Abstract
Assessing the bioavailability of non-heme iron and zinc is essential for recommending diets that meet the increased growth-related demand for these nutrients. We studied the bioavailability of iron and zinc from a rice-based meal in 16 adolescent boys and girls, 13–15 y of age, from 2 government-run residential schools. Participants were given a standardized rice meal (regular) and the same meal with 100 g of guava fruit (modified) with 57Fe on 2 consecutive days. A single oral dose of 58Fe in orange juice was given at a separate time as a reference dose. Zinc absorption was assessed by using 70Zn, administered intravenously, and 67Zn given orally with meals. The mean hemoglobin concentration was similar in girls (129 ± 7.8 g/L) and boys (126 ± 7.1 g/L). There were no sex differences in the indicators of iron and zinc status except for a higher hepcidin concentration in boys (P < 0.05). The regular and modified meals were similar in total iron (10–13 mg/meal) and zinc (2.7 mg/meal) content. The molar ratio of iron to phytic acid was >1:1, but the modified diet had 20 times greater ascorbic acid content. The absorption of 57Fe from the modified meal, compared with regular meal, was significantly (P < 0.05) greater in both girls (23.9 ± 11.2 vs. 9.7 ± 6.5%) and boys (19.2 ± 8.4 vs. 8.6 ± 4.1%). Fractional zinc absorption was similar between the regular and modified meals in both sexes. Hepcidin was found to be a significant predictor of iron absorption (standardized β = −0.63, P = 0.001, R2 = 0.40) from the reference dose. There was no significant effect of sex on iron and zinc bioavailability from meals. We conclude that simultaneous ingestion of guava fruit with a habitual rice-based meal enhances iron bioavailability in adolescents.
Introduction
The National Nutrition Council (India) has identified iron deficiency as a major public health problem in India. Widespread iron deficiency anemia is attributed to the habitual consumption of diets rich in inhibitors of non-heme iron absorption. Iron and zinc deficiencies usually occur concurrently in the Indian population because the dietary factors that impair iron absorption often will also adversely affect zinc absorption (1–3). This has a significant impact on adolescents due to their higher requirement of these minerals during their period of rapid growth. Furthermore, the bioavailability of iron and zinc from diets varies according to age and sex, leading to uncertainty about optimal nutrient requirements (3, 4).
A limited number of studies have been carried out in vulnerable segments of the population to assess the extent of iron and zinc bioavailability. A detailed iron absorption study with habitual Indian diets containing wheat, rice, ragi (millet), or sorghum as staples was performed with the use of radioisotopes in adult men (5). This study formed the basis for deriving the RDA of iron for Indians, wherein the following absorption rates were used: 3% for men, children, and adolescent boys; 5% for adult women, lactating women, and adolescent girls; and 8% for pregnant women (6). However, a subsequent iron absorption study from a single rice-based meal in normal and iron-deficient women, using stable isotopes of iron, reported mean absorption rates of 7.3 and 17.5%, respectively (7). These observations highlight the need to carry out further studies on iron absorption. In addition, adolescence is a unique period in life because of the growth spurt and the onset of menarche in girls, which both may increase requirements for dietary iron and zinc (8). During adolescence there is an increase in body mass of ∼4.3 kg/y in boys and 4 kg/y in girls (9). Similarly, an increase in hemoglobin of ∼20 g/L in adolescent boys and 10 g/L in girls further increases the iron requirements. Thus, 13–15-y adolescent boys and girls can be considered as a vulnerable group for having inadequately absorbed iron and zinc. The aim of the current study was to investigate whether dietary diversification with fruit rich in ascorbic acid can improve iron bioavailability from a regular Indian meal. Because information on the absorption of zinc from habitual diets in Indian adolescents is lacking, we simultaneously assessed zinc absorption along with iron. Furthermore, because it is also not known how the absorption of iron and zinc differ by sex, we examined the sex effect on bioavailability as a secondary objective. We believe that this is the first study to assess the absorption of iron and zinc simultaneously among adolescents from similar habitual and modified meals.
Participants and Methods
Participants and settings.
There are 296 residential schools for girls and boys in the state of Andhra Pradesh, India, that are funded by the Social Welfare Department, Government of Andhra Pradesh. At these schools, the students receive secondary, technical, and vocational education, along with co-curricular activities, food, and medical care. Food is prepared at in-house kitchens according to a standard menu and served 3 times daily. Therefore, the dietary intake of participants from these schools is uniform and comparable.
Study design.
A sample size of 14 participants for each sex for paired comparison was arrived at by assuming that the intervention would lead to a doubling of iron absorption from 3 to 6% (5), with a mean difference of 3% and an SD of 3%, and with an α error of 0.05 and power of 90%. The final sample size of 16 for enrollment was calculated considering a possible dropout rate of 20% during the study.
Ethical approvals were obtained from the institutional review board, National Institute of Nutrition, Hyderabad, India and Baylor College of Medicine, Houston, TX, in December 2008. Approvals from the Secretary, Andhra Pradesh Social Welfare Residential Educational Institutional Society, Andhra Pradesh, India, and the principals of the 2 selected schools from the state’s capital, Hyderabad, were also obtained in 2009. Girls and boys aged 13–15 y from single-sex residential schools were registered for the study. Written consent from parents and assent from the study participants were obtained. The study was carried out during the school sessions that occurred from April 2010 to March 2012.
