Abstract
Isoflavones have been associated with lower cardiovascular disease risk, but existing research focused on very high isoflavone intakes, as seen in Asian populations, as well as on risk factor reductions primarily in postmenopausal women. We investigated whether habitual low isoflavone intake among premenopausal women was associated with serum C-reactive protein (CRP) concentration, a commonly used biomarker associated with prediction of cardiovascular disease risk in healthy women. Between 2005 and 2007, 259 healthy, regularly menstruating women were enrolled in the BioCycle Study, and followed for up to 2 menstrual cycles. CRP was measured in serum at up to 16 clinic visits, timed to phases of the women’s menstrual cycle. Diet was assessed up to 4 times per cycle by using 24-h recalls. Marginal structural models with inverse probability of exposure weights estimated the association between CRP and quartiles of isoflavone intake adjusted for age, race, BMI, cycle phase, total energy intake, total fiber, total whole grains, and phase-specific hormone concentrations including estradiol, progesterone, luteinizing hormone, and follicle-stimulating hormone. Compared with the lowest quartile of total isoflavone intake, women in the highest quartile had, on average, 27% lower serum CRP concentrations (95% CI: –35, –21%). Our results suggest that dietary isoflavone intakes at levels characteristic of the U.S. population are associated with decreased serum CRP concentrations, a factor associated with beneficial effects on inflammation, and subsequently may have the potential to improve health status among young women.
Introduction
Dietary isoflavones, compounds having moderate estrogenic effects found in soy products, have long been regarded as potential preventive agents for various chronic diseases (1). Accumulating data from animal studies and human trials indicate that isoflavones may reduce chronic inflammation, a possible risk factor for diseases such as diabetes (2), various cancers (3), and cardiovascular disease (CVD)5 (4).
A number of cardioprotective benefits have been attributed to dietary isoflavones, including a reduction in LDL cholesterol, inhibition of proinflammatory cytokines and platelet aggregation, improvement in vascular reactivity, and decreased concentrations of C-reactive protein (CRP) (5). Circulating inflammatory markers, such as CRP, have recently emerged as prominent biomarkers of inflammation and chronic disease risk in both young and older women. In premenopausal women, elevated CRP is one of the most commonly used markers of inflammation and a validated predictor of vascular events even among low-risk subgroups of women with no readily apparent markers of disease (4). Clinical trials assessing the effect of isoflavones on CRP have yielded mixed results (6–10). However, CRP concentrations have been shown to fluctuate across the menstrual cycle (11), and prior intervention studies only reported up to 2 measurements of CRP (baseline and postintervention, not timed to menstrual cycle phase), which may not account for important sources of variability. Moreover, no previous studies examined the association between usual, unsupplemented dietary isoflavone consumption in reproductive-aged women and multiple measurements of circulating CRP concentrations.
The objective of this study was to assess the association between isoflavone intake and serum CRP concentrations among healthy, regularly menstruating women in the BioCycle Study, with multiple CRP measurements timed to menstrual cycle phase.
Participants and Methods
Study design.
The BioCycle Study was a prospective cohort study in 259 healthy, regularly menstruating women aged 18 to 44 y recruited from western New York and followed for up to 2 menstrual cycles. Exclusion criteria included the following: use of oral contraceptives during the past 3 mo, current use of vitamin and mineral supplements or prescription medications, pregnancy or breastfeeding in the past 6 mo, diagnosis of polycystic ovary syndrome, recent history of infections or diagnosis of chronic medical conditions, and self-reported BMI at screening of <18 or >35 kg/m2. Details of the study have been published elsewhere (12). Data collection occurred from 2005 to 2007 at the University at Buffalo. The University at Buffalo Health Sciences Institutional Review Board approved the study and served as the institutional review board designated by the NIH for this study under a reliance agreement. All participants provided written informed consent.
Study visits were scheduled 8 times per cycle, timed to occur during menses, mid- and late follicular phase, 2 d around expected ovulation, and the early, mid-, and late luteal phase in each cycle. Fertility monitors (Clearblue Easy fertility monitor; Inverness Medical) were used to assist in timing of visits and specimen collection to capture critical windows of hormonal variation, including the midcycle luteinizing hormone surge (13).
Dietary assessment.
Dietary intake was assessed by using multiple 24-h dietary recalls (24HDRs), with information collected up to 4 times/cycle, corresponding to visits during menses, midfollicular phase, ovulation, and midluteal phase. Dietary intake data were collected and analyzed by using the Nutrition Data System for Research (NDSR) software version 2005 developed by the Nutrition Coordinating Center, University of Minnesota. This program computed food sources (e.g., polyunsaturated vegetable fat–filled milk), food components (e.g., soy milk), and nutrients (e.g., isoflavones). The NDSR uses the USDA Database for the Isoflavone Content of Selected Foods, release 2.0, for food and nutrient composition of isoflavones (14). Values in the database have been converted to milligrams of aglycone of the following isoflavones: daidzein, genistein, glycitein, biochanin A, and formonontein. Total isoflavone intake refers to the sum of daidzein, genistein, glycitein, biochanin A, and formonontein. Ninety-six percent of the participants completed four 24HDRs in at least 1 of their cycles under study; 99% completed at least 3 24HDRs in both cycles, and all women completed at least 2 24HDRs in both cycles.
