Abstract
An objective dietary biomarker would help clarify the contribution of sugar-sweetened beverage (SSB) intake to obesity and chronic disease risk. Previous studies have proposed the carbon isotope ratio (δ13C) as a biomarker of SSB intake but found associations that were of modest size and confounded by other components of the diet. We investigated whether the δ13C values of nonessential amino acids (δ13CNEAA) in RBCs could provide valid biomarkers that are more specific to SSBs. We assessed the associations of RBC δ13CNEAA with SSB intake in a study population of 68 Yup’ik people, using gas chromatography/combustion/isotope ratio mass spectrometry to measure δ13CNEAA and four 24-h dietary recalls to assess intake. Among RBC nonessential amino acids, alanine δ13C (δ13Calanine) was strongly correlated with intake of SSBs, added sugar, and total sugar (r = 0.70, 0.59, and 0.57, respectively; P < 0.0001) but uncorrelated with other dietary sources of elevated δ13C. We also evaluated whether sweetener intake could be noninvasively assessed using hair δ13Calanine in a subset of the study population (n = 30). Hair δ13Calanine was correlated with RBC δ13Calanine (r = 0.65; P < 0.0001) and showed similar associations with SSB intake. These results show that δ13Calanine in RBCs provides a valid and specific biomarker of SSB intake for the Yup’ik population and suggest RBCs and hair δ13Calanine as candidate biomarkers of SSB intake for validation in the general U.S. population. Ultimately, these biomarkers could clarify our understanding of whether and how SSB intake contributes to chronic disease.
Introduction
The consumption of sugar-sweetened beverages (SSBs)6, including sodas, sports drinks, sweetened tea, and other sweetened beverages, has increased over the past 30 y in the United States (1, 2). SSB intake has been linked to obesity (3–5) and type 2 diabetes (6), although empirical support for these relationships is mixed (7–9). Associations between self-reported SSB consumption and disease are attenuated by measurement error (10, 11), and both energy and sugar intake are known to be significantly under-reported (12–14). Recently, measurements of 24-h urinary sucrose and fructose have been validated as biomarkers of sugar intake; however, due to high day-to-day variability, repeated collections are required to characterize usual intake (15–17). An objective biomarker that could capture usual intake from a single blood or hair sample would be a valuable addition to existing tools for evaluating the health effects of SSB intake (11, 18).
Several studies have evaluated whether the stable carbon isotope ratio (13C/12C; expressed as δ13C as defined in Participants and Methods) of blood samples could be used as a measure of sweetener and SSB intake (19–21). This measure is based on the naturally high δ13C of foods made from sugar cane and corn, which is generated by a distinct photosynthetic pathway (C4) shared by very few other U.S. food plants (22, 23). However, associations between serum and whole blood δ13C and sweetener intake are modest (19, 21), possibly due to stronger associations between δ13C and corn-fed meat and other animal products (21, 24). A fundamental limitation of blood δ13C as a measure of sweetener intake is that the carbon in blood occurs primarily in the form of proteins and thus tends to reflect the δ13C of dietary protein sources. Our group recently showed that using the nitrogen isotope ratio to control for the confounding effects of dietary protein on δ13C greatly improves its validity in an Alaska Native population (25). Although this approach dramatically improves the validity for sweeteners, it does not control for all dietary confounders, including commercial (corn-fed) meat intake.
Another approach to improving the specificity of δ13C for sweeteners is to measure the δ13C of molecules that favor the incorporation of glucose carbon, thus reducing or eliminating the influence of dietary confounders (26). For example, the nonessential (dispensable) amino acids (NEAAs) in protein have the potential to be synthesized from glucose carbon, whereas the essential (indispensable) amino acids do not (27, 28). When an NEAA is synthesized from glucose, its δ13C should reflect the δ13C of recent corn- and cane-based sweetener intake (26). In humans, glucose can significantly contribute to synthesis of NEAAs, particularly alanine (29, 30). Here, we propose that measurements of δ13C of individual NEAAs (δ13CNEAA) may provide more specific biomarkers of usual SSB intake than whole-protein δ13C.
The aim of this research is to identify whether δ13CNEAA can provide valid and specific biomarkers of SSB and sugar intake. We evaluate these biomarkers in a Yup’ik study population from Southwest Alaska, with whom we have an ongoing research relationship focused on diet and chronic disease risk. We evaluated the relationships between δ13CNEAA and self-reported intake of SSBs, added sugar, and total sugar as well as other foods having elevated δ13C: commercial (corn-fed) meats, corn products, and marine foods (fish and marine mammals), which have elevated δ13C from marine bicarbonate (25, 31). Intake measures were based on the mean of four 24-h recall dietary interviews (24HRs). As a preliminary assessment of whether hair could provide a less-invasive alternative to RBCs, we also assessed associations among hair δ13CNEAA, RBC δ13CNEAA, and SSB intake in a subset of the study population.
Participants and Methods
Recruitment and procedures.
Data are from the Center for Alaska Native Health Research Neqem Nallunailkutaa (“The Foods’ Marker”) study. This study was approved by the University of Alaska Fairbanks Institutional Review Board and the Yukon-Kuskokwim Health Corporation Human Studies Committee.
In 2008–2009, 68 participants aged 14–79 y were recruited from 2 coastal Yup’ik communities in Southwest Alaska. All participants completed a demographic questionnaire at enrollment and the first of four 24HRs. Three more dietary interviews were conducted over the next 4 wk, as described elsewhere (25). At ∼2 wk after the last dietary interview, fasting blood samples, hair samples, and basic anthropometric measurements were collected. Specimen collection was timed so that the mean age of RBCs (1.5 mo) would coincide with the midpoint of the period over which dietary interviews were conducted (32). Hair samples were collected only from participants with hair length >2 cm and had limited availability following previous analyses. Therefore, the sample size for hair analyses was 30.