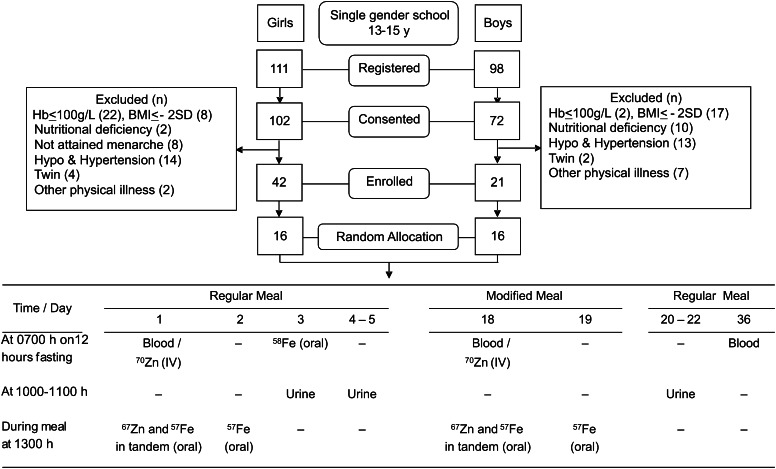
The participants were students in grades 7–9. They were introduced to the study through informal discussions followed by a formal presentation about the study. A total of 72 boys and 102 girls aged 13–15 y consented to participate in the study. They were screened for obvious clinical signs of nutritional deficiencies by a physician through a general health examination. Girls were asked whether they had attained menarche. Body weight of the participants were determined without shoes and heavy clothing by using a calibrated weighing scale (Seca), with a precision of 100 g. A portable anthropometric rod (Galaxy Scientifics) was used for measuring height, to the nearest of 0.1 cm, in standing posture. BMI was calculated according to the WHO global database on BMI (10). Potential participants were also screened for severe anemia by estimating hemoglobin in a finger-prick blood sample by using the cyanmethemoglobin method. We applied the following inclusion criteria: hemoglobin ≥100g/L, BMI > −2 SD and < +2 SD of age and sex averages, and absence of any acute or chronic illnesses; we then enrolled 21 boys and 42 girls, from whom 16 boys and 16 girls were randomly selected for the bioavailability study (Fig. 1). One month before the study commenced, a single dose of Albendazole (GlaxoSmithKline) was given to enrolled children for deworming.
FIGURE 1.
Study design, time points of dosing, and sample collection. Hb, hemoglobin.
Regular and modified meals.
In both schools, meals were prepared in the common kitchen. Because the quantity of food served to the participants was ad libitum, we assessed intake of a lunch meal among these participants and the quantity of regular meals consumed was standardized on the day before the bioavailability trial. The preferred menu by both sexes was chosen for the study period, and intake quantity was assessed by using a weighing balance (Seca Culina 852 digital scale; Seca). The average quantity of each of the cooked items was separately calculated for boys and girls. The standardized regular meal consisted of boiled rice; a potato-tomato vegetable preparation; a broth made with pigeon peas, vegetable, and tamarind, locally known as sambar; and plain yogurt (Table 1). The quantities of rice and yogurt served were different between the sexes due to consistency, cooking procedure, and preference of the participants. The regular meal was diversified to include 100 g of sliced guava fruit and served as the modified meal. During the bioavailability trial, weighed amounts of each item were served to the participants during lunch. The entire study, including providing the meals, the iron and zinc isotope dosing, and blood and urine sample collection, was carried out under direct supervision of the investigators.
TABLE 1.
Meal and nutrient composition of regular and modified meals1
| Girls |
Boys |
|||
| Details | Regular | Modified | Regular | Modified |
| Meal composition, g | ||||
| Rice2 | 450 | 450 | 400 | 400 |
| Vegetable curry3 | 100 | 100 | 100 | 100 |
| Sambar4 | 100 | 100 | 150 | 150 |
| Buttermilk/yogurt5 | 100 | 100 | 200 | 200 |
| Guava | — | 100 | — | 100 |
| Nutrient composition | ||||
| Protein, g/meal | 10.2 | 9.6 | 9.8 | 9.8 |
| Iron, mg/meal | 10.8 | 13.3 | 11.4 | 10.0 |
| Zinc, mg/meal | 2.6 | 2.7 | 2.8 | 2.8 |
| AA, mg/meal | 7.8 | 188.0* | 10.4 | 190.4* |
| Phytate, g / meal | 0.3 | 0.2 | 0.4 | 0.3 |
| Fe:PA:AA | 1:2.4:0.2 | 1:1.3:4.6 | 1:3:0.3 | 1:2.5:6 |
*Different from regular diet, P < 0.05 (t test). AA, ascorbic acid; Fe:PA:AA, ratio of non-heme iron to phytic acid to ascorbic acid.
Equivalent to dry weight, 130 g for boys and 150 g for girls.
80 g of potatoes and 20 g of tomatoes.
A broth prepared by combining 30 g dehusked split pigeon peas, tamarind pulp, and 15 g of bottle gourd. The other ingredients used in the preparation are onions, green and red chilies, turmeric, iodized salt, spice powder, and peanut oil to taste.
Buttermilk was served to boys by diluting yogurt in water as 1:1.
Iron and zinc isotope dosing.
