Abstract
Dietary factors, including dietary fat, may affect the biological aging process, as reflected by the shortening of telomere length (TL), by affecting levels of oxidative stress and inflammatory responses. We examined the direct relations of total and types of dietary fats and fat-rich foods to peripheral leukocyte TL. In 4029 apparently healthy postmenopausal women who participated in the Women’s Health Initiative, intakes of total fat, individual fatty acids, and fat-rich foods were assessed by a questionnaire. TL was measured by quantitative polymerase chain reaction. Intake of short-to-medium-chain saturated fatty acids (SMSFAs; aliphatic tails of ≤12 carbons) was inversely associated with TL. Compared with participants in other quartiles of SMSFA intake, women who were in the highest quartile (median: 1.29% of energy) had shorter TLs [mean: 4.00 kb (95% CI: 3.89, 4.11 kb)], whereas women in the lowest quartile of intake (median: 0.29% of energy) had longer TLs [mean: 4.13 kb (95% CI: 4.03, 4.24 kb); P-trend = 0.046]. Except for lauric acid, all other individual SMSFAs were inversely associated with TL (P < 0.05). In isoenergetic substitution models, the substitution of 1% of energy from SMSFAs with any other energy source was associated with 119 bp longer TLs (95% CI: 21, 216 bp). Intakes of nonskim milk, butter, and whole-milk cheese (major sources of SMSFAs) were all inversely associated with TL. No significant associations were found with long-chain saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids. In conclusion, we found that higher intakes of SMSFAs and SMSFA-rich foods were associated with shorter peripheral leukocyte TL among postmenopausal women. These findings suggest the potential roles of SMSFAs in the rate of biological aging.
Introduction
Telomeres are highly conserved regions of repetitive nucleotide sequences (TTAGGG) that protect the ends of chromosomes. Telomere length (TL)15 decreases over time due to cell division and may serve as a biomarker for the aging process and some age-related complex diseases (1). Whereas the exact mechanism determining TL remains incompletely understood, dietary factors may play an important role in influencing the rate of telomere shortening (2, 3).
Several reports have associated “dietary scores” with TL (4, 5), including a recent cross-sectional study in 1942 European men that linked intakes of total fat and SFAs to TL (6). In a small intervention trial in 47 women, changes in dietary fat intake appeared to also negatively affect telomerase activity (7). Moreover, higher intake of some types of dietary fat has been associated with increased risks of various age-related chronic diseases, including coronary heart disease, diabetes, and cancer (8–11). However, few studies have directly examined the relations between individual FAs and TL. We therefore analyzed data from the Women’s Health Initiative (WHI), a large population of multiethnic postmenopausal women, to examine whether and to what extent dietary intakes of total fat and individual FAs are associated with leukocyte TL.
Participants and Methods
Participants.
Participants of this study were from the WHI Observational Study (WHI-OS). The WHI study design has been described elsewhere (12, 13). In brief, the WHI-OS examines the relationship between lifestyle, environmental, medical, and molecular risk factors and specific measures of health or disease outcomes. The WHI-OS involves tracking the medical history and health habits of 93,676 women not participating in the clinical trials. In the present study, we used baseline data from a prospective case-control study of type 2 diabetes nested in the WHI-OS cohort (14). Control participants were matched to diabetes cases on age (±2.5 y), race-ethnicity, clinical center, time of blood draw, and length of follow-up. This study involved 4029 participants with successful TL measurements, including 2020 whites, 1213 blacks, 487 Hispanics, and 308 Asians/Pacific Islanders (one participant missing race-ethnicity information). All study participants provided informed consent before study enrollment in the WHI. The institutional review board at University of California, Los Angeles approved the current study.
Measurement and classification of dietary fat intake.
The methods of data collection and validation have been reported previously (12, 15). Participants completed a standardized semiquantitative FFQ developed for participants in the WHI to estimate average daily dietary intake over the previous 3-mo period. The FFQ was based on instruments used in the WHI feasibility studies (16, 17) and the original National Cancer Institute/Block FFQ (18). The 3 sections of the WHI FFQ included 19 questions related to type of fat intake and 122 questions on frequency of consumption and portion size of composite and single food items (19). The dietary database, linked to the University of Minnesota’s Nutrition Coordinating Center Nutrition Data System for Research (Nutrition Coordinating Center, Minneapolis, MN), is based on the USDA standard reference releases and manufacturer information (20). Dietary intakes of total fat and individual FAs were calculated from the information in the FFQ. FAs were categorized into SFAs, MUFAs, and PUFAs according to the number of double bonds in aliphatic tails. Values for SFAs, MUFAs, and PUFAs may include individual FAs not determined; thus, the sum of their values may exceed the sum of the individual FA. SFAs were further categorized into short-to-medium-chain SFAs (SMSFAs; SFAs with aliphatic tails of ≤12 carbons) and long-chain SFAs (LSFAs; SFAs with aliphatic tails of >12 carbons).
Measurement and classification of covariates.
Self-administrated questionnaires were used to collect information on demographic characteristics and lifestyle factors at study entry into the WHI. Participants were categorized into “never smokers,” “former smokers,” and “current smokers” according to their smoking history. Levels of alcohol intake (g/d) and total energy intake (kcal/d) were also calculated from the FFQ. Body weight and height were measured at baseline entry into the WHI, and BMI was calculated as body weight (kg) divided by height (m) squared. The level of physical activity in metabolic equivalent hours per week was estimated on the basis of self-reported duration of different types of exercise, weighted by their intensity levels.
Measurement of peripheral leukocyte TLs.
The measurement of TL has been described elsewhere (14). In brief, we applied the method proposed by O’Callaghan et al. (21) in a high-throughput, 384-well format using Applied Biosystems 7900HT PCR System (Applied Biosystems by Life Technologies Corporation). Mean TL per chromosome was calculated with the use of the following formula: (TL/copies of diploid genome)/(23 × 2). Ten percent of the samples were blinded reproducibility samples as part of routine quality control. The overall intraplate CV was 0.8%, and the interplate CV was 5.7%.
Statistical analysis.
Dietary intakes of total fat and individual FAs were expressed as nutrient density (% of total energy intake). To examine latent variables that may explain the correlations among individual FAs, we conducted an exploratory factor analysis by using data from all participants of WHI-OS (n = 93,676). We used principal components analysis to extract factors and performed varimax rotations (which are orthogonal). A cumulative proportion of the total variance of 80% was set as the criterion for the number of factors.
