Abstract
During pregnancy, the iodine requirement rises to meet demands for neurological development and fetal growth. If these requirements are not met, irreversible pathological cognitive and behavioral changes to the fetus may ensue. This study estimated the prevalence of iodine-containing dietary supplement (DS) use and intakes of iodine from DSs among pregnant women and nonpregnant women of reproductive age (15–39 y) who were interviewed and examined in NHANES 1999–2006 (n = 6404). Although 77.5% of pregnant women reported taking one or more DSs in the past 30 d, only 22.3% consumed an iodine-containing supplement. Most pregnant women reported using one DS and reported taking this product daily. The vast majority of iodine-containing DSs reported by pregnant women claimed an iodine content of 150 μg iodine/serving on the label. Pregnant women using at least one DS containing iodine had a mean daily iodine intake of 122 μg/d from supplements; the median value was 144 μg/d. Median urinary iodine concentrations (UICs) were similar for pregnant and nonpregnant women in the population aged 15–39 y. The median UIC was 148 μg/L for pregnant women and 133 μg/L for nonpregnant women. The WHO has established a cutoff for insufficient iodine intake at <150 μg/L for pregnant women and <100 mg/L for those who are not pregnant. This suggests that as a population, we may not be meeting adequate intakes of iodine for pregnant women. More research is needed on the iodine intakes of pregnant women and women of reproductive age on their total iodine intake from all sources, not just DSs.
Introduction
Iodine is a necessary component of the thyroid hormones thyroxine and triiodothyronine, which are essential for adequate fetal and postnatal central nervous system growth and development (1, 2). The iodine requirement increases by >50% during pregnancy because of fetal needs, alterations in maternal iodine metabolism, and greater urinary iodine loss due to its enhanced renal clearance. The fetus is completely dependent upon maternal iodine stores for the first few months of pregnancy, and an ample supply is critical, particularly early in pregnancy when the fetal brain is growing rapidly. Maternal iodine deficiency, particularly when it occurs during early pregnancy, can lead to irreversible neurological complications and profound mental retardation in the offspring (1). Therefore, it is important to determine the iodine intake and status of both pregnant and nonpregnant women of reproductive age, because the critical period of organogenesis comes before many women know that they are pregnant. It had been assumed that iodine deficiency in pregnant women was no longer a problem in the United States (3). However, as urinary iodine concentrations (UICs)6 have declined, concern has grown regarding the need to examine current intake levels, particularly for a vulnerable group like pregnant women (4–6).
UIC6 is a good indicator of recent intake, because iodine intake and excretion are in a steady state; ∼90% of dietary iodine is excreted in urine (2). The WHO defines iodine sufficiency in pregnancy as a median UIC of 150–249 μg/L, with concentrations <150 μg/L as insufficient (7). Very little is known about the iodine intakes of pregnant women in the US. Iodine is voluntarily added to table salt by some manufacturers in the US. It is estimated that only ∼7–8% of sodium intake in the US is contributed by table salt and only ∼70% of consumers choose iodized salt (8). Most salt ingestion is from processed foods and the salt used in food processing is typically not iodized (8). It is not possible to determine dietary exposure to iodine in the US, because the national food composition tables do not currently include iodine amounts from foods and beverages. However, databases do exist that permit estimation of iodine intake from dietary supplements (DSs). Prenatal DSs are encouraged during pregnancy and additionally the American Thyroid Association recommends supplemental iodine daily for all women who are pregnant, lactating, or planning a pregnancy (9). The objective of this study was to estimate the prevalence of iodine-containing DSs use and intake of iodine from DSs and to examine UICs in pregnant and nonpregnant women of reproductive age in the US in 1999–2006.
Materials and Methods
Survey description.
The NHANES is a nationally representative sample of the U.S. civilian, noninstitutionalized population sponsored by the National Center for Health Statistics (NCHS), CDC. This population-based survey uses a complex, stratified, multistage probability cluster sampling design to provide data that are representative of the civilian, noninstitutionalized U.S. population. There is oversampling of certain groups, including persons aged 60 y and older, Mexican American, and non-Hispanic black Americans in order to increase the precision of estimates for these groups. To improve the precision of the estimates for pregnant women, a supplemental sample of pregnant women was selected (10). Only women aged 15–39 y were eligible for this supplemental sample. Survey participants were asked to complete an in-person home interview and a health examination conducted in mobile examination centers. Written informed consent was obtained for all participants or proxies and the survey protocol was approved by the Research Ethics Review Board at the NCHS.
