Abstract
Background: Animal models show that periconceptional supplementation with folic acid, vitamin B-12, choline, and betaine can induce differences in offspring phenotype mediated by epigenetic changes in DNA. In humans, altered DNA methylation patterns have been observed in offspring whose mothers were exposed to famine or who conceived in the Gambian rainy season.
Objective: The objective was to understand the seasonality of DNA methylation patterns in rural Gambian women. We studied natural variations in dietary intake of nutrients involved in methyl-donor pathways and their effect on the respective metabolic biomarkers.
Design: In 30 women of reproductive age (18–45 y), we monitored diets monthly for 1 y by using 48-h weighed records to measure intakes of choline, betaine, folate, methionine, riboflavin, and vitamins B-6 and B-12. Blood biomarkers of these nutrients, S-adenosylhomocysteine (SAH), S-adenosylmethionine (SAM), homocysteine, cysteine, and dimethylglycine were also assessed monthly.
Results: Dietary intakes of riboflavin, folate, choline, and betaine varied significantly by season; the most dramatic variation was seen for betaine. All metabolic biomarkers showed significant seasonality, and vitamin B-6 and folate had the highest fluctuations. Correlations between dietary intakes and blood biomarkers were found for riboflavin, vitamin B-6, active vitamin B-12 (holotranscobalamin), and betaine. We observed a seasonal switch between the betaine and folate pathways and a probable limiting role of riboflavin in these processes and a higher SAM/SAH ratio during the rainy season.
Conclusions: Naturally occurring seasonal variations in food-consumption patterns have a profound effect on methyl-donor biomarker status. The direction of these changes was consistent with previously reported differences in methylation of metastable epialleles. This trial was registered at www.clinicaltrials.gov as NCT01811641.
See corresponding editorial on page 1157.
INTRODUCTION
The Developmental Origins of Health and Disease hypothesis is built on a substantial body of evidence that early life environment (especially nutritional) has lifelong effects on human health (1). The current challenge is to identify the underlying molecular mechanisms; epigenetic regulation is a strong candidate. Epigenetic modifications of DNA convey stable alterations in gene expression, not mediated by changes in DNA sequence. DNA methylation, which involves the addition of methyl groups (-CH3) to cytosine-phosphate-guanine dinucleotides, appears to be the most stable epigenetic modification (2) and, when established during early ontogeny, can persist for life (3).
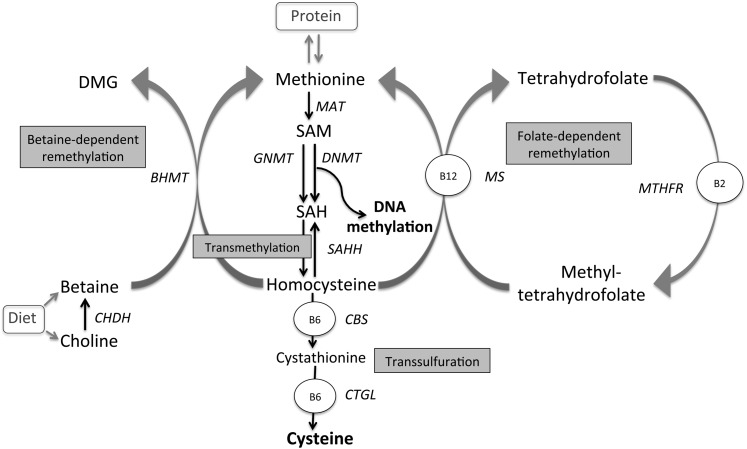
One-carbon metabolic pathways supply methyl groups for all biological methylation reactions (Figure 1). Methylated folate (5,10,methylene-tetrathydrofolate) and betaine, a metabolite of choline, are the immediate substrates providing methyl groups to remethylate homocysteine and form methionine (4). Methionine is adenylated to S-adenosylmethionine (SAM)4, which donates methyl groups for DNA methylation, converting to S-adenosylhomocysteine (SAH). SAH is then metabolized to homocysteine which can be remethylated to methionine or enter the transsulfuration pathway leading to cysteine. Dietary supplies of folate and choline, and of riboflavin and vitamins B-6 and B-12, acting as enzyme cofactors are key to these processes. It is recognized that methylation reactions are important in various aspects of embryogenesis and fetal development (5). Substrate restricted limitations in such pathways, whether driven by dietary deficits or competitive demand, could therefore influence diverse aspects of in utero programming (6) besides DNA methylation, which forms the focus of our interest here.
FIGURE 1.
One-carbon metabolism. Adapted from reference 4. BHMT, betaine-homocysteine methyltransferase; B2, riboflavin; B6, vitamin B-6; B12, vitamin B-12; CBS, cystathionine-β-synthase; CHDH, choline dehydrogenase; CTGL, cystathionine-γ-lyase; DMG, dimethylglycine; DNMT, DNA methyltransferases; GNMT, glycine-N-methyltransferase; MAT, methionine adenosyltrasferase; MTHFR, methylenetetrahydrofolate reductase; MS, methionine synthase; SAH, S-adenosylhomocysteine; SAHH, S-adenosylhomocysteine hydrolase; SAM, S-adenosylmethionine. Methyl groups released from conversion of SAM to SAH are also used for other methylation reactions (eg, proteins).
Animal studies have shown that periconceptional supplementation/restriction of the maternal diet with betaine, choline, folic acid, methionine, or vitamin B-12 can affect the establishment of DNA methylation patterns, altering gene expression and phenotype of the offspring (7, 8). In humans, such a causal association has not been described; however, periconceptional undernutrition during the Dutch Hunger Winter was associated with decreased DNA methylation of the insulin-like growth factor gene in adult offspring (9). In The Gambia, periconceptional maternal micronutrient supplementation influenced offspring DNA methylation, genome-wide and at imprinted loci (10, 11). Also, Gambian children conceived during the rainy season (August–September) had significantly greater methylation at 5 metastable epialleles than did those conceived in the dry season (12). Metastable epialleles are loci for which epigenotype is established stochastically in the early embryo and maintained thereafter across tissues.