Biological specimens.
Fasting serum samples were scheduled to be collected between 0700 and 0830 at each clinic visit, at up to 8 visits/cycle. Ninety-four percent of participants completed at least 7 clinic visits/cycle, and 100% completed ≥5 visits/cycle. High-sensitivity CRP was measured by solid-phase competitive chemiluminescent enzymatic immunoassay (Specialty Laboratories) on the Immulite 2000 analyzer (Diagnostic Products Corporation, now part of Siemens Medical Solutions Diagnostics) (sensitive to 0.3 mg/L) (11). Serum homocysteine was measured at midfollicular, ovulation, and midluteal visits by using an Immulite 2000 homocysteine competitive immunoassay (CV <10% at all concentrations). Serum total cholesterol, HDL cholesterol, and TGs were determined on a Beckman LX20 automated chemistry analyzer (<5% CV for all assays). LDL cholesterol was calculated using the Friedewald formula (15). Serum insulin was measured by using a solid-phase competitive Chemiluminscent Enzymatic Immunoassay by Specialty Laboratories on the DPC Immulite 2000 analyzer (<10% CV). Fasting plasma glucose was assayed using a hexokinase-based methodology on a Beckman LX20 autoanalyzer (Beckman: Brea, CA; CV<3%). Insulin resistance was calculated on the basis of the homeostatic assessment–insulin resistance model using the following equation: fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5 (16). All biomarkers were analyzed at the Kaleida Center for Laboratory Medicine, Buffalo, NY.
Covariate assessment.
At study enrollment, measured height and weight were obtained by trained study staff using standardized protocols and were used to calculate BMI, whereas data on potential confounders such as age, race, physical activity (17), past oral contraceptive use, education, smoking, and reproductive history were obtained via questionnaires (12). All covariates had <5% total missing data.
Statistical analysis.
Linear mixed models were used to compare dietary intake by visit to determine whether isoflavone intake changed significantly over the cycle. All significance was assessed by using an α level of 0.05. No significant differences in isoflavone intake were observed across each cycle; therefore, the mean intake per cycle was calculated for isoflavone intake and other dietary variables. Isoflavone intake was assessed continuously and as quartiles, with the lowest quartile as the reference category. Descriptive statistics for continuous and categorical covariates were compared between quartiles of intake across both cycles by using ANOVA, chi-square tests, or Fisher’s exact test, as appropriate.
Geometric means and IQRs for isoflavone intake across both cycles were calculated for the individual components of isoflavones as well as for the sum of the following components: daidzein, genistein, glycitein, biochanin A, and formononetin. Descriptive statistics were calculated for serum biomarkers and were compared between quartiles of total isoflavone intake. Specifically, serum biomarkers including homocysteine, cholesterol, insulin, and glucose; measures of insulin resistance (homeostatic assessment–insulin resistance); and blood pressure were averaged across both cycles per woman for comparisons. Geometric means and IQRs were calculated for CRP concentrations at each visit across both cycles. In all analyses, CRP concentrations were log-transformed for normality. Serum CRP concentrations >10 mg/L, which might indicate viral or bacterial infection, were excluded from the analyses (<1% of CRP measurements). Six percent of the measured CRP concentrations were below the limit of detection (0.1) and were included using multiple imputation, with values imputed between zero and the limit of detection (18). Women were classified according to the American Heart Association (AHA) CVD risk cutpoints as low (CRP <1 mg/L), moderate (CRP ≥1 and ≤3 mg/L), or elevated (CRP >3 mg/L) (19) according to the average CRP concentration across both cycles and compared between quartiles of total isoflavone intake.
Linear mixed models on the log scale were used to evaluate the association between CRP concentrations and quartile of isoflavone intake per cycle (20). Random intercepts accounted for the variation in baseline CRP concentrations between women and the correlation between visits of the same woman. Potential confounders were identified by using directed acyclic graphs and a change in point estimates approach (21). Potential confounders identified via literature review and directed acyclic graphs were included in the model if they changed the exposure coefficient by >15%. Age (continuous), BMI (continuous), race (white, black, Asian, other), cycle phase (menses, mid- and late follicular phase, 2 d around expected ovulation, and early, mid-, and late luteal phase), fiber intake (continuous), whole-grain intake (continuous), and total energy intake (continuous) were included in the final models. Additional covariates, including physical activity, past oral contraceptive use, dietary intakes such as animal protein, cholesterol, fat (PUFAs, MUFAs, SFAs), sodium intake, antioxidant vitamins (vitamin E, vitamin C, and carotenoids), were considered but did not appreciably alter the estimates.