Iron and zinc absorption was assessed from the regular and modified meals during a 36-d study period. Stable isotopes of iron (57Fe and 58Fe) and zinc (70Zn and 67Zn) (Trace Sciences International) were used for the study. On d 1 of the study, 10 mL of fasting (12 h) blood was collected at 0700 h into standard heparin-coated vials followed by i.v. administration of 70Zn (400 μg) to all participants. The participants were allowed to consume breakfast at 0800 h and had access to water only until lunch at 1300 h. Preweighed items of the regular lunch meal were provided to the participants. During the meal, 2 mL of 67Zn (1 mg) and 1.7 mL of 57Fe (3.4 mg) were given orally, in tandem, in glucose water (100 g/L). Dosing of iron (57Fe) was repeated the next day with an identical freshly cooked lunch meal. On d 3 at 0700 h, after 12 h of fasting, a reference dose of 0.8 mL of 58Fe (1.6 mg), mixed in 100 mL of commercial fruit juice (ascorbic acid, 100 mg/100 mL), was administered orally. Participants were allowed to have breakfast 2 h after the dosing. Provision of the modified meal was initiated on d 18 by using the above protocol, except that the reference dose was not administered on d 20. Urine samples (50 mL) were collected between 1000 and 1100 h on d 3–5 and on d 20–22. Ten milliliters of fasting blood was collected into standard heparinized vials by using mineral-free syringes on d 18 and also on d 36 (Fig. 1). Blood samples were brought to the laboratory and stored at −20°C until analysis. Samples of regular and modified meals served during the period were collected in triplicate. The composites were homogenized, lyophilized, and stored at −20°C for analysis of macro- and micronutrient content.
Laboratory analyses.
Hemoglobin concentrations were estimated in whole blood on the same day of blood collection by using the cyanmethemoglobin method (11). Ascorbic acid was estimated in plasma by using the α-α dipyridyl micro method (12) on the same day within 3 h of the sample collection. Ferritin was analyzed by using an in-house sandwich ELISA with an assay range of 5–100 μg/L and minimum detectable limit of 1 μg/L (13). Soluble transferrin receptor (sTfR) was analyzed by using sandwich ELISA, which had an assay range of 3–80 nmol/L, a minimum detectable limit of 0.5 nmol/L, and an inter- and intraassay variation of <10% (R&D Systems). Hepcidin (hepcidin-25) was analyzed by using ELISA with an assay range of 3.85–140 μg/L, an intraassay variation of <5%, an interassay variation of 7–11%, and analytical sensitivity of 0.9 μg/L (USCN Life Sciences). Zinc concentration was estimated by atomic absorption spectrophotometry (AA 7000 Series; Shimadzu) by using flame atomic absorption (14). C-reactive protein (CRP) was measured by using a commercial kit (minimum detectable limit of 0.3 μg/L; Alpha Diagnostic International). The determination of plasma retinol was carried out by HPLC (Thermo Finnigan) (15). Folate and vitamin B-12 were analyzed by dual RIA kit (Siemens). The in-house methods for the estimation of retinol and ferritin are under routine external quality control via the VITAL-EQA program (laboratory no. 34, CDC).
Normal cutoff values for hemoglobin (≥120 g/L for girls and boys), ascorbic acid (30–110 μmol/L), retinol (0.35–1.75 μmol/L), and ferritin (<12 μg/L) to define iron deficiency were used (16, 17).
Nutrient analysis in the composite diets was carried out in lyophilized samples, except for ascorbic acid, which was estimated in freshly homogenized samples (18). Total protein was estimated by the Kjeldahl method, iron and zinc by atomic absorption spectrophotometry (19, 20), and phytate by anion exchange followed by acid digestion and estimation of phosphorus (21). All of the laboratory analyses were performed at the collaborating center in India.
Calculation of iron bioavailability and fractional absorption of zinc.
The stable isotopes 57Fe and 58Fe in RBCs and 67Zn and 70Zn in urine were analyzed by inductively coupled plasma MS (Thermoquest Element 2, Thermoquest) at the Baylor College of Medicine (Houston, TX) (22). Briefly, iron absorption was evaluated by estimating the isotope fraction incorporated in RBCs while assuming an RBC incorporation rate of 90%. The ratio of 57Fe and 58Fe was determined relative to 56Fe in the same blood sample. The quantity of administered isotope incorporated into erythrocytes was determined from enriched and baseline isotope ratios. The isotope fraction incorporated in RBCs was calculated by dividing total isotope incorporation by the dose administered (23). Zinc absorption was calculated via the tracer/tracee method from the urine collected on d 3–5 after dosing. The relative recovery of the tracers in the urine was multiplied by 100 to calculate the percentage of zinc absorption (24, 25).
Statistical analyses.
Data were analyzed by using SPSS for Windows, version 19.0 (SPSS, Inc.). Variables were tested by using the Kolmogorov-Smirnov Z test to verify normal distribution. For variables that were normally distributed, Pearson’s product-moment correlation was used, and for the variables that were not normally distributed such as plasma ferritin, sTfR, hepcidin, folate, vitamin B-12, ascorbic acid, and CRP concentrations, Spearman’s rank correlation and Mann-Whitney U test were used. Student’s t test was used for comparing means. Paired t test was used to test the differences in absorption between the regular and modified meals, and 2-way repeated-measures ANOVA was used to determine the effects of diet (regular vs. modified) and sex (female vs. male) and their interaction on the absorption of iron and zinc. To test the relation between biomarkers and absorption, multiple linear regression modeling with adjustment for confounding variables was used. Pooled data of biomarkers and absorption were tested by Pearson correlation coefficient. The relation between phytate and mineral absorption was tested by using Spearman’s rank correlation. Values are presented as means ± SDs or medians (IQRs), and significance was set at P ≤ 0.05.
Results
Nutrient composition of meals.