Seasonal variations in food supply and metabolic demand in rural Gambia provide a natural experiment to study effects of maternal dietary intake on epigenetic programming in humans. Here, we evaluated whether seasonal variation in methyl-donor supply could explain the differences in DNA methylation previously reported (12), and ultimately as part of our wider program, using Gambian seasonality to describe links between maternal nutrition, infant epigenome, and phenotypic expression of affected pathways.
SUBJECTS AND METHODS
Study population
This observational study was conducted between July 2009 and June 2010. Nonpregnant women of reproductive age (18–45 y) from 3 villages in the rural area of West Kiang (Jiffarong, Janneh Kunda, and Keneba), The Gambia, were invited to participate. The West Kiang Demographic Surveillance System, run through the Medical Research Council (MRC) Keneba field station, was used as the sampling framework, and the population sample (10 per village) was obtained by random selection, proportional to 5 age groups (18–45 y) to ensure that all age groups were represented. Exclusion criteria included confirmed pregnancy, severe anemia (<70 g/L), menopause, contraceptive use, or plans to move away from the village during the course of the study. All blood samples were tested for hemoglobin concentration to determine anemic status and malaria parasites, and participants were treated at the MRC Keneba when necessary. Any woman excluded during the course of the study (eg, because they became pregnant or chose to withdraw) was replaced by a new participant from the same age group and village. Further details of the duration of subject participation are given elsewhere (see Supplemental Figure S1 under “Supplemental data” in the online issue). Thirty women were visited each month throughout a single calendar year. Each visit involved 2 d of dietary assessment and collection of a fasted blood sample on the third day.
The Scientific Coordinating Committee of MRC Unit, The Gambia, granted scientific approval, and the joint Gambian Government/MRC Ethics Committee (SCC/Ethics Committee 1151) and the London School of Hygiene and Tropical Medicine Ethics Committee (Ethics Committee 5525) granted ethical permission for this study. After community approval, informed written consent was obtained from each woman before participation.
Dietary intake measurement
Dietary records
The foods eaten in West Kiang are mostly grown locally. The diet typically consists of a staple (refined white rice, millet, or maize) with a sauce made from a limited number of ingredients, such as vegetable/palm oil, groundnuts, green leaves, fish, or vegetables (13). Full details of the local diet have been described elsewhere (14). Two main meals are eaten daily, one in the early afternoon and the second in the evening. Breakfast—often cereal porridge, leftovers from the previous day, or tea and bread in a more Westernized style—is eaten by some, but not all women. The food intake of each participant was determined by direct 24-h weighed dietary record on 2 consecutive days each month by using standardized procedures. On each day of assessment, women were visited in their household early in the morning by a trained fieldworker, who then stayed with the subject throughout the day to weigh and record a description of all foods eaten, including meals and intermeal snacks. Because families in this community eat from a common shared bowl, participants were supplied with a standard plastic container to separate and weigh their individual portions (Salter 1020 scale with Aquatronic Feature; 5 kg, 1 g accuracy). After taring the container, staples, sauces, and any other foods were added one-by-one, and the container was weighed after each addition and then reweighed to record the individual components of any leftover food at the end of the meal. Total food intake was thus calculated. Quantitative information on all recipes was obtained by weighing all individual ingredients, including water, as well as the cooked weight of the food (Salter Brecknell WS scale; 15 kg, 5 g accuracy). The food records and recipes also identified all of the different foods, including sauces, spices, and condiments consumed.
Food composition
Contemporaneously, samples of 98 of the most commonly eaten foods in West Kiang were collected at different times of the year from the local markets (see Supplemental Table S1 under “Supplemental data” in the online issue). Up to 8 samples of each food were combined in a single composite sample. Food samples were prepared as consumed (raw or cooked), blended and freeze-dried (Edwards Modulyo EF4) to constant weight before composite samples were prepared for analysis of the target nutrients. Total choline [coefficient of interassay variation (CVi) = 4.7%] and betaine (CVi = 3.5%) were determined by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry (15), including the following choline compounds: free choline, glycerophosphocholine, phosphocholine, phosphatidylcholine, and sphingomyelin, at the University of North Carolina–Chapel Hill Nutrition Obesity Research Center. Riboflavin (CVi = 4.4%), vitamin B-6 (CVi = 6.3%), and methionine (CVi = 4.3%) were determined by gradient HPLC with fluorescence detection against calibration standard solutions of known concentration (in-house methods), whereas folate (CVi = 7.6%) and vitamin B-12 (CVi = 7.8%) were analyzed by a surface plasmon resonance inhibition assay (Biacore Q assay) in a commercial laboratory (Eclipse Scientific Group Ltd).
Dietary intake calculation
The 2 sets of dietary data (dietary records and food composition) were entered into the Diet In Nutrients Out (DINO)—a dietary assessment software application specifically designed by MRC Human Nutrition Research (Cambridge, United Kingdom) to calculate dietary intakes of Gambian foods. Intakes of energy, folate, riboflavin, vitamin B-6, vitamin B-12, choline, betaine, and methionine among the women in this study were thus calculated.