We present 3 models: unadjusted (model 1), adjusted (model 2; adjusted for age, race, BMI, cycle phase, and intakes of fiber, whole grain, and total energy), and fully adjusted (model 3; adjusted for variables in model 2 as well as other sex hormones through the use of inverse probability of exposure weights). Due to the time-dependent confounding nature of sex hormones over the cycle, we used marginal structural models in the fully adjusted model to evaluate the association between isoflavone intake and log serum CRP concentrations after adjusting for the complex feedback mechanisms of the other hormones (22). Weighted linear mixed models with inverse probability of exposure weights were used to estimate the parameters of the marginal structural model. Stabilized weights for each cycle visit when dietary information was collected were obtained by using ordinal logistic regression for the exposure quartiles of isoflavone intake. Analyses were also performed with servings of soymilk as a continuous variable, as well as a categorical variable [0 servings/d, >0 to <0.5 servings/d (<118 mL), and ≥0.5 servings/d (≥118 mL)]. All analyses take multiple cycles as well as multiple imputation into account. All analyses were carried out by using SAS version 9.2 (SAS Institute).
Results
Women in the BioCycle Study had a mean age of 27.3 y and a mean BMI of 24.1 kg/m2, were moderately to highly physically active (90.5%), and were nonsmokers (96.1%) (Table 1). There were no significant differences in age, BMI, years of education, past oral contraceptive use, or history of smoking status across quartiles of isoflavone intake. Women who identified themselves as Asian were more likely to consume higher amounts of isoflavones.
TABLE 1.
Characteristics of women in the BioCycle Study according to quartiles of total isoflavone intake1
| Total | Q1 (0.0–0.3 mg/d) | Q2 (0.31–0.6 mg/d) | Q3 (0.61–1.6 mg/d) | Q4 (1.61–78.8 mg/d) | P value2 | |
| Age, y | 27.3 ± 8.2 | 27.1 ± 8.7 | 26.4 ± 7.9 | 28.4 ± 8.1 | 27.3 ± 8.1 | 0.59 |
| BMI, kg/m2 | 24.1 ± 3.9 | 24.4 ± 3.8 | 23.9 ± 3.7 | 24.5 ± 4.5 | 23.4 ± 3.3 | 0.32 |
| Race, n (%) | 0.02 | |||||
| White | 154 (59.4) | 32 (50.0) | 43 (66.1) | 41 (63.0) | 38 (58.4) | |
| Black | 51 (19.7) | 21 (32.8) | 13 (20.0) | 10 (15.4) | 7 (10.8) | |
| Asian | 39 (15.1) | 8 (12.5) | 7 (10.8) | 7 (10.8) | 17 (26.2) | |
| Other | 15 (5.8) | 3 (4.7) | 2 (3.1) | 7 (10.8) | 3 (4.6) | |
| Dietary intake3 | ||||||
| Total energy intake, kcal/d | 1610 ± 22 | 1530 ± 44 | 1610 ± 44 | 1600 ± 44 | 1680 ± 44 | 0.11 |
| Carbohydrate intake, g/d | 201 ± 1.7 | 201 ± 3.5 | 195 ± 3.4 | 201 ± 3.4 | 208 ± 3.4 | 0.06 |
| Total protein intake, g/d | 62.2 ± 0.7 | 58.9 ± 1.3 | 63.2 ± 1.3 | 63.6 ± 1.3 | 62.9 ± 1.3 | 0.05 |
| Animal protein | 40.7 ± 0.8 | 39.7 ± 1.5 | 43.7 ± 1.5 | 43.1 ± 1.5 | 36.1 ± 1.5 | 0.001 |
| Vegetable protein | 21.4 ± 0.4 | 19.1 ± 0.7 | 19.3 ± 0.7 | 20.3 ± 0.7 | 26.7 ± 0.7 | < 0.0001 |
| Total fat intake, g/d | 62.1 ± 0.6 | 64.1 ± 1.2 | 63.6 ± 1.1 | 60.7 ± 1.1 | 60.3 ± 1.2 | 0.04 |
| Total SFAs | 21.3 ± 0.3 | 22.2 ± 0.5 | 21.9 ± 0.5 | 21.0 ± 0.5 | 20.0 ± 0.5 | 0.02 |
| Total MUFAs | 23.1 ± 0.2 | 23.7 ± 0.5 | 23.7 ± 0.5 | 22.5 ± 0.5 | 22.5 ± 0.5 | 0.11 |
| Total PUFAs | 12.8 ± 0.2 | 13.0 ± 0.4 | 12.9 ± 0.4 | 12.2 ± 0.4 | 13.2 ± 0.4 | 0.31 |
| Sodium, g/d | 2.76 ± 0.3 | 2.60 ± 0.6 | 2.84 ± 0.6 | 2.85 ± 0.6 | 2.75 ± 0.6 | 0.008 |
| Vitamin E, mg/d | 9.8 ± 0.3 | 7.8 ± 0.6 | 8.8 ± 0.6 | 8.4 ± 0.6 | 14.3 ± 0.6 | <0.0001 |
| Vitamin C, mg/d | 69.7 ± 2.2 | 58.4 ± 4.4 | 74.6 ± 4.3 | 68.1 ± 4.3 | 77.4 ± 4.4 | 0.01 |
| β-Carotene, mg/d | 2.33 ± 0.12 | 1.75 ± 0.23 | 2.02 ± 0.23 | 2.31 ± 0.23 | 3.25 ± 0.23 | <0.0001 |
| β-Cryptoxanthin, μg/d | 138 ± 13 | 148 ± 26 | 143 ± 26 | 114 ± 26 | 146 ± 26 | 0.78 |
| Cholesterol intake, g/d | 210 ± 5 | 200 ± 11 | 234 ± 10 | 217 ± 10 | 187 ± 11 | 0.01 |
| Alcohol intake, g/d | 2.7 ± 0.3 | 1.5 ± 0.6 | 3.5 ± 0.6 | 3.7 ± 0.6 | 1.9 ± 0.6 | 0.02 |
| Fructose intake, g/d | 16.9 ± 0.5 | 18.6 ± 1.1 | 17.5 ± 1.0 | 16.2 ± 1.0 | 15.5 ± 1.1 | 0.17 |
| Total fiber, g/d | 13.6 ± 0.3 | 12.0 ± 0.6 | 12.3 ± 0.6 | 13.1 ± 0.6 | 16.8 ± 0.6 | <0.0001 |
| Soluble fiber | 3.8 ± 0.1 | 3.4 ± 0.1 | 3.5 ± 0.1 | 3.6 ± 0.1 | 4.5 ± 0.1 | <0.0001 |
| Insoluble fiber | 9.6 ± 0.2 | 8.4 ± 0.5 | 8.6 ± 0.5 | 9.3 ± 0.5 | 12.1 ± 0.5 | <0.0001 |
Values are means ± SDs (for demographic characteristics), means ± SEs (for dietary intake variables), or n (%) and are based on quartiles of isoflavone intake and mean serum CRP concentrations across both cycles. CRP, C-reactive protein; Q, quartile.
