Abstract
Selenoproteins are essential molecules for the mammalian antioxidant network. We previously demonstrated that targeted loss of all selenoproteins in mouse epidermis disrupted skin and hair development and caused premature death. In the current study we targeted specific selenoproteins for epidermal deletion to determine whether similar phenotypes developed. Keratinocyte-specific knockout mice lacking either the glutathione peroxidase 4 (GPx4) or thioredoxin reductase 1 (TR1) gene were generated by cre-lox technology using K14-cre. TR1 knockout mice had a normal phenotype in resting skin while GPx4 loss in epidermis caused epidermal hyperplasia, dermal inflammatory infiltrate, dysmorphic hair follicles and alopecia in perinatal mice. Unlike epidermal ablation of all selenoproteins, mice ablated for GPx4 recovered after 5 weeks and had a normal lifespan. GPx1 and TR1 were upregulated in the skin and keratinocytes of GPx4 knockout mice. GPx4 deletion reduces keratinocyte adhesion in culture and increases lipid peroxidation and COX-2 levels in cultured keratinocytes and whole skin. Feeding a COX-2 inhibitor to nursing mothers partially prevents development of the abnormal skin phenotype in knockout pups. These data link the activity of cutaneous GPx4 to the regulation of COX-2 and hair follicle morphogenesis and provide insight into the function of individual selenoprotein activity in maintaining cutaneous homeostasis.
Introduction
Selenoproteins are important antioxidant enzymes in mammals, harboring the 21st amino acid, selenocysteine (Sec), in their catalytic domain (Kryukov et al., 2003). As observed for all selenoproteins (Kumaraswamy et al., 2003 and references therein), knockout mouse models for individual selenoproteins like glutathione peroxidase 4 (GPx4) (Imai et al., 2003; Yant et al., 2003); thioredoxin reductase 1 (TR1) (Jakupoglu et al., 2005; Bondareva et al., 2007) and thioredoxin reductase 2 (TR2) (Conrad et al., 2004), are also embryonic lethal, establishing the importance of glutathione peroxidases and thioredoxin reductases in development.
Glutathione peroxidases react with H2O2 and fatty acid hydroperoxides (Brigelius-Flohé and Kipp, 2009), and among its family members, five are selenoproteins in human and four in mice (Kryukov et al., 2003). GPx4, or phospholipid hydroperoxide glutathione peroxidase (PHGPx), is the only member of the family directly reducing phospholipid hydroperoxides in membranes and lipoproteins at the expense of glutathione (Ursini et al., 1982; Thomas et al., 1990a) or protein-thiol groups (Conrad et al., 2005; Mauri et al., 2003). GPx4 functions as a redox sensor to induce cell death (Seiler et al., 2008), protects against lipid hydroperoxide damage in neurodegenerative diseases (Yoo et al., 2010; Wirth et al., 2010) and is regulated in cancer (Liu et al., 2006; Cejas et al., 2007). GPx4 overexpression in cells renders them more resistant to oxidative stress causing agents (Arai et al., 1999) and inhibits the enzymatic activity of many lipoxygenases and cyclooxygenases (Chen et al., 2003; Huang et al., 1999). GPx4 knockout embryos have disorganized germ layers lacking differentiation into structures (Imai et al., 2003; Yant et al., 2003), while cell lines generated from GPx4 null mouse embryos are susceptible to inducers of oxidative stress (Yant et al., 2003), confirming the importance of GPx4 in the antioxidant network.
Thioredoxin reductases in combination with thioredoxins, constitute an important oxidoreductase system in mammals (Nordberg and Arner, 2001), having cytosolic (TR1) (Gladyshev et al., 1996), mitochondrial (TR2) (Gasdaska et al., 1999), and testis-specific isoforms (Sun et al., 2001). The catalytically active penultimate Sec residue at the C-terminal domain gives uniqueness to their function (Gladyshev et al., 1996; Mustacich and Powis, 2000). TR1 is the most studied member of this group, owing to roles in redox regulation, cell proliferation, DNA repair, angiogenesis, cell signaling and antagonistic roles in preventing and promoting cancer (Hatfield et al., 2009). Knockout mouse models established the importance of TR1 in development, revealing embryonic lethality along with severe growth retardation and defective cell proliferation (Jakupoglu et al., 2005).
Antioxidant enzymes are essential to neutralize the damaging effects of reactive oxygen species (ROS) in mammalian skin generated through atmospheric oxygen, environmental toxins, pollutants and UV light, which may initiate several skin disorders including malignancies (Guyton and Kensler, 1993; Richelle et al., 2006). We recently demonstrated the importance of selenoproteins as antioxidants in skin, through their targeted removal in mouse epidermis, which generated mice with stunted growth, gross abnormalities of skin, hair loss and premature death, in addition to ROS accumulation and lipid peroxidation (Sengupta et al., 2010). This study also revealed GPx4 and TR1 as two of the most abundantly expressed selenoproteins in the epidermis of skin and cultured keratinocytes, corroborating earlier reports of functional significance of glutathione peroxidase and thioredoxin reductase protein families in skin (Richelle et al., 2006; Sohn et al., 2007). In light of these observations and earlier reports showing that knockout mouse models of GPx4 or TR1 are embryonic lethal, the present study was undertaken to examine whether one of these selenoproteins may be responsible, at least in part, for the many phenotypic and histological changes resulting from the ablation of selenoproteins in skin (Sengupta et al., 2010). Though no obvious phenotypic changes were observed for targeted removal of TR1 in skin, the lack of GPx4 modulated postnatal hair follicle morphogenesis, inflammatory cell infiltration and epidermal proliferation, associated with elevated expression of COX-2. With time, the phenotype resolved in association with compensatory upregulation of other selenoproteins including TR1 and GPx1.
Results
Deletion of GPx4 in mouse keratinocytes alters hair follicle morphogenesis
We generated two mouse models, with specific deletion of GPx4 or TR1 in K14 expressing cells of skin. Targeted removal of TR1 did not cause any apparent alteration in skin phenotype, life-span or weight of knockout mice, with both male and female knockouts being fertile (data not shown). Ablation of GPx4 in skin keratinocytes generated knockout pups, born with the expected Mendelian ratio (24.49%; Supplementary Figure S1a online), but exhibited visible skin abnormalities during the second week after birth. For all experimental purposes, knockout pups (K14-cre; GPx4fl/fl) were compared to control littermates (K14-cre; GPx4fl/+ or GPx4fl/fl) (Supplementary Figure S1b online). The extent of recombination of GPx4 in several tissues was determined in knockout and control (K14-cre; GPx4fl/+) littermates, where the ΔGPx4 allele was detected in skin of both genotypes and tongue of knockout mice (Supplementary Figure S1c online). Partial recombination in the skin of control heterozygous animals resulted due to the presence of K14-cre, and absence of ΔGPx4 allele in any other tissue established specificity of recombination. Subsequent breeding showed that both male and female GPx4 knockout mice were fertile.