Specimen collection.
Blood was collected into 10-mL EDTA tubes (15% solution, 0.117 mL, 17.55 mg) and centrifuged in the field. Samples were transported to the University of Alaska Fairbanks at −15°C and transferred to long term storage at −80°C.
A sample of ∼50 hairs/participant was clipped close to the scalp with scissors, just inferior to the post-occipital protuberance, and the proximal end of the section was marked with tape. Hair sections were placed into strips of aluminum foil, folded, and stored at room temperature. This study used the section of hair from 2–3 cm from the scalp, as the section from 0–2 cm from the scalp was used for a previous study. Hair was cleaned of tape and other residues with 2 successive 30-min washes with sonication in chloroform/methanol (2:1) and a final 30-min wash with sonication in distilled water (33).
Amino acid extraction and derivatization.
We extracted amino acids from RBCs and hair samples using protocols described by Popp et al. (34). RBCs (50 μg aliquots) and hair (∼1–1.5 mg) were hydrolyzed with 1 mL 6 M HCl (110°C, 20 h). Standard mixtures of 12 amino acids were prepared alongside samples (Sigma-Aldrich Chemie; Fluka Chemie); these underwent all steps including hydrolysis. To eliminate lipids from RBC samples, 2 mL n-hexane/dichloromethane (DCM) (6:5, v:v) was added to the hydrolysates and the lipid phase was removed. The solution was purified by filtration (0.22 μm Millex-GP, Millipore) followed by a rinse with 1 mL 0.01 N HCl (35). Hydrolysates from hair were not lipid-extracted. Norleucine was added to each sample as an internal standard. The hydrolysates were evaporated to dryness at 110°C in a heating block under a gentle stream of N2 for 30 min. After evaporation, samples were stored at 4°C for <2 wk.
Standard amino acid mixtures and isolated RBC and hair amino acids were derivatized to N-trifluoroacetic acid isopropyl esters as described elsewhere (36, 37). Each sample was propylated in a 0.8-mL solution of acidified 2-propanol (isopropanol:acetyl chloride, 4:1) for 1 h at 110°C, then dried under nitrogen. Trifluoroacetylation was performed by adding 1 mL DCM:trifluoroacetic anhydride (1:1, v:v, 10 min at 110°C). Following each reaction, reagents were evaporated under nitrogen and samples underwent successive washes and evaporation of DCM (2 × 250 μL). The samples were transferred to sealed vials in 500 μL DCM.
Analysis of amino acid δ13C.
δ13CNEAA were analyzed by GC/combustion/isotope ratio MS, using a Thermo Trace gas chromatograph, the GC-IsoLink combustion interface, and a Delta-V isotope ratio mass spectrometer (Thermo Fisher Scientific) at the Alaska Stable Isotope Facility (Fairbanks, Alaska). The conventional method of expressing δ13C at natural abundance is in permil (‰) abundance of 13C relative to an international standard (Vienna PeeDee Belemnite, 13C/12C = 0.011237), as follows:
Thus, a reported difference of 1‰ equals a difference in 13C/12Csample of 1/100,000, requiring isotope ratio MS or similarly precise techniques to measure. Because the standard contains more 13C than the samples in this study, all δ13C values reported here are negative.
Approximately 2 μg of amino acids (via l-μL injection) was injected on column in splitless mode at 75°C and separated on a 50-m HP Ultra-1 column (0.32-mm i.d., 0.25-μm film thickness). Amino acids were separated by GC, oxidized completely to CO2, and introduced into the isotope ratio mass spectrometer in a continuous stream of helium. Samples were analyzed in duplicate along with amino acid standards of known isotopic composition and each measured mean amino acid δ13C was corrected relative to the mean amino acid δ13C of standards to account for the exogenous carbon and kinetic fractionation introduced during derivatization (36, 37). Analytical errors (SDs) in measuring the derivatized amino acids ranged from 0.1 to 0.9‰ with a mean of ±0.2‰. Errors of corrected δ13C for each amino acid ranged from 0.1 to 1.5‰ with a mean of ±0.5‰.
We obtained δ13C values from 12 amino acids from each RBC sample and 13 amino acids from each hair sample. In this paper, we present data from 6 NEAAs that have the potential to be derived from sugar: alanine, glycine, serine, proline, aspartate/asparagine, and glutamate/glutamine. Asparagine and glutamine are converted to aspartate and glutamate, respectively, during acid hydrolysis; therefore, these amino acids are indistinguishable.
Assessment of dietary intake.
24HRs were collected from each participant by certified interviewers using computer-assisted software [Nutrition Data System for Research (NDSR) software 2008; University of Minnesota]. The majority of interviews were completed in person (93%, n = 261); however, some participants completed either 1 (n = 15) or 2 (n = 2) interviews over the telephone. Participants were asked to recall all food and beverages consumed the day prior to the interview using a multiple pass approach, which included a quick list, forgotten foods probe, detail cycle, and final probe. Interviewers used portion estimation tools, including measuring cups, rulers, bowls, mugs, drinking glasses, and food models. Participants completing phone recalls were given tool packs with a ruler, measuring cups and spoons, and a portion estimation guide (Fred Hutchinson Cancer Research Center). Although most participants were bilingual, an NDSR-certified native Yup’ik speaker conducted interviews for participants who did not speak English. Dietary interviews were 9 ± 5 d apart, with a minimum of 2 d between recalls. Intake of dietary components was calculated from the NDSR food and nutrient database using NDSR food codes (38).
In this study, sweetener intake is measured in 3 ways: as SSBs, added sugar, and total sugar. SSB intake was calculated as the sum of servings of sweetened soft drinks and sweetened fruit drinks [servings/d, 237 mL (8 fl oz)/serving]. Added sugar (g/d) is defined as the sum of sugars and syrups added to foods during food preparation or commercial food processing. Total sugar intake (g/d) is defined as the sum of all mono- and di-saccharides consumed, and included primarily sucrose, fructose, and glucose.