The NHANES data are publicly available as 2-y datasets to reduce the risk of disclosure. The NCHS recommends that analysts combine ≥4 y of data to improve the reliability and stability of statistical estimates (11). This analysis was completed using NHANES 1999–2000, 2001–2002, 2003–2004, and 2005–2006 datasets to increase the sample size of pregnant women. The response rate for examined women ages 12–39 y was 80.5% (6647 examined/8257 screened) in 1999–2006 (12). Lactating women were excluded from the analyses (26 women who were both pregnant and lactating and 180 women who were not pregnant but lactating). Lactating women were excluded, because NHANES has so few lactating females that a subgroup analysis for this group is not permissible. Lactating women have different iodine requirements and we could therefore not group them with the nonpregnant or pregnant group. Women were also excluded if they had missing data for DS use in the past 30 d or if they were missing data used to ascertain whether or not they took a DS containing iodine (n = 37). Missing data for the question that collected information on whether or not the participant had taken a DS in the past month/30 d excluded a small number of participants from the analysis (n = 8). Data may have been missing for this initial gate question because the participant did not actually have a chance to answer the question, did not know, or refused to answer it. Regardless, no further information was collected for that participant on DS use. Missing data were also due to missing DS product information, because the participants: 1) were unable to remember which product they had taken; 2) were unable to provide enough detail to find a match to a product on the market; 3) refused to answer; or 4) the interviewer did not completely record the name of the DS and so the name did not match to a product on the market. In such cases, these participants were excluded from the analyses, because there was not enough information to conclude that the participant took a DS containing iodine (n = 29).
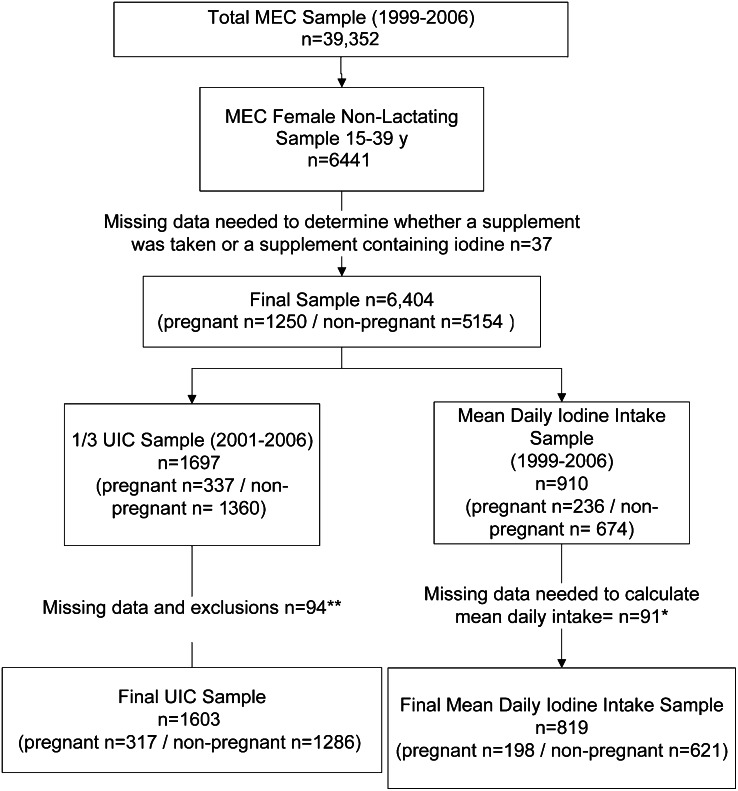
The final analytic sample was 6404, which included 1250 pregnant and 5154 nonpregnant women. Those women whose pregnancy status could not be determined were included in the group of nonpregnant women (10). Figure 1 provides information on the sample.
FIGURE 1.
Flow chart of women in analysis NHANES 1999–2006, ages 15–39 y. *Missing DS data and exclusions (n = 91): Missing both the frequency of use in the past 30 d and how much was taken on days used (n = 52), missing frequency of use in the past 30 d (n = 32), missing how much was taken on days used (n = 6), missing data because the DS product label was missing serving size and amount of iodine (n = 1). ** Missing data and exclusions (n = 94): Missing UIC (n = 49), excluded because of thyroid problem or use of thyroid medication (n = 36), missing DS data (n = 9). DS, dietary supplement; MEC, Mobile Examination Centers; UIC, urinary iodine concentration.
Demographic variables.
Age was examined as 3 age groups: 15–19, 20–29, and 30–39 y. Self-reported race/ethnic groups as defined in NHANES and used in the analysis were non-Hispanic white, non-Hispanic black, Mexican American, and “other.” Following NCHS recommendations, the “other” race/ethnic group was represented in the estimates for the total sample but is not presented separately as a group.
DS data.