Characteristics of the participants were summarized according to TL quartiles. Continuous variables were presented as means ± SDs, and categorical variables were presented in percentages. The significance of differences among TL quartiles was tested by ANOVA for continuous variables and by chi-square test for categorical variables. General linear models were used to estimate mean TL and their 95% CIs for different intake groups of fat and FAs while adjusting for covariates. The basic models were adjusted only for age at enrollment (y, continuous). The multivariable-adjusted models additionally adjusted for race-ethnicity (white, black, Hispanic, Asian/Pacific Islander), BMI (≤25, >25–30, >30–35, >35 kg/m2), smoking (never, former, current smoker), daily alcohol intake (≤0.01, >0.01–0.1, >0.1–2, >2 g/d), diabetes case in the primary case-control study (yes, no), physical activity (0, >0–5, >5–20, >20 metabolic equivalent hours/wk), daily energy intake (kcal, continuous), daily fruit and vegetable intake (medium serving [∼170 g], continuous), daily vitamin C intake (mg, continuous), daily vitamin E intake (IU, continuous), daily selenium intake (μg, continuous), and daily β-carotene intake (μg, continuous). Intake levels of total fat, individual FAs, and different combinations of FAs were categorized into quartiles. P values for linear trend were obtained by including the median intake levels of each group as continuous variables in the regression models. When different sets of variables of nutrient density from other sources are included in models as continuous variables, the coefficients from these nutrient-density models can be interpreted as the difference of TL by exchanging energy from a specific fat or FA for the same amount of energy from other sources (22). For the analysis of food items, intake levels were categorized into 4 groups for ease of conceptualization and to distribute similar numbers of participants across groups. P values for trend were obtained by including the median intake levels of each group as continuous variables in the regression models. To search for potential nonlinear relations between fat and FA intake and TL in this study, we performed LOESS (local regression analysis) (23, 24) of TL on these variables. Individuals with complete data were included in the analyses. Statistical analyses were repeated in the control population as sensitivity analyses. We conducted all statistical analyses with SAS software (version 9.3; SAS Institute). All P values are 2-sided, and P < 0.05 was considered significant.
Results
A total of 4029 WHI participants were included in this study. We retained 3 factors in the exploratory factor analysis that together accounted for 85.6% of the total variance in the original 23 variables (Supplemental Table 1). The first factor accounted mainly for SMSFAs [including butyric (4:0), hexanoic (6:0), octanoic (8:0), decanoic (10:0), and lauric acid (12:0)]. The second factor mainly represented plant source FAs [including primarily arachidic (20:0) and gadoleic acid (20:1), also with relatively large factor loadings of behenic (22:0), oleic (18:1), linoleic (18:2), and linolenic acid (18:3)]. The third factor almost exclusively accounted for marine-source FAs, including EPA (20:5n−3), docosapentaenoic acid (DPA, 22:5n−3), and DHA (22:6n−3). Participants’ characteristics according to quartiles of TL are summarized in Table 1. Participants with shorter TL tended to be white, older, and consumed more alcohol. Daily energy intake did not differ significantly across quartiles of TL.
TABLE 1.
Characteristics of the participants by quartiles of peripheral leukocyte telomere length in a subsample of postmenopausal women from the WHI1
| Leukocyte telomere length |
|||||
| Quartile 1 (n = 1007) | Quartile 2 (n = 1007) | Quartile 3 (n = 1007) | Quartile 4 (n = 1008) | P2 | |
| Median telomere length (IQR), kb | 2.60 (2.19–2.87) | 3.53 (3.32–3.73) | 4.32 (4.12–4.54) | 5.57 (5.14–6.30) | |
| Age, y | 63.4 ± 7.2 | 63.1 ± 7.1 | 62.2 ± 7.1 | 61.5 ± 6.7 | <0.001 |
| Race-ethnicity, % | <0.001 | ||||
| White | 55.2 | 55.5 | 49.9 | 40.0 | |
| Black | 28.2 | 25.0 | 30.5 | 36.7 | |
| Hispanic | 8.3 | 11.3 | 12.7 | 16.0 | |
| Asian/Pacific Islander | 8.2 | 8.1 | 6.9 | 7.3 | |
| Total energy intake, kcal/d | 1580 ± 890 | 1630 ± 950 | 1580 ± 900 | 1570 ± 830 | 0.43 |
| BMI, kg/m2 | 29.5 ± 6.5 | 29.3 ± 6.8 | 29.7 ± 6.8 | 29.5 ± 6.8 | 0.55 |
| Smoking, % | 0.53 | ||||
| Never | 51.2 | 54.1 | 55.2 | 55.0 | |
| Former | 40.9 | 38.4 | 37.3 | 38.5 | |
| Current | 8.0 | 7.5 | 7.4 | 6.5 | |
| Alcohol intake, g/d | 4.3 ± 12.2 | 3.1 ± 7.2 | 3.1 ± 8.3 | 2.7 ± 6.7 | <0.001 |
| Physical activity, MET-h/wk | 11.1 ± 12.9 | 12.3 ± 14.2 | 11.6 ± 13.3 | 11.2 ± 14.0 | 0.23 |
| Total fat intake, % of energy | 32.0 ± 9.1 | 31.2 ± 8.6 | 31.7 ± 8.6 | 31.6 ± 8.5 | 0.22 |
| SFAs, % of energy | 10.4 ± 3.3 | 10.2 ± 3.3 | 10.3 ± 3.1 | 10.1 ± 3.1 | 0.17 |
| MUFAs, % of energy | 12.1 ± 3.9 | 11.8 ± 3.6 | 12.1 ± 3.6 | 12.1 ± 3.5 | 0.23 |
| PUFAs, % of energy | 6.8 ± 2.5 | 6.6 ± 2.2 | 6.8 ± 2.4 | 6.8 ± 2.5 | 0.12 |
Continuous variables are shown as means ± SDs and categorical variables are shown as percentages. MET-h, metabolic equivalent hours; WHI, Women’s Health Initiative.
P values are based on ANOVA for continuous variables and on chi-square test for categorical variables.