Blood biomarker measurement
Blood biomarker status measurements included folate, riboflavin, vitamin B-6, vitamin B-12, active vitamin B-12 (holotranscobalamin), choline, betaine, and methionine as well as plasma total homocysteine (tHcy), SAM, SAH, and dimethylglycine. An overnight fasted blood sample was collected in the morning of the day after the 2-d dietary assessment. The samples were transported on ice to the MRC Keneba laboratory within an hour and immediately centrifuged, portioned into aliquots, and frozen at −70°C. Red blood cells (RBCs) were washed 3 times with physiologic saline solution (0.9% wt:vol NaCl) and stored at −40°C. Plasma tHcy (CVi = 1.7%), methionine (CVi = 1.1%), cysteine (CVi = 1.4%) (16), choline (CVi = 5.41%), betaine (CVi = 2.66%), dimethylglycine (CVi = 4.30%) (17), SAM (CVi = 1.0%), and SAH (CVi = 3.0%) were measured by liquid chromatography–tandem mass spectrometry in the Nutrition and Metabolism Laboratories of the Child and Family Research Institute at the University of British Columbia (CFRI/UBC), Canada, as previously reported (16, 17). Plasma vitamin B-6 (CVi of pyridoxal = 10.0%, CVi of pyridoxal phosphate = 8.0%, and CVi of pyridoxic acid = 13.0%) was also measured by liquid chromatography–tandem mass spectrometry at the CFRI/UBC based on the method of Midttun et al (18), and plasma vitamin B-12 (CVi = 3.3%), active vitamin B-12 (CVi = 4.5%), and folate (CVi = 7.4%) were measured by using a microparticle enzyme intrinsic factor assay and ion capture assay, respectively, with an AxSyM analyzer (Abbot Laboratories), also at the CFRI/UBC laboratory. Riboflavin status in RBCs was assessed by the erythrocyte glutathione reductase activation coefficient (EGRAC) assay, which measures the ratio of glutathione reductase activity in the presence and absence of added flavin adenine dinucleotide (CVi = 3.1%). EGRAC was performed on a microplate at MRC Human Nutrition Research (19). Higher EGRAC values denote greater riboflavin deficiency.
Statistical analysis
Statistical analyses were performed by using Stata 11.0 (StataCorp). All dietary intake variables and blood biomarkers showed evidence of a positively skewed distribution and were therefore logarithmically transformed and are reported as geometric means. Biomarkers of nutrient status were considered as falling into 2 main groups for analysis: 1) those that were consumed in the diet and also analyzed in blood (eg, folate, riboflavin, vitamin B-6, vitamin B-12, choline, betaine, and methionine) and 2) those that here are considered as functional biomarkers of one-carbon metabolism, namely SAM, SAH, tHcy, and dimethylglycine. Two additional variables were created, the SAM/SAH and betaine ratios, as indexes of methylation potential and the demand for betaine as a methyl donor, respectively. All methylation reactions are catalyzed by methyltransferase enzymes, with the use of SAM as substrate and inhibited by SAH. The SAM/SAH ratio determines the flux through the pathway, and a low SAM/SAH ratio indicates a decreased capacity for methylation, explained by functional inhibition of most cell methyltransferases by increased SAH (20–22). A high dimethylglycine/betaine ratio may indicate a higher rate of betaine methyl group transfer from remethylation of homocysteine (4). The cutoff for statistical significance was considered at P < 0.05.
To assess the reliability and stability of each dietary and biochemical (blood biomarker) variable, autocorrelations were calculated from multiple measurements of each woman 1–5 mo apart. These were performed on detrended values (ie, adjusted for seasonality so that the observed difference between the measurements represented the sum of real variations in intake and/or biomarkers over time and errors in measurement and was not reflecting an underlying seasonality).
Trends along the year were calculated for diet and biomarkers separately. These were established by using random-effects multiconcentration models through generalized least-squares regression to account for the repeat observations for each woman. The Fourier series approach was used to model patterns of seasonality (23). Two pairs of terms were judged to be sufficient for all variables, except for choline dietary intake and plasma vitamin B-12—each of which required 4 pairs of terms. The EGRAC values reflect riboflavin status and are expressed as 1/x for seasonal trend graphic representation. To express the effect size of the seasonality, a coefficient of cyclic variation (CCV) was calculated as the square root of half the sum of the squared coefficients of the Fourier terms. The differences in dietary intake and biomarker concentrations were also calculated by comparing the peak of the rainy/hungry (July–September) and dry/harvest (February–April) seasons.
Finally, dietary predictors of biomarker concentrations were investigated cross-sectionally across all months, by pairwise matching dietary intake with the specific blood biomarker status by simple regression analysis. Multiple linear regression was then used to assess the relation between dietary intake and the functional biomarkers (which had no direct equivalent measured in the diet).
RESULTS
Study population
A total of 86 women were approached; 62 of them consented and took part in the study. The mean age of the study participants was 31 y (range: 18–45 y). Results for the dietary assessments of women who became pregnant during the course of the study were retained, but all biochemical analysis of blood samples taken after conception were removed from the data set, because pregnancy can alter one-carbon metabolism (24). Therefore, there were complete dietary intake assessments for 28 to 30 women for each of the 12-mo and fasted blood samples for 20 to 29 women per month. The average duration of participation in the study was 6 mo.
