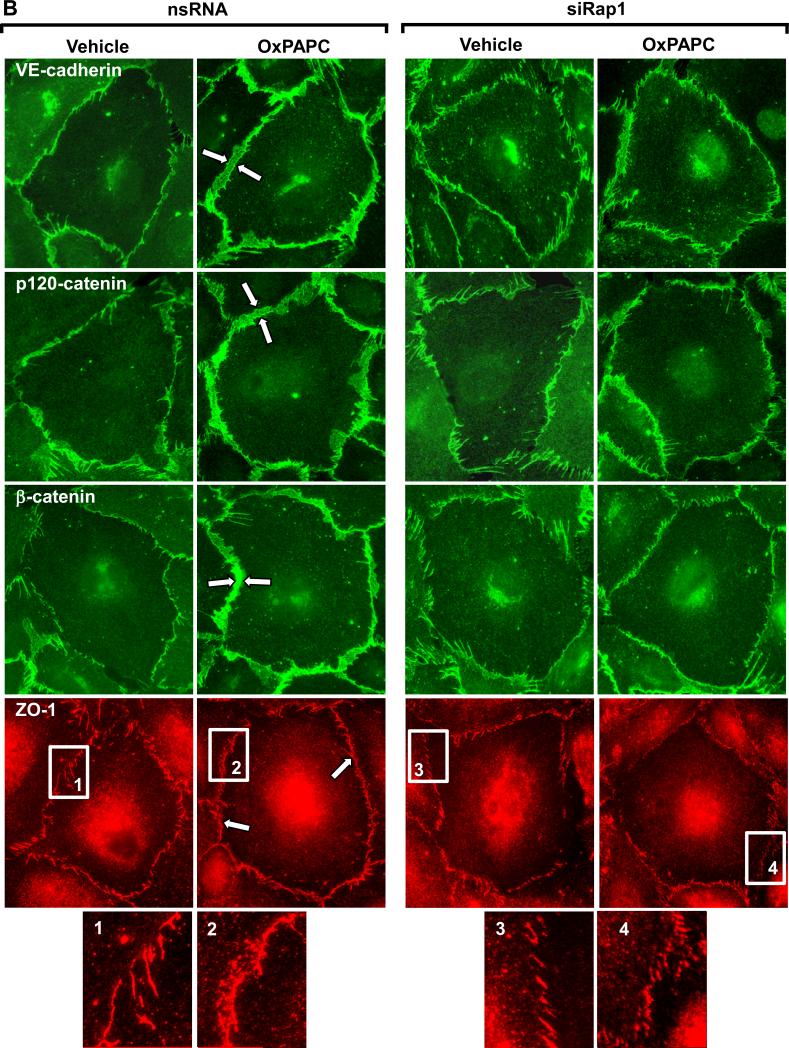
Figure 4. Effect of Rap1 depletion on OxPAPC-mediated adherens junction and tight junction remodeling.
Pulmonary EC were transfected with Rap1-specific siRNA, as described in Methods. Control cells were treated with non-specific RNA. A: HPAEC were stimulated with OxPAPC (10 μg/ml) or vehicle (indicated by arrow), and TER changes were monitored over 4 hrs. Inset depicts Rap1 protein depletion induced by specific siRNA duplexes, which was compared to treatment with non-specific RNA. Membrane reprobing with β-actin antibody was used as normalization control. Results are representative of five independent experiments. B: Cells were treated with OxPAPC for 30 min followed by immunofluorescence staining for VE-cadherin, p120-catenin, β-catenin, and ZO-1. High magnification insets depict ZO-1 staining of the cell-cell junctions in the nsRNA- and siRap1-treated cells. Shown are representative results of three independent experiments.
Our results show that OxPAPC-induced barrier enhancement is associated with peripheral accumulation of AJ and TJ complexes. The involvement of Rap1 in the OxPAPC-induced adherens junction and tight junction remodeling was further tested in knockdown experiments. Cells were transfected with Rap1-specific or non-specific RNA duplexes followed by OxPAPC treatment and analysis of AJ and TJ remodeling by immunofluorescence. Similar to OxPAPC-stimulated non-transfected cells (Figure 1), EC transfected with non-specific RNA responded to OxPAPC treatment by enlargement of AJ and TJ areas monitored by VE-cadherin, p120-catenin and β-catenin, and ZO-1 immunofluorescence staining (Figure 4B, left panels). In contrast, Rap1 knockdown abrogated OxPAPC-induced enlargement of AJ and TJ areas (Figure 4B, right panels).