We also present data on intake of other food items having elevated δ13C in this study population, including commercial meats (percent energy), fish and marine mammals (percent energy), and corn products (g/d) (31), because intake of these foods may also affect δ13CNEAA. Commercial meats were defined as meat products purchased from local stores and included poultry, eggs, pork, and beef products. Fish and marine mammals were harvested from the local environment and included both freshwater and marine fish species. Corn products included whole corn and foods made primarily from whole corn, including popcorn, corn cereal, corn chips, and corn tortillas (25).
To describe the diet pattern of the study population more generally, we present total energy intake (kcal), traditional and commercial intake (percent energy), and macronutrient intake (percent energy). Traditional and commercial intake includes all foods harvested from the local environment and purchased from stores, respectively. Access to commercial foods is limited, because the communities participating in this study are off the road system and must be reached by small plane. Each community has 2–3 small stores where limited commercial foods can be purchased, particularly nonperishable or frozen foods, and no restaurants. Traditional foods include fish, marine mammals, moose, caribou, waterfowl, and local greens and berries, with fish and marine mammals contributing >70% of traditional food energy (39). Major sources of commercial food energy include soda, fruit drinks, commercial meat, crackers, pasta, rice, and vegetable shortening (40).
Statistical analyses.
All statistical analyses were performed using JMP version 8 (SAS Institute). The following dietary intake variables were log-transformed for analyses: SSBs (servings/d + 1), added sugar (g/d), total sugar (g/d), and corn products (g/d + 1). Differences in age, sex, and BMI between the complete study sample (n = 68) and the subsample with hair analyses (n = 30) were assessed using Student’s t and χ2 tests. Associations of dietary intake variables with SSB intake were assessed using Pearson’s product moment correlations, as were associations of SSB intake with δ13CNEAA. Further analyses focused on the δ13C of alanine (δ13Calanine), because only δ13Calanine was strongly associated with SSB, added sugar, and total sugar intake. We used multiple regression models to examine whether intake of sugar (as SSBs, added sugar, or total sugar), commercial meat, fish and marine mammals, and corn products affected δ13Calanine independently. In these models, δ13Calanine was the dependent variable. We then assessed the ability of δ13Calanine to predict SSB intake with a linear regression model, in which SSB was the dependent variable. To test whether associations of δ13Calanine with SSBs differed by sex or BMI, we fit multiple regression models that included the interactions of sex and BMI with δ13Calanine (BMI groups = normal, ≥18.5 and ≤25 kg/m2, overweight >25 and ≤30 kg/m2, and obese >30 kg/m2). The association of δ13Calanine in RBCs and hair (n = 30) was assessed using both Pearson’s product moment correlation and the means and SDs of their differences (41). Lastly, we tested whether the regression coefficients for δ13C predicting SSB intake differed for hair and RBCs using a multiple regression model, which included the interaction of tissue type (RBC vs. hair) with δ13Calanine. Means are presented ±SDs and significance was set at 2-sided α = 0.05.
Results
The age, sex, and BMI distribution of the total study population (n = 68) and the subset of the population with hair samples (n = 30) are presented in Table 1. The distribution of age and BMI did not differ between the total study population and the subset with hair samples. A larger proportion of the subset were women, because many men had hair that was either too short to sample or was fully used in prior analyses.
TABLE 1.
Age, sex, and BMI distribution for the complete study sample and the subset of participants with hair samples12
| Complete sample, n = 68 | Hair subset, n = 30 | |
| Sex, y % female | 49 | 70* |
| Age, | 41 ± 18 | 37 ± 17 |
| 14–19 y, % | 16 | 17 |
| 20–39 y, % | 32 | 37 |
| 40–59 y, % | 40 | 37 |
| ≥60 y, % | 12 | 10 |
| BMI, kg/m2 | 27.2 ± 6.3 | 26.7 ± 6.5 |
| <18.5 kg/m2, % | 1 | 3 |
| ≥18.5 and ≤25 kg/m2, % | 44 | 40 |
| >25 and ≤30 kg/m2, % | 24 | 27 |
| >30 kg/m2, % | 31 | 30 |
Values are means ± SDs or percent of study sample. *Different from the complete sample, P < 0.05.
Differences in means between the study samples were assessed with t tests and differences in distribution between the study samples were assessed with chi-square tests.
The dietary characteristics of the study population and their associations with SSB, added sugar, and total sugar intake are presented in Table 2. SSB, added sugar, and total sugar intakes were positively associated with total commercial food intake and percent energy from carbohydrates (Table 2). SSB, added sugar, and total sugar intakes were negatively associated with total traditional intake, percent energy from dietary protein and fat, and fish and marine mammal intake. Intakes of added and total sugar, but not SSBs, were associated with total energy intake and corn product intake. None of the sugar intake variables were associated with percent energy from commercial meats. All sugar intake variables were strongly correlated.
TABLE 2.