Detailed information on the use of all types of prescription and no-prescription DS products was collected during the household interview (13). The scope of the collection included all vitamins, minerals, herbs, and botanicals and other types of DSs that were used during the past 30 d. Interviewers asked the participant to show them product containers if they were available. Interviewers had access to product containers for 80% of the reported DSs for nonlactating women ages 15–39 y. Information about product name, frequency of use, duration of use, and dosage was recorded for each DS reported during the interview. The mean daily iodine intake from DS products was derived for each participant based on the number of days the DS was used, the actual amount consumed based on the label serving size, and the iodine content of the product per serving as listed on the product label using methods previously reported (14–16). A total of 910 participants reported taking at least one DS containing iodine. If the question on how many days in the past month the DS was taken, the amount taken on those days, or product label information was missing, these participants were excluded, because mean daily intake, which is based on the information collected from these questions, could not be estimated. There were missing values for number of days the DS was used and the servings of product taken daily for 91 participants who reported taking a DS containing iodine. Fifty-two participants were missing data for both the frequency and amount used, 32 were missing just frequency of use, 6 were missing just amount used, and for 1 participant, the product label was missing the serving size and actual amount of iodine contained in the product. The final analytical sample for estimating mean daily iodine intake was 819 women.
UICs.
The UIC was measured on a one-third subsample consisting of 8012 participants, aged 6 y and older from 2001 to 2006. Although the other estimates presented in this report were collected in the NHANES 1999–2006, these data were collected only in NHANES 2001–2006. Women aged 15–39 y who provided complete information about DS use (n = 9 missing DS data), had urinary iodine data (n = 49 missing UIC), and did not have a current thyroid condition or were taking thyroid medication (n = 36 excluded) were included in the determination of UIC (17). The dataset for this analysis comprised 1603 participants. Determination of UIC was done by means of inductively coupled plasma dynamic reaction cell MS (18). Spot urine samples were used for the assessment of iodine nutritional status. Insufficiency was defined according to the WHO categories of <100 μg/L for nonpregnant women and <150 μg/L for pregnant women (19).
Statistical analysis.
All statistical analyses were performed using SAS (version 9.2, SAS Institute) and SAS-callable SUDAAN (version 10.0, Research Triangle Institute) software. All analyses were weighted using the examination weights for the DS analysis and the laboratory weights for the UIC analysis. Because pregnancy status was based on variables collected at the mobile examination center, the examination weights were used for all analyses. These sample weights account for the complex survey design (including oversampling) and survey nonresponse and are post-stratified to Census Bureau population estimates (11). Estimates on race/ethnicity groups were age-adjusted to the 2000 U.S. standard population using 3 age groups: 15–19, 20–29, and 30–39 y (20). Estimates of means and medians for mean daily iodine intake were expressed in μg/d and estimates for UIC were expressed in μg/L. Medians are more appropriate to use for UIC, because the population mean UIC is typically skewed in the direction of lower intakes (19). UICs were compared to cutoffs established by the WHO to determine whether the median UICs for pregnant women and nonpregnant women in the US were meeting the WHO cutoff. The SEs for all statistics of interest were approximated by Taylor Series Linearization, accounting for the complex design of NHANES. Contrasts were constructed to test for significant differences in prevalence estimates and mean intakes. Statistical hypotheses were tested using the t statistic and an α = 0.05 based on a 2-tailed test. The Bonferroni method was used to adjust for multiple comparisons by dividing the overall α level by the number of implied comparisons for the different prevalence estimates. For example, there were 18 implied comparisons for the percentage using any DS and an α of 0.003 was used. Estimates presented have a relative SE ≤30% and ≥12 df, unless otherwise noted.
Results
Overall, among nonpregnant reproductive-aged women, 41.3% used one or more DS and 18.5% used DS products containing iodine (Table 1). Supplement use was significantly higher for women aged 30–39 y compared with women aged 15–19 and 20–29 y.
TABLE 1.