P values for continuous variables calculated by using ANOVA and chi-square tests and for categorical variables by using Fisher’s exact test. All comparisons took repeated measures and correlations between cycles into account.
All dietary intake variables, in addition to total energy intake, were energy-adjusted.
Women in the BioCycle Study had an arithmetic mean total isoflavone intake of 2.9 mg/d (geometric mean: 0.86 mg/d; median: 0.55 mg/d). Geometric mean concentrations of isoflavones ranged from 0.17 mg/d in the lowest quartile to 6.31 mg/d in the highest quartile (Table 2). Overall, isoflavone intake was low, and daidzein and genistein were the primary components.
TABLE 2.
Constituents of isoflavones consumed by women in the BioCycle Study according to quartiles of total isoflavone intake1
| Total | Q1 (0.0–0.3 mg/d) | Q2 (0.31–0.6 mg/d) | Q3 (0.61–1.6 mg/d) | Q4 (1.61–78.8 mg/d) | P value2 | |
| Isoflavones, mg/d | ||||||
| Daidzein | 0.31 (0.11–0.69) | 0.06 (0.04–0.09) | 0.17 (0.13–0.23) | 0.33 (0.27–0.48) | 2.35 (1.24–4.43) | <0.0001 |
| Genistein | 0.34 (0.11–0.88) | 0.07 (0.05–0.12) | 0.19 (0.14–0.27) | 0.33 (0.24–0.55) | 3.02 (1.62–5.41) | <0.0001 |
| Glycitein | 0.06 (0.02–0.20) | 0.01 (0.01–0.02) | 0.02 (0.01–0.04) | 0.04 (0.02–0.09) | 0.50 (0.24–1.14) | <0.0001 |
| Biochanin | 0.11 (0.04–0.37) | 0.04 (0.02–0.01) | 0.06 (0.03–0.14) | 0.22 (0.09–0.64) | 0.16 (0.07–0.42) | <0.0001 |
| Formononetin | 0.005 (0.001–0.01) | 0.004 (0.001–0.01) | 0.004 (0.001–0.01) | 0.006 (0.002–0.01) | 0.007 (0.002–0.02) | 0.15 |
| Total isoflavones3, mg/d | 0.86 (0.33–1.90) | 0.17 (0.13–0.25) | 0.47 (0.39–0.58) | 1.02 (0.82–1.23) | 6.31 (3.18–10.49) | <0.0001 |
Values are geometric means (IQRs). Q, quartile.
P values for continuous variables calculated using ANOVA and chi square tests. All comparisons accounted for repeated measures and correlations between cycles.
Sum of daidzein, genistein, formononetin, and biochanin A.
The metabolic characteristics of women in the BioCycle Study by quartile of isoflavone intake are reported in Table 3. CRP concentrations varied significantly according to isoflavone intake, in which women in the highest quartile of intake (quartile 4) had decreased CRP concentrations compared with women in the lowest quartile (quartile 1) [1.2 mg/L (quartile 1) vs. 0.6 mg/L (quartile 4); P = 0.009]. More women in quartile 4 (79%) than in quartile 1 (55%) were at low risk of CVD (defined by CRP <1 mg/L; P = 0.03). Furthermore, more women in quartile 1 (11%) were at elevated risk of CVD compared with those in quartile 4 (2%). Homocysteine, cholesterol, insulin, glucose, and blood pressure were not significantly associated with isoflavone intake.
TABLE 3.