Dietary characteristics of the study population and associations with SSBs and added and total sugar intake1
| Associations with intake (r) |
||||
| Dietary intake variables | Intake values | SSB | Added sugar | Total sugar |
| General | ||||
| Total energy intake, kcal/d | 2055 ± 691 | 0.14 | 0.36** | 0.49*** |
| Total commercial intake, % energy | 78 ± 20 | 0.54*** | 0.54*** | 0.65*** |
| Total traditional intake, % energy | 22 ± 20 | −0.54*** | −0.54*** | −0.65*** |
| Macronutrients | ||||
| Protein, % energy | 18 ± 6 | −0.61*** | −0.66*** | −0.68*** |
| Fat, % energy | 38 ± 9 | −0.56*** | −0.48*** | −0.49*** |
| Carbohydrate, % energy | 44 ± 14 | 0.66*** | 0.64*** | 0.66*** |
| Sweeteners | ||||
| SSB intake,2 servings/d | 1.4 (1.1, 1.8) | — | 0.78*** | 0.75*** |
| Added sugars,2 g/d | 74 (62, 87) | 0.78*** | – | 0.92*** |
| Total sugars,2 g/d | 89 (77,104) | 0.75*** | 0.92*** | – |
| Other foods with elevated δ13C | ||||
| Commercial meat, % energy | 11 ± 8 | 0.13 | 0.05 | 0.23 |
| Fish and marine mammals, % energy | 18 ± 18 | −0.48*** | −0.44* | −0.56** |
| Corn products,2 g/d | 11 (8, 16) | 0.22 | 0.25* | 0.36** |
Values are means ± SDs or geometric mean (95% CI) for log-transformed variables and r, assessed with Pearson’s product moment correlation, n = 68. *P < 0.05, **P < 0.005, ***P < 0.0001. SSB, sugar-sweetened beverage; δ13C, carbon isotope ratio.
Variables were log-transformed for analysis.
The associations of RBC δ13CNEAA with intake of SSBs, added sugar, and total sugar are presented in Table 3. Only δ13Calanine was strongly associated with intake of SSBs, added sugar, and total sugar. The δ13C of proline was moderately associated with SSB intake, but not with added or total sugar. There were no associations between the δ13C of glutamate/glutamine, aspartate/asparagine, serine, or glycine and intake of SSBs, added sugar, or total sugar.
TABLE 3.
Associations of δ13CNEAA values with sweetener intake1
| Associations with intake (r) | ||||
| Amino acid | δ13C | SSB | Added sugar | Total sugar |
| Alanine | −16.9 ± 1.8 (−20.6, −12.4) | 0.70*** | 0.59*** | 0.57*** |
| Aspartate/asparagine | −18.3 ± 2.3 (−25.5, −14.9) | 0.23 | 0.15 | 0.15 |
| Glutamate/glutamine | −16.8 ± 2.4 (−23.4, −12.7) | 0.17 | 0.10 | 0.12 |
| Glycine | −8.8 ± 1.5 (−12.3, −5.6) | 0.05 | −0.07 | −0.13 |
| Proline | −16.1 ± 1.4 (−19.0, −13.3) | 0.31* | 0.14 | 0.10 |
| Serine | −8.6 ± 3.4 (−15.1, −2.0) | 0.04 | 0.07 | 0.09 |
Values are means ± SDs (range) and r, assessed with Pearson’s product moment correlation, n = 68. *P < 0.05, ***P < 0.0001. SSB, sugar-sweetened beverage; δ13C, carbon isotope ratio; δ13CNEAA, nonessential amino acid carbon isotope ratio.
The associations between δ13Calanine and SSBs, added sugar, and total sugar could be confounded by intake of other foods with elevated δ13C: commercial meat, fish and marine mammals, and corn products (25, 31). We tested the specificity of δ13Calanine for SSB intake using a linear model in which the dependent variable was δ13Calanine and the independent variables were SSB, commercial meat, fish and marine mammals, and corn product intake (Table 4). The only significant association was between δ13Calanine and SSB intake. We repeated this analysis using added sugar and total sugar, and again, only the sugar intake variables predicted δ13Calanine (data not shown).
TABLE 4.
| Dietary variable | βs | β (95% CI) | R2 |
| SSB, servings/d | 0.67 | 2.24 (1.56, 2.92)*** | 0.51 |
| Fish and marine mammals, % energy | −0.00 | −0.02 (−2.41, 2.38) | |
| Commercial meats, % energy | 0.12 | 2.85 (−2.25, 7.95) | |
| Corn products, g/d | 0.05 | 0.07 (−0.19, 0.34) |
Values are standardized (βs) and unstandardized (β) beta-coefficients and R2 for a linear model in which δ13Calanine was the dependent variable and dietary intake measures were the independent variables, n = 68. ***P < 0.0001. SSB, sugar-sweetened beverage; δ13C, carbon isotope ratio; δ13Calanine, alanine carbon isotope ratio.
1 serving SSB = 237 mL.
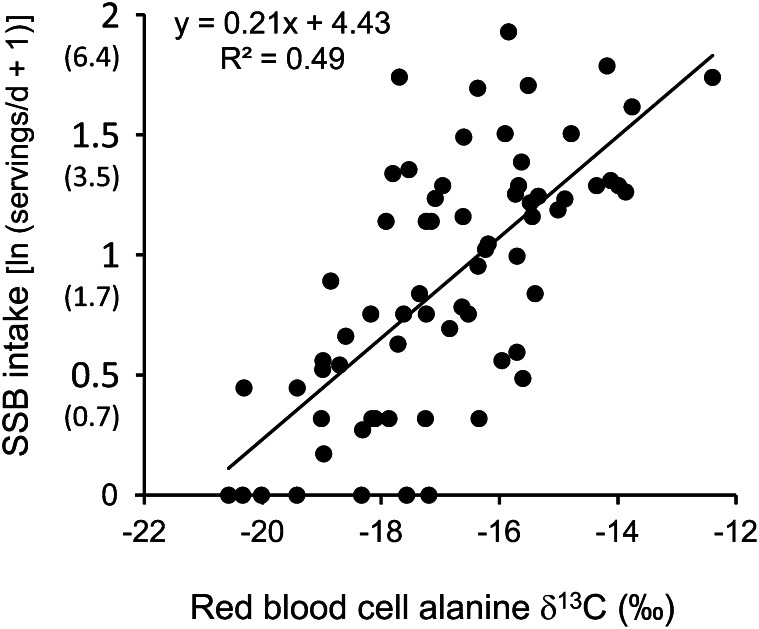
In a linear, predictive model, δ13Calanine explained 49% of the variation in SSB intake (Fig. 1; Table 5). Self-reported sugar intake may be differentially biased by sex and BMI; however, regression coefficients were similar and there were no significant differences across sex and BMI groups (Table 5).