Ablation of GPx4 did not lead to neonatal death but monitoring pups for 60 days post-birth showed that ~80% knockout mice survived to adulthood post-weaning (Figure 1a). The body weight of knockout pups was similar to control pups for about 1 week following birth, beyond which their weight gain was slower and a difference in body weight remained throughout the time observed (Figure 1b).
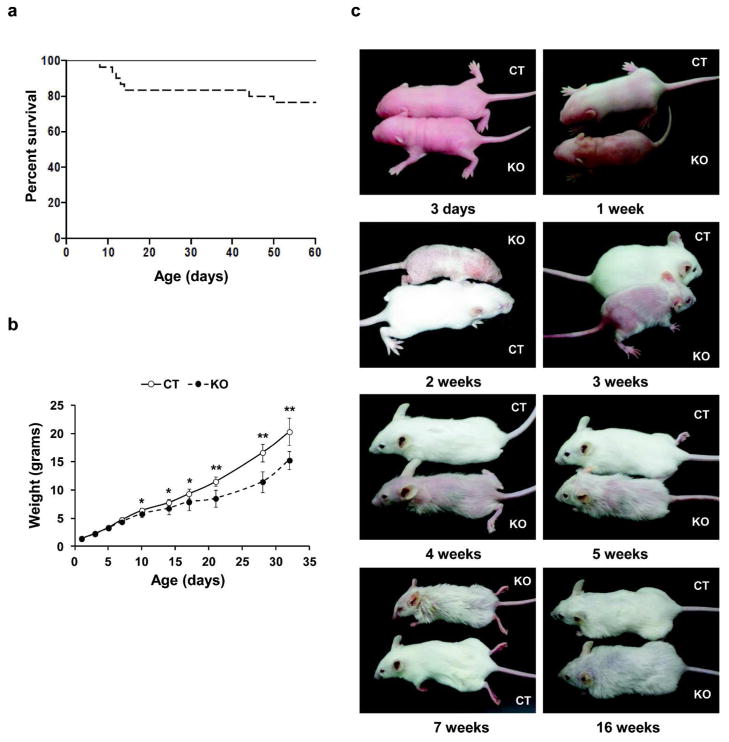
Figure 1. Survival rate, growth curve and phenotype of GPx4 knockout and control mice.
(a) Kaplan-Meier survival plot of GPx4 knockout (– – –, n=30) and control mice (——, n=54), with a cumulative survival proportion of 0.76 for knockout pups. (b) Growth curve of knockout (– – –, n=14) and control (——, n=23) littermates showing weight variations in the two mouse lines. (c) Phenotype of age matched knockout (KO) and control (CT) mice showing defective hair development, a less dense and furry hair coat in knockout offspring compared to control littermates. Error bars represent mean ± S.D. **, p ≤ 0.001; *, p ≤ 0.05.
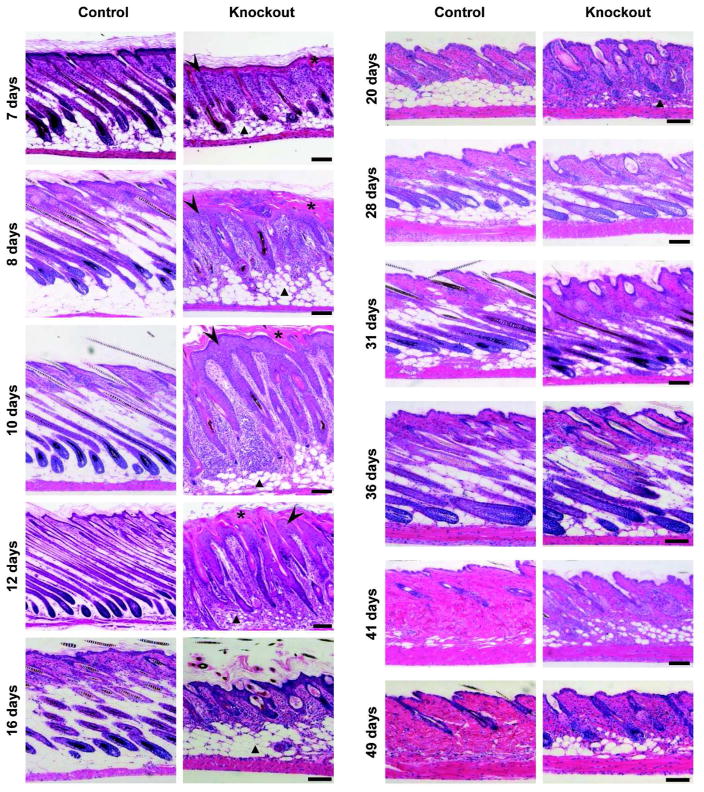
Skin of knockout mice was normal at birth (Supplementary Figure S2 online), but around 1 week of age, when control mice developed visible hair, the knockouts were still hairless over most of their bodies (Figure 1c) independent of skin color (Supplementary Figure S1d online). Over the next several weeks, when control pups had developed a uniform hair coat, hair growth on knockout mice was sparse, with areas of focal alopecia. This variation in coat quality continued as the mice aged. Histologically, knockout epidermis was hyperplastic (arrow head), and hair follicles were misaligned with marked outer root sheath (ORS) hyperplasia and abnormally shaped hair bulbs with little evidence of hair formation during the first hair cycle (Figure 2). The stratum corneum was thickened in young knockout mice (asterisk) along with a dense cellular dermis and decreased subcutaneous fat (triangle). While the hair cycle was in telogen in control mice at 3 weeks as expected, knockout mice had begun a second anagen. Accelerated entry into anagen was also observed in knockout mice in the next hair cycle at 49 days, with control follicles still in telogen. Nevertheless, by 28 days much of the alterations in histology had resolved in knockout skin, although visible hair remained sparse and sebaceous glands were enlarged. In contrast, hair follicle density was reduced in comparison to controls only during the perinatal period (Supplementary Figure S1e online), whereas hair follicle density was equivalent between knockout and control skin in older mice, suggesting the reduction in GPx4 impaired hair maturation. Histology of skin from newborn pups or tongue and whiskers from adult littermates did not reveal any significant change between the control and knockout pups (Supplementary Figure S2 online).
