Abstract
Serotonin (5-hydroxytryptamine; 5-HT) is an ancient signaling molecule that has a conserved role in modulating mood and behavior. Integral to its pleiotropic actions is the existence of multiple receptors, expressed in distinct but often overlapping patterns within the brain and the periphery. The existence of ~14 mammalian receptor subtypes, many of which possess similar pharmacological profiles, has made assigning functional roles for these receptors challenging; this challenge has been further compounded by the revelation that a single receptor can have several different functions depending upon where and when it is expressed and activated–i.e. in brain vs. periphery, or at different developmental time points. This review highlights the contribution of genetic techniques to dissect the specific function of distinct serotonin receptor populations across the life course, with an emphasis on the contribution of different serotonin 1A receptor populations to mood and behavior. Similar approaches hold the promise to elucidate the functional roles of other receptors, as well as the interaction of serotonin with other neuroendocrine modulators of mood and behavior.
Introduction
The complexity of the serotonergic system is both exciting and daunting from a scientific perspective. There are 14 known human 5-HT receptors, more than in any other neuromodulatory system; receptor diversity is further amplified by alternative splicing, RNA editing, and heterodimerization [1,2]. Multiple different receptors can be expressed in the same brain regions, and even within the same cells [2]. Functionally, 5-HT receptors often have similar pharmacological profiles, making it difficult to identify specific antagonists or agonists for each receptor. Likewise, there is a dearth of selective antibodies to effectively label the receptors. Many studies therefore depend upon autoradiographic techniques, which lack cellular resolution and can also, in some cases, lack specificity.
Given this complexity, genetic techniques have proven instrumental in determining the role of different 5-HT receptors in the brain. In particular, refined techniques have moved beyond general questions of receptor function to address how variation in levels of a particular receptor in different brain regions and/or at different developmental time points modulate mood and behavior.
In this review we discuss progress in genetic techniques used to develop mouse models of neuropsychiatric disorders over the last 15 years, beginning with global gene knockout. Using the serotonin 1A receptor (5-HT1A) as an example, we will discuss the benefits and pitfalls of different approaches and provide an overview of how refinement of genetic techniques has contributed to our understanding of the modulatory effects of 5-HT1A on mood and behavior. We also discuss the role of 5-HT1A in modulating the neural circuits involved in anxiety and stress response and briefly highlight future opportunities for use of advanced techniques to investigate complex social behaviors, including mating and aggression.
Serotonin 1A receptors
5-HT1A was the first serotonin receptor to be cloned, and has been intensively studied due to its potential involvement in psychiatric illness – anxiety disorders in particular [3–6]. 5-HT1A is expressed in the brain, spleen, and neonatal kidney [7–9]. Within the brain, it is expressed on neuronal somas and dendrites both as an autoreceptor and as a heteroreceptor. Its activation leads to hyperpolarization and reduced firing rates. As such, autoreceptors gate the firing of the dorsal and median raphe, impacting 5-HT release in forebrain projection areas [10]. Heteroreceptors exist in high density in limbic regions, including the hippocampus, septum, and cortex, as well as the hypothalamus [11,12]. Given the broad distribution of these receptors in behaviorally relevant brain regions, it has been difficult to determine which sub-population(s) of 5-HT1A are responsible for anxiety and other behavioral phenotypes.
Germline gene knockout
The use of homologous recombination to eliminate genes from the mouse genome provided an opportunity to selectively study the role of individual serotonergic receptors. Using this technique (Fig. 1A), three groups independently discovered that deletion of the serotonin 1A receptor gene in mice yielded increased anxiety levels. In addition, an “anti-depressed” phenotype was observed, characterized by increased active coping in the face of a stressor, such as increased struggling in the forced swim and tail suspension tests [13–15]. These studies are possibly consistent with the anxiolytic effects observed after 5-HT1A agonist administration, and led to further investigation of the potential role of 5-HT1A receptors in the pathophysiology and treatment of anxiety and depression [6].