FIGURE 1.
The linear relationship between δ13Calanine values in RBC and SSB intake, n = 68. Axis labels are also shown back-transformed (in parentheses) as servings/d. 1 serving SSB = 237 mL. SSB, sugar-sweetened beverage; δ13C, carbon isotope ratio; δ13Calanine, alanine carbon isotope ratio.
TABLE 5.
| Sample | n | β3 (95% CI) | Intercept | R2 | P |
| Total | 68 | 0.23 (0.17, 0.30) | 4.43 | 0.49 | <0.0001 |
| Male | 35 | 0.25 (0.15, 0.35) | 4.72 | 0.50 | <0.0001 |
| Female | 33 | 0.22 (0.14, 0.31) | 4.24 | 0.51 | <0.0001 |
| Normal weight | 29 | 0.22 (0.14, 0.30) | 4.26 | 0.57 | <0.0001 |
| Overweight | 17 | 0.30 (0.08, 0.57) | 5.20 | 0.38 | 0.0087 |
| Obese | 21 | 0.26 (0.09, 0.45) | 4.84 | 0.38 | <0.0027 |
| Overweight + obese | 38 | 0.25 (0.13, 0.38) | 4.58 | 0.34 | <0.0001 |
Values are n, model parameters, R2, and P for linear models in which SSB intake was the dependent variable and δ13Calanine was the independent variable. SSB, sugar-sweetened beverage; δ13Calanine, alanine carbon isotope ratio.
One underweight participant was excluded from BMI-stratified analyses.
β-Coefficients are back-transformed for ease of interpretation and are interpreted as proportional increase in SSB intake for every 1‰ increase in δ13Calanine.
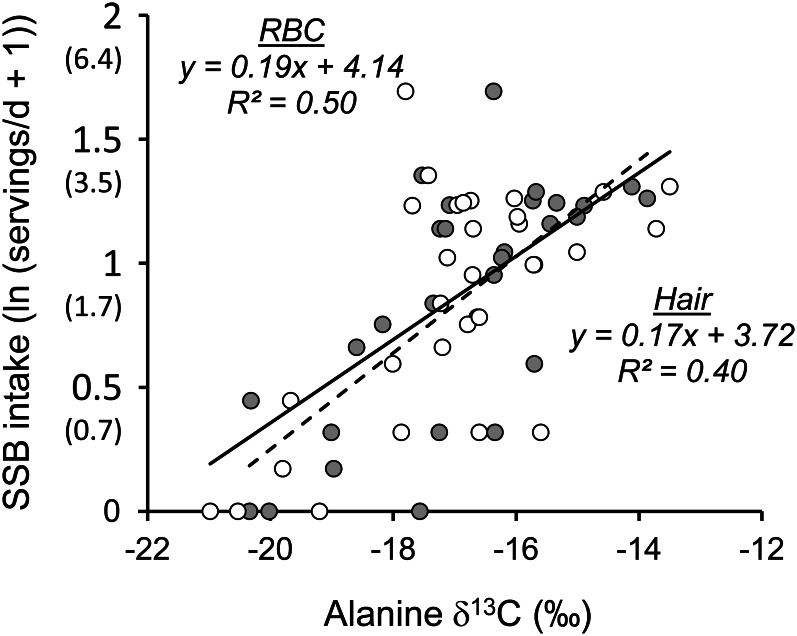
The correlation between RBC and hair δ13Calanine was r = 0.65 (P < 0.0001) and the measures generally showed good agreement, with a mean difference of 0.1 ± 1.5‰. Agreement was poor for 6 of the samples, with differences from 2.2 to 3.4‰. Figure 2 shows the linear associations between SSB intake and δ13Calanine in RBCs and hair for the subset of participants with both RBC and hair samples (n = 30). The slope of the linear relationship with SSB intake did not significantly differ for RBC and hair δ13Calanine; however, the R2 was higher for RBC δ13Calanine (0.50) than for hair δ13Calanine (0.40).
FIGURE 2.
The linear relationships between δ13Calanine values in RBCs (gray circles, solid line) and hair (white circles, dotted line) and SSB intake, n = 30. Axis labels are also shown back-transformed (in parentheses) as servings/d. 1 serving SSB = 237 mL. SSB, sugar-sweetened beverage; δ13C, carbon isotope ratio; δ13Calanine, alanine carbon isotope ratio.
Discussion
In this Yup’ik study population, we found that δ13Calanine, but not other NEAAs, was strongly correlated with intakes of SSB, added sugar, and total sugar. Furthermore, δ13Calanine was specific to sugar intake, as it was not associated with intake of other foods having elevated δ13C, including corn products, commercial meat, or fish and marine mammals. δ13Calanine predicted 49% of the variation in SSB intake, with each 1‰ increase in δ13Calanine associated with a 23% increase in SSB intake. The relationship of δ13Calanine with SSBs was consistent across sex and BMI classes, suggesting that SSB intake was not selectively under-reported in these groups. Finally, preliminary data suggested that hair δ13Calanine, a noninvasive measure, had a predictive relationship for SSB that was very similar to RBC δ13Calanine, although the R2 was higher for RBC δ13Calanine. These results show that δ13Calanine is a good biomarker for SSB and sugar intake in Yup’ik people and has promise as a new biomarker of sugar intake for the general population.