Figure 2. Postnatal hair follicle morphogenesis and cycling in GPx4 knockout mice.
Back skin sections from control and knockout mice at various ages were stained with H&E for histological examination. Sections from knockout mice show hair follicles of abnormal morphology that are misaligned and irregularly spaced.A thickened cornified layer (asterisk) above a hyperplastic epidermis (arrow head), dense cellular dermis and decreased number of fat cells (triangle) were observed in knockout mice until 3 weeks of age and then the phenotype resolves. Scale bar: 100 μm.
Lack of GPx4 alters keratinocyte proliferation, differentiation markers and induces infiltration of inflammatory cells
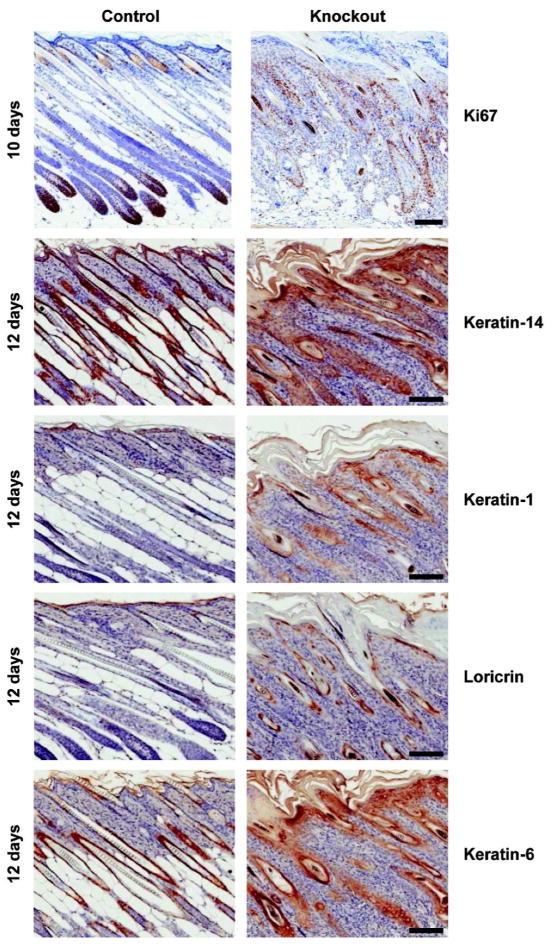
Using specific antibodies, we documented biochemical changes in perinatal GPx4 knockout skin epithelium in situ associated with the altered cutaneous morphology. Unlike compartmentalized proliferation (Ki67) in basal epidermis and hair follicle bulbs in control skin, proliferating epithelial cells were abundant throughout the skin of knockout mice, particularly in the ORS of follicles (Figure 3) and basal epidermal cells. Likewise, there was a marked expansion of keratin 14 (K14) positive cells and aberrant expression of keratin 6 (K6) in the epidermis and outer root sheath. Further, the epidermal terminal differentiation markers keratin 1 (K1) and loricrin were detected in hair follicle structures, suggesting an abnormal “epidermal like” differentiation in the absence of GPx4. Of interest is the “normalization” of these differentiation markers in knockout skin as a function of age, associated with the normalization of the morphological changes (Supplementary Figure S3 online). Using similar in situ staining, we explored the composition of the extensive dermal cellularity to determine whether these were inflammatory cells, as observed earlier with complete ablation of selenoproteins in skin epithelium (Sengupta et al., 2010). Supplementary Figure S4 online confirms the extensive infiltration of macrophages and granulocytes within the first 10 days of life for knockout pups and the gradual resolution of inflammation as morphological changes in skin resolve with aging.
Figure 3. Altered expression of proliferation and keratinocyte differentiation markers in skin sections of mice lacking GPx4 in keratinocytes.
Back skin sections from age matched control and knockout mice were stained for Ki67 to examine proliferation and for keratin-14, keratin-1, loricrin and keratin-6 to study differentiation. Increased Ki67 staining was detected in matrix cells of hair follicle bulbs, basal layer of epidermis and ORS of hair follicles of 10 day old knockout pups. Aberrant expression of markers of epidermal differentiation was noted in 12 day old knockout skin sections. Scale bar: 100 μm.
Deletion of GPx4 causes cell autonomous changes in isolated keratinocytes
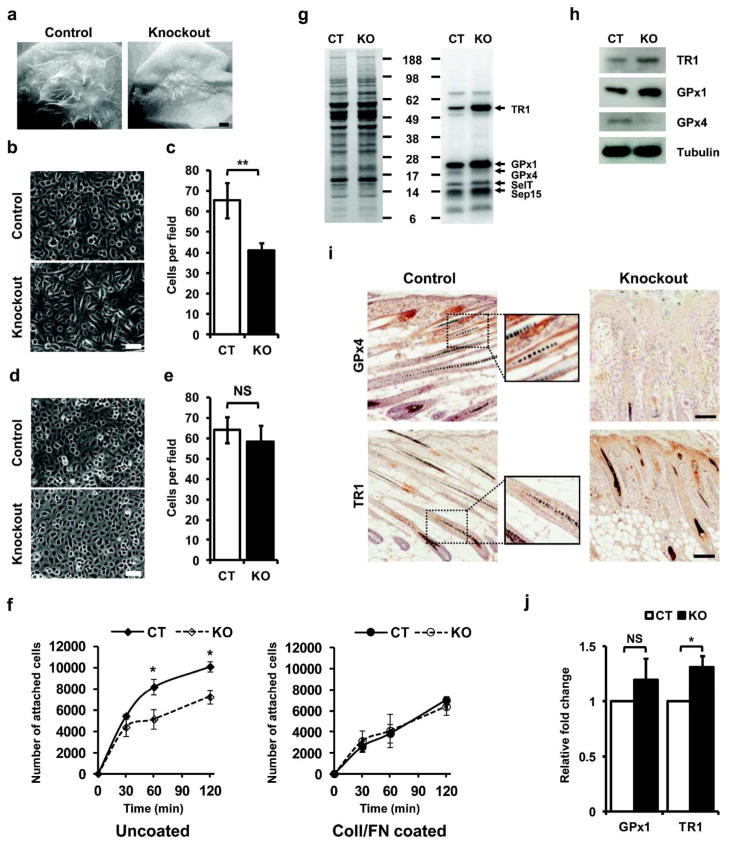
To rule out a systemic contribution to the altered skin phenotype and confirm the cell autonomous defect of GPx4 ablation in keratinocytes, we grafted freshly isolated primary keratinocytes and their corresponding dermal cells from each genotype onto the dorsum of nude mice. Grafts of control cells produced normal haired skin after 3 weeks, while knockout grafts were contracted with sparse hair growth (Figure 4a). Isolated cultured primary keratinocytes from knockout mice grew poorly on plastic culture dishes (Figure 4b, c), showing a 37% reduction in cell number in comparison to those from control mice. This could be corrected by coating plates with a mixture of collagen I and fibronectin (ColI/FN), where the knockout keratinocytes grew as well as control keratinocytes (Figure 4d, e). Hence, in subsequent experiments, cells were grown on such coated plates. The number of GPx4 keratinocytes that adhered to plastic over time was compromised but that improved when the plates were coated with ColI/FN (Figure 4f).