Dietary intake
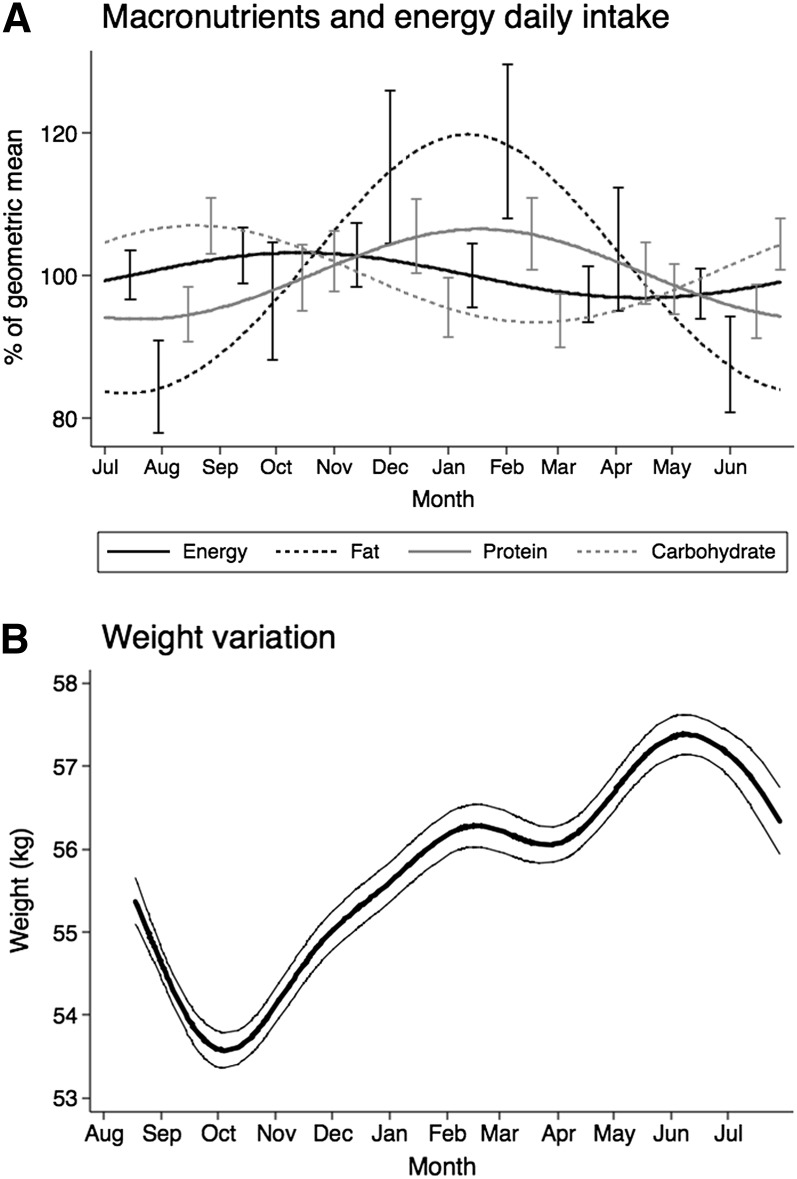
Dietary data were collected for a total of 706 d and included 1143 different recipes. The mean daily weight of food consumed (excluding water intake) was 1553 g (95% CI: 1489, 1619 g/d), with no significant variation over the year (P = 0.78). The overall mean energy intake was 1830 kcal/d (95% CI: 1712, 1816), with no significant variation over the year (P = 0.60). However, the dietary macronutrient composition did change across the year. The intake of fat (averaging 20.4% of the total energy intake), protein (11.7%), and carbohydrates (67.9%) varied significantly throughout the year, as shown in Figure 2A. Seasonal variation in body weights for all of the nonpregnant women in the study region exhibited seasonal variation as observed previously for this community, with a dramatic decrease during the rainy season (a period of heavy agricultural workload) and sustained weight gain during the dry season (Figure 2B).
FIGURE 2.
Seasonal trends in energy and macronutrient intakes and weight. A: n dietary intake measurements were made in an average of 29 women × 12 mo × 2 d. 95% CIs are shown with bars, at 2-mo intervals (different by substance to avoid excessive overlap and help visual clarity). Generalized least-squares regression with one pair of Fourier terms was used (P < 0.05 for fat, protein, and carbohydrate only). B: n = 517 subjects × (1–12 mo) = 2747 measure points. Thick line: arithmetic mean. Thin lines: 95% upper and lower CIs. Generalized least-squares regression with 4 pairs of Fourier terms was used.
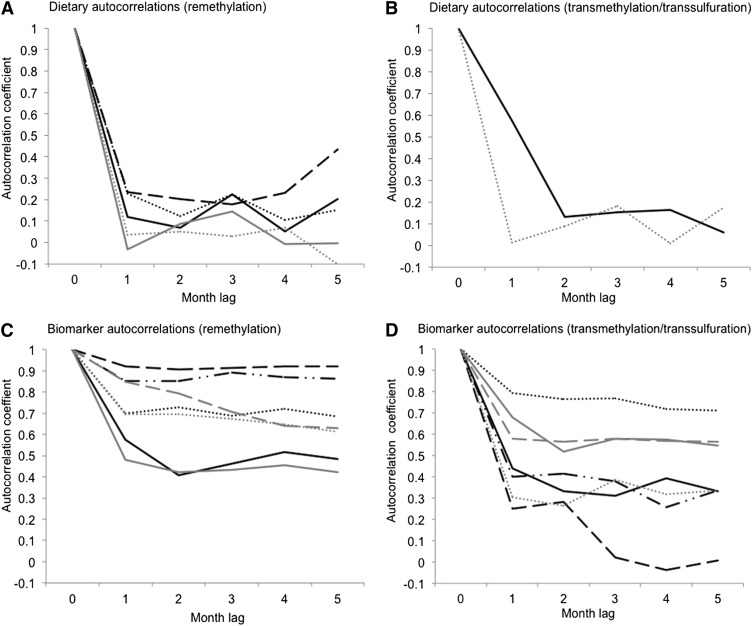
The results of the autocorrelations to establish the reliability (precision of a single measurement or extrapolation back to zero lag) and stability (variation with time not related to seasonality) of the 48-h dietary intakes are shown in Figure 3A. In addition, repeatability of measurements on 2 consecutive days ranged between 40.1% and 60.1%. CCVs are shown in Table 1. The dietary intakes of folate, riboflavin, vitamin B-6, and choline were significantly below the current international recommendations (Table 1), expressed as the Estimated Average Requirement (EAR). The EAR is the daily dietary intake of a nutrient expected to satisfy the needs of 50% of a population group. The dietary intakes surpassing these recommendations are also in Table 1 and were <30% for all intakes except vitamin B-12 (100%) and methionine (98.9%).
