Abstract
Prostate cancer (PC) patients once Paclitaxel (TAX) treatment responsive later develop hormone refractory PC, thus becoming TAX-insensitive. This underscores the urgent need to develop novel anti-PC therapies. Vernonia amygdalina (VA) could be one such candidate agent. We have shown that androgen-independent PC-3 cells are sensitive to VA treatment in-vitro. VA extract (0.01, 0.1 and 1mg/ml) inhibited DNA synthesis by 12%, 45%, (P<0.05), and 73% (P<0.01) respectively. In contrast, TAX (0.01, 0.1, and 1μM) failed to significantly affect cell growth, suggesting TAX resistance. We tested molecular mechanisms which may lend to the observed PC-3 cell VA sensitivity/TAX resistance. Though both VA and TAX stimulated MAPK activity, VA’s induction was more intense, but transient, compared to TAX’s sustained action. NF-κB activation was inhibited on average by 50% by either 1mg/ml VA or 1 μM TAX. VA extract caused 35% and 45% increases in c-Myc activity at 10 and 60 min intervals respectively, with the highest stimulation attained 1 hr after treatment. In contrast, similar levels were attained by TAX rapidly (within 5 min) and were sustained compared to the slow/multiphasic action of VA. VA extract treatments had no effect on AKT gene expression, while TAX treatments yielded a four-fold (P<0.01) increase; and P-glycoprotein (P-gp) activity was inhibited by VA and stimulated by TAX, compared to control (basal ATPase activity). This study shows that TAX-resistant PC-3 cells are sensitive to VA, perhaps explained by differential regulatory patterns of MAPK, c-Myc, AKT, and Pgp activities/expressions.
Keywords: Vernonia amygdalina, PC-3 cells, Chemotherapy, Signal transduction, Proto-oncogenes, P-glycoprotein activity
1. Introduction
Prostate cancer (PC), the most common type of cancer in American men, accounts for 10% of all cancer-related deaths in men (Jemal et al., 2008). Risk factors associated with PC include age, familial history, ethnicity and hormonal status, with seventy-five percent of all cases of PC found in men 65 years or older (Ramon and Denis, 2007). Treatment options available for PC are surgery, radiation therapy, hormone therapy, and chemotherapy (ACS, 2011). Paclitaxel (Taxol, TAX), a chemotherapeutic agent, is currently considered to be among the most effective anticancer agents used in the treatment of cancer (Miller and Ojima, 2001). Isolated from the needles and bark of the Pacific yew tree (Taxus brevifolia), this member of the taxane class of antitumor compounds (Huizing et al., 1995) inhibits mitosis by promoting both the inhibition and disassembly of stable microtubules. By binding to the beta-subunit of tubulin in the microtubules, TAX exposure leads to mitotic arrest and subsequently cell death (Kamath et al., 2005). Studies show that TAX is effective in the treatment of hormone-refractory prostate carcinoma. However, human cancerous cells acquire spontaneous mutations in the beta1-tubulin gene that cause resistance to TAX, suggesting that patients with some polymorphisms in the beta1-tubulin gene may require higher TAX concentration or alternative therapy (Obasaju and Hudes, 2001; Yin et al., 2010). In addition, multidrug resistance (MDR) and numerous side effects are associated with the treatment of PC with TAX (Miller and Ojima, 2001).
Still, given the significant toxicities associated with current treatments, scientifically validated efficacious natural supplements can serve as novel and effective alternative strategies for PC management (Kumar et al., 2010). In PC cells, aside from TAX, herbal agents including resveratrol, capsaicin, PC-SPES, Phellodendron amurense barks, Piper species, ginger and rosemary extracts have been observed to influence multiple mechanisms associated with PC (Kubota et al., 2000; Bonham et al., 2002; Bigler et al., 2003; Mori et al., 2006; Gill et al., 2007; Yesil-Celiktas 2010; Lopes et al., 2012; Oboh et al., 2012). In addition, the leaf extract of Vernonia amygdalina (VA) plant is gaining global attention as a possible anti-cancer agent. The VA plant shrub or small tree grows well in the tropical areas of Africa and is used for both therapeutic and nutritional purposes (Bonsi et al., 1995). VA leaves contain about 18% proteins and 8.5% fiber based on dry matter (Igile et al., 1994). Wild chimpanzees use VA for treatment of parasitic diseases (Huffman and Seifu, 1989). Other investigators have shown that VA extracts possess antimalarial and antihelmintic properties, and the sesquiterpene and steroidal constituents of VA have been reported as an effective treatment against Plasmodium falciparium in vitro, making VA suitable for use as an antiplasmodial agent (Abosi and Raseroka, 2003). Extracts of VA also exhibited hypoglycaemic and hypolipidaemic properties in experimental animals, and hence, may be used in the management of diabetes mellitus (Akah and Okafor, 1992; Uhegbu and Ogbuehi, 2004); and VA is reported to be hepatoprotective (Leonard et al., 2002; Babalola et al., 2001). Recent studies in our laboratories have shown that aqueous VA extract retards the proliferation of ER+ and ER− breast cancerous cells (Izevbigie, 2003; Gresham et al., 2008) and Opata et al showed that aqueous extract of VA alters cell membrane permeability and efflux in breast cancer cells (Opata and Izevbigie, 2006).