The iron and zinc contents of the regular and modified lunches were similar. Protein and phytate contents of the meals were also similar. The modified meal had ∼20-fold higher ascorbic acid content compared with the regular meal. The molar ratio of iron to phytic acid was >1:1 in both the meals, and the modified meal had 20 times greater ascorbic acid content than the regular meal (Table 1).
Participant characteristics and iron and zinc status.
The mean BMI of girls was significantly higher (P < 0.05) than that of boys. All of the participants had weight-for-age Z-scores between > −2 SD and < +1 SD, except for 1 girl with a score > +1 SD. Body weights were lower than the Indian reference body weights for adolescents. However, BMI in girls was similar and for boys it was lower than the Indian reference value (Table 2). No significant differences were found in iron status and plasma zinc between sexes. Among boys, 3 were anemic (hemoglobin < 120 g/L), 5 had low ferritin (<12 μg/L), and 14 had elevated sTfR (≥29.5 nmol/L). Among girls, 2 had anemia, 7 had low ferritin, and 13 had elevated sTfR. Plasma hepcidin concentrations were 2.5 times higher (P < 0.05) and ascorbic acid was significantly lower (P < 0.05) in boys than in girls. Mean CRP concentrations were comparable between sexes, and none of the participants had CRP concentrations >10 mg/L. There were no sex differences in serum folate, vitamin B-12, and retinol concentrations (Table 3).
TABLE 2.
Anthropometric characteristics of the participants and comparison with Indian and FAO/WHO reference values1
| Girls |
Boys |
|||||
| Characteristic | Present study | Indian reference value2 | FAO/WHO reference value3 | Present study | Indian reference value2 | FAO/WHO reference value3 |
| Age, y | 14.2 ± 0.82 | 13–15 | 11–14 | 13.9 ± 0.75 | 13–15 | 11–14 |
| Height, cm | 149 ± 4.9 | 156.3 | — | 157 ± 9.3 | 162.1 | — |
| Weight, kg | 43.3 ± 4.15 | 46.6 | 46.1 | 40.1 ± 4.85 | 47.6 | 45 |
| BMI4, kg/m2 | 19.5 ± 1.91 | 19.0 (16.8 ± 2.35) | — | 16.7 ± 0.97* | 18.1 (15.7 ± 2.15) | — |
Values are means ± SDs unless otherwise indicated, n = 16. All boys were normal weight (−2 to +1 Z-score) and only 1 girl showed a > +1 Z-score suggestive of overweight. *Different from girls, P < 0.05 (t test).
95th percentile values based on national reference data (9).
FAO/WHO mean values (26).
BMI-for-age calculated on the basis of WHO Z-score scale.
Based on national nutrition survey carried out in rural areas of India (27).
TABLE 3.
Biomarkers of iron status, plasma hepcidin, zinc, CRP, ascorbic acid, folate, vitamin B-12, and retinol in girls and boys at baseline1
| Biomarker | Girls | Boys |
| Hemoglobin2, g/L | 129 ± 7.8 | 126 ± 7.1 |
| Ferritin3, μg/L | 12.3 (9.3, 17.2) | 16.3 (9.3, 22.3) |
| sTfR3, nmol/L | 37.1 (30.3, 50.7) | 37.7 (33.4, 45.1) |
| Hepcidin3, μg/L | 7.5 (3.7, 11.0) | 19.9 (10.3, 33.1)* |
| Zinc2, μmol/L | 13.7 ± 1.0 | 14.0 ± 2.2 |
| CRP3, mg/L | 0.40 (0.40, 0.75) | 0.79 (0.40, 2.96) |
| Ascorbic acid3, μmol/L | 30.4 (21.6, 36.1) | 17.0 (12.2, 20.5)* |
| Folate3, nmol/L | 11.3 (10.0, 13.1) | 12.0 (10.9, 12.9) |
| Vitamin B-123, pmol/L | 127 (107, 150) | 140 (112, 183) |
| Retinol2, μmol/L | 1.0 ± 0.27 | 0.9 ± 0.24 |
Values are means ± SDs or medians (IQRs), n = 16. *Different from girls, P < 0.05. CRP, C-reactive protein; sTfR, soluble transferrin receptor.
Normally distributed variables; t test was applied.
Skewed variables; nonparametric Mann-Whitney U test was applied.
Iron and zinc absorption.
The modified meal enhanced iron bioavailability significantly compared with a regular meal in girls and in boys (P < 0.05). Fractional zinc absorption did not differ between regular and modified meals in either sex (Table 4).There was no sex difference in iron bioavailability. Fractional absorption of zinc tended to be greater in boys than in girls (P = 0.054).
TABLE 4.
Percentage of iron bioavailability and fractional zinc absorption from rice-based meals without (regular) and with (modified) guava in girls and boys1
| Girls |
Boys |
P value2 |
|||||
| Mineral | Regular | Modified | Regular | Modified | Diet | Sex | Diet × sex |
| Iron | 9.7 ± 6.5 | 23.9 ± 11.2 | 8.6 ± 4.1 | 19.2 ± 8.4 | 0.0001 | 0.125 | 0.459 |
| Difference | 14.2 ± 14.5* | 10.6 ± 10.4* | |||||
| Zinc | 26.9 ± 6.4 | 27.3 ± 7.4 | 32.5 ± 16.6 | 37.1 ± 3.0 | 0.122 | 0.054 | 0.208 |
| Difference3 | 0.5 ± 6.3 | 4.6 ± 10.9 | |||||
| Reference dose: 58Fe4 | 58.2 ± 22.2 | 48.9 ± 16.0 | |||||
Values are means ± SDs, n = 15 for girls and 14 for boys. *P < 0.05 (paired t test).