Rap1 knockdown attenuates OxPAPC-induced protein interactions in adherens junction and tight junction complexes
To examine a role of Rap1 in the OxPAPC-induced interactions between AJ and TJ, we depleted Rap1 in the pulmonary EC, as described above. AJ and TJ co-localization was monitored by double immunofluorescence staining for p120-catenin and ZO-1 followed by laser confocal microscopy analysis (Figure 5A). EC transfection with siRap1 attenuated OxPAPC-induced increase in the peripheral p120/ZO-1 immunoreactivity and disrupted continuous pattern of p120-catenin and ZO-1 peripheral localization (Figure 5A, lower panels), as compared to EC treated with non-specific RNA (Figure 5A, upper panels), or non-transfected cells (Figure 2D).
Figure 5. Role of Rap1 in OxPAPC-induced interactions between adherens junctions and tight junctions.
Cells were transfected with non-specific RNA or Rap1-specific siRNA followed by OxPAPC (10 μg/ml) stimulation for 30 min. A: Double immunofluorescence staining was performed with p120-catenin and ZO-1 antibodies to visualize AJ and TJ, respectively. Merged images depict increased areas of p120-catenin and ZO-1 co-localization in OxPAPC-stimulated EC monolayers, which appear in yellow and marked by arrows. This effect was abolished in Rap1-depleted EC. B and C: HPAEC were stimulated with OxPAPC (10 μg/ml, 30 min). Co-immunoprecipitation assays using p120-catenin (B) or ZO-1 (C) antibodies were performed, and associated proteins were detected by western blot analysis with corresponding antibodies. Result of densitometry shown as mean ± SD, * p<0.05
Rap1-dependent mechanism of OxPAPC-induced interactions between AJ and TJ was further studied by co-immunoprecipitation assays in siRNA transfected pulmonary EC using p120-catenin and ZO-1 antibodies.
Results of quantitative analysis of co-immunoprecipitation assays using antibodies to p120-catenin (Figure 5B) and ZO-1 (Figure 5C) show that p120-catenin/ZO-1, p120-catenin/VE-cadherin, and p120-catenin/β-catenin interactions induced by OxPAPC were attenuated by Rap1 knockdown, while changes in ZO-1/occludin interactions did not reach statistical significance.
Rap1 knockdown attenuates protective effects of OxPAPC in vivo
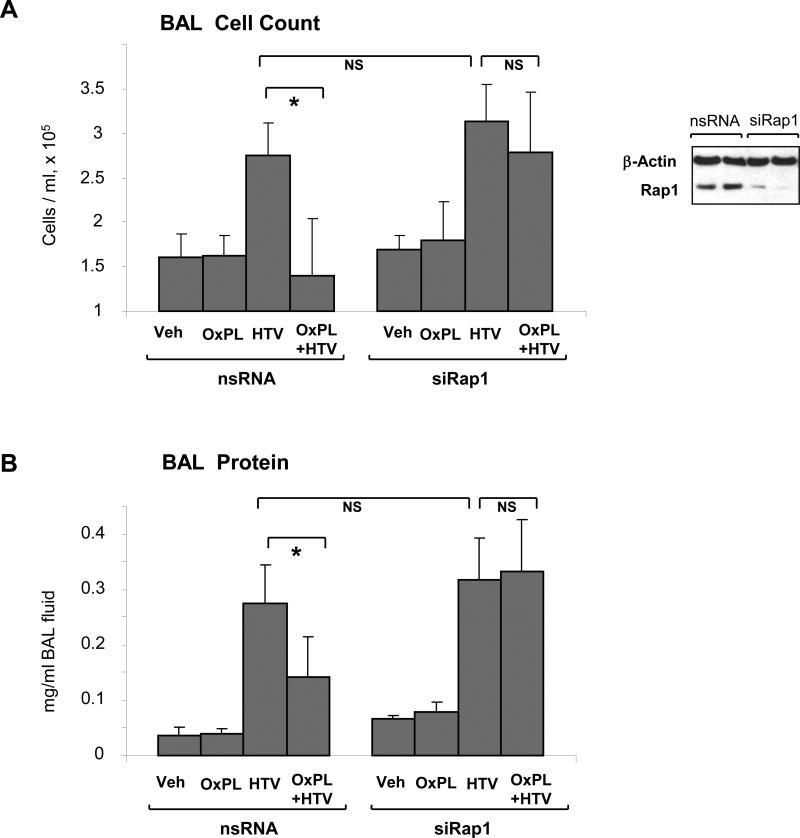
We have previously reported potent protective effect of OxPAPC in rat and murine models of ventilator induced lung injury (VILI). To test a Rap1 mechanism of the OxPAPC-mediated protective effects against lung injury induced by mechanical ventilation at high tidal volume (HTV), endogenous Rap1 was depleted using siRNA approach in vivo tested in our previous studies (Birukova et al., 2009; Singleton et al., 2009). Mice were transfected with non-specific or Rap1-specific siRNA for 72 hr followed by high tidal volume mechanical ventilation with or without OxPAPC treatment. After four hours of ventilation, bronchoalveolar lavage (BAL) fluid was collected, and BAL protein and cellular content was analyzed as indices of lung dysfunction. Rap1 protein depletion was confirmed by western blot analysis of lung tissue (Figure 6A, inset). Knockdown of Rap1 did not affect elevation of BAL cell count (Figure 6A) and protein content (Figure 6B) caused by HTV. However, Rap1 knockdown significantly attenuated protective effects of OxPAPC against HTV-induced increases in BAL cell count and protein content, as compared to control animals transfected with non-specific siRNA. Taken together, these data support a critical role of Rap1 in the OxPAPC-induced pulmonary barrier protection.