Prostate cancer (PC) is often caused by endogenous processes since there is no definitive exogenous carcinogen (Schultz, 2005). In the healthy prostate, the rate of proliferation and apoptosis are tightly regulated. However, in PC, the balance between proliferation and apoptosis is compromised, resulting in a greater proliferation than death rate, producing continuous net growth (Denmeade et al., 1996). In this study, we hypothesized that aqueous VA extract would inhibit the proliferative activity of TAX-resistant prostate adenocarcinoma cells (PC-3 cells) by mitigation of key regulatory patterns of MAPK and pro-tumor transcription factors/proto-oncogenes. Substantial evidence suggests that MAPK, NF-κB, c-Myc, and AKT are involved in tumor cell proliferation, survival, and metastasis (Spencer and Groudine, 1991; Teramoto and Gutkind, 1996; Dang, 1999; Huang et al., 2001; Chuang et al., 2002; Malik et al., 2002; Bernard et al., 2003; Cassinelli et al., 2004; Izevbigie et al., 2004; Ghosh et al., 2005; Lee et al., 2005; Laidler et al., 2007; Karin, 2009; Paur et al., 2010) and other parameters to broad to detail here. Mitogen activated protein kinases (MAPKs) or extracellular signal regulated kinases (ERKs) are essential components in transduction through their role in modulating gene transcription in the nucleus in response to changes in the cellular environment (Teramoto and Gutkind, 1996). Activation of ERK is instrumental in normal and aberrant cell growth, including malignant transformation (Teramoto and Gutkind, 1996; Izevbigie et al., 2004; Ghosh et al., 2005; Lee et al., 2005). The pro-inflammatory transcription factor, NF-κB provides a critical link between inflammation and cancer based on its ability to up-regulate the expression of tumor promoting cytokines such as IL-6 or TNF-α, and survival genes such as Bcl-XL (Karin, 2009). NF-κB has emerged as a vital player in the development and progression of malignant cancers, and it has been showed that blockade of NF-κB activity in human PC cells is associated with suppression of invasion and metastasis (Huang et al., 2001). Natural product agents that inhibit NF-κB have shown promise as a mode of chemoprevention (Chuang et al., 2002; Paur et al., 2010). Over-expression of c-Myc accounts for one-seventh of all cancer related deaths, leading to intensified efforts to investigate the function of the c-Myc protein in cancer biology and to test it as a therapeutic target (Spencer and Groudine, 1991; Dang, 1999; Bernard et al., 2003; Laidler et al., 2007). Cassinelli et al., 2004, investigated the role of c-Myc in the cellular response after treatment with TAX and found that hormone-refractory prostate carcinoma cells’ response to TAX involves over-expression of c-Myc. Finally, Malik et al., 2002, showed that advanced PC is accompanied by activation of ERKs and PTEN/P13K/AKT modulation and Ghosh et al., 2005, showed that AKT plays a critical role in prostate cancerous cell proliferation.
Multidrug resistance (MDR) is a major obstacle associated with the effectiveness of chemotherapies in the treatment of cancer (Gottesman and Pastan, 1993; Gonzalez-Mariscal et al., 2008). As a result of MDR, in vitro tumor cells exposed to a cytotoxic agent develop cross-resistance to a range of structurally and functionally unrelated compounds. Epithelium regulates the transportation of molecules in and out of the cells by two pathways: transcellular and paracellular. The former is utilized by hydrophobic, amphiphatic, natural product-derived compounds, such as taxanes (paclitaxel, docetaxel), vinca alkaloids (vinorelbine, vincristine, vinblastine), anthracyclines (doxorubicin, daunorubicin, epirubicin), epipodophyllotoxins (etoposide, tenipo-side), topotecan, dactinomycin, and mitomycin C, the cytotoxic drugs most associated with MDR (Mullin et al., 1986; Pauletti et al., 1997; Crowe, 2002; Jia et al., 2003). In contrast, hydrophilic molecules cannot cross biological membrane, and are restricted to paracellular pathways (Pauletti et al., 1997; Gonzalez-Mariscal et al., 2008). The development of drug resistance in cancerous cells often results from the over-expression of certain proteins, such as cell membrane transporters. These membrane proteins lead to an increased efflux of the cytotoxic drug from the cancerous cell, thus lowering their intracellular concentrations (Mullin et al., 1986). The increased efflux and subsequent low intracellular drug concentration are attributable (at least in part) to either over-expression and/or high activity of a particular member of a superfamily of ATP-dependent transport proteins known as P-glycoprotein (Pgp). Pgp is a 170 kDA ATP-dependent membrane transporter protein molecule which functions as an energy-dependent pump for the efflux of a myriad of anticancer drugs from MDR cells. Pgp-mediated MDR tumor cells play a major role in a patient’s response to chemotherapy. Conversely, chemosensitizers are compounds with the ability to reverse the MDR phenotype, thus providing new insights into improving efficacy for some nonresponsive malignancies (Mullin et al., 1986; Pauletti et al., 1997; Crowe, 2002; Jia et al., 2003; Thomas and Coley, 2003). We believe VA acts as a chemosensitizer. Hence, another clear objective of this study is to determine whether VA is able to reverse MDR phenotype by remaining within the cancerous cells at sufficient concentrations, and not affecting the ATPase activity of P-glycoprotein compared to TAX which we expect to increase P-glycoprotein ATPase activity in PC-3 cells.
2. Materials and methods
2.1. Cell culture
Hormone refractory or androgen independent prostate adenocarcinoma (PC-3 cells) were purchased from American Type Culture Collection (Manassas, VA). DMEM Medium, Fetal Bovine Serum (FBS), and Antibiotic/Antimycotic solution were purchased from Fisher Scientific (Houston, TX). NF-κB and c-Myc kits were purchased from Active Motif (Carlsbad, CA). AKT and NF-κB antibodies were purchased from Cell Signaling (Danvers, MA). P-glycoprotein kit was purchased from Fisher Scientific (Houston, TX). All other chemicals were obtained from Sigma (St. Louis, MO).
2.2. Aqueous Vernonia amygdalina extract preparation
Fresh pesticide-free Vernonia amygdalina (VA) leaves collected in Benin City, Nigeria, were rinsed with cold, distilled water. After rinsing, the leaves were spread out evenly on galvanized–wire screens with the edges bent upward 2 inches on all sides. For complete dryness, the galvanized–wire screens were placed in a specially-constructed dryer and heated to 130–140°F within 4h. Three hundred grams of dried leaves were soaked in 6 L of ddH2O (1:20 w/w) overnight at 4°C before squeezing by hand to a mixture. The mixture was filtered through a 0.45-μm filtration unit for sterilization after filtration through a clean white gauge to remove the particulate matter. The resulting sample solution was then lyophilized to a dry powder (30 g) on a SpeedVac Concentrator (Savant SC210A), transferred into a 50 ml centrifugation tube and stored at −20°C for bioactivity assays.
2.3. DNA synthesis determination
DNA synthesis was determined by [3H] thymidine incorporation assays. Prostate carcinoma cells (PC-3) were seeded at a density of 5 × 104 in 35 mm diameter plates. PC-3 cells were allowed to grow to 60% confluence before stimulating the cells with either VA or Paclitaxel (TAX) for 18 h. Treatments included 0.01, 0.1, and 1 mg/ml of VA and 0.001, 0.1, 1 and 10 μM TAX. Twenty microliters (2μCi/2 ml) of [3H] thymidine/35 mm well was added after 18 h of incubation and incubated again for 6 h at 37°C. All incubations were terminated by aspirating the DMEM medium and doing triplicate washes with 2 ml of cold PBS to remove residual [3H] thymidine. Two milliliters (2 ml) of 10% cold TCA was added to each well and incubated at 4°C for 10 min for cell fixation. Following fixation, the cells were washed three times with ddH2O and solubilized by incubation for 30 min with 0.5 M NaOH (2ml/35mm) at 37°C. Upon solubilization, 1 ml of cell solution was added to 5 ml of scintillation cocktail and mixed vigorously. A scintillation counter was used to determine radioactivity.