This study follows a recent publication from our group presenting a dual-isotope model of sweetener intake based on RBC δ13C and δ15N (25). In both cases, the isotopic measures explained a similar percentage of variation in sweetener intake: 49% of the variation in SSB intake for δ13Calanine and 48% of the variation in total sugar intake for the dual-isotope model (25). However, there were also differences in the performance of the 2 measures: δ13Calanine was not associated with any potential dietary confounders, including commercial meat and fish intake, whereas the dual-isotope model was associated with commercial meat intake as well as sweeteners. Measuring the δ13C of specific amino acids like alanine is significantly more time-consuming and analytically challenging than measuring δ13C and δ15N in whole tissue samples. We expect that δ13Calanine will be most useful as a calibration tool, either for self-report data or more high-throughput biomarkers of sweetener intake. Importantly, both isotopic approaches to measuring sweetener intake require further validation in a more representative U.S. population before they can be used more broadly in nutritional epidemiology. We expect those future studies will further clarify the costs and benefits of these 2 isotopic approaches.
Isotopic biomarkers of sweetener intake in RBC offer some advantages to existing biomarkers of sugar and sweetener intake, in particular, their potential to capture longer term or “usual” intake from a single sample. The time to 85% replacement of human RBCs takes ∼3 mo (32), and the amino acids used in RBC synthesis may derive in part from body proteins, which integrate dietary carbon over an even longer period. Thus, isotopic measures in RBCs reflect moderately long-term intake and can be measured in either fasting or nonfasting blood samples. Alternatively, daily extrinsic sugar intake can be measured from 24-h urinary sucrose and fructose (16, 17) or from the mean of several spot urines collected across the day (15). However, multiple days of sampling are required to estimate longer term or usual intake, which may present logistical difficulties for some studies. Isotopic measures like RBC δ13Calanine would be especially useful when SSB intake is of particular interest, when a single blood specimen is more feasible to collect, or when only stored, fasted specimens are available.
In the Yup’ik population, δ13Calanine was most strongly associated with SSBs but was also associated with added and total sugar. SSBs are primarily sweetened with corn syrup or cane sugar, both of which have highly elevated δ13C values (20). In contrast, added sugar may include beet sugar, honey, and maple syrup, which do not have elevated δ13C values. Total sugar includes added sugar as well as “intrinsic” sugars from milk, fruit, and other sources that do not have high δ13C values (17). For our study population, beet sugar and pure maple syrup were not available in local stores and our dietary assessments found limited use of honey (this study), low intake of dairy (except in youth), and relatively little consumption of fresh fruit (40). Added sugar made up 84% of total sugar intake and δ13Calanine was similarly correlated with both added and total sugar. In a non-Yup’ik population, we expect δ13Calanine would show the strongest associations with SSB intake, followed by added sugar intake, and weaker associations with total sugar intake, as has been found elsewhere (19).
Although alanine is an amino acid, its δ13C was not associated with intake of dietary protein sources having elevated δ13C, namely, commercial meats or fish and marine mammals. Alanine can be synthesized in a single reaction from pyruvate, the end product of glycolysis (42). Early work posited a “glucose-alanine cycle” in which pyruvate is converted to alanine and recycled back to glucose in the liver (43). In mice that were fed glucose with isotopically labeled carbon, circulating blood alanine was in isotopic equilibrium with blood glucose (44). Results from human studies also support a high level of alanine synthesis from blood glucose (29, 30). A recent controlled feeding study in humans found a strong but short-term effect of corn- and cane-based sweetener intake on the δ13C of blood glucose (26). We propose that these short-term effects on blood glucose δ13C are captured in a longer lasting isotopic “record” when glucose is converted to alanine and alanine is incorporated into proteins during protein synthesis. Our data support a substantial level of alanine synthesis from dietary sugar in human RBCs and suggest a lesser contribution of carbon directly from dietary proteins.
Measurements of δ13Calanine in RBCs and hair showed very similar relationships with SSB intake. However, RBC δ13Calanine explained more of the variation in SSB intake than hair δ13Calanine, and agreement in δ13Calanine between RBCs and hair was poor for several samples. The study was designed so that the midpoint of the period during which dietary data were being collected matched the mean age of RBCs at the time of specimen collection. Because hair grows at ∼1 cm/mo, we estimate that the hair section with the best temporal match to the dietary data would be 1–2 cm from the scalp. Unfortunately, this section was used for another study. Here, we used the section from 2–3 cm, which may have caused a small temporal mismatch between hair and RBC δ13C as well as with the dietary intake data. Nevertheless, these data show that hair δ13Calanine has promise to be a noninvasive biomarker of SSB intake and that further investigation of this marker is warranted.
The use of self-reported measures to validate δ13Calanine as a biomarker of SSB intake has limitations. Error and bias in self-reported dietary intake should attenuate relationships between biomarkers and diet; therefore, the associations presented in this study are conservative. 24HRs have 2 sources of error: first, that dietary recall is imperfect, and second, intake on the day of recall may not be representative of usual intake (45). These sources of error are mitigated by averaging intake across multiple, independently collected 24HRs, as was done here (10, 46). Another concern is that energy and sugar intake have been shown to be significantly under-reported (12–14, 47, 48), which could also attenuate the relationships observed between δ13Calanine and SSBs (48). In other studies, these biases have been shown to be associated with sex (47, 49) and BMI (12, 14). However, in this study, we found identical relationships between δ13Calanine and SSB intake in men and women and in normal weight, overweight, and obese participants.
The design of this study also has important advantages. Validation at the population level is needed to test the effects of dietary background on tissue stable isotope ratios, as multiple foods can affect isotope ratios and both the isotopic distribution among foods and dietary patterns may differ among populations. Here, the population level design allowed us to demonstrate that varying intake of fish and marine mammals and commercial meats did not affect δ13Calanine in this Yup’ik study population, which is an important finding. However, adoption of δ13Calanine as a biomarker of SSB intake in the general U.S. population will require validation in a more representative population. For example, in this study, we found that intake of corn products did not affect δ13Calanine; however, the Yup’ik population consumes relatively little corn (mean intake, 11 g/d). Further work is needed to demonstrate similar specificity in a population with higher and more variable corn intake, lower intake of fish, and higher intakes of meat from corn-fed animals.