Figure 4. Keratinocytes lacking GPx4 have altered adhesion properties and modified selenoprotein expression.
(a) Freshly isolated keratinocytes and corresponding dermal cells from control and knockout mice were grafted onto backs of nude mice and examined 21 days post grafting. Scale bar: 1 mm. (b, c) Keratinocytes from knockout pups attach poorly to plastic culture dishes and display elongated and refractile morphology. Scale bar: 50 μm. (c; n=5). (d, e) Keratinocytes from knockout pups attach to culture plates coated with ColI/FN identically as keratinocytes from control littermates and display cuboidal morphology. Scale bar: 50 μm. (e; n=5) (f) Adhesion differences for keratinocytes from control and knockout mice following 30, 60 and 120 min of attachment on uncoated and ColI/FN coated plates (n=3). (g) Expression of selenoproteins in keratinocytes labeled with [75Se]. Right panel shows incorporation of [75Se] into proteins and left panel, Coomassie blue staining. Identification of several selenoproteins is designated on the right of the figure. (h) Western blot for TR1, GPx1 and GPx4 in lysates of cultured keratinocytes, with tubulin as loading control. (i) Immunodetection of GPx4 (10 days old) and TR1 (12 days old) in back skin sections of control and knockout littermates. Sections from control mice showed GPx4 staining in outer root sheath, with no staining in knockout section. TR1 staining was mostly confined to the inner root sheath of control mice, while in knockouts, it was also observed in the hyperplastic epidermis. Enlarged areas of stained region in control sections are shown alongside. Scale bar: 100 μm. (j) q-PCR analysis showing relative fold change in GPx1 and TR1 in cultured keratinocytes from GPx4 knockout mice. CT and KO designate control and knockout mice respectively. Data represent the mean values ± S.D. **, p ≤ 0.001; *, p ≤ 0.01; NS, not significant.
Cultured keratinocytes were radiolabeled with selenium [75Se] to visualize the expression of selenoproteins upon GPx4 ablation. Though intensities of most selenoproteins remained unchanged, GPx1 and TR1 were elevated in cultures from knockout mice relative to control keratinocytes (Figure 4g). This observation was corroborated through immunodetection of GPx1 and TR1 in lysates from cultured keratinocytes (Figure 4h). Immunodetection and selenium [75Se]-labeling also revealed a faint band corresponding to GPx4 in keratinocyte cultures. This could result from contaminating cells other than keratinocytes (e.g. melanocytes, fibroblasts) that are known to be present in these cultures (Sengupta et al., 2010) or from incomplete recombination in the crossbreeding. The latter seems less likely as GPx4 immunostaining was not detected in epidermis or hair follicles from knockout mice (Figure 4i). In contrast, enhanced staining of TR1 was detected in knockout epidermis and hair follicles, consistent with the enhanced expression detected in isolated keratinocytes. Unfortunately, antibodies capable of detecting GPx1 in skin in vivo are not available, nonetheless, quantitative real-time PCR (q-PCR) revealed elevated levels of GPx1 and TR1 transcripts in cultured keratinocytes from knockout mice (Figure 4j).
COX-2 expression and lipid peroxidation increase in keratinocytes lacking GPx4
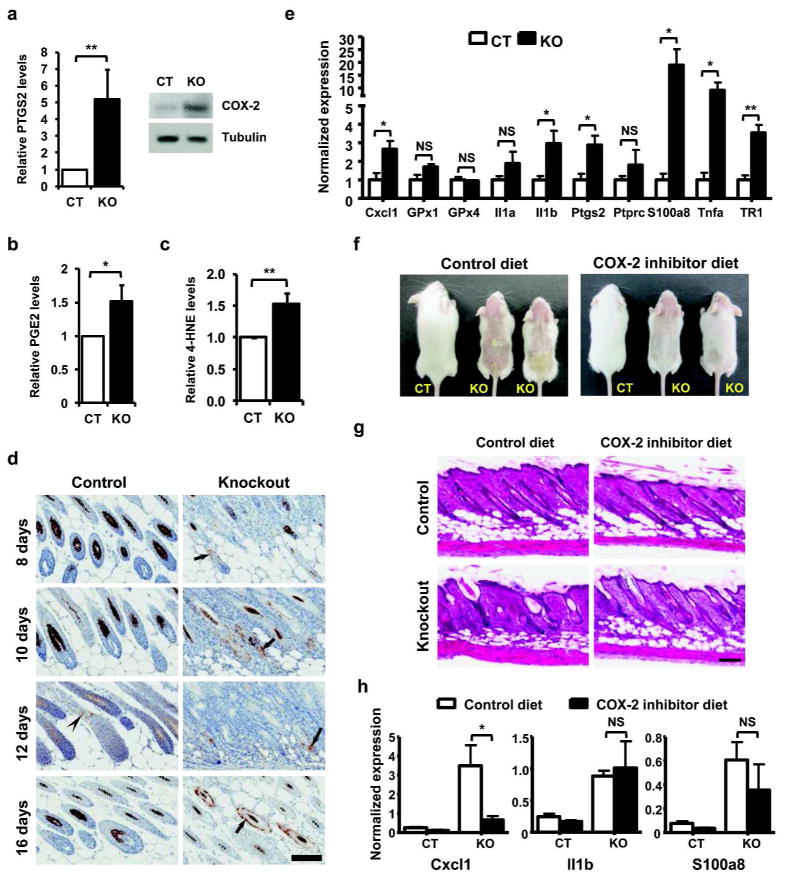
Cultured keratinocytes were examined for gene expression profile associated with targeted removal of GPx4 (GEO accession number GSE34215) and the top 25 genes displaying ≥ 2-fold variation (increase or decrease) and p value ≤ 0.05 (Supplementary Table S1 online) are depicted along with some major functional classes influenced by the loss of GPx4 (Supplementary Figure S5a online). Several candidate genes from the regulated functional pathways were examined through q-PCR (Supplementary Figure S5b online) and results corroborate changes observed in microarrays for most genes. Networks were generated for differentially regulated genes through Ingenuity Pathway Analysis (IPA), to explore pathway(s) and identify target gene(s), which were substantially affected by the loss of GPx4 in keratinocytes (Supplementary Figure S6 online). One key gene regulated through loss of GPx4 is PTGS2 (prostaglandin-endoperoxide synthase 2), or COX-2 (cyclooxygenase-2). Cyclooxygenases are proteins, whose enzymatic activity is inhibited by GPx4 (Imai and Nakagawa, 2003; Huang et al., 1999); hence, we examined the levels of COX-2 in GPx4 knockdown cells and observed an increase in COX-2 mRNA (Figure 5a, left panel), concurrent with elevated protein levels (Figure 5a, right panel) in cultured keratinocytes from knockout mice. Elevated COX-2 levels were also associated with increased levels of PGE2 in skin of knockout pups (Figure 5b). GPx4 protects cells by reducing membrane lipid