2.4. Assessment of MAPK activity
The cells were grown in DMEM medium supplemented with 10% FBS and 1% antibiotic/antimycotic solution. The medium was changed every 2–3 days until the cells were 70–80% confluent. The medium was aspirated and replaced with fresh medium before adding treatments of either VA or TAX for different time intervals (0, 5, 10, 20, 40, and 60 min). The cells were harvested under non-denaturing conditions (by removing the medium, rinsing the cells once with ice-cold PBS; adding 0.5 ml ice-cold 1X cell lysis buffer plus 1mM phenylmethylsulfonyl fluoride (PMSF); and incubating on ice for 5 minutes). The cells were scraped off the plate and transferred to the appropriate tubes kept on ice. The samples were microcentrifuged for 10 min at 4°C and the supernatants were transferred to other tubes. Protein content determination within cell lysates was made using the BCA method. After quantification, 200 μl of cell lysates were immunoprecipitated with immobilized p44/42 primary antibody by adding 15 μl of immobilized bead slurry. The tubes were incubated with gentle rocking overnight at 4°C then the cell lysate/immobilized antibody was microcentrifuged at 14,000 × g for 30 sec at 4°C. The pellet was washed with 500 μl 1X Cell Lysis Buffer kept on ice, followed by washing with 500 μl of 1X Kinase Buffer kept on ice, and then suspended in 50 μl of Kinase Buffer supplemented with 200 μM ATP and appropriated quantities of kinase substrate. The cocktails were incubated for 30 min at 30°C; the reactions were terminated with the addition of 25 μl of 3X SDS Sample Buffer; vortexed, and microcentrifuged for 30 sec before heating the samples to 95–100°C for 2–5 min. After heating, the samples were loaded on an SDS-PAGE gel for analysis.
2.5. NF-κB activity determination
The medium from confluent cells was replaced with fresh medium and the cells were treated with either VA or TAX at different time intervals (0, 5, 15, 30, 45, and 60 min). Wash Buffer and Binding Buffers were prepared according to manufacturer’s protocol. Two hundred microliters (200 μl) of 1X Wash Buffer was added to each well and incubated for 20 min at room temperature, followed by 3 washes of 1X Wash Buffer. The plates were inverted and tapped 3 times on absorbent paper towel. Forty microliters of Binding Buffer was used for each well. Ten microliters of the samples diluted in Nuclear Extract Dilution Buffer per well using 10 μg of nuclear or whole cell extract were added per well. The plate was sealed and incubated at room temperature for 1 hr on a plate rocker. The cells were washed 3 times with 200 μl 1X Wash Buffer, tapped 3 times on absorbent paper towel, and 100 μl of diluted NF-κB p50 antibody at a 1:200 dilution was added to each well. The plate was sealed and incubated for 1 hr on a plate rocker. The cells were washed 3 times with 200 μl 1X Wash Buffer and tapped 3 times on absorbent paper towel. After washing and tapping dry, 100 μl of diluted anti-rabbit antibody was added to each well and incubated for 1 hr at room temperature on rocking platform. During the incubation, the Developing Substrate Solution (DSS) was placed at room temperature, followed by washing and tapping dry, 100 μl of DSS was added to each well. The plates were incubated for 5–15 minutes at room temperature protected from direct light. Blue color development in sample and positive control wells was monitored until it turned medium to dark blue preventing overdevelopment. One hundred microliters (100 μl) of Stop Solution was added and the blue solution turned yellow once the reaction ceased. Absorbance was read within 5 min using a spectrophotometer at 450 nm with an optional reference wavelength at 655 nm.
2.6. c-Myc activity determination
Subconfluent cells were treated at different time intervals (0, 5, 10, 20, 40, and 60 min) previously mentioned. Nuclear extracts were prepared at each time point by aspirating medium, washing with 5 ml ice-cold PBS/Phosphatase inhibitors, and gently scraping the cells off with cell scrapers before transfer to pre-chilled 15 ml conical tubes. Followed by centrifugation at 500 rpm at 4°C, the supernatants were discarded while retaining the pellets kept on ice. For cytoplasmic fraction collection, the cells were resuspended in 500 μl 1 X hypotonic buffer by pipetting up and down several times before transferring the suspension to pre-chilled microcentrifuge tubes. The suspensions were incubated on ice for 15 min followed by the addition of 25 μl of detergent and vortexed. The suspension were centrifuged for 30 sec at 14, 000 × g in a microcentrifuge pre-cooled to 4°C. The supernatants were discarded and pellets were used for nuclear fraction collection, wherein pellets were resuspended in 50 μl Complete Lysis Buffer (CLB) by pipetting up and down and incubated for 30 min on ice on a rocking platform at 150 rpm. Next, the suspension was centrifuged for 10 min at 14, 000 × g in a microcentrifuge pre-cooled at 4°C. The supernatant was transferred to a pre-chilled microcentrifuge tube and quantified for protein contents using the BCA assay. Afterwards, 40 μl of Complete Binding Buffer (CBB) was added to the 96-well plate to which oligonucleotide containing a c-Myc consensus sequence had been immobilized. The samples were diluted to 5 μg/ml in CBB. Ten microliters of each diluted sample was added to each sample well. Five micrograms of provided Jurkat Nuclear Extract diluted in 10 μl of CBB was added to the positive control wells. Ten microliters of CBB was added to the blank wells. The plate was sealed and incubated for 1 hr at room temperature with mild agitation on a rocking platform. Each well was washed 3 times with 200 μl 1 X Wash Buffer. For each wash, the plate was flicked over a sink to empty the contents of the wells, then tapped and inverted 3 times on absorbent paper towels. For binding of the primary antibody, 100 μl of diluted c-Myc antibody (1:1000 of 1 X Antibody Binding Buffer) was added to all wells. The plate was covered and incubated for 1 hr at room temperature without agitation. The wells were washed 3 times with 200 μl of 1 X Wash Buffer. For binding of the secondary antibody, 100 μl of diluted HRP-conjugated antibody (1:1000 dilution in 1 X Antibody Binding Buffer) was added to each well. The plate was covered and incubated for 1 hr at room temperature without agitation. During the incubation, the developing solution was placed at room temperature. After 1 hr, the wells were washed with 200 μl 1 X Wash Buffer. Afterwards, 100 μl of developing solution was added for colorimetric reaction. The plate was incubated for 2–10 min protected from direct light until blue color development. One hundred microliters of Stop Solution was added turning the blue color yellow. Absorbance was read on spectrophotometer at 450 nm wavelength.