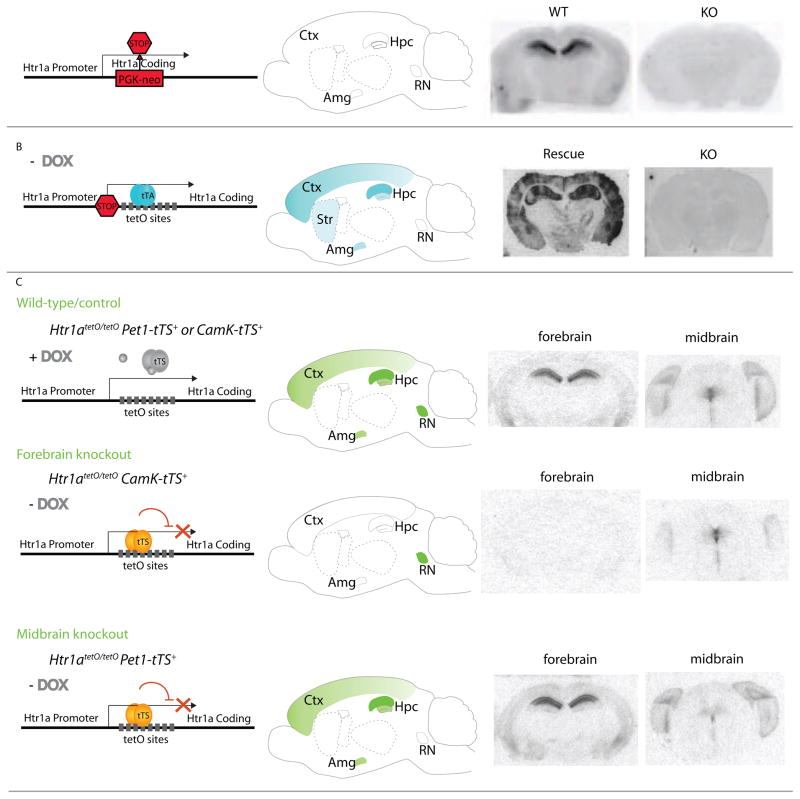
Figure 1. Evolution of genetic strategies for examination of 5-HT1A function.
A) Germline gene knockout. To constitutively eradicate 5-HT1AR gene expression, the PGK-neo gene was inserted into an AscI site located after the third transmembrane domain of the 5-HT1A gene [15]. This eliminated 5-HT1A expression in the whole brain throughout development. Ctx: cortex; Hpc: hippocampus; Amg: amygdala; RN: raphe nuclei; WT: wild-type; KO: knockout.
B) Forebrain-specific 5-HT1A rescue on a knockout background. A cassette containing a stop codon followed by a tetO binding site was inserted upstream of the mouse 5-HT1A gene, halting its transcription globally (KO). Upon addition of a second transgene encoding the tetracycline-transactivator protein (tTA), the stop codon was bypassed and expression of 5-HT1A was restored (Rescue). Because tTA was driven by the forebrain-specific α-calcium-calmodulin-dependent protein kinase II (αCaMKII) promoter, rescue of 5-HT1A was mainly restricted to the cortex and hippocampus, with low levels of expression in the striatum and amygdala (blue shading). The result was a mouse that lacked 5-HT1A everywhere except forebrain regions. Temporal regulation could be achieved through administration of doxycycline (DOX) in the food, which binds to tTA and inhibits its ability to activate transcription [19].
C) Tissue-specific knockout of 5-HT1AR. A dual transgene system was created by initially placing a tetO binding site into the 5′ leader sequence of the 5-HT1A gene [20,21]. This was then bred with different lines expressing a tetracycline-controlled transcriptional suppressor (tTS) protein under the control of various promoters. Like tTA, temporal control of this system occurs through administration of doxycycline. In the presence of DOX, tTS fails to bind the tetO site, and normal transcription occurs. As a result, 5-HT1A transcription is suppressed only where the tTS is present, while WT patterns exist either if tTS is absent or if tTS is prevented from binding tetO with DOX. Wild-type: tetO insertion between the 5-HT1AR promoter and coding regions did not affect protein expression. 5-HT1AR levels in forebrain and midbrain are thus comparable to wild-type levels. Forebrain knockout: The αCaMKII promoter was used to drive expression of tTS. In the absence of DOX, tTS bound to the tetO sites inserted between the 5-HT1A promoter and coding region, leading to specific knockout of forebrain 5-HT1A receptors. Midbrain knockout: The Pet1 promoter was used to drive expression of the tTS, leading to raphe-specific knockout of 5-HT1A receptors in the absence of DOX.
While induction of null mutations through the use of knock out technology has been a pivotal development, it also has significant limitations. Specifically, knock out of a gene from the germline results in its complete deletion. Thus, the technique cannot be used to assess the role of different regional populations of a given receptor, or whether its phenotypic impact differs across the life course. Additionally, instances of complete gene deletion in humans are usually rare, limiting the translational impact of findings from mice to humans. Many of these limitations have been subsequently addressed with newer genetic technologies.