Metabolic characteristics of women in the BioCycle Study according to quartiles of total isoflavone intake1
| Total | Q1 (0.0–0.3 mg/d) | Q2 (0.31–0.6 mg/d) | Q3 (0.61–1.6 mg/d) | Q4 (1.61–78.8 mg/d) | P value2 | |
| Participants, n | 259 | 64 | 65 | 65 | 65 | |
| Blood pressure, mm Hg | ||||||
| Systolic | 99.2 ± 8.8 | 100.1 ± 8.2 | 99.7 ± 8.6 | 99.9 ± 9.4 | 97.2 ± 8.8 | 0.12 |
| Diastolic | 61.2 ± 8.2 | 62.1 ± 9.2 | 60.9 ± 7.9 | 61.9 ± 8.3 | 59.9 ± 7.4 | 0.4 |
| Serum biomarkers3 | ||||||
| CRP4, mg/L | 0.9 (0.2–1.2) | 1.2 (0.3–1.5) | 1.0 (0.3–1.3) | 1.0 (0.3–1.3) | 0.6 (0.2–0.8) | 0.009 |
| Homocysteine, μmol/L | 6.0 ± 1.4 | 6.2 ± 1.5 | 5.8 ± 1.3 | 6.1 ± 1.4 | 5.8 ± 1.3 | 0.25 |
| Cholesterol5, mg/dL | 164 ± 27.1 | 164 ± 28.8 | 164 ± 24.3 | 167 ± 29.0 | 163 ± 26.8 | 0.88 |
| LDL cholesterol5, mg/dL | 101 ± 25 | 103 ± 28 | 99 ± 23 | 102 ± 25 | 99 ± 23 | 0.63 |
| HDL cholesterol5, md/dL | 52.3 ± 11.2 | 49.4 ± 9.5 | 54.2 ± 12.0 | 53.0 ± 12.6 | 52.8 ± 9.8 | 0.08 |
| TGs6, mg/dL | 59.7 ± 25.1 | 64.2 ± 24.5 | 55.9 ± 21.5 | 60.7 ± 30.4 | 58.3 ± 22.9 | 0.28 |
| Insulin7, μU/mL | 8.3 ± 5.2 | 8.9 ± 4.8 | 9.4 ± 7.2 | 7.7 ± 4.5 | 7.3 ± 3.5 | 0.08 |
| Insulin resistance (HOMA-IR) | 1.8 ± 1.3 | 1.9 ± 1.1 | 2.1 ± 2.0 | 1.7 ± 1.0 | 1.6 ± 0.8 | 0.11 |
| Glucose8, mg/dL | 86.8 ± 4.9 | 87.4 ± 5.0 | 86.9 ± 5.3 | 86.9 ± 4.6 | 86.0 ± 4.8 | 0.47 |
| CRP category9, n (%) | ||||||
| Low (CRP <1 mg/L) | 165 (63.7) | 35 (54.7) | 36 (55.4) | 43 (66.2) | 51 (78.5) | 0.03 |
| Moderate (CRP: 1–3 mg/L) | 80 (30.9) | 22 (34.4) | 26 (40.0) | 19 (29.2) | 13 (20.0) | |
| Elevated (CRP ≥3 mg/L) | 14 (5.4) | 7 (10.9) | 3 (4.6) | 3 (4.6) | 1 (1.5) |
Values are means ± SDs averaged across both cycles unless otherwise indicated. CRP, C-reactive protein; HOMA-IR, homeostatic model assessment–insulin resistance; Q, quartile.
P values for continuous variables calculated by using ANOVA and chi-square tests and for categorical variables by using Fisher’s exact test. All comparisons accounted for repeated measures and correlations between cycles.
All markers were measured in serum except for glucose which was measured in plasma.
Values are geometric means (IQRs) averaged across both cycles.
To convert cholesterol (total, HDL, and LDL) from mg/dL to mmol/L, multiply by 0.026.
To convert TGs from mg/dL to mmol/L, multiply by 0.011.
To convert insulin from μU/mL to pmol/L, multiply by 6.945.
To convert glucose from mg/dL to mmol/L, multiply by 0.0555.
CRP category defined according to American Heart Association cardiovascular disease risk cutpoints.
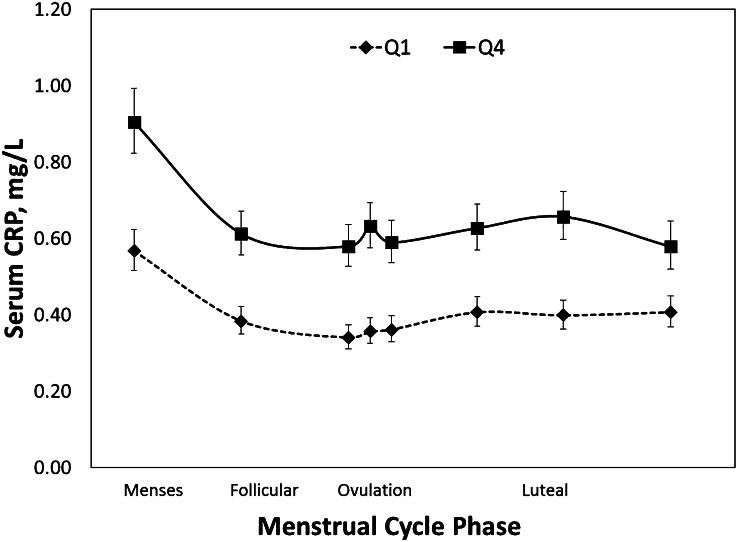
Isoflavone intake was inversely associated with serum CRP concentrations across the cycle, as shown in Figure 1, which displays the unadjusted mean serum CRP concentrations across the menstrual cycle by highest and lowest quartiles of isoflavone intake. Increased isoflavone intake was also significantly associated with decreased concentrations of CRP across the menstrual cycle in models adjusted for fiber intake, whole-grain intake, total energy intake, age, race, cycle phase, BMI, and other sex hormones through the use of marginal structural models with inverse probability of exposure weights (Table 4). In the fully adjusted model, women in quartile 4 had, on average, 27% lower CRP concentrations compared with those in quartile 1. Analysis of isoflavone intake according to servings of soymilk in adjusted models produced similar results in that women consuming >0 but <0.5 servings/d (<118 mL) had 27% lower CRP (95% CI: −57, −30%) and women consuming ≥0.5 servings/d (≥118 mL) had 45% lower CRP (95% CI: −38, −14%) compared with those who had 0 servings/d.