2.7. Western Blot analysis
Following the same culturing techniques and treatment intervals previously indicated, cells were harvested under non-denaturing conditions and lysed in order to extract the protein contents quantified and used to run Western blots. Two 12% SDS Resolving PAGE were prepared by adding 4.8 ml distilled deionized water, 3.75 ml 1.5 M Tris HCl pH 8.8, 6.25 ml Acrylamide stock, 150 μl 10% SDS, 75 μl 10% ammonium persulfate (APS), and 5 μl Temed. Following polymerization, two 4% stacking gels were prepared by adding 1.5 ml distilled deionized water, 625 μl 0.5 M Tris HCl pH 6.8, 335 μl acrylamide stock, 25μl 10 % SDS, 12.5 μl 10% APS, and 1.25 μl Temed.
2.8. P-glycoprotein activity assay
All reagents were prepared according to the manufacturer’s protocol. Twenty microliters of PgP-Glo Assay Buffer was added to wells labeled “no treatment” (NT). Twenty microliters of 0.25 mM Na3VO4 in the PgP-Glo Assay Buffer was added to the wells labeled Na3VO4 on the 96 well plate. Twenty microliters of 0.5 mM Verapamil in PgP-Glo Assay Buffer was added to the wells labeled “Ver” and 20 μl of 2.5 X concentrated test compounds was added to the experimental test compound wells. Afterwards, 20 μl of diluted PgP membranes was added to each well and incubated at 37°C for about 5 min (floating in 37°C water bath). The reactions were initiated by the addition 10 μl of 25 mM MgATP to all wells and mixed briefly by gentle tapping before placing in a 37°C incubator for 40 min. Fifty microliters of ATP Detection Reagent was added to each well after removing the plate from the heat source. The plate was mixed briefly and incubated at room temperature for 20 min to allow the luminescent signal to develop. Luminescence was read on a plate-reading luminometer.
2.9. Statistical analysis
The experimental replicates within individual experiments were averaged and expressed as mean ±SD. The comparisons between means were determined by Dunnett’s test, unpaired students t-test with 2 tailed P values reported, employing Graph Pad statistical software package. Each experiment was replicated three times with comparable results. Mean data were determined to be statistically significant if values were 0.05 or less.
3. Results
3.1. Effects on DNA synthesis
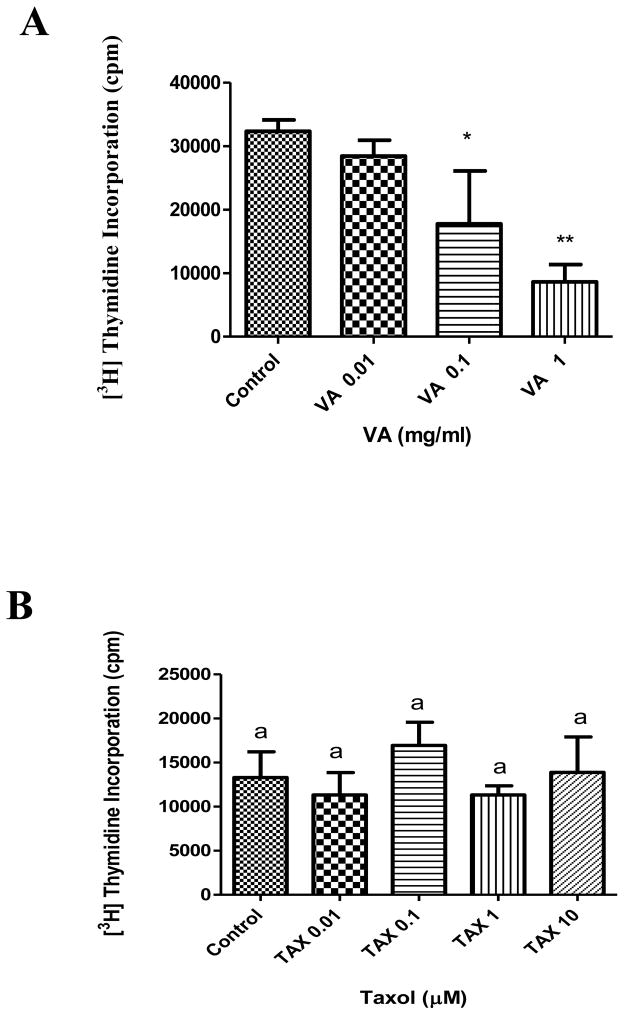
The result showed that VA extract, but not TAX, inhibits DNA synthesis in PC-3 cells in a concentration-dependent fashion (Figure 1). Treatment of cells with increasing concentrations of VA (0.01, 0.1, and 1 mg/ml) decreased DNA synthesis by 12%, 45% (P<0.05), and 73% (P<0.01) respectively compared to control. This is in agreement with previous studies conducted in our laboratory (Izevbigie, 2003; Opata and Izevbigie, 2006; Gresham et al., 2008). In contrast, neither TAX concentration (0.01, 0.1, 1 or 10 μM) treatment had any significant affect on DNA synthesis (Figure 1). Previous studies have reported an IC50 value at 17.4 nM and an inhibitory effect at 100 nM of TAX in PC-3 cells (Jia et al., 2003; Ping et al., 2010).
Figure 1.
Effect of VA extract (A) or TAX (B) treatment on DNA synthesis in PC-3 cells in vitro. PC-3 cells at the logarithmic growth phase were incubated with 0.01, 0.1, and 1 mg/ml VA or with 0.01, 0.1, and 1 mg/ml TAX for 18h before the addition of 1 μCi/ml [3H] thymidine. Each data point represents the mean of three independent experiments done in triplicates (n=9); *, P < 0.05; **, P < 0.01, data means represented by the same letter are not statistically different from each other. DNA synthesis was determined as described under Materials and Methods.
3.2. Effects on MAPK
VA treatment led to a time-dependent activation (3-fold) of MAPK in the first 10 min followed by a decline to a basal level compared to control treatment (Figure 2). This is consistent with previous finding in MCF-7 cells (Pauletti et al., 1997). TAX increased MAPK activity by approximately 50% (or 0.5 fold) up to 20 min after stimulation followed by a decline to a less than basal level in 40 min compared to control (Figure 2). Both VA and TAX stimulated MAPK activity, but VA-induced MAPK stimulation was more intense and short-lived. In contrast, TAX-induced MAPK activity was less intensive, but sustained. The observation that TAX activated MAPK in the present study is supported by previous studies in DU-145 and PC-3 cells (Lee et al., 2005).
Figure 2.