In comparison to women in the lower intake categories, those who were in the top quartile of total fat intake had shorter TLs, although the linear trend was not significant (Table 2; P-trend = 0.09). In particular, SFA intake (% of energy) was inversely associated with TL. Compared with women in the lower intake categories, those who consumed the highest percentage of energy from SFAs (median: 14.0%) also had shorter TLs [mean: 3.98 kb (95% CI: 3.86, 4.09 kb); P-trend = 0.017]. Intakes of MUFAs and PUFAs were not significantly associated with TL in the fully adjusted models.
TABLE 2.
Peripheral leukocyte telomere length according to quartiles of dietary fat intake in a subsample of postmenopausal women from the WHI1
| Dietary fat intake |
|||||
| Nutrient and telomere length | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend2 |
| Total fat, % of energy | 21.7 | 28.4 | 34.2 | 41.9 | |
| Telomere length, kb | |||||
| Model 1 | 4.07 (3.99, 4.16) | 4.05 (3.97, 4.14) | 4.11 (4.02, 4.19) | 3.99 (3.90, 4.07) | 0.26 |
| Model 2 | 4.12 (4.00, 4.23) | 4.12 (4.01, 4.23) | 4.16 (4.05, 4.26) | 3.98 (3.87, 4.09) | 0.09 |
| SFAs, % of energy | 6.6 | 9.0 | 11.1 | 14.0 | |
| Telomere length, kb | |||||
| Model 1 | 4.09 (4.00, 4.17) | 4.15 (4.06, 4.23) | 4.03 (3.95, 4.12) | 3.96 (3.87, 4.04) | 0.010 |
| Model 2 | 4.13 (4.01, 4.24) | 4.18 (4.07, 4.29) | 4.07 (3.96, 4.18) | 3.98 (3.86, 4.09) | 0.017 |
| MUFAs, % of energy | 7.9 | 10.6 | 13.1 | 16.3 | |
| Telomere length, kb | |||||
| Model 1 | 4.08 (3.99, 4.16) | 4.02 (3.93, 4.10) | 4.12 (4.03, 4.20) | 4.01 (3.93, 4.10) | 0.57 |
| Model 2 | 4.12 (4.00, 4.23) | 4.07 (3.96, 4.18) | 4.16 (4.05, 4.26) | 4.02 (3.91, 4.13) | 0.33 |
| PUFAs, % of energy | 4.4 | 5.8 | 7.1 | 9.3 | |
| Telomere length, kb | |||||
| Model 1 | 4.05 (3.96, 4.14) | 4.06 (3.98, 4.15) | 4.06 (3.97, 4.14) | 4.05 (3.96, 4.14) | 0.97 |
| Model 2 | 4.10 (3.98, 4.21) | 4.10 (3.99, 4.20) | 4.11 (4.00, 4.21) | 4.05 (3.95, 4.16) | 0.55 |
Values for telomere length are least-square means (95% CIs). Values for each nutrient are median intakes of the nutrient according to quartiles. Model 1: adjusted for age (y, continuous); model 2: adjusted for age (y, continuous), race-ethnicity (white, black, Hispanic, Asian/Pacific Islander), BMI (≤25, >25–30, >30–35, >35 kg/m2), smoking (never, former, current smoker), daily alcohol intake (≤0.01, >0.01–0.1, >0.1–2, >2 g/d), diabetes case in the primary case-control study (yes, no), physical activity (0, >0–5, >5–20, >20 metabolic equivalent hours/wk), and daily intakes of energy (kcal, continuous), fruit and vegetables (medium serving [∼170 g], continuous), vitamin C (mg, continuous), vitamin E (IU, continuous), selenium (μg, continuous), and β-carotene (μg, continuous). WHI, Women’s Health Initiative.
P values for linear trend were obtained by including the median intake levels of each group as continuous variables in the regression models.
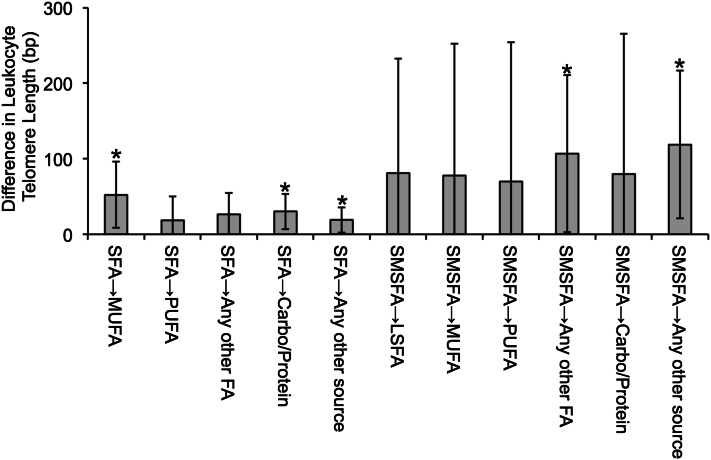
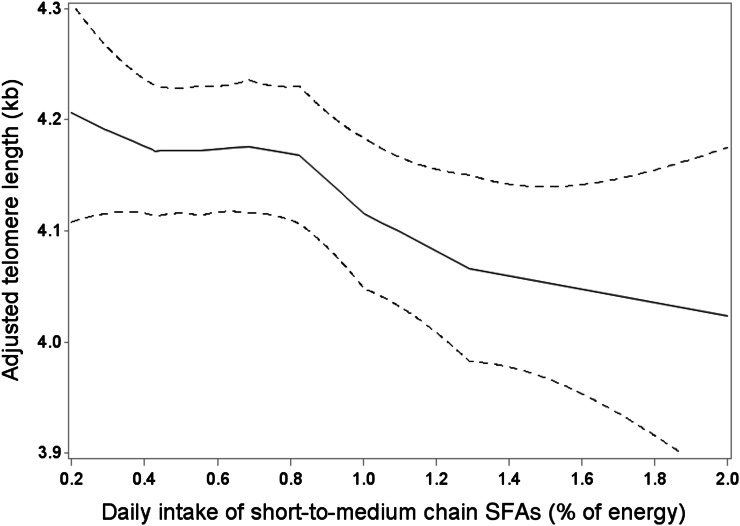
When individual FA types were investigated, intake levels of SMSFAs, rather than LSFAs, were inversely associated with TL (Table 3). Those who were in the highest quartile of SMSFA intake (median: 1.29% of energy) had shorter TL [mean: 4.00 kb (95% CI: 3.89, 4.11 kb); P-trend = 0.046]. Except for 12:0, all other individual SMSFAs, including 4:0, 6:0, 8:0, and 10:0 were all inversely associated with TL after multivariable adjustment (P-trend ≤ 0.05). Whereas the shortest LFSA members, myristic (14:0) and palmitic acid (16:0), were inversely associated with TL (P-trend = 0.020 and 0.031), other LSFAs, including margaric acid (17:0), stearic acid (18:0), 20:0, and 22:0, appeared to be not associated with TL. In the isoenergetic substitution models (Fig. 1), the substitution of 1% of energy of SFAs with MUFAs or carbohydrates/proteins was significantly associated with 52 bp (95% CI: 9, 96 bp) and 30 bp (95% CI: 7, 54 bp) longer TLs; the substitution of 1% of energy of SMSFAs with any other FA or any other energy source was significantly associated with 107 bp (95% CI: 3, 211 bp) and 119 bp (95% CI: 21, 216 bp) longer TLs, which is ∼5 times the average TL attrition due to aging. In a sensitivity analysis, we repeated the same analyses in the control population only (n = 2306) of our sample, and the association of SMSFAs to TL remained significant. In the LOESS analysis (Fig. 2), we observed that TL decreased steadily with increasing intake levels of SMSFAs. In addition, we investigated the association of individual MUFAs and PUFAs with TL (Supplemental Table 2), and no significant association was observed.