FIGURE 1.
Unadjusted geometric mean (95% CI) serum CRP concentrations over the menstrual cycle in the BioCycle Study by highest and lowest quartiles of isoflavone intake. The mean isoflavone intake per cycle was based on up to four 24-h dietary recalls. P values were calculated by using linear mixed models with random effects in which Q1 was the reference category. Q4 CRP concentrations were significantly lower than those at Q1 at all menstrual cycle phases, P < 0.0001. CRP, C-reactive protein; Q, quartile.
TABLE 4.
Linear mixed model results of the difference in log serum CRP concentrations by quartiles of total isoflavone intake1
| Variable and model | Q1 (0.0–0.3 mg/d) | Q2 (0.31–0.6 mg/d) | Q3 (0.61–1.6 mg/d) | Q4 (1.61–78.8 mg/d) |
| CRP (mg/L) | ||||
| Unadjusted | Ref | −1.7 (−11.7, 9.4) | −14.1 (−22.8, −4.4) | −38.1 (−44.4, −31.2) |
| Adjusted2 | Ref | −10.3 (−18.4, −1.5) | −25.8 (−32.4, −18.4) | −31.9 (−38.1, −25.0) |
| Fully adjusted3 | Ref | −8.3 (−16.3, 0.4) | −26.0 (−32.4, −19.0) | −27.3 (−33.7, −20.3) |
Values are percentage changes (95% CIs). CRP, C-reactive protein; Q, quartile; Ref, reference category.
Adjusted for age (continuous), race (white, black, Asian, other), BMI (continuous), cycle phase (menses, mid- and late follicular phase, 2 d around expected ovulation, and the early, mid-, and late luteal phase), fiber intake (continuous), whole-grain intake (continuous), and total energy (continuous).
Adjusted for age (continuous), race (white, black, Asian, other), BMI (continuous), cycle phase (menses, mid- and late follicular phase, 2 d around expected ovulation, and the early, mid-, and late luteal phase), fiber intake (continuous), whole-grain intake (continuous), and total energy (continuous) and relevant phase-specific hormone concentrations using marginal structural models with inverse probability of exposure weights.
Discussion
In this study in healthy women of reproductive age, we found a significant and inverse association between isoflavone intake and serum CRP concentrations after adjustment for potential confounders. These results highlight the potential benefits of increased isoflavone intake as part of a healthy diet and show that dietary isoflavones at intakes characteristic of the U.S. population (BioCycle women arithmetic mean of 2.8 mg/d vs. NHANES women arithmetic mean of 1.3 mg/d [23]) are associated with beneficial effects on CRP, a commonly used marker of inflammation, and subsequently may have the potential to improve health status among young women.
To the best of our knowledge, the present study is the first to assess habitual dietary intake of isoflavones among a cohort of healthy, premenopausal women through the use of multiple 24HDRs in relation to CRP. Findings from the present study are consistent with cross-sectional NHANES 1990–2002 data, in which total isoflavone intakes were inversely correlated with serum CRP concentrations (P < 0.01) (24). In addition, our results are in line with a recent meta-analysis of 14 trials (n = 603), which showed a slight, although nonsignificant reduction in CRP concentrations among postmenopausal women in response to soy isoflavone intake compared with control [of the 14 trials, sources of intake were isoflavone extracts (n = 6) and soy food (n = 8)] (25). However, our findings are in contrast with those from a 2-y dietary intervention in premenopausal women (n = 183) (26), which reported no effect of soy foods on CRP. A lack of intervention effect among women in this trial (mean age: 43.3 y; mean baseline CRP: 1.95 mg/L) could be explained by limited CRP measurements (5 blood samples in 2 y), lack of frequent hormone measurements (5 blood samples in 2 y), and timing of measurements (measurements occurred 4–6 d after ovulation as determined by an ovulation kit).
The potential antiinflammatory effect of isoflavones is biologically plausible. Isoflavones have been shown to suppress the synthesis of adhesion molecules (27–29), chemotactic factors (30), arachidonic acid metabolism, inducible NO synthase, and cyclooxygenase 2 (COX-2) (31) in in vitro and animal studies. Among human intervention studies, the data regarding cardiovascular health benefits of dietary isoflavones remain conflicting. Clinical trials evaluating soy isoflavone intake have reported improvements in systemic arterial compliance in postmenopausal women, increased plasma nitrite/nitrate and decreased endothelin-1 concentrations, and increased brachial artery flow-mediated dilation (32), although not all studies reported significant effects (6, 7, 9–10, 26, 33).