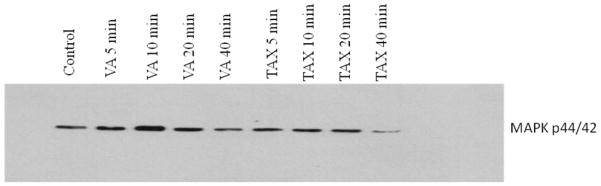
Effect of VA extract or TAX treatment on MAPK in vitro. PC-3 cells were treated with 1mg/ml VA or 1 μM TAX at different time intervals. The cells were lysed and the protein concentrations were determined by the BCA method followed by immunoprecipitation assays.
3.3. Effects on NF-κB activity
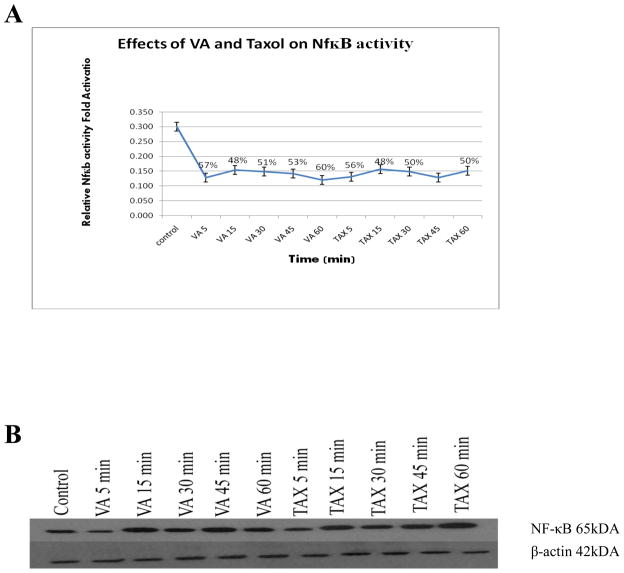
Exposure of cells to either 1 mg/ml VA extract or 1 μM TAX for 5 min yielded decreased NF-κB activity by approximately 50 %. Longer exposure times (15, 30, 45, and 60 min) with either VA extract or TAX, had no significant affect on NF-κB activity compared to control (Figure 3). This suggests that neither VA extract nor TAX-induced NF-κB activity is time-sensitive. These findings are in agreement with previous findings by Chuang et al (Chuang et al 2002).
Figure 3.
Effect of VA extract or TAX treatment on NF-κB activity in PC-3 cells. Cells were exposed to either 1 mg/ml VA extract or 1 μM TAX for 5, 15, 30, 45 or 60 min time intervals before extraction of protein. Protein was quantified and used for assessment of VA and TAX effects on NF-κB activity as compared to control as determined by Western blotting analysis. β-actin was used as a loading control.
3.4. Effects on NF-κB expression
Cells exposed to either VA (1 mg/ml) or 1 μM TAX at various intervals showed increased NF-κB expression 15 minutes after stimulation, which remained elevated (four-fold) for 60 minutes compared to control as determined by Western blots (Figure 3). This result suggests that VA and TAX regulation of NF-κB activity occurs at the activation level, not expression level.
3.5. Effects on c-Myc
Treatment of cells with the indicated doses of VA for 10 min caused modest and transient activation, of c-Myc, with a return to basal levels in 40 min and a doubling at 60 min. This revealed a biphasic effect of VA treatment (Figure 4). In contrast, TAX treatment yielded a twofold pattern of activation at 5 min with sustained elevation for 60 min (Figure 4). This is in agreement with previous findings by others (Bernard et al., 2003; Laidler et al., 2007) which showed a positive correlation between c-Myc activity and PC-3 cell proliferation.
Figure 4.
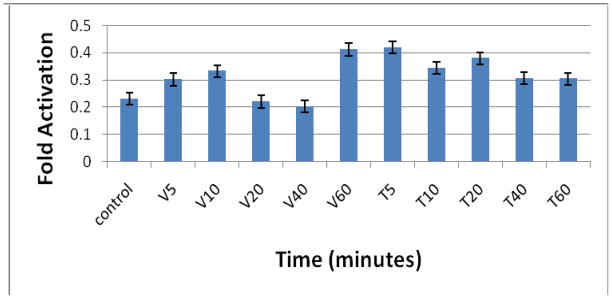
Effect of VA extract or TAX treatment on c-Myc activity in PC-3 cells. Subconfluent cells plated in 100 mm tissue culture plates were treated at different time intervals (0, 5, 10, 20, 40, and 60 min) and absorbance was read on a spectrophotometer at 450 nm wavelength. The cells were lysed and treatment protein quantified using the BSA method. c-Myc activities were assessed following protocol in the Materials and Methods section.
3.6. Effects on AKT expression
Treatment of cells with 1mg/ml VA extracts for intervals (5, 10, 20, 40, and 60 min) did not statistically affect AKT gene expression as revealed by Western Blot analysis shown in Figure 5. In a sharp contrast, TAX (1μM) at 5 min caused an average five-fold increase (P<0.01) in the AKT gene expression of (Figure 5). AKT expression or over-expression has been implicated in tumorigenesis (Ghosh et al., 2005).
Figure 5.
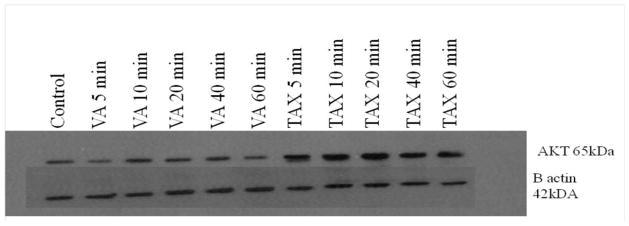
Effects of VA or TAX treatment on AKT regulation. Cells were lysed after treatments at different time intervals, and protein was extracted and quantified using the BCA assay as described under the Materials and Methods section. β-actin was used as a loading control for the Western blots.