TABLE 3.
Peripheral leukocyte telomere length according to quartiles of individual dietary SFA intake in a subsample of postmenopausal women from the WHI1
| Dietary SFA intake |
|||||
| SFA and telomere length | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend2 |
| SMSFAs, % of energy | 0.29 | 0.55 | 0.83 | 1.29 | |
| Telomere length, kb | |||||
| Model 1 | 4.11 (4.03, 4.20) | 4.09 (4.01, 4.18) | 4.06 (3.98, 4.15) | 3.95 (3.86, 4.04) | 0.005 |
| Model 2 | 4.13 (4.03, 4.24) | 4.11 (4.00, 4.21) | 4.10 (3.99, 4.21) | 4.00 (3.89, 4.11) | 0.046 |
| Butyric acid (4:0), % of energy | 0.08 | 0.15 | 0.22 | 0.36 | |
| Telomere length, kb | |||||
| Model 1 | 4.12 (4.04, 4.21) | 4.09 (4.00, 4.17) | 4.09 (4.00, 4.17) | 3.92 (3.84, 4.01) | <0.001 |
| Model 2 | 4.12 (4.02, 4.23) | 4.12 (4.01, 4.22) | 4.12 (4.01, 4.23) | 3.98 (3.87, 4.09) | 0.025 |
| Hexanoic acid (6:0), % of energy | 0.03 | 0.07 | 0.11 | 0.18 | |
| Telomere length, kb | |||||
| Model 1 | 4.12 (4.03, 4.20) | 4.13 (4.04, 4.21) | 4.06 (3.98, 4.15) | 3.91 (3.83, 4.00) | <0.001 |
| Model 2 | 4.12 (4.02, 4.23) | 4.14 (4.04, 4.25) | 4.11 (4.00, 4.23) | 3.96 (3.85, 4.07) | 0.005 |
| Octanoic acid (8:0), % of energy | 0.03 | 0.06 | 0.09 | 0.13 | |
| Telomere length, kb | |||||
| Model 1 | 4.13 (4.04, 4.21) | 4.12 (4.04, 4.21) | 4.05 (3.97, 4.14) | 3.92 (3.83, 4.00) | <0.001 |
| Model 2 | 4.15 (4.04, 4.26) | 4.13 (4.02, 4.23) | 4.09 (3.98, 4.20) | 3.97 (3.86, 4.08) | 0.005 |
| Decanoic acid (10:0), % of energy | 0.06 | 0.11 | 0.17 | 0.26 | |
| Telomere length, kb | |||||
| Model 1 | 4.11 (4.02, 4.19) | 4.13 (4.04, 4.21) | 4.08 (3.99, 4.16) | 3.91 (3.82, 3.99) | <0.001 |
| Model 2 | 4.13 (4.02, 4.24) | 4.13 (4.03, 4.24) | 4.12 (4.01, 4.23) | 3.95 (3.84, 4.07) | 0.005 |
| Lauric acid (12:0), % of energy | 0.08 | 0.15 | 0.23 | 0.38 | |
| Telomere length, kb | |||||
| Model 1 | 4.11 (4.03, 4.20) | 4.05 (3.97, 4.14) | 4.05 (3.97, 4.14) | 4.00 (3.92, 4.09) | 0.10 |
| Model 2 | 4.14 (4.03, 4.25) | 4.07 (3.96, 4.18) | 4.09 (3.99, 4.20) | 4.04 (3.93, 4.15) | 0.23 |
| LSFAs, % of energy | 6.03 | 8.18 | 10.10 | 12.60 | |
| Telomere length, kb | |||||
| Model 1 | 4.08 (3.99, 4.16) | 4.13 (4.05, 4.22) | 4.02 (3.94, 4.11) | 3.98 (3.90, 4.07) | 0.044 |
| Model 2 | 4.12 (4.01, 4.23) | 4.17 (4.06, 4.28) | 4.05 (3.95, 4.16) | 4.00 (3.89, 4.12) | 0.06 |
| Myristic acid (14:0), % of energy | 0.39 | 0.65 | 0.86 | 1.23 | |
| Telomere length, kb | |||||
| Model 1 | 4.11 (4.03, 4.20) | 4.10 (4.01, 4.18) | 4.07 (3.98, 4.15) | 3.94 (3.85, 4.02) | 0.003 |
| Model 2 | 4.13 (4.02, 4.24) | 4.13 (4.02, 4.23) | 4.10 (3.99, 4.21) | 3.98 (3.87, 4.09) | 0.020 |
| Palmitic acid (16:0), % of energy | 3.74 | 4.99 | 6.08 | 7.51 | |
| Telomere length, kb | |||||
| Model 1 | 4.08 (4.00, 4.17) | 4.11 (4.03, 4.20) | 4.05 (3.97, 4.14) | 3.97 (3.88, 4.05) | 0.036 |
| Model 2 | 4.13 (4.01, 4.24) | 4.16 (4.05, 4.27) | 4.08 (3.97, 4.19) | 3.98 (3.87, 4.10) | 0.031 |
| Margaric acid (17:0), % of energy | 0.01 | 0.02 | 0.03 | 0.06 | |
| Telomere length, kb | |||||
| Model 1 | 4.04 (3.96, 4.13) | 4.18 (4.09, 4.26) | 4.02 (3.94, 4.11) | 3.98 (3.89, 4.06) | 0.049 |
| Model 2 | 4.04 (3.93, 4.14) | 4.20 (4.10, 4.31) | 4.06 (3.96, 4.17) | 4.04 (3.93, 4.15) | 0.40 |
| Stearic acid (18:0), % of energy | 1.71 | 2.40 | 3.03 | 3.84 | |
| Telomere length, kb | |||||
| Model 1 | 4.07 (3.98, 4.15) | 4.13 (4.05, 4.22) | 4.02 (3.94, 4.11) | 3.99 (3.91, 4.08) | 0.10 |
| Model 2 | 4.11 (3.99, 4.22) | 4.18 (4.07, 4.29) | 4.05 (3.95, 4.16) | 4.02 (3.90, 4.13) | 0.12 |
| Arachidic acid (20:0), % of energy | 0.02 | 0.03 | 0.04 | 0.07 | |
| Telomere length, kb | |||||
| Model 1 | 4.03 (3.95, 4.12) | 4.06 (3.97, 4.14) | 4.04 (3.96, 4.13) | 4.09 (4.00, 4.17) | 0.41 |
| Model 2 | 4.05 (3.94, 4.16) | 4.08 (3.97, 4.19) | 4.08 (3.97, 4.18) | 4.13 (4.02, 4.24) | 0.25 |
| Behenic acid (22:0), % of energy | 0.01 | 0.02 | 0.03 | 0.08 | |
| Telomere length, kb | |||||
| Model 1 | 3.98 (3.90, 4.07) | 4.10 (4.02, 4.19) | 4.06 (3.98, 4.15) | 4.08 (3.99, 4.16) | 0.37 |
| Model 2 | 3.98 (3.87, 4.09) | 4.14 (4.03, 4.24) | 4.11 (4.00, 4.22) | 4.13 (4.02, 4.24) | 0.13 |