In 2006 the AHA Scientific Advisory for Professionals Committee concluded that “direct cardiovascular health benefits of soy protein or isoflavone supplements is minimal at best” (34). The AHA committee suggested that prior studies that found cardioprotective effects of isoflavones are likely to be explained by other nutrients commonly found in foods high in isoflavones, such as polyunsaturated fats, fiber, vitamins, and minerals. However, in the present study the association between isoflavone intake and serum CRP concentrations remained significant even after controlling for these dietary covariates, although the impact of residual confounding cannot be excluded completely.
The present study had a number of strengths. The 24HDRs included detailed information on almost all major food sources of isoflavones in Western diets, including bread, nut and seed products, and vegetable and fruit items. The use of multiple validated 24HDRs improved our ability to assess usual intake, reducing the potential for exposure misclassification. In addition, our study used the NDSR, which matched food consumption data to the USDA–Iowa State University Database on the Isoflavone Content of Foods, which was generated by extensive sampling of 108 soy-containing foods. Intensive monitoring throughout 2 cycles of a relatively large number of women, with multiple clinic visits timed by using fertility monitors, allowed us to ascertain up to 8 measurements of CRP/cycle, standardized to the menstrual cycle. Given that concentrations of CRP in premenopausal women have been shown to vary significantly across the menstrual cycle and are associated with reproductive hormones including estradiol and progesterone (11), we were able to account for this source of variability. The use of a high-sensitivity CRP assay allowed us to measure very small amounts of CRP in the blood, which is necessary to assess potential risks among seemingly healthy individuals. In addition, standardized assessment of a wide variety of participant and dietary characteristics allowed for control for potential confounding variables, including time-dependent confounders such as other hormones, and other dietary components with known impacts on CRP concentrations. Finally, this study reports dietary intakes of isoflavones at levels characteristic of the American population (23), making results highly generalizable.
Nevertheless, there are several limitations of this study. Usual isoflavone consumption was relatively low in this study, and only a small number of women were consuming diets rich in isoflavones, which limits our ability to assess dietary intakes of women consuming isoflavone-rich diets. However, even among women who consumed dietary isoflavones at intakes characteristic of the U.S. population, we observed a significant and inverse association with CRP concentrations. It should be noted that results from the present study are limited in assessing causality because this was not an intervention study. In addition, estimates of dietary isoflavone intake are based on food composition data, which have limited comprehensiveness in capturing all food sources (35). However, intakes using similar dietary recall methods in NHANES compared with urinary isoflavone concentrations have been found to correlate in a dose-response manner with the major sources of isoflavones (e.g., genistein, daidzein) (36). We also did not measure urinary metabolites of isoflavones and were unable to validate dietary isoflavone consumption data. In addition, although CRP concentration is a reliable biomarker of chronic inflammation (19), we were unable to directly evaluate CVD outcomes and thus were only able to assess common markers of disease risk. Residual confounding cannot be excluded, although associations remained after adjustment for other dietary factors.
In conclusion, in the present study, higher isoflavone consumption among young, healthy women was significantly associated with lower concentrations of CRP. Given the central role of inflammation in the progression of atherosclerotic vascular disease and other chronic diseases (37), and the significant predictive value of serum CRP concentration for CVD risk, findings from the present study indicate that dietary isoflavones may have the potential to improve health status among young women. Further studies to confirm these findings and to extend these results to assess the impact of levels of isoflavone intake among larger populations of women are warranted.
Acknowledgments
E.F.S. and J.W.-W. formulated the study concept and design, supervised the study, and assisted in the acquisition of data; A.C.F., S.L.M., C.Z., E.H.Y., and E.F.S. analyzed and interpreted the data; A.C.F., S.L.M and E.F.S. drafted the manuscript and performed the statistical analyses; E.H.Y., A.Z.P., C.Z., J.W.-W., and N.J.P. critically revised the manuscript for important intellectual content; and A.C.F. and E.F.S. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: AHA, American Heart Association; CVD, cardiovascular disease; COX-2, cyclooxygenase 2; CRP, C-reactive protein; NDSR, Nutrition Data System for Research; 24HDR, 24-hour dietary recall.