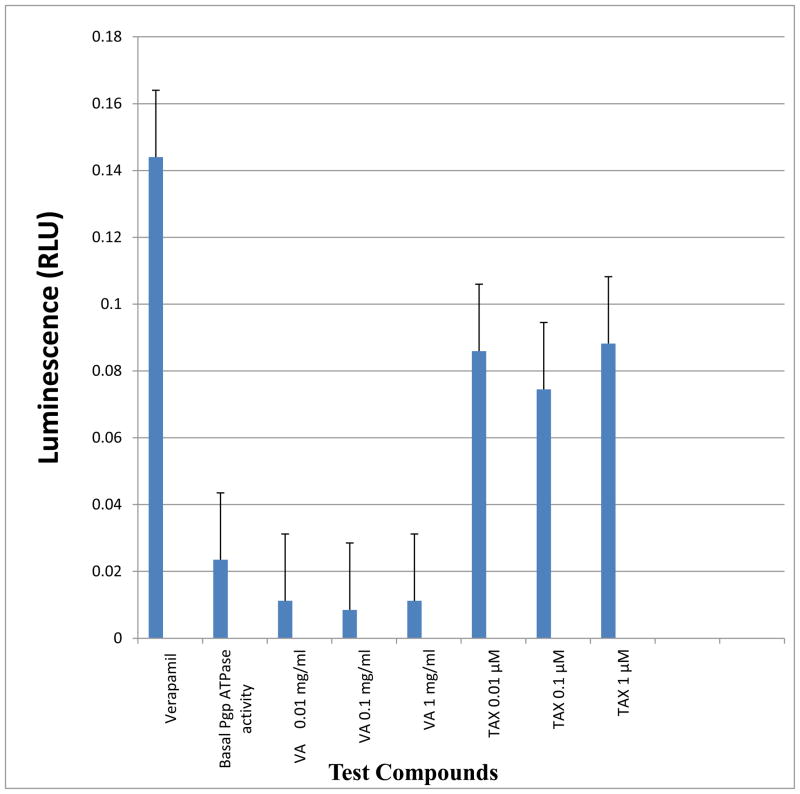
3.7. Effects on P-glycoprotein activity
Neither concentrations of VA extracts (0.01, 0.1, and 1 mg/ml) statistically (P>0.05) affected the ATPase activity of P-glycoprotein compared to basal level control. Again, in sharp contrast, TAX treatment at all concentrations (0.01, 0.1, and 1 μM) resulted in an average fourfold increase in the ATPase activity of P-glycoprotein compared to control (Figure 6).
Figure 6.
Effects of VA or TAX treatment on stimulation of P-glycoprotein (Pgp) ATPase activity. Treatments using VA or TAX at the indicated concentrations, yielded luminescence readings which were measured against Na3VO4 – treated samples RLU (Na3VO4). ΔRLU basal reflects basal Pgp ATPase activity. Differences between average luminescent signals from RLU (Na3VO4) and test compounds RLU TC were used to determine Pgp ATPase activity in the presence of a test compounds.
4. Discussion
Prostate Cancer (PC) is the second leading cause of cancer-related deaths amongst American men. Estimated new cases and deaths from PC in the U.S. in 2012 are 241,740 and 28,170 respectively (ACS, 2011). Vernonia amygdalina (VA) extract, given alone or in combination with conventional therapies is likely a beneficial regimen for PC patients. Here, we have shown that TAX-insensitive PC-3 cells (used in these studies and exposed to up to 10 μM TAX) were sensitive to VA treatment. Our observation that the PC-3 cells were insensitive to TAX treatment is at variance with a previous report that TAX exhibited an IC50 value of 100 nM in hormone-refractory PC cell growth inhibition (Ping et al., 2010). Treatment of cells with 1 mg/ml VA inhibited DNA synthesis by 73% (P<0.01) (Fig. 1). Next, we investigated the mechanisms that underlie VA-sensitivity and TAX-insensitivity of these cells, and our findings show that both VA and TAX stimulated MAPK activity in a similar fashion but at different intensity. Previous investigators have shown that TAX stimulated MAPK activity in DU-145 and PC-3 cells (Lee et al., 2005) which is in agreement with the present studies.
NF-κB, a transcription factor, regulates gene expression involved in cell proliferation and survival, inflammatory responses, and other functions. Evidence suggests that NF-κB is involved in the development of drug resistance in cancerous cells. Chuang et al examined the baseline levels of NF-κB activity of carcinoma cell lines and the alteration of NF-κB activity in response to anticancer drugs including TAX (Chuang et al 2002). Their findings showed that carcinoma cell lines responded with a transient activation of NF-κB followed by a decline to basal level despite variation in the concentration of the agent and the duration of the treatment (Chuang et al 2002). In contrast, in the present study, we found that both VA and TAX down- regulated NF-κB activation by approximately 50%.
In a sharp contrast, VA caused a modest and transient activation of c-Myc, with a return to basal level in 40 min; but TAX treatment yielded a two-fold, rapid, sustained increase in c-Myc activity. c-Myc is a proto-oncogene whose over-expression has been implicated in hematopoietic tumors and other types of tumors (Spencer and Groudine, 1991; Bernard et al., 2003; Laidler et al., 2007). It has been demonstrated that over-expression of c-Myc in mouse prostate and normal epithelial resulted in tumor transformation with invasive phenotype (Spencer and Groudine, 1991). In addition, both human androgen independent and dependent PC cells expressing c-Myc grew in the absence of androgen and presented tumoregenic properties suggesting that c-Myc is required for by both androgen receptor (AR) AR+ and AR− PC cells (Bernard et al., 2003).
Many factors, including multi-drug resistance (MDR), contribute to PC incidence and deaths. MDR, commonly found in tumor cells, is a direct limitation of conventional cancer chemotherapies (Gonzalez-Mariscal et al., 2008). MDR can be achieved by the active efflux of a broad range of anticancer drugs through the plasma membrane by MDR proteins, including the ATP binding cassette transporter family of proteins (Mullin et al., 1986; Pauletti et al., 1997; Crowe, 2002; Jia et al., 2003; Gonzalez-Mariscal et al., 2008). It causes the cells to rapidly clear out anticancer drugs faster that they can elicit their therapeutic actions and subsequently renders the drug ineffective. Therefore, MDR contributes to poor prognosis and treatment outcome in PC patients (Spencer and Groudine, 1991; Dang, 1999; Bernard et al., 2003; Cassinelli et al., 2004; Laidler et al., 2007). The P-glycoprotein molecule (Pgp), a MDR protein, is a member of the ATP-binding cassette-transporter family (Gonzalez-Mariscal et al., 2008). Generally, cells utilize two mechanisms for the permeation of drugs across the cell membrane, lipophilic and hydrophilic drugs pathways (Gonzalez-Mariscal et al., 2008). Hydrophilic drugs are unable to cross the cell plasma membrane. Therefore, they are restricted to the paracellular pathway, which consists of aqueous pores created by tight junctions (Mullin et al., 1986; Gonzalez-Mariscal et al., 2008). Suppression of Pgp activity may improve cancerous cell sensitivity and improve treatment outcome (Gottesman and Pastan, 1993; Mullin et al., 1986; Crowe, 2002; Jia et al., 2003; Gonzalez-Mariscal et al., 2008). Novel therapies that could mitigate MDR by the inhibition of Pgp activity and render hormone-refractory PC more sensitive to chemotherapies, and thus improve treatment outcome and save lives, will be welcome allies for the fight against cancer. In the present study, we found that all concentrations of TAX tested stimulated Pgp ATPase activity (P<0.05) above basal level. Interestingly, in a sharp contrast, VA treatment elicited an inhibitory effect on basal Pgp ATPase activity suggesting that, besides the anticancer property, VA is a chemosensitizer that may be helpful in the treatment of PC. In summary, the TAX-resistant, but VA-sensitive nature of these PC-3 cells may be explained by differential regulatory pattern of MAPK, c-Myc, AKT, and Pgp activities and/or expression.