Genetic rescue
Perturbations that affect the serotonin system have different effects during development versus adulthood, indicating that sensitive windows may exist for the effects of serotonin on various behavioral phenotypes. Transient pharmacological blockade of the serotonin transporter or of 5-HT1A during post-natal development has long lasting behavioral effects, demonstrating a critical role for serotonin in the maturation of emotional function [16–18]. As such, genetic techniques that enable not only spatial, but also temporal control of gene expression, were essential for identifying the necessity and sufficiency of different receptor populations at different time points in the modulation of serotonin-mediated behaviors.
Among the first approaches to parse the role of specific 5-HT1A subpopulations across development were selective forebrain rescue techniques. Specifically, Gross and colleagues generated a tetracycline operator (tetO) mouse that enabled both spatial and temporal control of expression of 5-HT1A on a knockout background (Fig. 1B) [19]. Studies of these mice demonstrated that selective expression of 5-HT1A in the forebrain, with the tetracycline-controlled transactivator (tTA) under the control of the αCaMKII promoter, was sufficient to reverse the anxiety phenotype observed in 5-HT1A knock out mice. In addition, they found that rescue of the anxiety phenotype required forebrain 5-HT1A expression during development but not during adulthood. Specifically, blocking 5-HT1A transcription until post-natal day (PND) 21 produced increased anxiety levels despite fully rescued forebrain receptors in adulthood. In contrast, inhibiting 5-HT1A production in adulthood beginning on PND80 did not influence anxiety, further indicating that the effects of 5-HT1A on anxiety represented a developmental phenotype [19].
Experiments in which receptor levels are rescued in a subset of cells or brain regions are an ideal way to determine the behavioral sufficiency of different receptor sub-populations. However, there are a number of limitations to the specific approach employed here. Specifically, αCaMKII-tTA-driven 5-HT1A expression resulted in forebrain patterns that were different from endogenous forebrain 5-HT1A receptor expression. This raises two complementary concerns 1) ectopic expression resulting in aberrant 5-HT1A mediated inhibition of cells that would not ordinarily express the receptor; and 2) lack of 5-HT-mediated inhibition in cells that would normally express 5-HT1A. Therefore, another interpretation of these studies is that developmental inhibition of forebrain structures has an important impact on anxiety-related phenotypes, but the observed 5-HT1A mediated effects may not be prominent under natural conditions.
Determining the necessity of receptor sub-populations
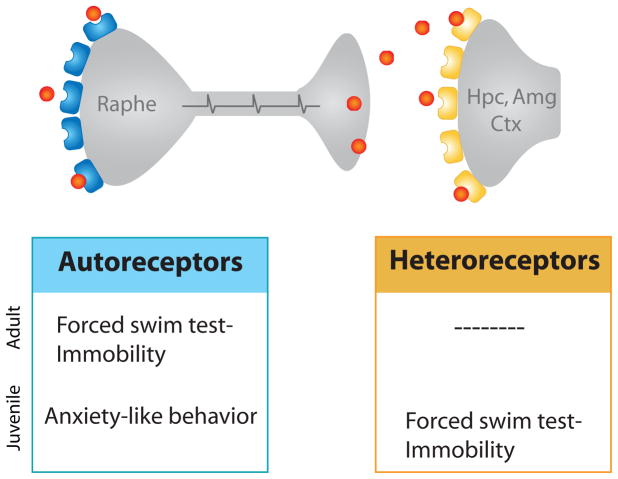
Newer strategies are currently being used to determine the necessity of different receptor sub-populations in the development and expression of anxiety behavior. Similar to the strategy outlined above, Richardson-Jones et al. [20,21]developed a tetracycline sensitive transgenic system in which selected receptor populations could be suppressed in a temporally-selective manner (Figure 1C). This technique was used to assess the role that both 5-HT1A auto- and heteroreceptors in anxiety and depression-related behaviors. These experiments revealed that whole life suppression of autoreceptors increased anxiety but did not affect helplessness behavior in the forced swim test, which is used as a measure of behavioral despair. On the other hand, whole life suppression of heteroreceptors increased learned helplessness behavior in the forced swim test, implicating heteroreceptors in depressive-like behaviors. Further, the increased behavioral despair or anxiety exhibited by mice with knockdown of the heteroreceptor or autoreceptor populations, respectively, was not evident when the receptors were reduced only in adulthood, indicating that these are developmentally-mediated phenotypes (Fig 2) [21].
Figure 2. Schematic diagram depicting developmental and adult impact of 5-HT1A receptors on anxiety and depression-related behavior.