Literature Cited
- 1.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129 Suppl:758S–67S. [DOI] [PubMed] [Google Scholar]
- 2.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. [DOI] [PubMed] [Google Scholar]
- 3.Heikkilä K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, Ben-Shlomo Y, Ebrahim S, Lawlor DA. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15–26. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. [DOI] [PubMed] [Google Scholar]
- 5.Rimbach G, Boesch-Saadatmandi C, Frank J, Fuchs D, Wenzel U, Daniel H, Hall WL, Weinberg PD. Dietary isoflavones in the prevention of cardiovascular disease—a molecular perspective. Food Chem Toxicol. 2008;46:1308–19. [DOI] [PubMed] [Google Scholar]
- 6.D’Anna R, Baviera G, Corrado F, Cancellieri F, Crisafulli A, Squadrito F. The effect of the phytoestrogen genistein and hormone replacement therapy on homocysteine and C-reactive protein level in postmenopausal women. Acta Obstet Gynecol Scand. 2005;84:474–7. [DOI] [PubMed] [Google Scholar]
- 7.Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Consumption of isoflavone-rich soy protein does not alter homocysteine or markers of inflammation in postmenopausal women. Eur J Clin Nutr. 2008;62:1419–25. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins DJ, Kendall CW, Connelly PW, Jackson CJ, Parker T, Faulkner D, Vidgen E. Effects of high- and low-isoflavone (phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle-aged men and women. Metabolism. 2002;51:919–24. [DOI] [PubMed] [Google Scholar]
- 9.Nikander E, Metsa-Heikkila M, Tiitinen A, Ylikorkala O. Evidence of a lack of effect of a phytoestrogen regimen on the levels of C-reactive protein, E-selectin, and nitrate in postmenopausal women. J Clin Endocrinol Metab. 2003;88:5180–5. [DOI] [PubMed] [Google Scholar]
- 10.Ryan-Borchers TA, Park JS, Chew BP, McGuire MK, Fournier LR, Beerman KA. Soy isoflavones modulate immune function in healthy postmenopausal women. Am J Clin Nutr. 2006;83:1118–25. [DOI] [PubMed] [Google Scholar]
- 11.Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J, Perkins NJ, Schisterman EF. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Liu A, Trevisan M; BioCycle Study Group. BioCycle Study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23:171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169:105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.USDA, Agricultural Research Service. USDA-Iowa State University database on the isoflavone content of selected foods, release 2.0. Washington: Nutrient Data Laboratory; 2008 [cited 2013 Mar 1]. Available from: http://www.ars.usda.gov/nutrientdata.
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 17.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 18.Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley & Sons; 1987.
- 19.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. ; Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice. A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 20.Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–31. [DOI] [PubMed] [Google Scholar]
- 21.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169:1182–90. [DOI] [PubMed] [Google Scholar]
- 22.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. 2007;137:1244–52. [DOI] [PubMed] [Google Scholar]
- 24.Chun OK, Chung SJ, Claycombe KJ, Song WO. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J Nutr. 2008;138:753–60. [DOI] [PubMed] [Google Scholar]
- 25.Dong JY, Wang P, He K, Qin LQ. Effect of soy isoflavones on circulating C-reactive protein in postmenopausal women: meta-analysis of randomized controlled trials. Menopause. 2011;18:1256–62. [DOI] [PubMed] [Google Scholar]
- 26.Maskarinec G, Steude JS, Franke AA, Cooney RV. Inflammatory markers in a 2-year soy intervention among premenopausal women. J Inflamm (Lond). 2009;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke-Gaffney A, Hellewell PG. Tumour necrosis factor-alpha-induced ICAM-1 expression in human vascular endothelial and lung epithelial cells: modulation by tyrosine kinase inhibitors. Br J Pharmacol. 1996;119:1149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata M, Sedgwick JB, Busse WW. Synergistic activation of eosinophil superoxide anion generation by VCAM-1 and GM-CSF: involvement of tyrosine kinase and protein kinase C. Int Arch Allergy Immunol. 1997;114 Suppl 1:78–80. [DOI] [PubMed] [Google Scholar]
- 29.Weber C. Involvement of tyrosine phosphorylation in endothelial adhesion molecule induction. Immunol Res. 1996;15:30–7. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe J, Watanabe M, Kondoh S, Mue S, Ohuchi K. Possible roles of protein kinases in neutrophil chemotactic factor production by leucocytes in allergic inflammation in rats. Br J Pharmacol. 1994;113:1480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corbett JA, Kwon G, Marino MH, Rodi CP, Sullivan PM, Turk J, McDaniel ML. Tyrosine kinase inhibitors prevent cytokine-induced expression of iNOS and COX-2 by human islets. Am J Physiol. 1996;270:C1581–7. [DOI] [PubMed] [Google Scholar]
- 32.Siow RC, Li FY, Rowlands DJ, de Winter P, Mann GE. Cardiovascular targets for estrogens and phytoestrogens: transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radic Biol Med. 2007;42:909–25. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins DJ, Kendall CW, Jackson CJ, Connelly PW, Parker T, Faulkner D, Vidgen E, Cunnane SC, Leiter LA, Josse RG. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr. 2002;76:365–72. [DOI] [PubMed] [Google Scholar]
- 34.Sacks FM, Lichtenstein A, Van HL, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–44. [DOI] [PubMed] [Google Scholar]
- 35.Institute of Medicine, Food and Nutrition Board. Reference Intakes: proposed definition and plan for review of dietary antioxidants and related compounds. Washington: National Academies Press; 1998.
- 36.Chun OK, Chung SJ, Song WO. Urinary isoflavones and their metabolites validate the dietary isoflavone intakes in US adults. J Am Diet Assoc. 2009;109:245–54. [DOI] [PubMed] [Google Scholar]
- 37.Anthony MS, Clarkson TB, Williams JK. Effects of soy isoflavones on atherosclerosis: potential mechanisms. Am J Clin Nutr. 1998;68:1390S–3S. [DOI] [PubMed] [Google Scholar]