Prevalence of use of DSs and supplemental iodine by women 15–39 y, by pregnancy status, age, and race/ethnicity: United States, 1999–20061
| n | Percentage using any DS | Percentage using any DS with iodine | |
| Age group | |||
| All women (15–39 y) | 6404 | 44.4 ± 1.0 | 18.7 ± 0.8 |
| 15–19 y | 2717 | 29.8 ± 1.5c | 12.8 ± 1.0b |
| 20–29 y | 1991 | 43.7 ± 1.5b | 16.2 ± 1.2b |
| 30–39 y | 1696 | 52.0 ± 1.8a | 24.2 ± 1.4a |
| All pregnant | 1250 | 77.5 ± 2.1 | 22.3 ± 2.2 |
| 15–19 y | 194 | 63.8 ± 5.4b | 11.8 ± 4.1b |
| 20–29 y | 691 | 73.2 ± 2.7b | 18.1 ± 2.1b,a |
| 30–39 y | 365 | 88.3 ± 3.0a | 32.2 ± 4.4a |
| All nonpregnant | 5154 | 41.3 ± 1.0 | 18.5 ± 0.8 |
| 15–19 y | 2523 | 28.4 ± 1.5c | 12.8 ± 1.0b |
| 20–29 y | 1300 | 39.4 ± 1.6b | 16.0 ± 1.2b |
| 30–39 y | 1331 | 49.3 ± 1.8a | 23.6 ± 1.4a |
| Race/ethnicity2 | |||
| All women | 6404 | 44.4 ± 1.0 | 18.7 ± 0.8 |
| Non-Hispanic white | 2373 | 50.8 ± 1.4a | 22.3 ± 1.1a |
| Non-Hispanic black | 1598 | 30.0 ± 1.6b | 11.3 ± 1.1b |
| Mexican American | 1833 | 30.4 ± 1.4b | 10.8 ± 0.9b |
| All pregnant | 1250 | 77.5 ± 2.1 | 22.3 ± 2.2 |
| Non-Hispanic white | 545 | 89.0 ± 1.9a | 27.6 ± 3.4a |
| Non-Hispanic black | 200 | 58.7 ± 4.8b | 9.1 ± 2.9b |
| Mexican American | 374 | 65.0 ± 3.4b | 20.9 ± 2.4a,b |
| All nonpregnant | 5154 | 41.3 ± 1.0 | 18.5 ± 0.8 |
| Non-Hispanic white | 1828 | 47.8 ± 1.5a | 21.9 ± 1.2a |
| Non-Hispanic black | 1398 | 26.7 ± 1.8b | 11.3 ± 1.2b |
| Mexican American | 1459 | 25.5 ± 1.5b | 9.5 ± 1.0b |
Data are percentages ± SEs. Labeled percentages in a column with superscripts without a common letter differ for age overall and age by pregnancy status and for race/ethnicity overall and by pregnancy status, P < 0.003. DS, dietary supplement.
Race/ethnicity estimates age adjusted by direct method to the year 2000 projected U.S. population using 3 age groups: 15–19, 20–29, and 30–39 y.
Use of any DS was common among pregnant women; 77.5% reported DS use, which differed by age group (Table 1). Younger pregnant women reported less use (63.8%) compared with the oldest group, 30–39 y olds (88.3%). The use of iodine-containing DSs followed a similar pattern by age group, with 22.3% of pregnant women reporting the use of one or more iodine-containing DSs. Iodine-containing DSs were reported by 32.2% of pregnant women aged 30–39 y, which was significantly higher than women aged 15–19 y (11.8%) and women aged 20–29 y (18.1%). Among all women of reproductive age, non-Hispanic white women reported greater use both of any DS and of iodine-containing DSs than non-Hispanic blacks or Mexican Americans, regardless of their pregnancy status.
Among reproductive-aged women who reported using supplements with iodine, the mean daily iodine intake from supplements was 107 μg/d (Table 2). The Estimated Average Requirement (EAR) is 160 μg/d for pregnant women and 95 μg/d for nonpregnant women, regardless of age (21, 22). Comparing the population’s total intake of iodine to the established Dietary Reference Intakes would be very important to determine the percentage of the population not meeting the recommendations. However, because only supplement information is available, we were not able to estimate total iodine intake from foods, beverages, and DSs. For both groups, the median was slightly higher than the mean intake. The vast majority of iodine-containing DSs used by pregnant women contained 150 μg/serving (90%), with the primary source of the iodine in these supplements from potassium iodide (90%); another 5% of the iodine was contributed by kelp (data not shown). Among pregnant women, 64.6% took a DS labeled as a “prenatal,” which included over-the-counter (OTC) prenatals or prenatals prescribed by a doctor or other health care provider (Table 3). An estimated 37.1% of pregnant women reported use of a prescription product compared with 27.9% who reported use of OTC prenatals. Among pregnant women, 15.8% (Table 3) took a DS labeled as a prenatal that contained iodine. Iodine-containing prescription prenatals were taken by 10.9% of pregnant women and iodine-containing OTCs were taken by 4.9% of pregnant women. After prenatals, the next most commonly taken DSs for pregnant women were multivitamin/multimineral products (which were defined as those containing ≥3 vitamins and ≥1 minerals) (16), which was reported by 12.1% of pregnant women. Additionally, 3.0% of nonpregnant women reported taking a DS labeled as a prenatal, with older women (30–39 y) more likely to take such a product than the youngest women (15–19 y).
TABLE 2.