Acknowledgments
Our sincere appreciation goes to Alphonsus Obayuwana, MD., Department of Obstetrics and Gynecology, St. Vincent Medical Center, Toledo, OH; and the library staff with the Office of the Deputy Vice Chancellor at Benson Idahosa University, PMB 1100, Benin City, Edo State, Nigeria for technical assistance toward preparation of this manuscript. The research was supported by grants from the following agencies: US Dept of Education, Strengthening Environmental Science Ph D Programs Through Research (grant #P031E090210); National Center for Research Resources (grant #5 G12 RR013459-15) and the National Institute on Minority Health and Health Disparities (grant #8 G12 MD007581-15) from the National Institutes of Health; and Transforming the Climate and Advancing STEM Women at JSU, an HBCU in the South (JSUAdvance) from the National Science Foundation (NSF Award #1008708). Any opinions, findings, and conclusions or recommendations are those of the authors and do not reflect the view of NSF.
Footnotes
Author agreement: All authors contributed to and participated in the experimental design, analyses and interpretation of the data. Professor Ernest B. Izevbigie, and Drs. Carolyn B. Howard and Keyuna S. Cameron contributed to the formatting, editing and preparation of this manuscript. This work has not been published before (except as a part of the thesis for Keyuna S. Cameron, then a PhD student in the laboratory of Professor Ernest Izevbigie a Jackson State University (JSU). This publication is not under consideration elsewhere and its publication has been approved by all co-authors as well as all authorities at JSU.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abosi AO, Raseroka BH. In vivo antimalarial activity of Vernonia amygdalina. Br J Biomed Sci. 2003;60(2):89–91. doi: 10.1080/09674845.2003.11783680. [DOI] [PubMed] [Google Scholar]
- Akah PA, Okafor CI. Blood sugar lowering effect of Vernonia amygdalina (del) in an experimental rabbit model. Phytotherapy Res. 1992;6:171–3. [Google Scholar]
- American Cancer Society. Prostate cancer treatments. 2011. [Google Scholar]
- Babalola OO, Anetor JI, Adeniyi FA. Amelioration of carbon tetrachloride induced hepatotoxicity of terpenoid extract from leaves of Vernonia amygdalina. Afr J Med Sci. 2001;30(1–2):91–3. [PubMed] [Google Scholar]
- Bernard D, Paurtier-Manzando A, Gil J, Bleach DH. cMyc confers androgen-independent prostate cancer cell growth. J Clin Ivest. 2003;112(11):1724–31. doi: 10.1172/JCI19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler D, Gulding KM, Dann R, et al. Gene profiling and promoter reporter assays: novel tools for comparing the biological effects of botanical extracts on human prostate cancer cells and understanding their mechanisms of action. Oncogene. 2003;22:1261–72. doi: 10.1038/sj.onc.1206242. [DOI] [PubMed] [Google Scholar]
- Bonham MJ, Galkin A, Montgomery B, et al. Effects of the herbal extract PC-SPES on microtubule dynamics and paclitaxel-mediated prostate tumor growth inhibition. JNCI Journal of NCI. 2002;94(21):1641–7. doi: 10.1093/jnci/94.21.1641. [DOI] [PubMed] [Google Scholar]
- Bonsi MLK, Osuji PO, Tuah AK, Umunna MN. Vernonia amygdalina as a supplement of teff straw (Eragrostis tef) fed to Ethiopian menz sheep. Agroforestry systems. 1995;31(3):229–44. [Google Scholar]
- Cassinelli G, Supino R, Zuco V, et al. Role of c-myc protein in hormone refractory prostate carcinoma: cellular response to paclitaxel. Biochem Pharmacol. 2004;68(5):923–31. doi: 10.1016/j.bcp.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Chuang SE, Yeh PY, Lu YS, et al. Basal levels and patterns of anticancer drug-induced activation of nuclear factor-kappaB (NF-kappaB), and its attenuation by tamoxifen, dexamethasone, and curcumin in carcinoma cells. Biochem Pharmacol. 2002;63(9):1709–16. doi: 10.1016/s0006-2952(02)00931-0. [DOI] [PubMed] [Google Scholar]
- Crowe A. The influence of p-glycoprotein on morphine transport in Caco-2 cells: comparison with paclitaxel. Eur J Pharmacol. 2002;440(1):7–16. doi: 10.1016/s0014-2999(02)01366-3. [DOI] [PubMed] [Google Scholar]
- Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol and Cell Biol. 1999;19(1):1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28(4):251–65. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ghosh PM, Malik SN, Bedolla RG, et al. Signal transduction pathways in androgen-dependent and -independent prostate cancer cell proliferation. Endocr Relat Cancer. 2005;12(1):119–34. doi: 10.1677/erc.1.00835. [DOI] [PubMed] [Google Scholar]
- Gill C, Walsh SE, Morrissey C, et al. Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate. 2007;67(15):1641–53. doi: 10.1002/pros.20653. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Hernández S, Vega J. Inventions designed to enhance drug delivery across epithelial and endothelial cells through the paracellular pathway. Recent Pat Drug Deliv Formul. 2008;2(2):145–76. doi: 10.2174/187221108784534117. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gresham LJ, Ross J, Izevbigie EB. Vernonia amygdalina: anticancer activity, authentication, and adulteration detection. Int J Environ Res Public Health. 2008;5(5):342–8. doi: 10.3390/ijerph5050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Pettaway C, Uehara H, et al. Blockade of NF-kB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–97. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- Huffman MA, Seifu M. Observations on the illness and composition of a possibly medicinal plant Vernonia amygdalina (del) by a wild chimpanzee in the Mohale Mountains National Park, Tanzania. Primates. 1989;30(1):51–63. [Google Scholar]
- Huizing MT, Misser VH, Pieters RC, et al. Taxanes: a new class of antitumor agents. Cancer Invest. 1995;13(4):381–404. doi: 10.3109/07357909509031919. [DOI] [PubMed] [Google Scholar]
- Igile GO, Oleszek W, Jurzysta M, et al. Flavonoids from Vernonia amygdalina and their antioxidant activities. J Agric Food Chem. 1994;42 (11):2445–8. [Google Scholar]