Values for telomere length are least-square means (95% CIs). Values for each SFA are median intakes of the SFA according to quartiles. Model 1: adjusted for age (y, continuous); model 2: adjusted for age (y, continuous), race-ethnicity (white, black, Hispanic, Asian/Pacific Islander), BMI (≤25, >25–30, >30–35, >35 kg/m2), smoking (never, former, current smoker), daily alcohol intake (≤0.01, >0.01–0.1, >0.1–2, >2 g/d), diabetes case in the primary case-control study (yes, no), physical activity (0, >0–5, >5–20, >20 metabolic equivalent hours/wk), and daily intakes of energy (kcal, continuous), fruit and vegetables (medium serving [∼170 g], continuous), vitamin C (mg, continuous), vitamin E (IU, continuous), selenium (μg, continuous), and β-carotene (μg, continuous). LSFA, long-chain SFA; SMSFA, short-to-medium-chain SFA; WHI, Women’s Health Initiative.
P values for linear trend were obtained by including the median intake levels of each group as continuous variables in the regression models.
FIGURE 1.
Estimated changes in leukocyte telomere length (bp) associated with isoenergetic substitution of 1% of energy in a subsample of postmenopausal women from the Women’s Health Initiative. Values are mean differences ± 95% CIs. Mean differences were adjusted for age (y, continuous), race-ethnicity (white, black, Hispanic, Asian/Pacific Islander), BMI (≤25, >25–30, >30–35, >35 kg/m2), smoking (never, former, current smoker), daily alcohol intake (≤0.01, >0.01–0.1, >0.1–2, >2 g/d), diabetes case in the primary case-control study (yes, no), physical activity (0, >0–5, >5–20, >20 metabolic equivalent hours/wk), and daily intakes of energy (kcal, continuous), fruit and vegetables (medium serving [∼170 g], continuous), vitamin C (mg, continuous), vitamin E (IU, continuous), selenium (μg, continuous), and β-carotene (μg, continuous). *P < 0.05. Carbo, carbohydrates; LSFA, long-chain SFAs; SMSFA, short-to-medium-chain SFAs.
FIGURE 2.
Local regression of the association between leukocyte telomere length (kb) and daily intake of short-to-medium-chain SFAs (% of energy) in a subsample of postmenopausal women from the Women’s Health Initiative. Adjusted covariates: age (y, continuous), race-ethnicity (white, black, Hispanic, Asian/Pacific Islander), BMI (≤25, >25–30, >30–35, >35 kg/m2), smoking (never, former, current smoker), daily alcohol intake (≤0.01, >0.01–0.1, >0.1–2, >2 g/d), diabetes case in the primary case-control study (yes, no), physical activity (0, >0–5, >5–20, >20 metabolic equivalent hours/wk), and daily intakes of energy (kcal, continuous), fruit and vegetables (medium serving [∼170 g], continuous), vitamin C (mg, continuous), vitamin E (IU, continuous), selenium (μg, continuous), and β-carotene (μg, continuous). The solid curve shows the expected means of adjusted telomere length (kb) in local regression models. The dashed curves show the pointwise 95% CIs.
Associations between intake of SMSFA-abundant foods and TL are presented in Table 4. In general, daily intake of milk was not associated to TL in multivariable-adjusted models (P-trend = 0.37). However, when participants with different preferences of milk intake were examined, we found that among women who usually chose nonskim milk (whole milk or reduced-fat milk; n = 1481), compared with those who usually chose skim milk (n = 1228), milk intake was inversely associated with TL (P-trend = 0.036). The amount of fat added after cooking was not significantly associated with TL (P-trend = 1.00). For those who used butter only (n = 330), however, the amount added was inversely associated to TL in the age-adjusted model (P-trend = 0.029) but missed the significance level in the multivariable-adjusted model (P-trend = 0.20). The amount of fat added on bread was not significantly associated with TL (P-trend = 0.28). Total cheese intake was not significantly associated with TL in the multivariable-adjusted model (P-trend = 0.46). When we examined the intakes of different types of cheese, we found that low-/no-fat cheese was not associated with TL (P-trend = 0.80), whereas the intakes of other cheese (fat-containing) was inversely associated with TL (P-trend = 0.038).
TABLE 4.