hydroperoxides, which are key activators of lipoxygenase and cyclooxygenase. Hence, we explored whether the loss of GPx4 influenced lipid peroxidation by examining levels of 4-hydroxynonenal (4-HNE), a marker of lipid peroxidation, and observed ~1.6 fold increase in keratinocytes derived from knockout mice in comparison to their control counterpart (Figure 5c). In vivo, COX-2 is detected in a few hair follicle cells of normal mice (Figure 5d). In contrast, COX-2 positive cells are relatively abundant in skin from knockout mice, where they are localized particularly in the prominent K14 positive expanded follicle epithelium and the ORS of more organized follicles at day 16 (Figure 5d). Total skin from control and knockout littermates were examined for selected selenoproteins and genes involved in inflammatory response by q-PCR. A significant increase in transcripts for inflammatory response related genes (Cxcl1, Il1b, S100a8, Tnfa, Ptgs2) along with an increase in TR1 and to a lesser extent GPx1 transcripts, similar to cultured keratinocytes, were detected (Figure 5e). The connection of selenoprotein deficiency and COX-2 upregulation is seen most prominently in mice lacking all selenoproteins in the skin epithelium (Supplementary Figure S7 online). Under these conditions COX-2 is highly upregulated in both epidermis and abnormal hair follicles. To determine the contribution of COX-2 to the hair and skin phenotype, we added a COX-2 inhibitor, celecoxib, to the diet of nursing mothers giving rise to control and knockout pups and examined the phenotype of the offspring (Figure 5f). Control pups were not affected by exposure to celecoxib in milk, but the skin phenotype of knockout littermates was substantially improved by 17 days (Figure 5f). Histological examination revealed a significant decrease in aberrant follicles, with skin histology of knockouts resembling those of control pups (Figure 5g). When we examined the skin of control and knockout pups from litters where the nursing mother was treated with a celecoxib diet (Figure 5h), there was a reduction in the elevated transcripts for Cxcl1 and S100a8, suggesting that at least part of the inflammatory response is mediated by the increased activity of COX2. These results support an active contribution of COX-2 and prostaglandins to the GPx4 depleted skin phenotype.
Figure 5. COX-2 and other inflammatory mediators are elevated in keratinocytes and skin of GPx4 knockout pups and mediate the altered skin phenotype.
(a) Left panel, q-PCR analysis of COX-2 expression in cultured keratinocytes from control and knockout littermates. (n=3). Right panel, immunodetection of COX-2 in lysates of cultured keratinocytes, with tubulin as loading control. (b) PGE2 levels in skin from knockout pups relative to control littermates (n=4). (c) ELISA assay for 4-hydroxynonenal (4-HNE) in protein lysates from cultured keratinocytes to assess lipid peroxidation (n=3). (d) Immunodetection of COX-2 in back skin sections of age matched control and knockout mice. Faint staining was noted in hair bulbs of 12 d old control mice (arrowhead), while scattered staining was observed in ORS of misshapen hair bulbs (arrows) of various ages for knockout mice. Scale bar: 100 μm. (e) q-PCR analysis from skin biopsies of 15 day old control and knockout mice for selected selenoproteins and genes involved in inflammatory response (n=4). (f) Representative phenotype of 17d old control and knockout pups nursed by mothers fed either control or celecoxib (COX-2 inhibitor) containing diets beginning 24h after birth (n=5, for each phenotype per diet). (g) Histology of back skin of 17d old control and knockout littermates, nursed by mothers fed either control or celecoxib diets. Scale bar: 100 μm. (h) q-PCR analysis from skin biopsies of 15 day old control and knockout mice from litters where nursing mothers were fed control diet or diet containing celecoxib. CT and KO designate control and knockout mice respectively. Bars represent mean values ± S.D. **, p ≤ 0.01; *, p ≤ 0.05; NS, not significant.
Discussion
GPx4 and TR1 are important regulators of cellular ROS (Nordberg and Arner, 2001; Hatfield et al., 2006), with their ablation being embryonic lethal in mice (Imai et al., 2003; Yant et al., 2003; Jakupoglu et al., 2005). Hence, conditional knockout mouse models were generated to elucidate their functions in various organs and tissues (Conrad, 2009). However, skin was not examined in previous targeted deletion studies and in light of their critical roles as antioxidants and the severe developmental outcome due to lack of all selenoproteins in skin epithelia (Sengupta et al., 2010), this study was undertaken to evaluate the requirement for either GPx4 or TR1 as individual selenoproteins known to be expressed prominently in skin.