Daily intake of iodine from DSs among reproductive-aged women (15–39 y) who reported using a DS containing iodine, by pregnancy status in the United States, 1999–20061
| n | Mean2 | 25th percentile | Median3 | 75th percentile | |
| μg/d | |||||
| All women | 819 | 107 (102–113) | 60 | 124 (100–146) | 149 |
| Pregnant | 198 | 122 (110–134) | 75 | 144 (138–148) | 149 |
| Nonpregnant | 621 | 106 (100–112)* | 50 | 112 (99–146) | 149 |
Estimates of iodine intake are only from DSs. *Different from pregnant, P < 0.05. DS, dietary supplement.
Data are means and 95% CIs.
Data are medians and 95% CIs.
TABLE 3.
Prevalence of use of Rx and OTC prenatal DSs and supplemental iodine by women 15–39 y, by pregnancy status, age, and race/ethnicity: United States, 1999–20061–3
| Prenatal DS |
Prenatal DS containing iodine |
||||||
| n | Overall | Rx prenatal | OTC prenatal | Overall | Rx prenatal | OTC prenatal | |
| Age group | |||||||
| All women (15–39 y) | 6404 | 8.2 ± 0.5 | 4.2 ± 0.4 | 4.0 ± 0.3 | 1.8 ± 0.2 | 1.3 ± 0.2 | 0.5 ± 0.1 |
| 15–19 y | 2717 | 3.1 ± 0.4b | 1.7 ± 0.4b | 1.4 ± 0.2b | 0.5 ± 0.24b | – | – |
| 20–29 y | 1991 | 10.5 ± 0.7a | 5.1 ± 0.5a | 5.2 ± 0.6a | 2.0 ± 0.3a | 1.3 ± 0.2a | 0.8 ± 0.2 |
| 30–39 y | 1696 | 8.6 ± 0.8a | 4.5 ± 0.7a | 4.1 ± 0.5b | 2.2 ± 0.4a | 1.7 ± 0.4a | 0.5 ± 0.24 |
| All pregnant | 1250 | 64.6 ± 2.1 | 37.1 ± 2.7 | 27.9 ± 2.3 | 15.8 ± 2.2 | 10.9 ± 1.9 | 4.9 ± 1.4 |
| 15–19 y | 194 | 56.0 ± 5.5 | 34.5 ± 5.7 | 21.9 ± 4.4 | – | – | – |
| 20–29 y | 691 | 63.4 ± 2.5 | 33.2 ± 3.0 | 30.2 ± 2.7 | 14.5 ± 2.2 | 9.1 ± 1.6 | 5.4 ± 1.84 |
| 30–39 y | 365 | 68.9 ± 3.4 | 44.3 ± 5.0 | 25.8 ± 3.9 | 19.8 ± 4.3 | 14.9 ± 4.1 | – |
| All nonpregnant | 5154 | 3.0 ± 0.3 | 1.1 ± 0.2 | 1.8 ± 0.3 | 0.5 ± 0.1 | 0.4 ± 0.1 | – |
| 15–19 y | 2523 | 1.0 ± 0.3b | – | 0.6 ± 0.24b | – | 0 | – |
| 20–29 y | 1300 | 2.8 ± 0.5a,b | 1.0 ± 0.3 | 1.5 ± 0.4a,b | – | – | – |
| 30–39 y | 1331 | 4.1 ± 0.6a | 1.6 ± 0.4 | 2.5 ± 0.5a | 0.9 ± 0.34 | – | – |
| Race/ethnicity5 | |||||||
| All women | 6404 | 8.2 ± 0.5 | 4.2 ± 0.4 | 4.0 ± 0.3 | 1.8 ± 0.2 | 1.3 ± 0.2 | 0.5 ± 0.1 |
| Non-Hispanic white | 2373 | 8.9 ± 0.7 | 4.6 ± 0.5 | 4.2 ± 0.4a,b | 1.9 ± 0.3 | 1.3 ± 0.3 | 0.6 ± 0.24 |
| Non-Hispanic black | 1598 | 6.8 ± 0.7 | 4.0 ± 0.5 | 3.0 ± 0.5b | 1.3 ± 0.44 | 1.1 ± 0.44 | – |
| Mexican American | 1833 | 8.3 ± 0.8 | 3.0 ± 0.5 | 5.3 ± 0.6a | 1.9 ± 0.4 | 1.1 ± 0.44 | 0.8 ± 0.34 |
| All pregnant | 1250 | 64.2 ± 2.3 | 38.2 ± 2.8 | 26.6 ± 2.4 | 15.8 ± 2.4 | 11.2 ± 2.2 | 4.6 ± 1.3 |
| Non-Hispanic white | 545 | 72.6 ± 2.8a | 45.0 ± 3.7a | 27.9 ± 3.2 | 18.1 ± 3.5 | 11.9 ± 3.0 | 6.2 ± 2.24 |
| Non-Hispanic black | 200 | 48.3 ± 4.9b | 31.5 ± 4.3a,b | 19.3 ± 4.1 | 8.4 ± 2.94 | 7.2 ± 2.84 | – |
| Mexican American | 374 | 50.1 ± 4.3b | 18.4 ± 3.1b | 32.2 ± 4.3 | 12.0 ± 2.3 | 6.9 ± 2.64 | – |
| All nonpregnant | 5154 | 3.0 ± 0.3 | 1.1 ± 0.2 | 1.8 ± 0.3 | 0.5 ± 0.1 | 0.4 ± 0.1 | – |
| Non-Hispanic white | 1828 | 3.7 ± 0.5 | 1.4 ± 0.3 | 2.2 ± 0.4 | 0.6 ± 0.24 | 0.5 ± 0.24 | – |
| Non-Hispanic black | 1398 | 2.0 ± 0.4 | 0.8 ± 0.34 | 1.2 ± 0.3 | – | – | 0 |
| Mexican American | 1459 | 2.1 ± 0.5 | – | 1.4 ± 0.3 | – | – | – |
Prenatal DS is defined as a product that is labeled as a “prenatal” on the front label. DS, dietary supplement; OTC, over-the-counter; Rx, products that were prescribed by a doctor or other health care provider.