FIGURE 3.
Reliability and stability of dietary intakes and blood biomarker concentrations for substances under study. Black lines: solid (A and C, folate; B and D, methionine), dot (A and C, riboflavin; D, SAM), dash (A and C, vitamin B-12; D, SAH), dash-dot-dot (C, active vitamin B-12; D, SAM/SAH). Gray lines: solid (A and C, choline; D, homocysteine), dot (A and C, betaine; B and C, vitamin B-6), dash (C, DMG; D, cysteine). Autocorrelation (internal correlation) of each variable measurement with measurements for the same woman 1 to 5 months apart (lag: 1–5), adjusted by seasonality. Dietary intake measurements were made in an average of 29 women × 12 mo × 2 d. Number of biomarker measurements = 316 (293 for riboflavin). DMG, dimethylglycine; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
TABLE 1.
Dietary intakes of one-carbon metabolites in Gambian women of reproductive age and international intake recommendations for women1
| Nutrient | 12-mo intake2 | EAR (prevalence of adequacy)3 | Rainy season intake (hungry: July–September)2 | Dry season intake (harvest: February–April)2 | Difference between seasons | Repeatability of measurement on 2 consecutive days4 | CCV5 (95% CI) |
| % | % | % | |||||
| Folate (μg/d) | 131.7 (69.2, 251.5) | 320 (1.2) | 120.5 (112.3, 129.2) | 138.4 (129.0, 148.6) | −14.9 | 52.1 | 6.6 (3.3, 9.9) |
| Riboflavin (mg/d) | 0.30 (0.08, 1.18) | 0.9 (6.2) | 0.27 (0.23, 0.31) | 0.30 (0.26, 0.35) | −12.1 | 60.1 | 12.3 (5.4, 19.2) |
| Vitamin B-12 (μg/d) | 2.7 (0.4, 21.0) | 2 (100) | 2.8 (2.2, 3.5) | 2.6 (2.1, 3.3) | 5.8 | 44.9 | 14.7 (4.7, 24.7) |
| Total choline (mg/d) | 155.26 (60.6, 396.7) | 4257 (2.8) | 158.4 (142.0, 176.7) | 158.4 (141.9, 176.7) | 0.1 | 52.9 | 15.1 (10.4, 19.8) |
| Betaine (mg/d) | 33.5 (6.9, 163.3) | NA | 42.3 (35.3, 50.6) | 28.6 (23.9, 34.2) | 32.4 | 48.8 | 31.8 (24.2, 39.4) |
| Vitamin B-6 (mg/d) | 0.92 (0.49, 1.75) | 1.1 (28.7) | 0.88 (0.81, 0.95) | 0.93 (0.86, 1.01) | −6.4 | 40.1 | 4.3 (1.0, 7.6) |
| Methionine (g/d) | 2.03 (1.23, 3.38) | 0.88 (98.9) | 2.06 (1.94, 2.18) | 2.11 (1.99, 2.24) | −2.7 | 44.7 | 2.4 (0.1, 4.8) |
n = average of 29 women × 12 mo × 2 d. NA, not available.
Values are geometric means; 95% CIs in parentheses (geometric mean ± 2 SD).
Estimated Average Requirement (25, 26); the daily dietary intake of a nutrient expected to satisfy the needs of 50% of a population group; percentages of the observations achieving the EAR in parentheses.
Defined as measurement of the reliability calculated on how similar the intakes between 2 consecutive 24-h weighed record measurements were in each subject.
Coefficient of cyclic variation, calculated as the square root of half the sum of the squared coefficients of the Fourier terms.
Percentage contribution to total choline: 52.6% from free choline, 15.6% from glycerophosphocholine, 3.3% from phosphocholine, 37.6% from phosphatidylcholine, and 0.8% from sphingomyelin.
Adequate Intake: dietary intake believed to be adequate for everyone in the demographic group to maintain health; established when no sufficient data to establish EAR were available. Only an Adequate Intake has been set for choline (26).
Recommendations for methionine + cysteine are 19 mg/kg per day, calculated for an average weight of 55 kg.