Peripheral leukocyte telomere length according to intake levels of short-to-medium-chain SFA–abundant foods in a subsample of postmenopausal women from the WHI1
| Short-to-medium-chain SFA–abundant foods intake |
|||||
| Food item and telomere length | Group 1 | Group 2 | Group 3 | Group 4 | P-trend2 |
| Milk3, medium servings/d | 0 | 0 to <0.25 | 0.25 to <0.75 | ≥0.75 | |
| Telomere length, kb | |||||
| Model 1 | 4.13 (4.02, 4.25) | 4.09 (4.01, 4.16) | 4.07 (3.98, 4.15) | 3.98 (3.90, 4.05) | 0.014 |
| Model 2 | 4.13 (3.99, 4.26) | 4.08 (3.98, 4.18) | 4.11 (4.00, 4.21) | 4.05 (3.94, 4.16) | 0.37 |
| Usually nonskim milk (n = 1481) | |||||
| Telomere length, kb | |||||
| Model 1 | 4.43 (4.11, 4.75) | 4.12 (3.99, 4.25) | 4.10 (3.98, 4.22) | 3.89 (3.78, 4.01) | <0.001 |
| Model 2 | 4.43 (4.09, 4.78) | 4.06 (3.90, 4.23) | 4.13 (3.97, 4.29) | 3.95 (3.79, 4.12) | 0.036 |
| Usually skim milk (n = 1228) | |||||
| Telomere length, kb | |||||
| Model 1 | 4.19 (3.81, 4.57) | 4.10 (3.91, 4.29) | 4.00 (3.84, 4.15) | 4.03 (3.92, 4.14) | 0.65 |
| Model 2 | 4.17 (3.76, 4.59) | 4.11 (3.87, 4.35) | 4.05 (3.84, 4.27) | 4.16 (3.96, 4.36) | 0.44 |
| Fat added after cooking4, medium servings/d | 0 | 0 to <0.1 | 0.1 to <0.5 | ≥0.5 | |
| Telomere length, kb | |||||
| Model 1 | 4.09 (4.01, 4.17) | 4.11 (4.02, 4.20) | 4.00 (3.93, 4.07) | 4.02 (3.89, 4.15) | 0.18 |
| Model 2 | 4.08 (3.98, 4.18) | 4.13 (4.02, 4.24) | 4.04 (3.94, 4.14) | 4.12 (3.96, 4.27) | 1.00 |
| Use butter only (n = 330) | |||||
| Telomere length, kb | |||||
| Model 1 | 4.42 (3.91, 4.93) | 4.17 (3.88, 4.46) | 3.86 (3.64, 4.07) | 3.73 (3.43, 4.03) | 0.029 |
| Model 2 | 4.33 (3.74, 4.93) | 4.15 (3.76, 4.55) | 3.79 (3.46, 4.12) | 3.81 (3.40, 4.22) | 0.20 |
| Use other fat only (n = 2142) | |||||
| Telomere length, kb | |||||
| Model 1 | 4.20 (4.06, 4.34) | 4.19 (4.07, 4.30) | 4.03 (3.94, 4.13) | 4.12 (3.96, 4.28) | 0.38 |
| Model 2 | 4.20 (4.03, 4.37) | 4.25 (4.10, 4.40) | 4.13 (3.99, 4.27) | 4.27 (4.07, 4.48) | 0.70 |
| Fat added on bread4, medium servings/d | 0 | 0 to <0.2 | 0.2 to <0.5 | ≥0.5 | |
| Telomere length, kb | |||||
| Model 1 | 4.06 (3.97, 4.14) | 4.13 (4.05, 4.21) | 4.06 (3.98, 4.15) | 3.92 (3.82, 4.02) | 0.005 |
| Model 2 | 4.03 (3.93, 4.14) | 4.16 (4.05, 4.26) | 4.10 (4.00, 4.21) | 4.01 (3.89, 4.14) | 0.28 |
| Use butter only (n = 612) | |||||
| Telomere length, kb | |||||
| Model 1 | 3.71 (3.43, 3.99) | 4.10 (3.90, 4.30) | 4.01 (3.83, 4.20) | 3.94 (3.72, 4.15) | 0.89 |
| Model 2 | 3.59 (3.25, 3.92) | 4.04 (3.79, 4.29) | 4.02 (3.78, 4.26) | 3.98 (3.69, 4.27) | 0.57 |
| Use other fat only (n = 2298) | |||||
| Telomere length, kb | |||||
| Model 1 | 4.10 (3.96, 4.23) | 4.16 (4.06, 4.27) | 4.09 (3.99, 4.19) | 3.95 (3.83, 4.08) | 0.020 |
| Model 2 | 4.11 (3.95, 4.27) | 4.22 (4.08, 4.35) | 4.15 (4.02, 4.29) | 4.06 (3.90, 4.22) | 0.21 |
| Cheese5, medium servings/d | 0 | 0 to <0.1 | 0.1 to <0.5 | ≥0.5 | |
| Telomere length, kb | |||||
| Model 1 | 4.16 (4.02, 4.30) | 4.12 (4.03, 4.21) | 4.04 (3.98, 4.10) | 3.97 (3.88, 4.06) | 0.011 |
| Model 2 | 4.15 (3.99, 4.30) | 4.10 (3.99, 4.22) | 4.07 (3.98, 4.17) | 4.05 (3.93, 4.18) | 0.46 |
| Low-/no-fat cheese | |||||
| Telomere length, kb | |||||
| Model 1 | 4.13 (4.04, 4.21) | 4.06 (3.98, 4.13) | 4.01 (3.93, 4.08) | 4.01 (3.87, 4.14) | 0.14 |
| Model 2 | 4.11 (4.01, 4.22) | 4.08 (3.99, 4.18) | 4.06 (3.96, 4.17) | 4.09 (3.93, 4.24) | 0.80 |
| Other cheese | |||||
| Telomere length, kb | |||||
| Model 1 | 4.18 (4.09, 4.27) | 4.06 (3.99, 4.12) | 4.00 (3.92, 4.08) | 3.84 (3.67, 4.02) | <0.001 |
| Model 2 | 4.19 (4.08, 4.30) | 4.07 (3.98, 4.17) | 4.04 (3.93, 4.14) | 3.92 (3.72, 4.13) | 0.038 |
Values for telomere length are least-square means (95% CIs). Values for each food item are intake ranges according to categories. Model 1: adjusted for age (y, continuous); model 2: adjusted for age (y, continuous), race-ethnicity (white, black, Hispanic, Asian/Pacific Islander), BMI (≤25, >25–30, >30–35, >35 kg/m2), smoking (never, former, current smoker), daily alcohol intake (≤0.01, >0.01–0.1, >0.1–2, >2 g/d), diabetes case in the primary case-control study (yes, no), physical activity (0, >0–5, >5–20, >20 metabolic equivalent hours/wk), and daily intakes of energy (kcal, continuous), fruit and vegetables (medium serving [∼170 g], continuous), vitamin C (mg, continuous), vitamin E (IU, continuous), selenium (μg, continuous), and β-carotene (μg, continuous). WHI, Women’s Health Initiative.