Figure 6. Rap1 depletion in vivo abolishes protective effects by OxPAPC in the murine model of ventilator induced lung injury.
A and B: Mice were transfected with non-specific or Rap1-specific siRNA (72 hrs) followed by high tidal volume mechanical ventilation (HTV; 30 ml/kg, 4 hrs) with or without OxPAPC treatment. Cell count (A) and protein concentration (B) in BAL samples were analyzed. n=4-6 per condition; *p<0.05. Inset depicts Rap1 depletion in lung samples assessed by western blot. Probing of the membrane with β-actin antibody served as a loading control.
DISCUSSION
The major finding of this study is coordinated enhancement of tight junction and adherens junction complexes in OxPAPC-stimulated pulmonary EC, and increased interaction between p120-catenin and ZO-1. These molecular events contribute to enhancement of pulmonary endothelial barrier and are regulated by OxPAPC-induced activation of small GTPase Rap1.
Prominent effects of OxPAPC on adherens junctions remodeling have been described in our previous works (Birukov et al., 2004a; Birukova et al., 2007d; Birukova et al., 2007e). In addition to enhancement of individual focal adhesions and adherens junction complexes, OxPAPC promoted interactions between focal adhesion adaptor protein paxillin and adherens junction protein β-catenin (Birukova et al., 2007e). Interestingly, knockdown of paxillin attenuated OxPAPC-induced enhancement of adherens junctions, while inhibition of β-catenin reduced peripheral translocation of focal adhesions. These results indicate that interactions between different classes of cell adhesive structures play an important role in endothelial responses to barrier-protective stimuli. The results of this study show a novel type of interactions between AJ and TJ in pulmonary endothelium, which are induced by OxPAPC and regulated by Rap1 GTPase. These data are in apparent conflict with other report showing reduction of protein expression and mRNA levels of tight junction protein occludin and increased endothelial permeability in OxPAPC-stimulated bovine endothelial cells (DeMaio et al., 2006). Increased production of reactive oxygen species observed in that study was implicated in reduced total occludin protein and mRNA levels, increased occludin phosphorylation, and reduced the immunoreactivity of zonula occludens-1 at the cell-cell contacts. (DeMaio et al., 2006). The differences between these results may be explained by different extent of PAPC oxidation, or used OxPAPC doses. It has been demonstrated that OxPAPC at high concentrations may cause endothelial barrier disruption and increased permeability (Birukov et al., 2004a; Birukova et al., 2007c).