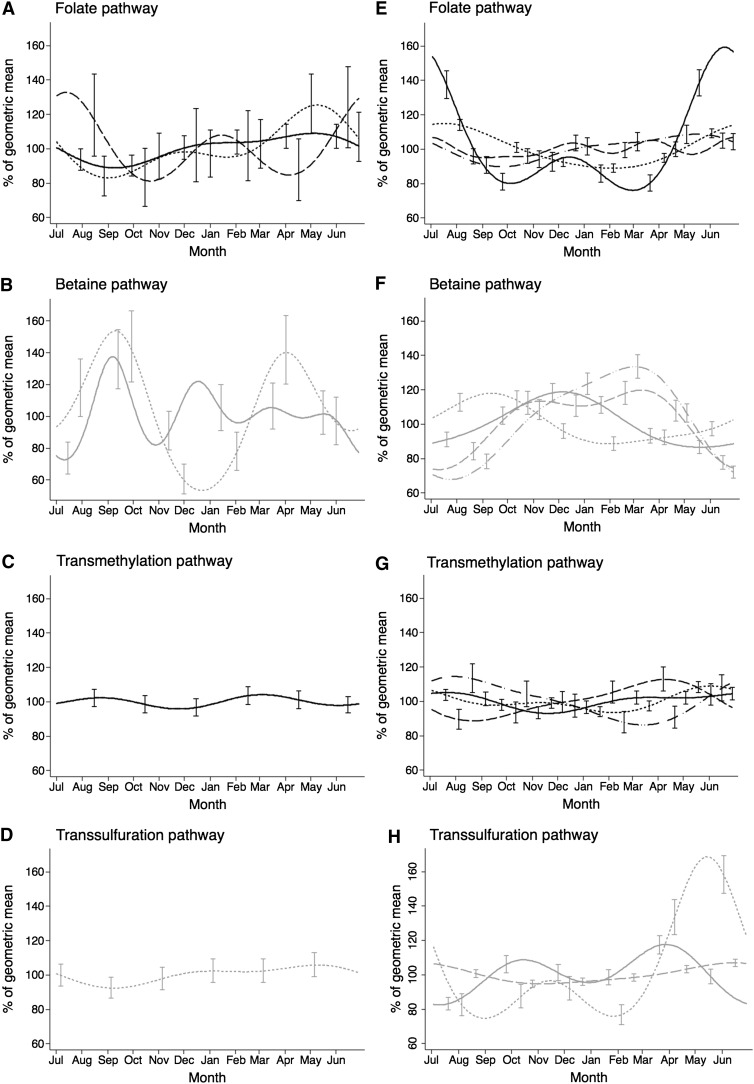
The monthly variation in dietary intakes, expressed as percentage deviation from the overall geometric mean, are illustrated in Figure 4 (A, B, C, and D). The annual variation in the intakes of folate (P = 0.0038), riboflavin (P = 0.016), and choline and betaine (P < 0.0001 for both) was statistically significant. The results show that the intakes of riboflavin and folate decreased over the rainy season until September, followed by an increase, and reached their highest intake toward the end of the dry season peak (May). Choline and betaine intakes were highest in September, with second peaks in December and April, respectively. Methionine, vitamin B-6, and B-12 intakes, conversely, did not vary significantly throughout the year (P > 0.05). Of those nutrient intakes that showed significant variation over the year, substantial differences between both seasons were also shown for betaine, folate, and riboflavin (Table 1) but not for total choline. Riboflavin and folate were higher during the peak of the dry season (February to April; 12.1% and 14.9%, respectively), and betaine was higher during the peak of the rainy season (July to September; 32.4%).
FIGURE 4.
Seasonal trends of methyl donors and cofactors as per dietary intake and blood biomarker concentrations expressed as a percentage of the geometric mean of all measurements (between July 2009 and June 2010). Black lines: solid (A and E, folate; C and G, methionine), dot (A and E, riboflavin; G, SAM), dash (A and E, vitamin B-12; G, SAH), dash-dot-dot (E, active vitamin B-12; G, SAM/SAH). Gray lines: solid (B and F, choline; H, homocysteine), dot (B and F, betaine; D and H, vitamin B-6), dash (F, DMG; H, cysteine), dash-dot-dot (F, DMG/betaine). Dietary intake measurements were made in an average of 29 women × 12 mo × 2 d. Number of biomarker measurements = 316 (293 for riboflavin). Bars indicate 95% CIs at 2-mo intervals (different by substance to avoid excessive overlap and for visual clarity). Generalized least-squares regression with 2 pairs of Fourier terms was used, except for choline dietary intake and plasma vitamin B-12—each of which required 4 pairs of terms. Riboflavin status is shown as the inverse of EGRAC assay results. DMG, dimethylglycine; EGRAC, erythrocyte glutathione reductase activation coefficient; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
Blood biomarkers
During the 12 mo of study, 316 plasma samples were obtained for analyses. Fewer RBC samples (a total of 293) were prepared for analysis of riboflavin, because it was not always possible to obtain the second heparinized blood sample required. The autocorrelation calculations for biomarkers showed that, with the exception of SAH and dimethylglycine, all were stable (ie they correlate almost as well at 5 mo as at 1 mo lag) (Figure 3B). Different degrees of reliability were apparent: the measurements of vitamins B-12 and active vitamin B-12 were highly reliable, whereas others, particularly SAH, showed poorer reliability (ie, the correlation was weak, even at 1 mo lag).
The geometric means and CCVs of biomarker concentrations for this group of Gambian women over the 1-y study period are shown in Table 2. The annual variation in blood biomarker concentrations, as a percentage variation from the geometric mean, are illustrated in Figure 4 (E, F, G, and H). All blood biomarkers showed large, significant annual variation (vitamin B-12, P = 0.03; all the other biomarkers, P < 0.001). However, the actual seasonal pattern of variation differed in timing and amplitude (magnitude of variation from the geometric mean, as indicated by the CCV) between the various biomarkers measured, with the highest seasonal variation for plasma folate, vitamin B-6, and the ratio of dimethylglycine to betaine. The status of plasma folate and vitamin B-6 was highest from May to June, at the end of the dry season, and was lowest from September to March (Figure 4, E and H). Riboflavin status declined progressively from the beginning of the rainy season peak (July) until January and then improved again. Betaine reached its peak later in the rainy season (September; Figure 4E). Plasma choline showed higher concentrations in November, whereas vitamin B-12 and methionine, in contrast with the other nutrients, remained relatively stable over the year (Figure 4, E, F, and G, respectively). Regarding the functional biomarkers, the SAM/SAH ratio was highest in July and lowest in March, and the seasonal fluctuation in the ratio was explained largely by the seasonal change in SAH. Plasma tHcy showed an opposite change; the lowest and highest plasma tHcy concentrations were found in July and April, respectively. Plasma dimethylglycine concentrations were highest between November and March, at which time the ratio of dimethylglycine to betaine reached the highest peak.