Data are percentages and SEs. Labeled percentages in a column with alphabetical superscripts without a common letter differ for age overall and age by pregnancy status and for race/ethnicity overall and by pregnancy status, P < 0.003.
Dashes indicate the relative SE >40% (data not shown).
The relative SE is >30% but ≤40% and may be statistically unreliable. The NHANES guidelines recommend a relative SE ≤30% and df ≥12.
Race/ethnicity estimates age adjusted by direct method to the year 2000 projected U.S. population using 3 age groups: 15–19, 20–29, and 30–39 y.
Median UICs were similar for pregnant and nonpregnant women (Table 4). Median UICs were also similar for pregnant users and nonusers of iodine-containing DSs. The median UIC was 125 μg/L for nonpregnant women not taking a supplement that contained iodine and 156 μg/L for nonpregnant women who were taking at least one DS containing iodine; the majority of labels claimed 150 μg/serving (Table 4). The median UIC was 150 μg/L for pregnant women not taking a supplement that contained iodine and 139 μg/L for pregnant women taking at least one DS containing iodine (also generally containing 150 μg/serving).
TABLE 4.
UICs among reproductive-aged women by pregnancy status and iodine-containing supplement use in the United States, 2001–2006
| n | Median1 | Q1 (25th percentile), Q3 (75th percentile) | |
| μg/L | |||
| All women | 1603 | 134 (120–145) | 72, 233 |
| Pregnant | 317 | 148 (101–198) | 72, 266 |
| Nonpregnant | 1286 | 133 (120–143) | 72, 230 |
| All nonusers2 | 1372 | 127 (115–140) | 68, 217 |
| Pregnant | 260 | 150 (104–204) | 60, 260 |
| Nonpregnant | 1112 | 125 (114–139) | 68, 215 |
| All users of DS with iodine3 | 231 | 153 (133–205) | 85, 299 |
| Pregnant | 57 | 139 (74–259) | 76, 289 |
| Nonpregnant | 174 | 156 (130–206) | 84, 300 |
Data are medians and 95% CIs. DS, dietary supplement; UIC, urinary iodine concentration.
Nonusers are defined as participants who did not use a DS that contained iodine but may or may not have used a DS.
Users of DS with iodine are defined as participants who used a DS that contained iodine.
Discussion
Many DSs used by pregnant women do not contain iodine. Although 77.5% of pregnant women reported taking one or more DSs, only 22.3% were taking an iodine-containing DS. Studies conducted in Europe have indicated that a large proportion of women in general also receive a prenatal supplement, with those taking products containing iodine ranging from 13 to 50% (23). The median UIC among pregnant women in this study was 148 μg/L (95% CI: 101–198). Iodine deficiency is the most frequent cause of preventable mental retardation globally. Although the burden of iodine deficiency in the US is lower than that in the developing world (24), it is still an important public health issue, because some subgroups of pregnant and reproductive-aged women are still at risk for mild deficiency (17). It has been difficult to assess pregnant women in nationally representative samples worldwide because of limited data on UIC, so therefore more studies are also need to measure this vulnerable group (24).