Rac GTPase is involved in the OxPAPC-induced assembly of cell adhesions and endothelial barrier enhancement (Birukov et al., 2004a; Birukova et al., 2008a; Birukova et al., 2007d; Birukova et al., 2007e), and Rac knockdown abolishes OxPAPC-induced barrier enhancement and protein interactions in the focal adhesion and adherens junction complexes (Birukova et al., 2007e). This study shows alternative mechanism of OxPAPC-induced EC barrier enhancement and cell adhesion regulation by Rap1. Rap1 may become activated upon elevation of intracellular cAMP levels via cAMP-dependent Rap1-specific GEF, Epac (Bos, 2003). Such cAMP-dependent Rap1 activation is involved in endothelial barrier protective responses to prostaglandins, atrial natriuretic peptide and stable cAMP analogs (Birukova et al., 2010; Birukova et al., 2008b; Birukova et al., 2007f; Cullere et al., 2005; Fukuhara et al., 2005; Kooistra et al., 2005). OxPAPC-induced activation of cAMP-dependent signaling in endothelial cells has been previously described (Birukov et al., 2004b; Birukova et al., 2007b; Cole et al., 2003), which provides a plausible mechanism of Rap1 activation observed in this study. Independently of cAMP regulation, Rap1 becomes activated at early stages of junctional assembly or after restoration of the cell-cell junctions in endothelial cells (Wittchen et al., 2005), but precise mechanisms of local Rap1 activation in cell junctions remain unclear. Although upstream mechanisms of Rap1 activation by OxPAPC were not in the focus of this work, they remain and intriguing topic of the future studies.
Our results suggest involvement of Rap1 in enhancement of TJ-AJ and barrier protection in pulmonary endothelium. Previous studies in epithelial cells show that inhibition of Rap1 generates immature adherens junctions, whereas activation of Rap1 tightens cell-cell junctions (Kooistra et al., 2007). In turn Rap1 activation promotes its interaction with cell junction associated regulatory proteins such as afadin. Afadin/Rap1-GTP complex may interact with p120-catenin (Ikeda et al., 1999; Sato et al., 2006; Takai and Nakanishi, 2003) leading to enhancement of epithelial cadherin. It remains to be investigated whether similar events occur in endothelial cells. Furthermore, Rap1-activated interactions between adherens junction and tight junction proteins have not been described before. This study shows for the first time Rap1-dependent formation of the protein complex containing p120-catenin and ZO-1 in pulmonary endothelium, which is induced by barrier protective oxidized phospholipids.
A role of Rap1 signaling in OxPAPC protective effects has been further tested in animal studies. OxPAPC markedly attenuated lung barrier dysfunction caused by high tidal volume mechanical ventilation, as monitored by protein content and cell counts in BAL samples from control and experimental animals. Protective effects of OxPAPC were attenuated by Rap1 knockdown. These results are highly consistent with cell culture experiments which showed suppression OxPAPC-induced barrier enhancement by Rap1 knockdown.
Why does Rap1 knockdown in vivo cause no increase in lung permeability in control or mechanically ventilated lungs when used without OxPAPC? The exact explanation of this result is not completely clear. Possibly, basal barrier regulation in the lung is compensated by additional mechanisms. In support of this notion, Rap1a knockout mice have only a mild defect in leukocyte adhesion and normal immune function and basal vascular permeability (Duchniewicz et al., 2006). Interestingly, expressing RapGAP or siRap1A does not perturb a confluent monolayer (Hogan et al., 2004). These observations suggest that the primary function of Rap1 is in junction formation rather than junction maintenance. In contrast, under pathologic conditions of high tidal volume mechanical ventilation, additional protective signaling triggered by OxPAPC becomes essential for the lung permeability control, and knockdown of Rap1 pathway impairs lung barrier protective responses to OxPAPC treatment.