TABLE 2.
Mean concentrations of blood biomarkers throughout the year and by season1
| Biomarkers | 12-mo intake2 | Cutoff for adequacy (prevalence of adequacy)3 | Rainy season intake (hungry: July–September)2 | Dry season intake (harvest: February–April)2 | Difference between seasons | CCV (95% CI)4 |
| % | % | |||||
| Folate (nmol/L) | 14.9 (6.6, 33.4) | >10 (82.3)5 | 13.4 (12.2, 14.9) | 12.5 (11.4, 13.9) | 6.7 | 23.8 (20.5, 26.5) |
| Riboflavin (EGRAC coefficient)6 | 2.4 (1.5, 3.7) | <1.37 (0.7) | 2.3 (2.1, 2.4) | 2.6 (2.5, 2.7) | −14.16 | 9.0 (7.6, 10.4) |
| Vitamin B-12 (pmol/L) | 342.3 (139.1, 842.2) | >2208 (80.7) | 333.7 (285.9, 389.6) | 353.1 (302.5, 412.1) | −5.8 | 3.5 (1.9, 5.1) |
| Active vitamin B-12 (pmol/L) | 70.9 (20.0, 251.5) | >379 (85.8) | 67.2 (55.9, 80.8) | 76.3 (63.5, 91.7) | −13.5 | 6.2 (3.7, 8.8) |
| Choline (μmol/L) | 8.2 (4.7, 14.0) | >1010 (20.9) | 8.2 (7.6, 8.8) | 8.3 (7.7, 8.9) | −1.3 | 11.1 (8.9, 13.4) |
| Betaine (μmol/L) | 40.4 (20.9, 78.4) | —11 | 45.7 (41.7, 50.1) | 36.5 (33.3, 39.9) | 20.1 | 9.8 (7.6, 12.0) |
| DMG (μmol/L) | 3.3 (1.1, 9.3) | — | 2.9 (2.5, 3.4) | 3.4 (2.9, 4.0) | −18.4 | 16.3 (13.4, 19.3) |
| DMG/betaine | 0.08 (0.03, 0.26) | — | 0.06 (0.05, 0.07) | 0.09 (0.08, 0.11) | −49.0 | 23.3 (20.6, 25.9) |
| Methionine (μmol/L) | 29.1 (19.6, 43.3) | — | 28.9 (27.3, 30.7) | 29.4 (27.7, 31.1) | −1.5 | 3.9 (2.0, 5.8) |
| SAM (nmol/L) | 90.3 (59.1, 137.8) | — | 90.3 (85.3, 95.5) | 86.3 (81.6, 91.2) | 4.4 | 4.6 (3.0, 6.1) |
| SAH (nmol/L) | 12.2 (6.8, 21.9) | — | 11.1 (10.5, 11.9) | 12.9 (12.1, 13.7) | −15.9 | 7.8 (4.7, 10.9) |
| SAM:SAH | 7.42 (3.8, 14.5) | — | 8.1 (7.6, 8.7) | 6.7 (6.2, 7.2) | 17.6 | 9.4 (5.8, 12.9) |
| Homocysteine (μmol/L) | 8.03 (4.2, 15.3) | <1512 (98.8) | 7.9 (7.2, 8.7) | 8.1 (7.4, 9.0) | −2.9 | 10.2 (7.9, 12.5) |
| Vitamin B-6 (nmol/L) | 39.7 (14.3, 110.6) | — | 33.8 (29.8, 38.2) | 36.0 (31.8, 40.7) | −6.6 | 26.2 (22.4, 30.0) |
| Cysteine (μmol/L) | 224.9 (170.8, 296.1) | — | 217.1 (207.9, 226.7) | 219.0 (209.9, 228.6) | −0.9 | 4.1 (3.0, 5.2) |
n = 316 (293 for riboflavin). CCV, coefficient of cyclic variation; DMG, dimethylglicine; EGRAC, erythrocyte glutathione reductase activation coefficient; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
Values are geometric means; 95% CIs in parentheses (geometric mean ± 2 SD).
Percentages of the observations beyond the cutoff in parentheses.
Calculated as the square root of half the sum of the squared coefficients of the Fourier terms.
From reference 27.
For the riboflavin assay, higher EGRAC values reflect higher riboflavin deficiency.
From reference 28.
From reference 29.
From reference 30.
From reference 26.
Indicates that no suitable cutoff could be identified.
From reference 31.
As for dietary intake, the concentration differences between the peak of the rainy and dry seasons for each biomarker are shown in Table 2. Comparison of the biomarker concentrations according to these divisions showed that plasma SAM, betaine, and folate concentrations and SAM/SAH ratio were higher, whereas plasma SAH, tHcy, dimethylglycine, the ratio of dimethylglycine to betaine, vitamin B-12, and vitamin B-6 were lower in the rainy than in the dry season (Table 2). A lower EGRAC value during the rainy season denotes a less-deficient riboflavin status. Cysteine, choline, and methionine exhibited some variation between seasons, but this represented a difference between seasons of <1.5%. Differences among villages, possibly mediated by subtle differences in wealth and dietary habits within this generally homogeneous environment, was significant for intakes of vitamin B-12 (P = 0.0023) and betaine (P = 0.0024) and for plasma concentrations of choline (P value= 0.0039), folate (P = 0.0131), methionine (P = 0.0022) and the SAM/SAH ratio (P = 0.0038). However, the effect of village did not confound other effects.
Dietary intake and blood biomarkers
Next, the relations between the dietary intakes of the nutrients under study and their blood concentrations were regressed cross-sectionally to determine the predictive ability of dietary intake on blood biomarkers. Additional comparisons were made between selected nutrients to investigate interactions in specific pathways.