Targeted removal of TR1 in keratinocytes did not cause apparent changes in skin phenotype or host growth, suggesting that TR1 may not be critical for skin function in the resting state or its absence was compensated by other proteins. However, additional studies on these mice will be required to determine whether keratinocyte TR1 function contributes to cutaneous homeostasis under conditions of stress. In contrast, lack of GPx4 in keratinocytes caused a major alteration in hair follicle morphogenesis, focal alopecia, epidermal hyperplasia and a marked dermal inflammatory infiltrate of neutrophils and macrophages. This phenotype is virtually identical to that described for complete knockout of selenoproteins reported previously with the same K14 targeting vector (Sengupta et al., 2010). This suggests that GPx4 is a major regulator of the oxidant environment required for the normal development and maturation of hair follicles in the first hair cycle. In contrast to the fatal outcome from loss of all cutaneous epithelial selenoproteins, individual loss of GPx4 can be compensated by elevation of GPx1 and TR1 and possibly others that were not examined. These compensations were not observed in mouse fibroblasts lacking GPx4 (Yoo et al., 2010), suggesting overlapping functional similarity of GPx1 and TR1 with GPx4 in keratinocytes. However, GPx1/GPx2 double knockout mice do not have a similar skin phenotype (Esworthy et al., 2001) further suggesting that GPx4 has a major anti-oxidant function in the skin. Of interest is the striking development of colitis in the GPx1/GPx2 double knockout mice indicating organ specific function for the GPx family of selenoproteins. GPx4 is important during early stages of development, as germ layers in embryos from GPx4 knockout mice lack differentiation into structures (Imai et al., 2003; Yant et al., 2003). Reduction in cell attachment to non-physiological substrates and elevated membrane lipid peroxides could contribute to this.
While reversal of extensive alopecia occurs after several weeks of life, a sparse hair coat persists even though hair follicle density is restored. This could be the result of telogen follicles failing to reenter the hair cycle. The physiological basis for the failure is unclear but could be related to the extensive inflammatory infiltrate that occurs around damaged hair follicles and the follicular hyperkeratosis in the first hair cycle. Post inflammatory alopecia is a well known skin disorder in humans and the consequence of many underlying inflammatory diatheses (Harries and Paus, 2010), with the loss of follicle stem cells as a result of inflammation being a contributing factor (Zhou et al., 2012).
We employed a multi-technique approach to understand the influence of GPx4 ablation on other genes in keratinocytes and identified COX-2 as a potential candidate, which was elevated in keratinocyte cultures and skin epithelium from knockout mice. Previous studies have linked downregulation of selenoproteins (GPx2) in intestinal cells or (DIO2 in chondrocytes with upregulation of COX-2 (Brigelius-Flohe and Kipp, 2012; Cheng et al., 2012). An important enzyme in the prostaglandin synthesis pathway, COX-2, is almost undetectable in skin under physiological conditions, but is induced in response to stimuli (Müller-Decker and Fürstenberger, 2007). It is noteworthy that transgenic mice over-expressing COX-2 in keratinocytes show delayed morphogenesis of pelage hair follicles, aberrant hair follicles, hyperplasia of tail epidermis and lifelong alopecia (Neufang et al., 2001; Bol et al., 2002), features also observed in the current study. However, mice over-expressing COX-2 did not exhibit inflammation. GPx4 protects cells by reducing hydroperoxides, which activate lipoxygenase and cyclooxygenase, causing inflammation, apoptosis and altered cellular signaling (Brigelius-Flohé and Kipp, 2009; Imai and Nakagawa 2003; Conrad et al., 2010). Membrane-bound hydroperoxides can only be reduced by GPx4 and its absence leads to accumulation of hydroperoxides, noted through increased 4-HNE in GPx4 depleted keratinocytes, highlighting the role of GPx4 in repairing membrane lipid hydroperoxides in skin (Thomas et al., 1990b; Sattler et al., 1994). Elevated 4-HNE levels can induce COX-2 expression in mammalian cells by stabilizing its mRNA (Kumagai et al., 2000; Kumagai et al., 2004). In light of current observations, we presume that loss of GPx4 in keratinocytes leads to increased lipid peroxidation, accumulation of hydroperoxides and its by-products like 4-HNE, which in turn could elevate COX-2 and prostaglandins in the skin (Supplementary Figure S8 online). It has been reported previously that topical application of lipid peroxides causes apoptosis of the hair follicle matrix cells (Naito et al., 2008). The mixed phenotype of inflammation and alopecia we observed is likely to involve apoptosis and direct necrotic damage to hair follicles as a result of lipid peroxides and pro-inflammatory cytokines and chemokines, both elevated in GPx4 depleted epidermis and keratinocytes. Undoubtedly, elevated COX2 and prostaglandins contribute to the hair follicle phenotype as demonstrated by the partial resolution of hair follicle abnormalities, resumption of hair growth and reduction of some inflammatory cytokines in knockout pups exposed to celecoxib through the nursing mother (Figure 5g, h). It should be noted that COX-2 may not be the only molecule contributing to the phenotype, but seems to be a key player in the process. Interestingly, even more intense COX-2 expression was detected in skin sections from mice with complete lack of selenoproteins in keratinocytes, suggesting that the presence of some selenoproteins in skin epithelium can moderate COX-2 levels. Since dietary selenium can modulate the levels of selenoproteins in vivo and selenium is known to protect keratinocytes from oxidizing events such as UVB radiation (Rafferty et al., 1998), illuminating the functions of individual selenoproteins in skin keratinocytes may reveal fundamental principles of environmental protection provided by the most important organ that interfaces with the outside world.
Materials and Methods
Generation of knockout mice
Mice floxed for GPx4 (GPx4fl/fl; Seiler et al., 2008) or TR1 (TR1fl/fl; Jakupoglu et al., 2005) were crossed with K14-cre transgenic mice to generate fertile heterozygous offspring bearing K14-cre. The resultant heterozygous male offspring bearing K14-cre (K14-cre; GPx4fl/+ or K14-cre; TR1fl/+) were crossed with floxed females to obtain knockout pups.
Histological and immunochemical analysis
Littermates were sacrificed at various ages by CO2 inhalation and samples from identical regions of back skin were processed for molecular, histological and histopathological examinations as described (Sengupta, et al., 2010). Paraffin-embedded tissue sections were used for immunohistochemistry by deparaffinizing in xylene, followed by alcohol rehydration. After quenching endogenous peroxidases, slides were rinsed in PBS, and when required, an antigen retrieval step was carried out for 10 min in preheated citrate buffer (pH 6.0). Slides were subsequently incubated overnight with the required primary antibody at 4°C (with the exception of COX-2 antibodies, which was done at room temperature), followed by incubation with biotin-conjugated secondary antibody. Vectastain Elite ABC kit and DAB were used for detection, following manufacturer’s instructions and slides were counterstained with hematoxylin. Appropriate pre-immune sera controls were used to rule out nonspecific immunostaining of tissue sections; however, some nonspecific staining was seen in sebaceous glands.