Autoreceptors (blue) are located in raphe nucleus, and heteroreceptors (yellow) are localized to forebrain regions including hippocampus (Hpc), amygdala (Amg), and cortex (Ctx). Endogenous autoreceptors, which limit levels of serotonin released by raphe neurons, affect anxiety-related circuitry in development, and circuitry mediating forced swim test behavior in the adult. Heteroreceptors affect forced swim test in development, but not anxiety-related behavior. Adapted from [21]
The complementarity of the tTA and tTS systems provide valuable insights via their ability to determine sufficiency and necessity, respectively. Synthesis of the above findings indicates that developmental 5-HT1A autoreceptors are necessary for the establishment of normal anxiety behaviors. Likewise, endogenous hippocampal/cortical 5-HT1A heteroreceptors are necessary for normal stress coping. While “rescuing” forebrain 5-HT1A appears to be sufficient to rescue the heightened anxiety levels in the 5-HT1A KO, these populations are not necessary for the establishment of normal anxiety patterns when other 5-HT1A populations are intact. This may suggest that there is an important balance of auto- and heteroreceptors in the development of normal anxiety behaviors. Alternatively, as discussed above, forebrain rescue may be mediated by ectopic expression of 5-HT1A receptors and may be unrelated to the normal function of these receptors.
Genetic strategies to model natural variation
Null mutations in serotonergic genes in the human population are rare. Manipulations of gene levels within physiologically relevant limits may therefore yield more insight into individual differences and disease risk, and thus provide greater translational impact. Since some natural human genetic variants lead to lower expression levels of genes of interest, the effects of these variants have been indirectly modeled by decreasing, but not eliminating, expression of target genes. In early studies, this involved use of mice that were heterozygous for the serotonin transporter as a model for the low-expressing human allelic variant [22]. More recently, partial 5-HT1A autoreceptor suppression has been used as a model for natural variation in 5-HT1A receptor levels. To achieve partial suppression, Richardson-Jones et al. [20] used the relatively weak Pet-1-driven tTS to decrease adult autoreceptor levels by ~30% beginning at PND50. This attenuated suppression results in 5-HT1A autoreceptor knock-down mice that mimic the natural variation observed in human 5-HT1A levels [23]. The authors found that animals with lower expression of autoreceptors were less vulnerable to stress and more responsive to antidepressant treatment. This would suggest that inherent differences in human autoreceptor levels may be an important predictor of both antidepressant efficacy and resilience to depression. Additionally, their results may account for the “anti-depressed” phenotype observed in the 5-HT1A constitutive knockout mice. Their findings further suggest that augmentation of classical antidepressant therapies with drugs that antagonize 5-HT1A autoreceptor function may be a promising treatment option [24,25].
New work is also beginning to directly model human genetic variation in mouse models to determine the complex effects of specific polymorphisms. To date, such work has focused primarily on variants that alter protein structure. For example, introduction of the human SERT Gly56Ala mutation into mice impacts homeostatic regulation of 5-HT [26]. Mice carrying this variant exhibit elevated p38 Map Kinase-dependent transporter phosphorylation, enhanced 5-HT clearance rates, and peripheral hyperserotonemia. These mice thus represent a model of hyperserotonemia observed in ~30% of autism spectrum disorder cases.
With advances in various genetic technologies, we are now moving beyond variants that affect protein structure and function to investigate human regulatory variants that have been implicated in mood and behavior. This includes the well-known serotonin transporter polymorphism (5-HTTLPR) [27], and the 5-HT1A G(-1019)C regulatory polymorphism (rs6295), which has been associated with differences in 5-HT1A levels in the raphe, depression risk, and antidepressant responsiveness [23,28]. Mouse models of such polymorphisms will elucidate their specific temporal and regional effects on gene expression, revealing the impact of common genetic variation in shaping individual differences in behavior and disease susceptibility.
Circuitry underlying effects of 5-HT1A on mood and anxiety
Genetic techniques have also begun to shed light on how 5-HT1A affects the circuits underlying mood and anxiety. A more in depth description of these circuits has been reviewed in detail elsewhere [29]. Substantial work has focused on structural and functional connections between the dorsal raphe nucleus (DRN), medial prefrontal cortex (mPFC) including the anterior cingulate cortex, and the amygdala, although the mesencephalic tectum system and the hippocampus have also been implicated in the modulation of stress responses and anxiety [29–31]. Variation in serotonergic modulation of thiscorticolimbic circuitry is associated with individual differences in personality measures and sensitivity to environmental threat, reflecting a related risk for psychopathology [32].