Derived by 2-way repeated-measures ANOVA. The effect of diet on iron bioavailability was significant and showed a trend of higher fractional zinc absorption in boys.
No significant difference in fractional zinc absorption between regular and modified diets.
No significant difference in percentage bioavailability of iron.
Iron and zinc status and absorption.
Pooled data of iron status biomarkers did not show any relation with iron bioavailability, except for a positive correlation (r = 0.56, P = 0.001) between sTfR concentration and iron bioavailability from the reference dose. However, in girls, log plasma ferritin and iron bioavailability showed a negative correlation when a modified meal was consumed (r = −0.61, P = 0.016). Hepcidin and iron absorption showed a significant correlation (standardized β = −0.63, P = 0.001, R2 = 0.40) after adjusting for hemoglobin and log values of ferritin, sTfR, ascorbic acid, vitamin B-12, folate, and CRP as confounders [unstandardized β = −31.4 (95% CI: −49, −13.9)]. There was no significant association between fractional zinc absorption and plasma zinc concentration in either sex. There was no significant relation between intakes of phytate and iron and zinc absorption.
Discussion
The absorption of dietary iron and zinc is an important nutritional issue during adolescence. This study demonstrated the effects of simple dietary diversification on iron and zinc bioavailability. The inclusion of 100 g of guava fruit as part of the regular meal was found to enhance iron bioavailability by >100%. Furthermore, we demonstrated that there was no sex difference in either iron or zinc absorption.
One of the major determinants for developing RDAs is the factorial derivation of nutrient requirements. This uses body weight and percentage absorption of iron and zinc. Participants of the study were from the rural areas of Andhra Pradesh, India, and their body weight was lower compared with the Indian and the FAO/WHO reference values (9, 26). On the other hand, BMIs were comparable to the Indian reference value in girls whereas it was lower in boys. However, BMIs in both sexes were higher than the mean values reported from rural India (27). Mean hemoglobin values in girls were higher than published national values of 112 ± 17.2 g/L for girls aged 12–14 y (28).
Although there are studies on the bioavailability of iron and zinc in other age groups, there is a paucity of information on absorption of these minerals in adolescents. Various studies in different age and sex groups in adults have reported iron bioavailability ranging from 3.5 to 13.7% (29–32). One study that shared the same geographical and cultural background as that of the present study reported mean iron absorption of 5.2–9.4% in non–iron-deficient and 15.6–19.7% in iron-deficient 18–35-y-old women from a typical rice meal (7). We report an iron bioavailability of 9.7% (95% CI: 6.1, 13.3) in girls and 8.6% (95% CI: 6.2, 11.0) in boys, which is similar to the range of values reported above in women. In our study, there were 7 girls and 5 boys who were iron deficient (plasma ferritin <12 μg/L), and this could have led to a higher absorption percentage. Zinc absorption of 26.9% (95% CI: 23.5, 30.3) in girls and 32.5% (95% CI: 22.9, 40.0) in boys is also within the range of 26–34% reported in women from other countries using radio- and stable isotopes (33–37).
There are no studies to suggest that the sex difference in requirements in fact modifies absorption rates of iron and zinc. The target groups selected for the study were girls and boys aged 13–15 y and the requirement of these 2 minerals are different between sexes. The iron requirement of Indian adolescents of aged 10–12 to 13–15 y is estimated to increase from 1.05 to 1.60 mg/d in boys and from 1.33 to 1.36 mg/d in girls (9). The sharp increase in the requirement for boys is mainly due to the growth spurt that overrides the increased iron demands after menarche in girls (26). These results are in agreement with the mean iron requirement for males and the lower range of values for females suggested by Beard (8). It is estimated that among U.S. children, the iron requirement increases from 1.39 to 2.54 mg/d in 11–14-y-old males and increases in females who attained menarche by ~1.68 mg/d (8, 26, 37).
Similarly, the pubertal growth spurt substantially increases the physiologic zinc requirements; the requirements are 1.47 and 1.70 mg/d for 12–15-y-old girls and boys, respectively (26). Therefore, these reports suggest that there exist sex differences in dietary requirements during adolescence for iron and zinc. On the contrary, we did not observe any sex difference in iron and zinc absorption, indicating that meeting these requirements must be done by dietary means rather than by expecting the physiologic response to any given iron or zinc intake to differ by sex.
There are many factors that can contribute to the variations in absorption. The 2 major contributory factors are iron or zinc status and the meal composition of these minerals. In the absence of any significant differences in meal composition, it is important to consider other contributory factors such as physiologic status and the role of hepcidin, the iron hormone. There is a higher accretion of muscle mass in boys compared with girls, which is the most important physiologic factor that needs to be considered. This could override the iron loss in girls during menstruation, thereby balancing the sex differences in iron requirements and its absorption, which would become apparent only during adulthood (30, 32). The other possibility could be the fact that the participants of the study were iron deficient, which may nullify the effect of sex on absorption. Thus, both girls and boys in this age group are equally vulnerable to increased physiologic demands of iron and zinc.
The absorption of iron and zinc is always influenced by mineral status (38, 39). Among these, body iron stores, usually defined by serum ferritin, are considered to be a good predictor of iron status and show an inverse relation with absorption (40, 41). Similarly, the concentration of circulating transferrin receptor, which is a predictor of tissue iron deficiency, may be directly related to absorption of iron (42). In this study, although there was no difference between sexes in the biomarkers of iron status, we observed a negative relationship between ferritin and iron absorption. However, this occurred only in girls and only with the modified diet. On the other hand, sTfR exhibited a positive correlation with iron bioavailability from the reference dose of iron. These results indicate that iron status can influence iron absorption, although the effects were not large in this study. There was no significant relationship between plasma zinc concentration and fractional absorption of zinc, which may be due to the limited validity of plasma zinc as a biomarker of zinc status (43).