The EAR established for pregnant women by the Institute of Medicine is 160 μg/d. The majority of iodine-containing DSs reported in the NHANES contained 150 μg/serving and we estimated the mean daily intake from pregnant women taking an iodine-containing DS to be 107 μg/d. However, with only 22.3% of pregnant women taking an iodine-containing DS, it is clear that iodine is being consumed through other sources. It is not possible to specifically address dietary adequacy of iodine in NHANES, because total dietary iodine intakes are not available. The Total Diet Study (TDS), conducted by the FDA, estimated mean dietary iodine intakes of the U.S. population from analyses of the iodine content of food samples collected in late 2003 and 2004 that were representative of mean food intakes. For reproductive-aged women in the 25–30 y age group, the estimated lower and upper bound mean intakes from foods only were 148–196 μg/(person · d). The TDS also found that the majority of dietary intake of iodine came from the dairy and grain food groups (25). Although the TDS provides helpful baseline data on the dietary intake for the U.S. population, the estimates do not account for iodized salt intake and further research is needed (25). Also, efforts to add iodine to food composition tables should be expanded, because food composition tables in the US and Canada do not currently provide information on iodine due in large part to the difficulty in providing reliable estimates, because the iodine concentration in foods in highly variable (19, 26). Other sources of iodine include seaweed, saltwater fish, and seafood. Their contributions to the diet vary depending on whether or not these foods are consumed regularly. Fruits and vegetables provide iodine, but the amounts can vary depending on the region and season (19, 26). The iodine content of many foods is dependent upon the soil concentration of the element, which varies considerably from place to place and influences the amount of iodine found in plants. Furthermore, iodine in table salt can sublimate after time, decreasing the amount found in the food. Perrine et al. (17) found that the UIC among pregnant women was related to intake of dairy products. In general, information on dietary exposure to iodine in pregnant women in the US is very limited.
The median UIC among pregnant women was 148 μg/L. The WHO has established a cutoff for insufficient iodine intake for pregnant women at <150 μg/L, suggesting that as a population, we may not be meeting adequate intakes of iodine for pregnant women. However, the cut point did fall within our IQR. This was also true for both pregnant women that reported taking a DS containing iodine and for those that did not report taking an iodine-containing DS (Table 4). Median UICs were similar between pregnant women who used and who did not use iodine-containing DSs. It may be that dietary exposure to foods was much greater in nonusers of supplements. Because the analysis is based on the labeled amount of iodine, there is still uncertainty about the true iodine content of the iodine-containing DSs reported by pregnant women. Additionally, DS use was collected as a 30-d frequency type questionnaire that was administered before participants had provided urine samples. In the future, the use of DSs should be assessed at the same time as urine collection.
It is also important to mention the much higher proportion of nonpregnant women who are receiving iodine through usage of DSs. Although only 41.3% of nonpregnant women are taking one or more DSs compared with 77.6% of pregnant women, almost one-half of them are getting iodine from DSs. This is most likely because multivitamin/multimineral products are the most commonly reported product taken by this group and the most commonly reported multivitamin/multimineral products do contain iodine.
The methods used in NHANES to accurately identify and record the specific DSs reported by participants ensures high quality data. Although the DS data are self-reported, 80% of the time, NHANES interviewers saw the DS bottles and labels that participants reported using to verify accuracy. One limitation is that the NHANES DS database relied on manufacturers’ label declarations of the amounts of iodine in the products. The accuracy of these declarations can vary compared with analytically derived nutrient estimates. It has been reported that iodine contained in a product can exceed label declarations by an average of 26% (27). The UIC is thought to be a good marker of iodine sufficiency for population-level analysis, although the concentrations are dependent on recent intake. The strengths of this study include the large supplemental sample of pregnant women from 1999 to 2006 and the analysis of several years of nationally representative data, which allowed us to estimate both the prevalence of use of any DS and the use of iodine-containing DSs in pregnant women in the US. This, to our knowledge, this is the first such analysis using the NHANES data.
There is a need to further examine UIC in pregnancy, particularly with larger sample sizes than are currently available in the NHANES. Although urinary iodine is a good acute indicator of intake, it is important to monitor and assess the population’s chronic dietary exposure to iodine. Estimates of UICs from this report and others suggest that pregnant women in particular may not be consuming adequate iodine. It is important to understand the sources of iodine in order to make recommendations concerning how to reach adequate intake. Because iodine is available in very few foods, DSs may play a key role in ensuring that the population, particularly pregnant and nonpregnant women of child-bearing age as well as other subgroups at risk in the population, receives enough iodine for optimal health and optimal fetal development. Currently, the Office of Dietary Supplements at the NIH is evaluating iodine assessment methods to better determine iodine intakes in the US.
Acknowledgments
J.J.G., R.L.B., and J.T.D. contributed to the concept development and manuscript preparation; J.J.G., R.L.B., and L.B.M. analyzed data; J.J.G. and R.L.B. wrote the paper; and J.J.G., R.L.B., and J.T.D. reviewed the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: DS, dietary supplement; EAR, Estimated Average Requirement; NCHS, National Center for Health Statistics; OTC, over-the-counter; TDS, Total Diet Study; UIC, urinary iodine concentration.