Serotonergic neurons within the raphe, especially the dorsal raphe, project to diverse forebrain regions, including the key corticolimbic structures involved in the regulation of stress, such as the mPFC, septum, extended amygdala, and hippocampus. Within the DRN, further topological organization suggests that specific sub-regions are preferentially involved in stress and anxiety [33]. Serotonergic tone, established through firing of serotonergic raphe neurons, is under tonic inhibitory control via somatodendritic 5-HT1A autoreceptors [34], and constitutive deletion of all 5-HT1A receptors increases basal extracellular 5-HT levels in some brain structures [35–37]. Specific reduction or removal of 5-HT1A from raphe neurons leads to heterogeneous, region-specific effects on both basal 5-HT levels, and fluoxetine-induced 5-HT release [20,21]. For example, whole life knock-out of the 5-HT1A autoreceptors increases basal 5-HT in the frontal cortex but not the ventral hippocampus [21]. More subtle reductions in adult autoreceptor levels, which may more accurately model naturally-occurring variation, increase tonic firing rates of DRN neurons but do not affect basal levels of 5-HT in either the mPFC or ventral hippocampus [20]. In addition, activation of 5-HT1A heteroreceptors in the frontal cortex results in inhibition of serotonergic neurons in the raphe while activation of 5-HT1A in the hippocampus does not [34,38,39]. In 5-HT1A autoreceptor knockout or knock down, fluoxetine challenge results in much greater elevation of 5-HT in the vHPC than mPFC [20,21]. This suggests that heteroreceptor feedback contributes substantially to buffering local 5-HT homeostasis in the mPFC, but autoreceptor feedback plays a greater role in the hippocampus. These findings exemplify the importance of genetic techniques in elucidating complex circuit level effects of specific receptor populations.
The role of forebrain 5-HT1A heteroreceptors is less well understood, partially due to their complexity of action and a lack of region-specific knockouts. As previously mentioned, mPFC heteroreceptors serve an important role in negative feedback on the DRN [40], but heteroreceptor activation also affects glutamate-receptor mediated firing of other major mPFC neuron projections, including those to the amygdala [41–45]. Within the basolateral amygdala (BLA), an area critical for anxiety and fear processing, 5-HT1A activation inhibits both excitatory neurons and GABA interneurons [46]. As a result, the net effect of 5-HT release in this area is thought to be determined by the relative balance of 5-HT1A actions on excitatory and inhibitory neurons. Human studies have also suggested 5-HT1A levels can modulate amygdala reactivity, although it remains unclear which population(s) of receptors mediate this effect [47,48]. Finally, 5-HT1A activation in the hippocampus is hypothesized to gate environmental information regarding the precise stimulus features of threatening stimuli [49–51], and many parts of this circuit can also affect stress-related phenotypes via indirect actions on the hypothalamic-pituitary-adrenal axis [52]. We have also shown that 5-HT1A is required for the effects of SSRIs on behavior and hippocampal neurogenesis [53], and our preliminary results indicate that 5-HT1A receptors located in the dentate gyrus are critical for these effects.
It is important to keep in mind that the effects of 5-HT1A activation on DRN-mPFC-amygdala circuitry are further influenced by both developmental plasticity and environmental sensitivity. In particular, neonatal blockade leads to increased anxiety during adulthood [17,18]. This suggests that activation of 5-HT1A during a developmental sensitive period plays a long-term role in establishment of anxiety circuitry. In addition, 5-HT1A receptor levels and sensitivity are influenced by exposure to stressful environments. For instance, social stressors, such as isolation, maternal separation, or peer rearing, have been associated with alteration of 5-HT1A levels in both rodents and primates [54–56]. In addition, chronic mild stress desensitizes 5-HT1A autoreceptor control of DRN firing via a glucocorticoid receptor-dependent mechanism [57–60]. Chronic antidepressants such as SSRIs have also been shown to desensitize 5-HT1A autoreceptors, and this desensitization has been proposed to contribute to the delayed onset of therapeutic efficacy of these compounds[24,25,61]. An active area of investigation is determining how these modifications to 5-HT1A contribute to complex, circuit level effects on behavior.
Expanding the scope of genetic studies
Although advanced genetic examination of the serotonin system has focused primarily on its role in mood and anxiety, serotonin is implicated in a wide range of behaviors [62]. Refinement of these techniques [63] will be instrumental for identifying the receptor populations and temporal dynamics of serotonin’s effects on complex behaviors, such as aggression and sexual behaviors. For instance, substantial pharmacological evidence suggests that activation of 5-HT1A receptors facilitates male sexual behavior, and genetic techniques will elucidate the role of different 5-HT1A populations within the neural circuits underlying sexual behavior [64,65]. Conversely, germ-line knock-out of the serotonin 1B receptor (5-HT1B) results in a highly aggressive phenotype [66]; the genetic techniques discussed above can now be applied to dissect the spatial and temporal role of 5-HT1B receptors in the expression of aggressive behavior.