P values for linear trend were obtained by including the median intake levels of each group as continuous variables in the regression models.
One medium serving of milk ∼ 227 mL.
One medium serving of fat ∼ 9.5 g.
One medium serving of cheese ∼ 57 g.
Discussion
Limited studies have evaluated the potential associations between dietary components and TL, and fewer have investigated the direct role of dietary fat intake in relation to TL. The current study comprehensively assessed the relation of total and types of dietary fats and individual FAs with TL in peripheral leukocytes among a large multiethnic sample of WHI participants. There was a significant inverse association of SMSFA intake level with TL. On average, the substitution of 1% of energy intake from SMSFAs with other energy sources was directly associated with 119 bp longer TL (which is ∼5 times the mean telomere attrition of 22 bp/y in our samples). In particular, food items rich in SMSFAs (including butter and fat-containing milk and cheese) were consistently and inversely associated with TL.
Previous studies have shown that higher SFA intake was associated with shorter TL (6, 25–27). Our analysis using a nutrient-density model reaches a similar conclusion that indicates that total SFA intake was inversely associated with TL. Nettleton et al. (28) showed that, among intake levels of all food items assessed by an FFQ, only processed meat, which is a major source of SFAs, was associated with TL after adjustment for covariates. PUFAs have been associated with TL in previous studies (25, 29), although others have not confirmed this association (26). Our results also did not show significant associations between PUFAs and TL. A previous study implicated that, compared with an SFA diet and a low-fat and high-carbohydrate diet, the Mediterranean diet may induce lower intracellular reactive oxidative species production, cellular apoptosis, and percentage of cells with telomere shortening in human umbilical endothelial cells (30). Therefore, increased oxidative stress and inflammation could be one of the underlying causes linking SFA intake to TL. However, few studies have directly investigated the effects of different SFAs to TL. The current study was the first, to our knowledge, to investigate specific effects of individual FAs on TL in a multiethnic sample of postmenopausal women. We found that both total and individual SMSFA intakes were inversely associated to TL, but no significant association was found for LSFA intake. These findings are consistent with previous studies that have shown different associations of SMSFAs and LSFAs with disease outcomes of interest. For example, Epstein et al. (31) reported that intake of shorter chain SFAs (4:0 to 10:0) and 14:0, rather than LSFAs, was inversely associated with prostate cancer–specific survival in localized cases. Karupaiah et al. (32) showed that plasma TGs peaked significantly earlier after shorter-SFA–rich meal compared with a longer-SFA–rich meal, and plasma total cholesterol response was also increased significantly by a shorter-SFA–rich meal. Other studies also suggested similar unfavorable effects of medium-chain FAs on lipid profiles (33, 34).
The major dietary source of SMSFAs is dairy products. SMSFAs account for ∼20% of SFAs found in whole milk, butter, and whole-milk cheese but account for a very low percentage of SFAs in other foods with animal fat and almost none in fat from plant sources (35). Previous studies indicated the potential risk of whole milk and potential protective effects of skim milk on various diseases (36–39). In our study, total milk or cheese intake was not significantly associated with TL after multivariable adjustment. When we excluded skim milk and low-/no-fat cheese, both associations became significant. The primary difference between nonskim milk/cheese and skim milk/cheese is the fat content. Thus, the fat contained is very likely to be at least partly responsible for the specific associations observed in our analysis. A previous study in 56 apparently healthy Italians also showed a borderline-significant inverse association of dairy food intake to TL but no associations between total oils and butter intake with TL (40). We also assessed the amount of fat added after cooking. The amount of fat other than butter was not associated with TL, whereas the amount of butter used was inversely associated in these postmenopausal women. The multivariable-adjusted model of butter used after cooking missed the significance level probably because of the small sample size of participants who used butter only (n = 330), which resulted in limited statistical power. It is possible that SMSFAs play a mechanistic role in this association, although further research is warranted to elucidate their contribution to telomere attrition. Recent studies can shed some light on the potential mechanism of this association. Liberato et al. (41) showed that medium-chain FAs are selective peroxisome proliferator-activated receptor (PPAR) γ activators and pan-PPAR partial agonists, and PPAR has been related to inflammatory reactions (42), which may lead to the accelerated telomere attrition.
One limitation that needs to be kept in mind when interpreting findings in the current study is its cross-sectional design, which makes direct causal inference difficult. Nevertheless, our participants were asked to complete the FFQ and other questionnaires on lifestyle factors before the time of blood draw and before TL measurement. Prospective studies are clearly warranted to further investigate the role of SMSFAs in affecting changes in TL among postmenopausal women, and the possible role of TL in mediating subsequent disease occurrence.
Supplementary Material
Acknowledgments
The authors thank Yilin Chen for the laboratory work of leukocyte TL measurement. S.L. and Yan S. designed the research; Yan S. analyzed data and drafted the manuscript; S.L. and N.-C.Y.Y. generated the telomere length data, supervised the analysis, and edited the manuscript; L.H., R.W., C.B.E., L.F.T., and S.L. participated in the original WHI study and contributed to data collection; and N.-C.Y.Y., Yiqing S., M.K.K., L.H., R.W., C.B.E., and L.F.T. revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: LSFA, long-chain SFA; PPAR, peroxisome proliferator activated receptor; SMSFA, short-to-medium-chain SFA; TL, telomere length; WHI, Women’s Health Initiative; WHI-OS, Women’s Health Initiative Observational Study.