It is interesting to note that, despite similar biomarkers of iron status, hepcidin concentration was higher in boys than in girls. However, a study carried out in a healthy adult population reported that men have more than twice the hepcidin concentration (median) compared with women. This was attributed to differences in iron status as reflected by serum ferritin (44). In the present study, hepcidin concentration in girls (7.5 μg/L) was similar, whereas that in boys (19.9 μg/L) was lower than that reported for a healthy adult population aged 18–24 y (7.2 and 26 μg/L, respectively). The influence of pubertally regulated sex steroid hormones cannot be ruled out (45). Hepcidin in isolation significantly predicted interindividual variation in iron absorption and is in line with the findings in healthy young women (46).
The absorption of non-heme iron and zinc is also regulated by dietary factors (26, 39). In this context, the role of fruit rich in ascorbic acid has been previously demonstrated to improve iron, but not zinc, absorption. Ascorbic acid supplementation (200 mg) to preschool children given daily for 60 d demonstrated an improvement in hematologic variables (47). However, it has also been suggested that long-term ascorbic acid supplementation negatively affects absorption of other minerals such as copper and selenium (48, 49). Previous studies have documented the importance of consuming ascorbic acid–rich fruit to increase iron bioavailability. It has been reported that 150 g of papaya containing 75 mg ascorbic acid increased non-heme iron absorption from a meal by almost 5 times (50). The consumption of fresh guava (100 g) along with a meal consisting of cooked rice, margarine, and sucrose has been tested in Durban-Indian women aged 22–74 y. An increase in iron absorption from 2.5 to 12.6% was reported with the inclusion of guava (51). Intakes of ascorbic acid in the form of guava and other locally grown seasonal fruit have been shown to be high among nonanemic Tanzanian schoolchildren aged 7–12 y on the basis of FFQs (52). A recent study reported that the consumption of guava juice showed only marginal beneficial effects on hemoglobin and ferritin concentrations in iron-deficient anemic children (53). Our results are in agreement with the above literature. However, the meal served in our study was inhibitory with respect to Fe:phytic acid molar ratio (>1:1), and 100 g of whole, fresh guava fruit, which provided ∼190 mg of ascorbic acid, increased iron absorption by almost 2 times in both sexes. On the other hand, fractional zinc absorption was similar between the meals in both sexes. These results suggest that diversification of a habitual diet with guava is a promising approach to improve dietary non-heme iron absorption in adolescents. This finding therefore suggests that there is a need to consider the inclusion of locally available, ascorbic acid–rich foods as part of every meal, which then forms an effective and suitable option to enhance dietary iron absorption (54).
This study had some potential limitations. It was designed to detect a 2-fold enhancement in iron bioavailability due to dietary modification and may not have had enough participants to detect the same for zinc and sex differences in absorption. The participants could not be given the exact same diet because the study involved 2 different locations, food being prepared fresh every day, and participants’ food habits. However, to a large extent, we were able to maintain uniformity in the food cooked and served during the trial.
In summary, dietary diversification of a habitual rice-based meal with 100 g of guava enhanced the bioavailability of non-heme iron but not zinc. There was no sex difference in the absorption of iron and zinc. Hepcidin concentration significantly predicted iron absorption. Long-term studies are needed to assess the impact of daily consumption of fruit rich in ascorbic acid along with nutrition education to translate these findings into a national strategy to control iron deficiency anemia.
Acknowledgments
The authors thank Dr. David C. Hilmers of the Baylor College of Medicine for his suggestions on protocols for isotope administration; Mr. J. S. Acharya and Mr. V. Vikas Rao for their expert assistance in laboratory analysis; and Dr. B. Sesikeran, former Director, National Institute of Nutrition, and Dr. Vasuprada Iyengar for their critical input. K.M.N. and S.A.A. conceived the overall project, obtained funding, provided study oversight, and had primary responsibility for the final content; G.N.V.B. was responsible for recruitment and screening and administration of doses; M.R. and D.R.C. conducted research; P.R. was responsible for laboratory analysis; N.B. performed statistical analysis; Z.C. performed analysis of iron and zinc isotopes in the samples; K.M.H. was responsible for preparing the protocols; and K.M.N. and D.R.C. wrote the manuscript. All authors read and approved the final manuscript.
Literature Cited
- 1.Vijayaraghavan K. Control of micronutrients deficiencies in India. Nutr Rev. 2002;60:73S–6S. [DOI] [PubMed] [Google Scholar]
- 2.Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91 Suppl:1461S–7S. [DOI] [PubMed] [Google Scholar]
- 3.Hunt JR. Bioavailability of iron, zinc and other trace minerals from vegetarian diets. Am J Clin Nutr. 2003;78 Suppl:633S–9S. [DOI] [PubMed] [Google Scholar]
- 4.Hunt JR, Roughead ZK. Adaptation of iron absorption in men consuming diets with high or low iron bioavailability. Am J Clin Nutr. 2000;71:94–102. [DOI] [PubMed] [Google Scholar]
- 5.Narasinga Rao BS, Vijayasarathy C, Prabhavathi T. Iron absorption from habitual diets of Indians studied by the extrinsic tag technique. Indian J Med Res. 1983;77:648–57. [PubMed] [Google Scholar]
- 6.Indian Council of Medical Research. Nutrient requirements and recommended dietary allowances for Indians. A Report of the Expert Working Group of the Indian Council of Medical Research. Hyderabad (India): National Institute of Nutrition; 1990.