Conclusions
To date, we have made key advances towards understanding the necessity and sufficiency of various serotonin receptor populations that impact mood and anxiety, and have gained insight into the importance of investigating sensitive periods of gene action. However, these technological and scientific advances remain only a subset of those required to decode the complex evolutionary history underlying 5-HT’s myriad roles. We still know little about the role of different receptor populations in modulating complex behaviors, or how 5-HT interacts with other neuromodulatory systems, including the endocrine and immune systems. Such work has implications not only for targeted therapies and our understanding of complex diseases, but also for providing policy guidelines for the use of serotonin-related drugs in pregnant mothers and young children.
Highlights.
Genetic approaches elucidate functional roles for specific serotonin receptors
Advances enable both spatially and temporally-specific receptor manipulation
Serotonin 1A receptors have distinct developmental and adult roles
Acknowledgments
The authors would like to acknowledge funding from National Institutes of Health T32 MH015144 (ZRD), R37 MH068542 (RH), K08 MH087718 (SEA), Louis V. Gerstner, Jr Scholars Program (SEA), HDRF (RH), and NYSTEM (C026430). R. H. is a consultant to Lundbeck and Roche.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 2.Nichols DE, Nichols CD. Serotonin receptors. Chemical reviews. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 3.Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Annals of the New York Academy of Sciences. 1998;861:204–216. doi: 10.1111/j.1749-6632.1998.tb10192.x. [DOI] [PubMed] [Google Scholar]
- 4.Gardier AM, Malagie I, Trillat AC, Jacquot C, Artigas F. Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundamental & clinical pharmacology. 1996;10:16–27. doi: 10.1111/j.1472-8206.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 5.Kobilka BK, Frielle T, Collins S, Yang-Feng T, Kobilka TS, Francke U, Lefkowitz RJ, Caron MG. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature. 1987;329:75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- 6.De Vry J. 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacology. 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- 7.Raymond JR, Fargin A, Lohse MJ, Regan JW, Senogles SE, Lefkowitz RJ, Caron MG. Identification of the ligand-binding subunit of the human 5-hydroxytryptamine1A receptor with N-(p-azido-m-[125I] iodophenethyl)spiperone, a high affinity radioiodinated photoaffinity probe. Molecular pharmacology. 1989;36:15–21. [PubMed] [Google Scholar]
- 8.Raymond JR, Kim J, Beach RE, Tisher CC. Immunohistochemical mapping of cellular and subcellular distribution of 5-HT1A receptors in rat and human kidneys. The American journal of physiology. 1993;264:F9–19. doi: 10.1152/ajprenal.1993.264.1.F9. [DOI] [PubMed] [Google Scholar]
- 9.Aghajanian G. Electrophysiology of serotonin receptor subtypes and signal transduction mechanisms. In: Bloom F, Kupfer D, editors. Psychopharmacology (The Fourth Generation of Progress) Raven Press; 1995. pp. 451–460. [Google Scholar]
- 10.Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain research. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- 11.Barone P, Jordan D, Atger F, Kopp N, Fillion G. Quantitative autoradiography of 5-HT1D and 5-HT1E binding sites labelled by [3H]5-HT, in frontal cortex and the hippocampal region of the human brain. Brain research. 1994;638:85–94. doi: 10.1016/0006-8993(94)90636-x. [DOI] [PubMed] [Google Scholar]
- 12.Kung MP, Frederick D, Mu M, Zhuang ZP, Kung HF. 4-(2′-Methoxy-phenyl)-1-[2′-(n-2″-pyridinyl)-p-iodobenzamido]-ethyl- piperazine ([125I]p-MPPI) as a new selective radioligand of serotonin-1A sites in rat brain: in vitro binding and autoradiographic studies. The Journal of pharmacology and experimental therapeutics. 1995;272:429–437. [PubMed] [Google Scholar]
- 13.Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 17.Lo Iacono L, Gross C. Alpha-Ca2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:6250–6257. doi: 10.1523/JNEUROSCI.5219-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinkers CH, Oosting RS, van Bogaert MJ, Olivier B, Groenink L. Early-life blockade of 5-HT(1A) receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biological Psychiatry. 2010;67:309–316. doi: 10.1016/j.biopsych.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 20**.Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. This paper is an excellent use of the tTS/tetO system to model physiologically-relevant variation in autoreceptors and identifies a previously uncharacterized role for 5-HT1A autoreceptors in stress responsiveness and anti-depressant efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, David DJ, Guiard BP, Beck SG, Hen R, et al. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. The Journal of Neuroscience. 2011;31:6008–6018. doi: 10.1523/JNEUROSCI.5836-10.2011. This paper functionally dissects the developmental and adult roles of both the 5-HT1A auto and hetero-receptor populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carola V, Gross C. Mouse Models of the 5-HTTLPR x Stress Risk Factor for Depression. Current topics in behavioral neurosciences. 2012;12:59–72. doi: 10.1007/7854_2011_190. [DOI] [PubMed] [Google Scholar]