Literature Cited
- 1.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. [DOI] [PubMed] [Google Scholar]
- 2.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–82. [PMC free article] [PubMed] [Google Scholar]
- 3.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4. [DOI] [PubMed] [Google Scholar]
- 4.Shiels PG, McGlynn LM, MacIntyre A, Johnson PC, Batty GD, Burns H, Cavanagh J, Deans KA, Ford I, McConnachie A, et al. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS ONE. 2011;6:e22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Q, Shi L, Prescott J, Chiuve SE, Hu FB, De Vivo I, Stampfer MJ, Franks PW, Manson JE, Rexrode KM. Healthy lifestyle and leukocyte telomere length in U.S. women. PLoS ONE. 2012;7:e38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiainen AM, Mannisto S, Blomstedt PA, Moltchanova E, Perala MM, Kaartinen NE, Kajantie E, Kananen L, Hovatta I, Eriksson JG. Leukocyte telomere length and its relation to food and nutrient intake in an elderly population. Eur J Clin Nutr. 2012;66:1290–4. [DOI] [PubMed] [Google Scholar]
- 7.Daubenmier J, Lin J, Blackburn E, Hecht FM, Kristeller J, Maninger N, Kuwata M, Bacchetti P, Havel PJ, Epel E. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37:917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–9. [DOI] [PubMed] [Google Scholar]
- 9.Bowers K, Tobias DK, Yeung E, Hu FB, Zhang C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am J Clin Nutr. 2012;95:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmerón J, Hu FB, Manson JE, Stampfer MJ, Colditz GA, Rimm EB, Willett WC. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73:1019–26. [DOI] [PubMed] [Google Scholar]
- 11.Willett WC. Dietary fat intake and cancer risk: a controversial and instructive story. Semin Cancer Biol. 1998;8:245–53. [DOI] [PubMed] [Google Scholar]
- 12.Women’s Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 13.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. [DOI] [PubMed] [Google Scholar]
- 14.You NC, Chen BH, Song Y, Lu X, Chen Y, Manson JE, Kang M, Howard BV, Margolis KL, et al. A prospective study of leukocyte telomere length and risk of type 2 diabetes in postmenopausal women. Diabetes. 2012;61:2998–3004. [DOI] [PMC free article] [PubMed]
- 15.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. [DOI] [PubMed] [Google Scholar]
- 16.Henderson MM, Kushi LH, Thompson DJ, Gorbach SL, Clifford CK, Insull W, Jr, Moskowitz M, Thompson RS. Feasibility of a randomized trial of a low-fat diet for the prevention of breast cancer: dietary compliance in the Women's Health Trial Vanguard Study. Prev Med. 1990;19:115–33. [DOI] [PubMed] [Google Scholar]
- 17.Kristal AR, Feng Z, Coates RJ, Oberman A, George V. Associations of race/ethnicity, education, and dietary intervention with the validity and reliability of a food frequency questionnaire: the Women's Health Trial Feasibility Study in Minority Populations. Am J Epidemiol. 1997;146:856–69. [DOI] [PubMed] [Google Scholar]
- 18.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. [DOI] [PubMed] [Google Scholar]
- 19.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- 20.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–71. [PubMed] [Google Scholar]
- 21.O'Callaghan N, Dhillon V, Thomas P, Fenech M. A quantitative real-time PCR method for absolute telomere length. Biotechniques. 2008;44:807–9. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC. Nutritional epidemiology. 3rd ed. New York: Oxford University Press; 2013.
- 23.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:8. [Google Scholar]
- 24.Cleveland WS, Devlin SJ. Locally-weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:15. [Google Scholar]
- 25.Cassidy A, De Vivo I, Liu Y, Han J, Prescott J, Hunter DJ, Rimm EB. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr. 2010;91:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kark JD, Goldberger N, Kimura M, Sinnreich R, Aviv A. Energy intake and leukocyte telomere length in young adults. Am J Clin Nutr. 2012;95:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirabello L, Huang WY, Wong JY, Chatterjee N, Reding D, Crawford ED, De Vivo I, Hayes RB, Savage SA. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR., Jr Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2008;88:1405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marin C, Delgado-Lista J, Ramirez R, Carracedo J, Caballero J, Perez-Martinez P, Gutierrez-Mariscal FM, Garcia-Rios A, Delgado-Casado N, Cruz-Teno C, et al. Mediterranean diet reduces senescence-associated stress in endothelial cells. Age (Dordr). 2012;34:1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epstein MM, Kasperzyk JL, Mucci LA, Giovannucci E, Price A, Wolk A, Hakansson N, Fall K, Andersson SO, Andren O. Dietary fatty acid intake and prostate cancer survival in Orebro County, Sweden. Am J Epidemiol. 2012;176:240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karupaiah T, Tan CH, Chinna K, Sundram K. The chain length of dietary saturated fatty acids affects human postprandial lipemia. J Am Coll Nutr. 2011;30:511–21. [DOI] [PubMed] [Google Scholar]
- 33.Tholstrup T, Ehnholm C, Jauhiainen M, Petersen M, Hoy CE, Lund P, Sandstrom B. Effects of medium-chain fatty acids and oleic acid on blood lipids, lipoproteins, glucose, insulin, and lipid transfer protein activities. Am J Clin Nutr. 2004;79:564–9. [DOI] [PubMed] [Google Scholar]
- 34.Temme EH, Mensink RP, Hornstra G. Effects of medium chain fatty acids (MCFA), myristic acid, and oleic acid on serum lipoproteins in healthy subjects. J Lipid Res. 1997;38:1746–54. [PubMed] [Google Scholar]
- 35.USDA National Agricultural Library. USDA National Nutrient Database for Standard Reference; 2011 [cited 2013 Jan 8]. Available from: http://ndb.nal.usda.gov.
- 36.Song Y, Chavarro JE, Cao Y, Qiu W, Mucci L, Sesso HD, Stampfer MJ, Giovannucci E, Pollak M, Liu S, et al. Whole milk intake is associated with prostate cancer-specific mortality among U.S. male physicians. J Nutr. 2013;143:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51:1073–9. [DOI] [PubMed] [Google Scholar]
- 38.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–11. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Choi HK, Ford E, Song Y, Klevak A, Buring JE, Manson JE. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care. 2006;29:1579–84. [DOI] [PubMed] [Google Scholar]
- 40.Marcon F, Siniscalchi E, Crebelli R, Saieva C, Sera F, Fortini P, Simonelli V, Palli D. Diet-related telomere shortening and chromosome stability. Mutagenesis. 2012;27:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liberato MV, Nascimento AS, Ayers SD, Lin JZ, Cvoro A, Silveira RL, Martinez L, Souza PC, Saidemberg D, Deng T, et al. Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR) gamma activators and pan-PPAR partial agonists. PLoS ONE. 2012;7:e36297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.