In summary, this study describes a novel Rap1 GTPase-dependent mechanism of lung barrier protection by oxidized phospholipids in the model of ventilator induced lung injury and demonstrates novel Rap1-dependent interactions between adherens junction and tight junction protein complexes, which may contribute to the vascular endothelial barrier enhancement in response to OxPAPC. Our results suggest that therapeutic strategies aimed at lung specific regulation of Rap1 activity is a promising approach for prevention of lung vascular barrier dysfunction associated with ALI/ARDS.
Acknowledgments
Supported by National Heart, Lung, and Blood Institutes grants HL87823, HL76259, and HL58064 for KGB; HL89257 and the American Heart Association Midwest Affiliate Grant-in-Aid for AAB
REFERENCES
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61(4):514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100(2):158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Anastasiadis PZ, Reynolds AB. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol. 2001;13(5):604–610. doi: 10.1016/s0955-0674(00)00258-1. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167(1):111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuri S, Yan J, Paranavitana NC, Quilliam LA. E-cadherin dis-engagement activates the Rap1 GTPase. J Cell Biochem. 2008;105(4):1027–1037. doi: 10.1002/jcb.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 2000;275(27):20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 2002;26(4):453–464. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004a;95(9):892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Leitinger N, Bochkov VN, Garcia JG. Signal transduction pathways activated in human pulmonary endothelial cells by OxPAPC, a bioactive component of oxidized lipoproteins. Microvasc Res. 2004b;67(1):18–28. doi: 10.1016/j.mvr.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AA. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006;290(3):L540–548. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Alekseeva E, Cokic I, Turner CE, Birukov KG. Cross talk between paxillin and Rac is critical for mediation of barrier-protective effects by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2008a;295(4):L593–602. doi: 10.1152/ajplung.90257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. HGF attenuates thrombin-induced permeability in the human pulmonary endothelial cells by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J. 2007a;21(11):2776–2786. doi: 10.1096/fj.06-7660com. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Burdette D, Moldobaeva N, Xing J, Fu P, Birukov KG. Rac GTPase is a hub for protein kinase A and Epac signaling in endothelial barrier protection by cAMP. Microvasc Res. 2010;79(2):128–138. doi: 10.1016/j.mvr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Chatchavalvanich S, Oskolkova O, Bochkov VN, Birukov KG. Signaling pathways involved in OxPAPC-induced pulmonary endothelial barrier protection. Microvasc Res. 2007b;73(3):173–181. doi: 10.1016/j.mvr.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007c;292(4):L924–935. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Fu P, Xing J, Birukov KG. Rap1 mediates protective effects of iloprost against ventilator induced lung injury. J Appl Physiol. 2009;107(6):1900–1910. doi: 10.1152/japplphysiol.00462.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol. 2007d;211(3):608–617. doi: 10.1002/jcp.20966. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin - {beta}-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2007e;293(1):L199–211. doi: 10.1152/ajplung.00020.2007. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67(1):64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol. 2008b;215(3):715–724. doi: 10.1002/jcp.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Alekseeva E, Fu P, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007f;313(11):2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419(6902):77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4(9):733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Bos JL, de Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol. 2001;2(5):369–377. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23(8):1384–1390. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105(5):1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5(4):261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- DeMaio L, Rouhanizadeh M, Reddy S, Sevanian A, Hwang J, Hsiai TK. Oxidized phospholipids mediate occludin expression and phosphorylation in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2006;290(2):H674–683. doi: 10.1152/ajpheart.00554.2005. [DOI] [PubMed] [Google Scholar]
- Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 2006;26(2):643–653. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Birukov KG. Oxidized phospholipids in control of inflammation and endothelial barrier. Transl Res. 2009;153(4):166–176. doi: 10.1016/j.trsl.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Birukova AA, Xing J, Sammani S, Murley JS, Garcia JG, Grdina DJ, Birukov KG. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J. 2009;33(3):612–624. doi: 10.1183/09031936.00014808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25(1):136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama T, Ogita H, Kawakatsu T, Inagaki M, Takai Y. Activation of Rac by cadherin through the c-Src-Rap1-phosphatidylinositol 3-kinase-Vav2 pathway. Oncogene. 2006;25(1):8–19. doi: 10.1038/sj.onc.1209010. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108(5):689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schutze S, Gulbins E, Uhlig S. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med. 2004;10(2):155–160. doi: 10.1038/nm977. [DOI] [PubMed] [Google Scholar]
- Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114(Pt 4):695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24(15):6690–6700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, Mizoguchi A, Nishikawa S, Takai Y. Afadin: A key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146(5):1117–1132. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadl A, Huber J, Gruber F, Bochkov VN, Binder BR, Leitinger N. Analysis of inflammatory gene induction by oxidized phospholipids in vivo by quantitative real-time RT-PCR in comparison with effects of LPS. Vascul Pharmacol. 2002;38(4):219–227. doi: 10.1016/s1537-1891(02)00172-6. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B. Nitrated lipids: a class of cell-signaling molecules. Proc Natl Acad Sci U S A. 2004;101(32):11527–11528. doi: 10.1073/pnas.0404309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579(22):4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120(Pt 1):17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- Leitinger N, Watson AD, Faull KF, Fogelman AM, Berliner JA. Monocyte binding to endothelial cells induced by oxidized phospholipids present in minimally oxidized low density lipoprotein is inhibited by a platelet activating factor receptor antagonist. Adv Exp Med Biol. 1997;433:379–382. doi: 10.1007/978-1-4899-1810-9_82. [DOI] [PubMed] [Google Scholar]
- Li R, Mouillesseaux KP, Montoya D, Cruz D, Gharavi N, Dun M, Koroniak L, Berliner JA. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ Res. 2006;98(5):642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- Ma Z, Li J, Yang L, Mu Y, Xie W, Pitt B, Li S. Inhibition of LPS- and CpG DNA-induced TNF-alpha response by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L808–816. doi: 10.1152/ajplung.00220.2003. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev. 2005;57(6):815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am J Respir Crit Care Med. 2002;166(12 Pt 2):S25–30. doi: 10.1164/rccm.2206011. [DOI] [PubMed] [Google Scholar]
- Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care. 2008;12(1):R27. doi: 10.1186/cc6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonas SA, Miller I, Kawkitinarong K, Chatchavalvanich S, Gorshkova I, Bochkov VN, Leitinger N, Natarajan V, Garcia JG, Birukov KG. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med. 2006;173(10):1130–1138. doi: 10.1164/rccm.200511-1737OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O'Brien K, Geary RL, Heinecke JW. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279(41):42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- Qiao J, Huang F, Naikawadi RP, Kim KS, Said T, Lum H. Lysophosphatidylcholine impairs endothelial barrier function through the G protein-coupled receptor GPR4. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L91–101. doi: 10.1152/ajplung.00508.2005. [DOI] [PubMed] [Google Scholar]
- Quinlan GJ, Lamb NJ, Evans TW, Gutteridge JM. Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Crit Care Med. 1996;24(2):241–246. doi: 10.1097/00003246-199602000-00010. [DOI] [PubMed] [Google Scholar]
- Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol. 2003;160(4):487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Fujita N, Yamada A, Ooshio T, Okamoto R, Irie K, Takai Y. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J Biol Chem. 2006;281(8):5288–5299. doi: 10.1074/jbc.M510070200. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res. 2009;104(8):978–986. doi: 10.1161/CIRCRESAHA.108.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbanagounder G, Leitinger N, Shih PT, Faull KF, Berliner JA. Evidence that phospholipid oxidation products and/or platelet-activating factor play an important role in early atherogenesis : in vitro and In vivo inhibition by WEB 2086. Circ Res. 1999;85(4):311–318. doi: 10.1161/01.res.85.4.311. [DOI] [PubMed] [Google Scholar]
- Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116(Pt 1):17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- Thomas M, Ge Q, Lu JJ, Chen J, Klibanov AM. Cross-linked small polyethylenimines: while still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharm Res. 2005a;22(3):373–380. doi: 10.1007/s11095-004-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A. 2005b;102(16):5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usatyuk PV, Natarajan V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J Biol Chem. 2004;279(12):11789–11797. doi: 10.1074/jbc.M311184200. [DOI] [PubMed] [Google Scholar]
- Waters CM. Reactive oxygen species in mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2004;287(3):L484–485. doi: 10.1152/ajplung.00161.2004. [DOI] [PubMed] [Google Scholar]
- Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272(21):13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127(5):1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280(12):11675–11682. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]