- Izevbigie EB. Discovery of water-soluble anticancer agents (edotides) from a vegetable found in Benin City, Nigeria. Exp Biol Med (Maywood) 2003;228(3):293–8. doi: 10.1177/153537020322800308. [DOI] [PubMed] [Google Scholar]
- Izevbigie EB, Bryant JL, Walker A. Natural inhibitor of extracelular signal-regulated kinases and human breast cancer cells. Exp Biol & Med. 2004;229:163–9. doi: 10.1177/153537020422900205. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics. Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jia L, Schweizer J, Wang Y, Cerna C, Wong H, Revilla M. Effect of nitric oxide on cytotoxicity of Taxol: enhanced Taxol transcellular permeability. Biochemical Pharmacology. 2003;66(11):2193–9. doi: 10.1016/j.bcp.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Kamath K, Wilson L, Cabral F, Jordan MA. Beta III-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem. 2005;280(13):12902–7. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]
- Karin M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Hisatake J, Hisatake Y, et al. PC-SPES: a unique inhibitor of proliferation of prostate cancer cells in vitro and in vivo. Prostate. 2000;42(3):163–71. doi: 10.1002/(sici)1097-0045(20000215)42:3<163::aid-pros1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kumar AP, Graham H, Robson C, et al. Natural products: potential for developing Phellodendron amurense bark extract for prostate cancer management. Mini Rev Med Chem. 2010;10(5):388–97. doi: 10.2174/138955710791330936. [DOI] [PubMed] [Google Scholar]
- Laidler P, Dulinska J, Mrozicki S. Does the inhibition of c-Myc expression mediate the antitumor activity of PPAR’s ligand in prostate cell lines? Arch Biochem Biophys. 2007;462(1):1–12. doi: 10.1016/j.abb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Lee JT, Jr, Steelman LS, McCabrey JA. Modulation of Raf/MEK/ERK kinase activity does not affect the chemoresistance profile of advanced prostate cancer cells. Int J Oncol. 2005;26(6):1637–44. [PubMed] [Google Scholar]
- Leonard BS, Karen LL, Bruce B, et al. Complementary and alternative medicine in chronic liver disease. Hepatology. 2002;34(3):595–603. doi: 10.1053/jhep.2001.27445. [DOI] [PubMed] [Google Scholar]
- Lopes JJ, Marx C, Ingrassia R, Picada JN, Pereira P, Ferraz A. Neurobehavioral and toxicological activities of two potentially CNS-acting medicinal plants of Piper genus. Exp Toxicol Path. 2012;64:9–14. doi: 10.1016/j.etp.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Malik SN, Brattain M, Ghosh PM, et al. Immunohistochemical demonstration of phospho-AKT in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8(4):1168–71. [PubMed] [Google Scholar]
- Miller ML, Ojima I. Chemistry and chemical biology of taxanes anticancer agents. Chem Rec. 2001;1(3):195–211. doi: 10.1002/tcr.1008. [DOI] [PubMed] [Google Scholar]
- Mori A, Lehmann S, O’Kelly J, et al. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006;66(6):3222–9. doi: 10.1158/0008-5472.CAN-05-0087. [DOI] [PubMed] [Google Scholar]
- Mullin JM, Fluk L, Kleinzeller A. Basal-lateral transport and transcellular flux of methyl alpha-D-glucoside across LLC-PK1 renal epithelial cells. Biochim Biophys Acta. 1986;885(3):233–9. doi: 10.1016/0167-4889(86)90237-5. [DOI] [PubMed] [Google Scholar]
- Oboh G, Akinyemi AJ, Ademiluyi AO. Antioxidant and inhibitory effect of red ginger (Zingiber officinale var. Rubra) and white ginger (Zingiber officinale Roscoe) on Fe2+ induced lipid peroxidation in rat brain in vitro. Exp Toxicol Path. 2012;64:31–6. doi: 10.1016/j.etp.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Obasaju C, Hudes GR. Paclitaxel and docetaxel in prostate cancer. Hematol Oncol Clin 2001; North Am. (3):525–45. doi: 10.1016/s0889-8588(05)70230-6. [DOI] [PubMed] [Google Scholar]
- Opata MM, Izevbigie EB. Aqueous Vernonia amygdalina extracts alter MCF-7 cell membrane permeability and efflux. Int J Environ Res Public Health. 2006;3(2):174–9. doi: 10.3390/ijerph2006030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauletti GM, Okumu FW, Borchardt RT. Effect of size and charge on the passive diffusion of peptides across Caco-2 cell monolayers via the paracellular pathway. Pharm Res. 1997;14(2):164–8. doi: 10.1023/a:1012040425146. [DOI] [PubMed] [Google Scholar]
- Paur I, Balstad TR, Kolberg M, et al. Extract of oregano, coffee, thyme, clove, and walnuts inhibits NF B in monocytes and in transgenic reporter mice. Cancer Prev Res. 2010;3(5):653–63. doi: 10.1158/1940-6207.CAPR-09-0089. [DOI] [PubMed] [Google Scholar]
- Ping SY, Hour TC, Lin SR, Yu DS. Taxol synergizes with antioxidants in inhibition of hormonal refractory prostate cancer cell growth. Urol. 2010;2:170–9. doi: 10.1016/j.urolonc.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Ramon J, Denis LJ. Prostate cancer. Vol. 174. Springer; 2007. [Google Scholar]
- Schultz WA. Molecular biology of human cancers: an advanced student textbook. Springer; 2005. [Google Scholar]
- Spencer CA, Groudine M. Control of cMyc regulation in normal and neoplastic cells. Adv Cancer Res. 1991;(56):1–48. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- Teramoto H, Gutkind JS. Mitogen-Activated protein kinase family. Encyclopedia of biological chemistry. 1996;271(44):27225–8. doi: 10.1074/jbc.271.44.27225. [DOI] [PubMed] [Google Scholar]
- Thomas H, Coley H. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10(2):159–65. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- Uhegbu FO, Ogbuehi KJ. Effect of aqueous extract (crude) of leaves of Vernonia amygdalina (del) on blood glucose, serum albumin and cholesterol levels in diabetic albino rats. Global J of Pure and Applied Sci. 2004;10(1):189–94. [Google Scholar]
- Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum Nutr. 2010;65(2):158–63. doi: 10.1007/s11130-010-0166-4. [DOI] [PubMed] [Google Scholar]
- Yin S, Bhattacharya R, Cabral F. Human mutations that confer paclitaxel resistance. Mol Cancer Ther. 2010;9(2):327–35. doi: 10.1158/1535-7163.MCT-09-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]