- 23.Albert PR, Lemonde S. 5-HT1A Receptors, Gene Repression, and Depression: Guilt by Association. The Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- 24.Bortolozzi A, Castane A, Semakova J, Santana N, Alvarado G, Cortes R, Ferres-Coy A, Fernandez G, Carmona MC, Toth M, et al. New antidepressant strategy based on acute siRNA silencing of 5-HT1A autoreceptors. Molecular Psychiatry. 2012;17:567. doi: 10.1038/mp.2012.52. [DOI] [PubMed] [Google Scholar]
- 25.Blier P, Bergeron R. The use of pindolol to potentiate antidepressant medication. The Journal of clinical psychiatry. 1998;59(Suppl 5):16–23. discussion 24–15. [PubMed] [Google Scholar]
- 26**.Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. This paper is among the first to model human serotonin-related genetic variation directly in a mouse model. Their careful work identifies the functional impact of the Gly56Ala mutation in the serotonin transporter and implicates mutations, such as this one, in the 30% of autism cases that present with hyperserotonemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stober G, Heils A, Lesch KP. Serotonin transporter gene polymorphism and affective disorder. Lancet. 1996;347:1340–1341. [PubMed] [Google Scholar]
- 28.Albert PR. Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2012;367:2402–2415. doi: 10.1098/rstb.2011.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nature neuroscience. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graeff FG. Serotonin, the periaqueductal gray and panic. Neuroscience and biobehavioral reviews. 2004;28:239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 32*.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neuroscience and biobehavioral reviews. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. This paper provides an excellent review of additional factors that influence cortico-amygdala circuits to modulate in serotonin-related disease risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cellular and molecular neurobiology. 2012;32:695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haddjeri N, Lavoie N, Blier P. Electrophysiological evidence for the tonic activation of 5-HT(1A) autoreceptors in the rat dorsal raphe nucleus. Neuropsychopharmacology official publication of the American College of Neuropsychopharmacology. 2004;29:1800–1806. doi: 10.1038/sj.npp.1300489. [DOI] [PubMed] [Google Scholar]
- 35.He M, Sibille E, Benjamin D, Toth M, Shippenberg T. Differential effects of 5-HT1A receptor deletion upon basal and fluoxetine-evoked 5-HT concentrations as revealed by in vivo microdialysis. Brain research. 2001;902:11–17. doi: 10.1016/s0006-8993(01)02271-5. [DOI] [PubMed] [Google Scholar]
- 36.Parsons LH, Kerr TM, Tecott LH. 5-HT(1A) receptor mutant mice exhibit enhanced tonic, stress-induced and fluoxetine-induced serotonergic neurotransmission. Journal of neurochemistry. 2001;77:607–617. doi: 10.1046/j.1471-4159.2001.00254.x. [DOI] [PubMed] [Google Scholar]
- 37.Richer M, Hen R, Blier P. Modification of serotonin neuron properties in mice lacking 5-HT1A receptors. European journal of pharmacology. 2002;435:195–203. doi: 10.1016/s0014-2999(01)01607-7. [DOI] [PubMed] [Google Scholar]
- 38.Ceci A, Baschirotto A, Borsini F. The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotoninergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–713. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 39.Casanovas JM, Hervas I, Artigas F. Postsynaptic 5-HT1A receptors control 5-HT release in the rat medial prefrontal cortex. Neuroreport. 1999;10:1441–1445. doi: 10.1097/00001756-199905140-00010. [DOI] [PubMed] [Google Scholar]
- 40.Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 41.Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine(1A) receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- 42.Ashby CR, Edwards E, Wang RY. Electrophysiological evidence for a functional interaction between 5- HT(1A) and 5-HT(2A) receptors in the rat medial prefrontal cortex: An iontophoretic study. Synapse. 1994;17:173–181. doi: 10.1002/syn.890170306. [DOI] [PubMed] [Google Scholar]
- 43.Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT 1A receptors regulate AMPA receptor channels through inhibiting Ca 2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. Journal of Biological Chemistry. 2002;277:36553–36562. doi: 10.1074/jbc.M203752200. [DOI] [PubMed] [Google Scholar]