- 7.Thankachan P, Walczyk T, Muthayya S, Kurpad A, Hurrell R. Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. Am J Clin Nutr. 2008;87:881–6. [DOI] [PubMed] [Google Scholar]
- 8.Beard JL. Iron requirement in adolescent females. J Nutr. 2000;130 Suppl:440S–2S. [DOI] [PubMed] [Google Scholar]
- 9.Indian Council of Medical Research. Nutrient requirements and recommended dietary allowances for Indians. A Report of the Expert Working Group of the Indian Council of Medical Research. Hyderabad (India): National Institute of Nutrition; 2010.
- 10.World Health Organization. Growth reference 5–19 years [cited 2012 Oct 3]. Available from: http://whqlibdoc.who.int/hq/2001/WHO_NHD_01.3.pdf.
- 11.Cook JD, Dallman PR, Bothwell TH, Lynch SR, Covell AM, Worwood MA, Reusser ME. Measurement of iron status. A report of International Nutritional Anemia Consultative Group. Washington: The Nutrition Foundation; 1985.
- 12.Zannoni V, Lynch M, Gokdstein S, Sato P. A rapid micro-method for the determination of ascorbic acid in plasma and tissues. Biochem Med. 1974;11:41–8. [DOI] [PubMed] [Google Scholar]
- 13.Pawashe AB, Raman L, Nair M, Sarma J. Validity of using capillary blood for the measurement of plasma ferritin. Clin Chim Acta. 1987;163:119–20. [DOI] [PubMed] [Google Scholar]
- 14.Perry DF. Flame atomic absorption spectrometric determination of serum zinc: a collaborative study. J Assoc Off Anal Chem. 1990;73:619–21. [PubMed] [Google Scholar]
- 15.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of α-tocopherol and retinol in plasma or red cell by high pressure liquid chromatography. Am J Clin Nutr. 1979;32:2143–9. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. 2001 [cited 2012 Oct 3]. Available from: http://whqlibdoc.who.int/hq/2001/WHO_NHD_01.3.pdf.
- 17.Young DS. Implementation of SI units for clinical laboratory data, style specifications and conversion tables. Ann Intern Med. 1987;106:114–29. [DOI] [PubMed] [Google Scholar]
- 18.Roe JH, Kuether CA. The determination of ascorbic acid in whole blood and urine through 2,4-DNP derivative of dehydro ascorbic acid. J Biol Chem. 1943;147:399–407. [Google Scholar]
- 19.Horwitz W, editor. Methods of analysis of Association of Analytical Chemists International. 17th ed. Vol I. Gaithersburg(MD): AOAC International Publications; 2000.
- 20.Jorhem L, Engman J. Determination of lead, cadmium, zinc, copper and iron in foods by atomic absorption spectrophotometry after microwave digestion: NMKL Collaborative Study. J AOAC Int. 2000;83:1189–203. [PubMed] [Google Scholar]
- 21.Harland BF, Oberleas D. Anion-exchange method for determination of phytate in foods: collaborative study. J Assoc Off Anal Chem. 1986;69:667–70. [PubMed] [Google Scholar]
- 22.Lowe NM, Shames DM, Woodhouse LR, Matel JS, Roehl R, Saccomani MP, Toffolo G, Cobelli C, King JC. A compartment model of zinc metabolism in healthy women using oral and intravenous stable isotope tracers. Am J Clin Nutr. 1997;65:1810–9. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Griffin IJ, Plumlee LM, Abrams SA. High resolution inductively couples plasma mass spectrometry allows rapid assessment of iron absorption in infants and children. J Nutr. 2005;135:1790–5. [DOI] [PubMed] [Google Scholar]
- 24.Abrams SA, Wen J, Stuff JE. Absorption of calcium, zinc and iron from breast milk by five to seven month old infants. Pediatr Res. 1997;41:384–90. [DOI] [PubMed] [Google Scholar]
- 25.Griffin IJ, Abrams SA. Methodological issues in stable isotope-based kinetic studies in children. Adv Exp Med Biol. 2003;537:117–30. [DOI] [PubMed] [Google Scholar]
- 26.FAO/WHO. Joint report: human vitamin and mineral requirements. Rome: FAO/WHO Food and Nutrition Division; 2001.
- 27.National Nutrition Monitoring Bureau. Diet and nutritional status of population and prevalence of hypertension among adults in rural areas. NNMB Technical Report No. 24. Hyderabad (India): National Institute of Nutrition, Indian Council of Medical Research; 2006 [cited 2013 Jan 17]. Available from: http://www.nnmbindia.org/NNMBReport06Nov20.pdf.
- 28.National Nutrition Monitoring Bureau. Prevalence of micronutrient deficiencies. NNMB Technical Report No. 22. Hyderabad (India): National Institute of Nutrition, Indian Council of Medical Research; 2003 [cited 2013 Jan 17]. Available from: http://www.nnmbindia.org/NNMB%20MND%20REPORT%202004-Web.pdf.