Primary keratinocyte culture, adhesion assay and molecular analysis
Primary keratinocytes were isolated and cultured from newborn mice as described, with all media; both for preparation and culture of primary keratinocytes, being supplemented with 100 nM sodium selenite (Sengupta et al., 2010). In some experiments, culture plates pre-coated with attachment factors collagen IV, Matrigel, or a mixture of collagen I and fibronectin (ColI/FN) were used for analysis (Sengupta et al., 2010; Lichti et al., 2008). Equal number of Mouse Equivalent (ME) cells from control and knockout mice was plated for experimental purposes and cultures were generally analyzed 2–3 days after plating. To determine the relative confluency following growth, images of cells from 4–5 fields of respective plates were captured, cells in each field counted using the ImageJ software and average count per field (under identical magnification) for each genotype was plotted as a bar-graph. Grafting of keratinocytes onto nude mice and cell adhesion studies in 12-well tissue culture plates were performed as described (Lichti et al., 2008; Sengupta et al., 2010).
Labeling of selenoproteins in cultured keratinocytes
Primary keratinocytes (5x105 cells/well) from control and knockout mice were seeded onto a 6-well plate and cultured for 3 days. Cells were washed once with PBS and labeled for 12 h with 50 μCi/ml of [75Se] in LoCa medium at pH 7.2. Following incubation, labeled cells were harvested and processed as described (Sengupta et al., 2010).
Microarray and quantitative real-time PCR (q-PCR)
Detailed protocol for microarray analysis and q-PCR are described in Supplementary Materials and Methods online.
Diet study
To study the effect of COX-2 selective inhibitor celecoxib on skin phenotype induced by knocking down GPx4 in keratinocytes, standard purified AIN-93G rodent diet without or with 500 ppm Celecoxib were made to order by Dyets, Inc. (Bethlehem, PA, USA). Inhibitor and control diet pellets were fed to separate nursing females starting on day 1 after giving birth. The pups were sacrificed between 17–19 days after birth for histological examination. In cases of large litters, littermates were genotyped on day 1 after birth in order to allow identification of control pups with the aim of reducing their numbers, and thus reducing the competition during feeding.
Prostaglandin assay
The entire skin from control and knockout mice was quickly removed following sacrifice by CO2 asphyxiation, and rapidly frozen in liquid nitrogen. Comparable regions of skin from control and knockout pups were analyzed for PGE2 content as described earlier (Ansari et al., 2007).
Supplementary Material
Acknowledgments
We would like to thank Histoserv Inc., Germantown, MD, USA for preparation of tissue sections and members of the Pathology/Histotechnology Laboratory, Center for Cancer Research, NCI, Frederick, MD, USA for their help with immunohistochemical analysis. The help from Raghunath Chatterjee and Petra Tsuji, NCI, NIH, with the Ingenuity Pathway Analysis is also acknowledged.
Funding
This research was supported by the Intramural Research Program at the Center for Cancer Research, National Cancer Institute, National Institutes of Health (to DLH and SHY) and by the Deutsche Forschungsgemeinschaft (DFG) CO 291/2-3 and the DFG-Priority Programme SPP1087 (MC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- Ansari KM, Sung YM, He G, et al. Prostaglandin receptor EP2 is responsible for cyclooxygenase-2 induction by prostaglandin E2 in mouse skin. Carcinogenesis. 2007;28:2063–2068. doi: 10.1093/carcin/bgm011. [DOI] [PubMed] [Google Scholar]
- Arai M, Imai H, Koumura T, et al. Mitochondrial phospholipid hydroperoxide glutathione peroxidase plays a major role in preventing oxidative injury to cells. J Biol Chem. 1999;274:4924–4933. doi: 10.1074/jbc.274.8.4924. [DOI] [PubMed] [Google Scholar]
- Bol DK, Rowley RB, Ho CP, et al. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 2002;62:2516–2521. [PubMed] [Google Scholar]
- Bondareva AA, Capecchi MR, Iverson SV, et al. Effects of thioredoxin reductase-1 deletion on embryogenesis and transcriptome. Free Radic Biol Med. 2007;43:911–923. doi: 10.1016/j.freeradbiomed.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohé R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790:1555–1568. doi: 10.1016/j.bbagen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohé R, Kipp AP. Physiological functions of GPx2 and its role in inflammation-triggered carcinogenesis. Ann N Y Acad Sci. 2012;1259:19–25. doi: 10.1111/j.1749-6632.2012.06574.x. [DOI] [PubMed] [Google Scholar]
- Cejas P, García-Cabezas MA, Casado E, et al. Phospholipid hydroperoxide glutathione peroxidase (PHGPx) expression is downregulated in poorly differentiated breast invasive ductal carcinoma. Free Radic Res. 2007;41:681–687. doi: 10.1080/10715760701286167. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Huang HS, Chang WC. Depletion of phospholipid hydroperoxide glutathione peroxidase up-regulates arachidonate metabolism by 12S-lipoxygenase and cyclooxygenase 1 in human epidermoid carcinoma A431 cells. FASEB J. 2003;17:1694–1696. doi: 10.1096/fj.02-0847fje. [DOI] [PubMed] [Google Scholar]
- Cheng AW, Bolognesi M, Kraus VB. DIO2 modifies inflammatory responses in chondrocytes. Osteoarthritis Cartilage. 2012;20:440–445. doi: 10.1016/j.joca.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M. Transgenic mouse models for the vital selenoenzymes cytosolic thioredoxin reductase, mitochondrial thioredoxin reductase and glutathione peroxidase 4. Biochim Biophys Acta. 2009;1790:1575–1585. doi: 10.1016/j.bbagen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Conrad M, Jakupoglu C, Moreno SG, et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M, Moreno SG, Sinowatz F, et al. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol Cell Biol. 2005;25:7637–7644. doi: 10.1128/MCB.25.17.7637-7644.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M, Sandin A, Förster H, et al. 12/15-lipoxygenase-derived lipid peroxides control receptor tyrosine kinase signaling through oxidation of protein tyrosine phosphatases. Proc Natl Acad Sci USA. 2010;107:15774–15779. doi: 10.1073/pnas.1007909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esworthy RS, Aranda R, Martín MG, et al. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G848–G855. doi: 10.1152/ajpgi.2001.281.3.G848. [DOI] [PubMed] [Google Scholar]