- 44.Hamon M, Lanfumey L, el Mestikawy S, Boni C, Miquel MC, Bolanos F, Schechter L, Gozlan H. The main features of central 5-HT1 receptors. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 1990;3:349–360. [PubMed] [Google Scholar]
- 45.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 46.Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. Journal of neurophysiology. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- 47.Fisher PM, Meltzer CC, Ziolko SK, Price JC, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nature neuroscience. 2006;9:1362–1363. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- 48.Fisher PM, Price JC, Meltzer CC, Moses-Kolko EL, Becker C, Berga SL, Hariri AR. Medial prefrontal cortex serotonin 1A and 2A receptor binding interacts to predict threat-related amygdala reactivity. Biology of mood & anxiety disorders. 2011;1:2. doi: 10.1186/2045-5380-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 50.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 51.Gray JA, McNaughton N. The neuropsychology of anxiety: reprise. Nebraska Symposium on Motivation Nebraska Symposium on Motivation. 1996;43:61–134. [PubMed] [Google Scholar]
- 52.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary- adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 54.Wang SH, Zhang ZJ, Guo YJ, Teng GJ, Chen BA. Decreased expression of serotonin 1A receptor in the dentate gyrus in association with chronic mild stress: a rat model of post-stroke depression. Psychiatry Research. 2009;170:245–251. doi: 10.1016/j.psychres.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Spinelli S, Chefer S, Carson RE, Jagoda E, Lang L, Heilig M, Barr CS, Suomi SJ, Higley JD, Stein EA. Effects of Early-Life Stress on Serotonin1A Receptors in Juvenile Rhesus Monkeys Measured by Positron Emission Tomography. Biological Psychiatry. 2010;67:1146–1153. doi: 10.1016/j.biopsych.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iyo AH, Kieran N, Chandran A, Albert PR, Wicks I, Bissette G, Austin MC. Differential regulation of the serotonin 1 A transcriptional modulators five prime repressor element under dual repression-1 and nuclear-deformed epidermal autoregulatory factor by chronic stress. Neuroscience. 2009;163:1119–1127. doi: 10.1016/j.neuroscience.2009.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanfumey L, Pardon MC, Laaris N, Joubert C, Hanoun N, Hamon M, Cohen-Salmon C. 5-HT(1A) autoreceptor desensitization by chronic ultramild stress in mice. Neuroreport. 1999;10:3369–3374. doi: 10.1097/00001756-199911080-00021. [DOI] [PubMed] [Google Scholar]
- 58.Laaris N, Haj-Dahmane S, Hamon M, Lanfumey L. Glucocorticoid receptor-mediated inhibition by corticosterone of 5-HT(1A) autoreceptor functioning in the rat dorsal raphe nucleus. Neuropharmacology. 1995;34:1201–1210. doi: 10.1016/0028-3908(95)00095-n. [DOI] [PubMed] [Google Scholar]
- 59.Laaris N, Le Poul E, Hamon M, Lanfumey L. Stress-induced alterations of somatodendritic 5-HT(1A) autoreceptor sensitivity in the rat dorsal raphe nucleus - In vitro electrophysiological evidence. Fundamental and Clinical Pharmacology. 1997;11:206–214. doi: 10.1111/j.1472-8206.1997.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 60.Gartside SE, Johnson DA, Leitch MM, Troakes C, Ingram CD. Early life adversity programs changes in central 5-HT neuronal function in adulthood. European Journal of Neuroscience. 2003;17:2401–2408. doi: 10.1046/j.1460-9568.2003.02668.x. [DOI] [PubMed] [Google Scholar]
- 61.Blier P. Altered function of the serotonin 1A autoreceptor and the antidepressant response. Neuron. 2010;65:1–2. doi: 10.1016/j.neuron.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 62.Lucki I. The spectrum of behaviors influenced by serotonin. Biological Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 63*.Tanaka KF, Ahmari SE, Leonardo ED, Richardson-Jones JW, Budreck EC, Scheiffele P, Sugio S, Inamura N, Ikenaka K, Hen R. Flexible Accelerated STOP Tetracycline Operator-knockin (FAST): a versatile and efficient new gene modulating system. Biological Psychiatry. 2010;67:770–773. doi: 10.1016/j.biopsych.2009.12.020. This paper provides a state of the art conditional knock-out/knock-in strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahlenius S, Larsson K, Arvidsson LE. Effects of stereoselective 5-HT1A agonists on male rat sexual behavior. Pharmacology, biochemistry, and behavior. 1989;33:691–695. doi: 10.1016/0091-3057(89)90408-5. [DOI] [PubMed] [Google Scholar]
- 65.Guptarak J, Sarkar J, Hiegel C, Uphouse L. Role of 5-HT(1A) receptors in fluoxetine-induced lordosis inhibition. Hormones and behavior. 2010;58:290–296. doi: 10.1016/j.yhbeh.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT(1B) receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]