In summary, this study proposes a new candidate biomarker of SSB intake based on a promising new methodology for nutritional epidemiology: stable isotope analysis of individual biochemical compounds. We find that the δ13C of RBC alanine performs very well as an objective biomarker of usual SSB and added and total sugar intake in the Yup’ik population. We suggest that δ13Calanine is a very promising candidate biomarker of usual SSB intake for the general U.S. population, because δ13Calanine appears to be more sensitive to intake of sugar than that of protein sources. However, because the dietary context of the general U.S. population differs significantly from that of the Yup’ik population studied here, validation in a more representative group is needed to more broadly apply this marker of SSB intake. Ultimately, measurement of δ13Calanine could help refine and clarify our understanding of how SSB intake contributes to obesity and chronic disease.
Acknowledgments
The authors thank E. Orr, K. Niles, and J. Black for assistance with data collection, and T. Howe and M. Wooller for assistance with the stable isotope analyses. This manuscript was improved by comments from T. Lee and C. van Hemert. D.M.O., A.R.K., and B.B.B. designed research; K.C., S.H.N., D.M.O., S.H., and B.B.B. conducted research; K.C. performed laboratory analyses; K.C. and D.M.O. performed statistical analyses; K.C., S.H.N., A.R.K., and D.M.O. wrote the paper; and D.M.O. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: DCM, dichloromethane; NDSR, Nutrition Data System for Research; NEAA, nonessential amino acid; SSB, sugar-sweetened beverage; 24HR, 24-h recall dietary interview; δ13C, carbon isotope ratio; δ13Calanine, alanine carbon isotope ratio; δ13CNEAA, nonessential amino acid carbon isotope ratio.
Literature Cited
- 1.French SA, Lin BH, Guthrie JF. National trends in soft drink consumption among children and adolescents age 6 to 17 years: prevalence, amounts, and sources, 1977/1978 to 1994/1998. J Am Diet Assoc. 2003;103:1326–31. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res. 2002;10:370–8. [DOI] [PubMed] [Google Scholar]
- 3.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–22. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–8. [DOI] [PubMed] [Google Scholar]
- 5.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- 7.Allison DB, Mattes RD. Nutritively sweetened beverage consumption and obesity: the need for solid evidence on a fluid issue. JAMA. 2009;301:318–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forshee RA, Anderson PA, Storey ML. Sugar-sweetened beverages and body mass index in children and adolescents: a meta-analysis. Am J Clin Nutr. 2008;87:1662–71. [DOI] [PubMed] [Google Scholar]
- 9.van Baak MA, Astrup A. Consumption of sugars and body weight. Obes Rev. 2009;10:9–23. [DOI] [PubMed] [Google Scholar]
- 10.Thompson FE, Subar AF. Dietary assessment methodology. : Coulston AM, Boushey CJ, Nutrition in the prevention and treatment of disease. 2nd ed San Diego: Academic Press; 2008. [Google Scholar]
- 11.Bingham SA. Biomarkers in nutritional epidemiology. Public Health Nutr. 2002;5:821–7. [DOI] [PubMed] [Google Scholar]
- 12.Bingham S, Luben R, Welch A, Tasevska N, Wareham N, Khaw KT. Epidemiologic assessment of sugars consumption using biomarkers: comparisons of obese and nonobese individuals in the European Prospective Investigation of Cancer Norfolk. Cancer Epidemiol Biomarkers Prev. 2007;16:1651–4. [DOI] [PubMed] [Google Scholar]
- 13.Poppitt SD, Swann D, Black AE, Prentice AM. Assessment of selective under-reporting of food intake by both obese and non-obese women in a metabolic facility. Int J Obes Relat Metab Disord. 1998;22:303–11. [DOI] [PubMed] [Google Scholar]
- 14.Prentice RL, Mossavar-Rahmani Y, Huang Y, Van Horn L, Beresford SA, Caan B, Tinker L, Schoeller D, Bingham S, et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol. 2011;174:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luceri C, Caderni G, Lodovici M, Spagnesi MT, Monserrat C, Lancioni L, Dolara P. Urinary excretion of sucrose and fructose as a predictor of sucrose intake in dietary intervention studies. Cancer Epidemiol Biomarkers Prev. 1996;5:167–71. [PubMed] [Google Scholar]
- 16.Tasevska N, Runswick SA, McTaggart A, Bingham SA. Urinary sucrose and fructose as biomarkers for sugar consumption. Cancer Epidemiol Biomarkers Prev. 2005;14:1287–94. [DOI] [PubMed] [Google Scholar]
- 17.Tasevska N, Runswick SA, Welch AA, McTaggart A, Bingham SA. Urinary sugars biomarker relates better to extrinsic than to intrinsic sugars intake in a metabolic study with volunteers consuming their normal diet. Eur J Clin Nutr. 2009;63:653–9. [DOI] [PubMed] [Google Scholar]
- 18.Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet. 2009;125:507–25. [DOI] [PubMed] [Google Scholar]
- 19.Davy BM, Jahren AH, Hedrick VE, Comber DL. Association of δ13C in fingerstick blood with added-sugar and sugar-sweetened beverage intake. J Am Diet Assoc. 2011;111:874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahren AH, Saudek C, Yeung E, Kao W, Kraft R, Caballero B. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr. 2006;84:1380–4. [DOI] [PubMed] [Google Scholar]