- Gasdaska PY, Berggren MM, Berry MJ, et al. Cloning, sequencing and functional expression of a novel human thioredoxin reductase. FEBS Lett. 1999;442:105–111. doi: 10.1016/s0014-5793(98)01638-x. [DOI] [PubMed] [Google Scholar]
- Gladyshev VN, Jeang KT, Stadtman TC. Selenocysteine, identified as the penultimate C-terminal residue in human T-cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc Natl Acad Sci USA. 1996;93:6146–6151. doi: 10.1073/pnas.93.12.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton KZ, Kensler TW. Oxidative mechanisms in carcinogenesis. Br Med Bull. 1993;49:523–544. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- Harries MJ, Paus R. The pathogenesis of primary cicatricial alopecias. Am J Pathol. 2010;177:2152–2162. doi: 10.2353/ajpath.2010.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield DL, Berry MJ, Gladyshev VN. Selenium: Its Molecular Biology and Role in Human Health. 2. New York: Springer Science+Business Media; 2006. p. 327. [Google Scholar]
- Hatfield DL, Yoo MH, Carlson BA, et al. Selenoproteins that function in cancer prevention and promotion. Biochim Biophys Acta. 2009;1790:1541–1545. doi: 10.1016/j.bbagen.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Chen CJ, Suzuki H, et al. Inhibitory effect of phospholipid hydroperoxide glutathione peroxidase on the activity of lipoxygenases and cyclooxygenases. Prostaglandins Other Lipid Mediat. 1999;58:65–75. doi: 10.1016/s0090-6980(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Imai H, Hirao F, Sakamoto T, et al. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem Biophys Res Commun. 2003;305:278–286. doi: 10.1016/s0006-291x(03)00734-4. [DOI] [PubMed] [Google Scholar]
- Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- Jakupoglu C, Przemeck GK, Schneider M, et al. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryukov GV, Castellano S, Novoselov SV, et al. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Kawamoto Y, Nakamura Y, et al. 4-hydroxy-2-nonenal, the end product of lipid peroxidation, is a specific inducer of cyclooxygenase-2 gene expression. Biochem Biophys Res Commun. 2000;273:437–441. doi: 10.1006/bbrc.2000.2967. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Matsukawa N, Kaneko Y, et al. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- Kumaraswamy E, Carlson BA, Morgan F, et al. Selective removal of the selenocysteine tRNA [Ser]Sec gene (Trsp) in mouse mammary epithelium. Mol Cell Biol. 2003;23:1477–1488. doi: 10.1128/MCB.23.5.1477-1488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Du J, Zhang Y, et al. Suppression of the malignant phenotype in pancreatic cancer by overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum Gene Ther. 2006;17:105–116. doi: 10.1089/hum.2006.17.105. [DOI] [PubMed] [Google Scholar]
- Mauri P, Benazzi L, Flohe L, et al. Versatility of selenium catalysis in PHGPx unraveled by LC/ESI-MS/MS. Biol Chem. 2003;384:575–588. doi: 10.1515/BC.2003.065. [DOI] [PubMed] [Google Scholar]
- Müller-Decker K, Fürstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog. 2007;46:705–710. doi: 10.1002/mc.20326. [DOI] [PubMed] [Google Scholar]
- Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346:1–8. [PMC free article] [PubMed] [Google Scholar]
- Naito A, Midorikawa T, Yoshino T, et al. Lipid peroxides induce early onset of catagen phase in murine hair cycles. Int J Mol Med. 2008;22:725–729. [PubMed] [Google Scholar]
- Neufang G, Furstenberger G, Heidt M, et al. Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proc Natl Acad Sci USA. 2001;98:7629–7634. doi: 10.1073/pnas.121574098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Rafferty TS, McKenzie RC, Hunter JA, et al. Differential expression of selenoproteins by human skin cells and protection by selenium from UVB-radiation-induced cell death. Biochem J. 1998;332:231–236. doi: 10.1042/bj3320231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelle M, Sabatier M, Steiling H, et al. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227–238. doi: 10.1079/bjn20061817. [DOI] [PubMed] [Google Scholar]
- Sattler W, Maiorino M, Stocker R. Reduction of HDL- and LDL-associated cholesterylester and phospholipid hydroperoxides by phospholipid hydroperoxide glutathione peroxidase and Ebselen (PZ 51) Arch Biochem Biophys. 1994;309:214–221. doi: 10.1006/abbi.1994.1105. [DOI] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Förster H, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Lichti UF, Carlson BA, et al. Selenoproteins are essential for proper keratinocyte function and skin development. PLoS One. 2010;5:e12249. doi: 10.1371/journal.pone.0012249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn KC, Jang S, Choi DK, et al. Effect of thioredoxin reductase 1 on glucocorticoid receptor activity in human outer root sheath cells. Biochem Biophys Res Commun. 2007;356:810–815. doi: 10.1016/j.bbrc.2007.03.065. [DOI] [PubMed] [Google Scholar]
- Sun QA, Kirnarsky L, Sherman S, et al. Selenoprotein oxidoreductase with specificity for thioredoxin and glutathione systems. Proc Natl Acad Sci USA. 2001;98:3673–3678. doi: 10.1073/pnas.051454398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JP, Geiger PG, Maiorino M, et al. Enzymatic reduction of phospholipid and cholesterol hydroperoxides in artificial bilayers and lipoproteins. Biochim Biophys Acta. 1990a;1045:252–260. doi: 10.1016/0005-2760(90)90128-k. [DOI] [PubMed] [Google Scholar]
- Thomas JP, Maiorino M, Ursini F, et al. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J Biol Chem. 1990b;265:454–461. [PubMed] [Google Scholar]
- Ursini F, Maiorino M, Valente M, et al. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta. 1982;710:197–211. doi: 10.1016/0005-2760(82)90150-3. [DOI] [PubMed] [Google Scholar]
- Wirth EK, Conrad M, Winterer J, et al. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 2010;24:844–852. doi: 10.1096/fj.09-143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant LJ, Ran Q, Rao L, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- Yoo MH, Gu X, Xu XM, et al. Delineating the role of glutathione peroxidase 4 in protecting cells against lipid hydroperoxide damage and in Alzheimer’s disease. Antioxid Redox Signal. 2010;12:819–827. doi: 10.1089/ars.2009.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Jiang X, Gu P, Chen W, Zeng X, Gao X. Gsdma3 mutation causes bulge stem cell depletion and alopecia mediated by skin inflammation. Am J Pathol. 2012;180:763–774. doi: 10.1016/j.ajpath.2011.10.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.