- 29.Walczyk T, Davidsson L, Rossander-Hulthen L, Hallberg L, Hurrell RF. No enhancing effect of vitamin A on iron absorption in humans. Am J Clin Nutr. 2003;77:144–9. [DOI] [PubMed] [Google Scholar]
- 30.Turnlund JR, Michel MC, Keyes WR, King JC, Margen S. Use of enriched stable isotopes to determine zinc and iron absorption in elderly men. Am J Clin Nutr. 1982;35:1033–40. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien KO, Zavaleta N, Caulfield LE, Yang DX, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell iron incorporation, and iron status in pregnant Peruvian women. Am J Clin Nutr. 1999;69:509–15. [DOI] [PubMed] [Google Scholar]
- 32.King JC, Raynolds WL, Margen S. Absorption of stable isotopes of iron, copper and zinc during oral contraceptive use. Am J Clin Nutr. 1978;31:1198–203. [DOI] [PubMed] [Google Scholar]
- 33.Hunt JR, Matthys LA, Johnson LK. Zinc absorption, mineral balance, and blood lipids in women consuming controlled lacto ovovegetarian and omnivorous diets for 8 weeks. Am J Clin Nutr. 1998;67:421–30. [DOI] [PubMed] [Google Scholar]
- 34.Petterson DS, Sandstrom B, Cederblad A. Absorption of zinc from lupin (Lupinus angustifolius)- based foods. Br J Nutr. 1994;72:865–71. [DOI] [PubMed] [Google Scholar]
- 35.Hunt JR, Gallagher SK, Johnson LK, Lykken GI. High versus low-meat diets: effects on zinc absorption, iron status, and calcium, copper, iron, magnesium, manganese, nitrogen, phosphorus, and zinc balance in postmenopausal women. Am J Clin Nutr. 1995;62:621–32. [DOI] [PubMed] [Google Scholar]
- 36.Sian L, Mingyan X, Miller LV, Tong L, Krebs NF, Hanbidge KM. Zinc absorption and intestinal losses or endogenous zinc in young Chinese women with marginal zinc intakes. Am J Clin Nutr. 1996;63:348–53. [DOI] [PubMed] [Google Scholar]
- 37.Fomon SJ, Drulis JM, Nelson SE, Serfass RE, Woodhead JC, Ziegler EE. Inevitable iron loss by human adolescents, with calculations of the requirement for absorbed iron. J Nutr. 2003;133:167–72. [DOI] [PubMed] [Google Scholar]
- 38.The National Academy of Sciences. Dietary reference intakes. Washington: National Academies Press; 2002. [Google Scholar]
- 39.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–95. [DOI] [PubMed] [Google Scholar]
- 40.Finch C. Regulators of iron balance in humans. Blood. 1994;84:1697–702. [PubMed] [Google Scholar]
- 41.Hallberg L, Hulthen L. Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability of dietary iron. Am J Clin Nutr. 2000;71:1147–60. [DOI] [PubMed] [Google Scholar]
- 42.Cook JD, Dassenko S, Skikne BS. Serum transferrin receptor as an index of iron absorption. Br J Haematol. 1990;75:603–9. [DOI] [PubMed] [Google Scholar]
- 43.Sonja HY, Janet M, King JC, Kenneth BH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull. 2007;28:403S–29S. [DOI] [PubMed] [Google Scholar]
- 44.Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, Tienoven D, Wetzels JF, Kiemeney LALM, Sweep FC, Heijer MD. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood 2011;117:e218–25. [DOI] [PubMed] [Google Scholar]
- 45.Bachman E, Feng R, Travison T, Li M, Olbina G, Ostland V, Ullor J, Zhang A, Basaria S, Ganz T, et al. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95:4743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young MF, Glahn RP, Ariza-Nieto M, Inglis J, Olbina G, Westerman M, O’Brien KO. Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women. Am J Clin Nutr. 2009;89:533–8. [DOI] [PubMed] [Google Scholar]
- 47.Seshadri S, Shah A, Bhade S. Haematologic response of anaemic preschool children to ascorbic acid supplementation. Hum Nutr Appl Nutr. 1985;39:151–4. [PubMed] [Google Scholar]
- 48.Levander OA. A global view of human selenium nutrition. Annu Rev Nutr. 1987;7:227–50. [DOI] [PubMed] [Google Scholar]
- 49.Robinson MF, Thomson CD, Huemmer PK. Effect of a megadose of ascorbic acid, a meal an orange juice on the absorption of selenium as sodium selenite. N Z Med J. 1985;98:627–9. [PubMed] [Google Scholar]
- 50.Layrisse M, Martinez-Torres C, Gonzalez M. Measurement of the total daily dietary iron absorption by extrinsic tag model. Am J Clin Nutr. 1974;27:152–62. [DOI] [PubMed] [Google Scholar]
- 51.Ballot D, Baynes RD, Bothwell TH, Gillooly M, Macfarlane J, Macphail AP, Lyons DP, Derman WR, Bezwoda WR, Torrance JD, et al. The effect of fruit juices and fruits on the absorption of iron from a rice meal. Br J Nutr. 1987;57:331–43. [DOI] [PubMed] [Google Scholar]
- 52.Tatala S, Ndossi G. Impact of dietary iron intake on anaemia in Tanzanian school children. SAJCN. 2004;17:94–100. [Google Scholar]
- 53.Monarrez-Espino J, Lopez-Alarcon M, Greiner T. Randomized placebo-controlled trial of guava juice as a source of ascorbic acid to reduce iron deficiency in Tarahumara indigenous school children on Northern Mexico. J Am Coll Nutr. 2011;30:191–200. [DOI] [PubMed] [Google Scholar]
- 54.Sandström B. Micronutrient interactions: effects on absorption and bioavailability. Br J Nutr. 2001;85:S181–5. [PubMed] [Google Scholar]