Literature Cited
- 1.Zimmermann MB. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr. 2009;89:S668–72. [DOI] [PubMed] [Google Scholar]
- 2.Tayie FA, Jourdan K. Hypertension, dietary salt restriction, and iodine deficiency among adults. Am J Hypertens. 2010;23:1095–102. [DOI] [PubMed] [Google Scholar]
- 3.Hollowell JG, Staehling NW, Hannon WH, Flanders DW, Gunter EW, Maberly GF, Braverman LE, Pino S, Miller DT, Garbe PL, et al. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994). J Clin Endocrinol Metab. 1998;83:3401–8. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell KL, Jones R, Hollowell JG. Urinary iodine concentration: United States National Health and Nutrition Examination Survey 2001–2002. Thyroid. 2005;15:692–9. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid. 2011;21:419–27. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell KL, Miller GA, Wang RY, Jain RB, Jones RL. Iodine status of the U.S. population, National Health and Nutrition Examination Survey 2003–2004. Thyroid. 2008;18:1207–14. [DOI] [PubMed] [Google Scholar]
- 7.WHO. United Nations Children's Fund and International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination, a guide for programme managers. 3rd ed.; 2007 [cited 2012 Dec 19]. Available from: http://www.unicef.org/ukraine/2_Guide_for_IDD_managers_eng.pdf.
- 8.Public Health Committee of the American Thyroid Association, Becker DV, Braverman LE, Delange F, Dunn JT, Franklyn JA, Hollowell JG, Lamm SH, Mitchell ML, Pearce E, et al. Iodine supplementation for pregnancy and lactation-United States and Canada: recommendations of the American Thyroid Association. Thyroid. 2006;16:949–51. [DOI] [PubMed] [Google Scholar]
- 9.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirel LB. Characteristics of pregnant women from the 2001–06 National Health and Nutrition Examination Survey. Joint Statistical Meeting; 2009; Washington, DC; 2009. p. 3592–2602.
- 11.National Center for Health Statistics. National Health and Nutrition Examination Survey Analytic Guidelines; 2004 [cited 2012 December 19]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_general_guidelines_june_04.pdf.
- 12.National Center for Health Statistics. National Health and Nutrition Examination Survey response rates and current population survey totals. 1999–2006 [cited 2011 Sep 1]. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm.
- 13.National Center for Health Statistics. National Health and Nutrition Examination Survey data sets and related documentation, dietary supplement. 1999–2006 [cited 2011 Sep 1]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 14.Bailey RL, McDowell MA, Dodd KW, Gahche JJ, Dwyer JT, Picciano MF. Total folate and folic acid intakes from foods and dietary supplements of US children aged 1–13 y. Am J Clin Nutr. 2010;92:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrine CG, Herrick K, Serdula MK, Sullivan KM. Some subgroups of reproductive age women in the United States may be at risk for iodine deficiency. J Nutr. 2010;140:1489–94. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. The NHANES urinary iodine laboratory protocol; 2001–2006 [cited 2012 Jan 1]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2001–2002/L06UIO_B.htm#Description_of_Laboratory_Methodology.
- 19.Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev. 2012;70:553–70. [DOI] [PubMed] [Google Scholar]
- 20.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2000. Stat Notes. 2001;20:1–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11503781. [PubMed]
- 21.Institute of Medicine Food and Nutrition Board. Dietary reference intakes applications in dietary assessment. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 22.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann M, Delange F. Iodine supplementation of pregnant women in Europe: a review and recommendations. Eur J Clin Nutr. 2004;58:979–84. [DOI] [PubMed] [Google Scholar]
- 24.Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr. 2012;142:744–50. [DOI] [PubMed] [Google Scholar]
- 25.Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US Food and Drug Administration's Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol. 2008;18:571–80. [DOI] [PubMed] [Google Scholar]
- 26.Swanson CA, Zimmermann MB, Skeaff S, Pearce EN, Dwyer JT, Trumbo PR, Zehaluk C, Andrews KW, Carriquiry A, Caldwell KL, et al. Summary of an NIH workshop to identify research needs to improve the monitoring of iodine status in the United States and to inform the DRI. J Nutr. 2012;142:S1175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.USDA, Agricultural Research Service. USDA Dietary Supplement Ingredient Database Release 2.0 DSID-2 adult MVM research summary; 2012 [cited 2013 Mar 13]. Available from: http://dsid.usda.nih.gov/dsid_database/ResSummAdultMVM-3–22–12.pdf.