- 21.Yeung EH, Saudek CD, Jahren AH, Kao WHL, Islas M, Kraft R, Coresh J, Anderson CAM. Evaluation of a novel isotope biomarker for dietary consumption of sweets. Am J Epidemiol. 2010;172:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:503–37. [Google Scholar]
- 23.O'Leary MH. Carbon isotopes in photosynthesis. Bioscience. 1988;38:328–36. [Google Scholar]
- 24.Petzke KJ, Boeing H, Klaus S, Metges CC. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr. 2005;135:1515–20. [DOI] [PubMed] [Google Scholar]
- 25.Nash SH, Kristal A, Bersamin A, Hopkins SE, Boyer BB, O'Brien DM. Carbon and nitrogen isotope ratios predict intake of sweeteners in a Yup'ik study population. J Nutr. 2013;143:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook CM, Alvig AL, Liu YQ, Schoeller DA. The natural 13C abundance of plasma glucose is a useful biomarker of recent dietary caloric sweetener intake. J Nutr. 2010;140:333–7. [DOI] [PubMed] [Google Scholar]
- 27.Jackson AA. Amino acids: essential and non-essential? Lancet. 1983;1:1034–7. [DOI] [PubMed] [Google Scholar]
- 28.Reeds PJ. Dispensable and indispensable amino acids for humans. J Nutr. 2000;130:S1835–40. [DOI] [PubMed] [Google Scholar]
- 29.Perriello G, Jorde R, Nurjhan N, Stumvoll M, Dailey G, Jenssen T, Bier DM, Gerich JE. Estimation of glucose-alanine-lactate-glutamine cycles in postabsorptive humans: role of skeletal muscle. Am J Physiol. 1995;269:E443–50. [DOI] [PubMed] [Google Scholar]
- 30.Waterhouse C, Keilson J. The contribution of glucose to alanine metabolism in man. J Lab Clin Med. 1978;92:803–12. [PubMed] [Google Scholar]
- 31.Nash SH, Bersamin A, Kristal AR, Hopkins SE, Church RS, Pasker RL, Luick BR, Mohatt GV, Boyer BB, et al. Stable nitrogen and carbon isotope ratios indicate traditional and market food intake in an indigenous circumpolar population. J Nutr. 2012;142:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, Ciraolo PJ, Palascak MB, Joiner CH. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112:4284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connell TC, Hedges REM, Healey MA, Simpson AHRW. Isotopic comparison of hair, nail and bone: modern analyses. J Archaeol Sci. 2001;28:1247–55. [Google Scholar]
- 34.Popp B, Graham B, Olson R, Hannides C, Lott M, Lopez-Ibarra G, Galvan-Magana F, Fry B. Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of proteinaceous amino acids. In: Dawson T, Siegwolf R, editor. Stable isotopes as indicators of ecological change. Amsterdam: Academic Press; 2007. p. 174–90. [Google Scholar]
- 35.Takano Y, Kashiyama Y, Ogawa NO, Chikaraishi Y, Ohkouchi N. Isolation and desalting with cation-exchange chromatography for compound-specific nitrogen isotope analysis of amino acids: application to biogeochemical samples. Rapid Commun Mass Spectrom. 2010;24:2317–23. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien DM, Fogel ML, Boggs CL. Renewable and nonrenewable resources: amino acid turnover and allocation to reproduction in Lepidoptera. Proc Natl Acad Sci USA. 2002;99:4413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silfer JA, Engel MH, Macko SA, Jumeau EJ. Stable carbon isotope analysis of amino-acid enantiomers by conventional isotope ratio mass-spectrometry and combined gas-chromatography isotope ratio mass-spectrometry. Anal Chem. 1991;63:370–4. [Google Scholar]
- 38.Schakel SF. Maintaining a nutrient database in a changing marketplace: keeping pace with changing food products: a research perspective. J Food Compost Anal. 2001;14:315–22. [Google Scholar]
- 39.Bersamin A, Zidenberg-Cherr S, Stern JS, Luick BR. Nutrient intakes are associated with adherence to a traditional diet among Yup'ik Eskimos living in remote Alaska Native communities: The Canhr Study. Int J Circumpolar Health. 2007;66:62–70. [DOI] [PubMed] [Google Scholar]
- 40.Bersamin A, Luick BR, Ruppert E, Stern JS, Zidenberg-Cherr S. Diet quality among Yup'ik Eskimos living in rural communities is low: The Center for Alaska Native Health Research pilot study. J Am Diet Assoc. 2006;106:1055–63. [DOI] [PubMed] [Google Scholar]
- 41.Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 42.McGrane M. Carbohydrate metabolism-synthesis and oxidation. Philadelphia: WB Saunders Company; 2000. [Google Scholar]
- 43.Felig P. The glucose-alanine cycle. Metabolism. 1973;22:179–207. [DOI] [PubMed] [Google Scholar]
- 44.Pascual M, Jahoor F, Reeds PJ. Dietary glucose is extensively recycled in the splanchnic bed of fed adult mice. J Nutr. 1997;127:1480–8. [DOI] [PubMed] [Google Scholar]
- 45.Dodd KW, Guenther PM, Freedman LS, Subar AF, Kipnis V, Midthune D, Tooze JA, Krebs-Smith SM. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc. 2006;106:1640–50. [DOI] [PubMed] [Google Scholar]
- 46.Ma Y, Olendzki BC, Pagoto SL, Hurley TG, Magner RP, Ockene IS, Schneider KL, Merriam PA, Hebert JR. Number of 24-hour diet recalls needed to estimate energy intake. Ann Epidemiol. 2009;19:553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: The OPEN Study. Am J Epidemiol. 2003;158:1–13. [DOI] [PubMed] [Google Scholar]
- 48.Tasevska N, Midthune D, Potischman N, Subar AF, Cross AJ, Bingham SA, Schatzkin A, Kipnis V. Use of the predictive sugars biomarker to evaluate self-reported total sugars intake in the Observing Protein and Energy Nutrition (OPEN) Study. Cancer Epidemiol Biomarkers Prev. 2011;20:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab. 2001;281:E891–9. [DOI] [PubMed] [Google Scholar]