Positive associations between dietary intakes and respective plasma biomarkers were not observed for folate, choline (with total choline or any of its components), or methionine (data not shown). Significant positive associations were found between the dietary intake and blood biomarkers for vitamin B-6 (β: 0.166 nmol/L per mg/d; 95% CI: 0.017, 0.316), betaine (β: 0.037 μmol/L per mg/d; 95% CI: 0.006, 0.069), and riboflavin (β: −0.038 EGRAC points per mg/d; 95% CI: −0.064, −0.0112, where a higher EGRAC indicates greater deficiency and thus explained the negative coefficient). Dietary vitamin B-12 showed no significant association with plasma total vitamin B-12, but was positively associated with plasma active vitamin B-12 (β: 0.043 pmol/L per μg/d; 95% CI: 0.015, 0.071). Plasma concentrations of folate were negatively associated with the combined intake of choline and betaine (β: −0.214 μmol/L per mg/d; 95% CI: −0.297, −0.131). Plasma concentrations of choline and betaine were not, however, associated with folate intake, but were inversely associated with the intake of riboflavin [choline (β: −0.065 μmol/L per mg/d; 95% CI: −0.103, −0.028); betaine (β: −0.058, 95% CI: −0.096, 0.021)].
To explore the potential effect of the dietary intake of the selected nutrients on one-carbon metabolism more generally, we also determined the strength of the associations between dietary intake and functional blood biomarker status. No statistically significant associations were found between the plasma SAM/SAH ratio or plasma SAM concentration and the intake of any of the nutrients under study. Plasma SAH showed an association with vitamin B-12 intake (β = 0.039 nmol/L per μg/d; 95% CI: 0.006, 0.072). Plasma tHcy and dimethylglycine concentrations were correlated with the combined intake of choline (β = 0.121 μmol/L per mg/d; 95% CI: 0.060, 0.182) and betaine (β= 0.088 μmol/L per mg/d; 95% CI: 0.004, 0.172). As found for plasma choline and betaine, the plasma dimethylglycine was also inversely associated with the dietary intake of riboflavin (β = −0.086 μmol/L per mg/d; 95% CI: −0.152, −0.020).
DISCUSSION
To our knowledge, this was the first study to comprehensively address dietary intakes and blood biomarkers relevant to one-carbon metabolism in an African population. As hypothesized, dietary intakes and blood biomarkers of one-carbon–related nutrients and metabolites varied widely across the year. Our results confirm that seasonal fluctuations in one-carbon–related nutrients and metabolites provide a natural experiment to address the biological underpinnings of the season-of-conception–dependent changes in DNA methylation of metastable epialleles recently reported by us in a retrospective study in this population (12).
Macronutrient intakes and energy balance
Overall energy intake was constant across the year. This is contrary to studies conducted several decades ago, which found that energy intakes were considerably lower during the rainy/hungry season (32). Several factors may contribute to this, including now-common financial remittances from family members living abroad that permit purchase of staple foods in the rainy/hungry season. However, because of differences in agricultural workload (33), body weights showed the customary annual fluctuation with a loss of ∼3.5 kg between June and October with a gradual replenishment over the rest of the year. The macronutrient composition of the diets also changed, most notably for fat, which was lowest from May to September and highest from December to March. Protein intake was ∼10% lower in the rainy season (June to October) than in the dry season.
Seasonal variations in physical work, energy balance, and nutrient intake may affect the supply and demand of methyl groups and the metabolic pathways through which methyl nutrients are used. De novo synthesis of phosphatidylcholine (34) and creatine (35) have been suggested to be major net consumers of methyl groups. The demand for de novo phosphatidylcholine synthesis, however, is complex and depends on the dietary supply of preformed choline, the demand for choline as a source of methyl groups for methylation of homocysteine, and the needs of phosphatidylcholine and sphingomyelin for membrane, bile, and plasma phospholipid synthesis and turnover. Creatine, on the other hand, likely depends on muscle mass and muscle energy metabolism because creatine phosphate is the major high energy store in muscle (34, 35). The current study design, which involved seasonal fluctuations in both body weight and physical work, do not allow us to draw clear inferences about how changes in utilization of methyl groups for de novo phosphatidylcholine or creatine synthesis affected the measures of biomarker status. Although it is reasonable to speculate that creatine synthesis and utilization may be highest in the rainy season, when physical workload is highest, this also coincides with a loss of body weight, which may ameliorate the effects somewhat.
Micronutrient and methyl donor intakes
The dietary intakes of riboflavin, folate, choline, and betaine show clear seasonality, whereas methionine, vitamin B-6, and vitamin B-12 intakes remain stable over the year (Figure 4). A possible explanation is that the major dietary source of vitamin B-12 (fish), which is also the second major source of methionine after rice, remained relatively constant month by month. Vitamin B-6 is nearly ubiquitous in foods. Ramadan, which fell between 20 August and 20 September 2009 during our study, may also have contributed to the peaks in dietary intake of betaine and choline from foods such as bread, potatoes, and eggs, which are consumed more frequently when breaking the fast (20). The median dietary intakes of folate, riboflavin, and choline were <50%, and vitamin B-6 intake was 84% of the current EAR.
Data on seasonal variation in the intake of one-carbon–related nutrients in this population have been reported only for riboflavin and folate from 1979 to 1981 (36). The deficient intake of riboflavin in previous work (29) and in the current study is consistent with the scarcity of milk and meat in the diet (14). Riboflavin intake in the current study was lowest during the rainy season, which is also consistent with dietary patterns found 3 decades ago (36). The current seasonal variability in folate intake, however, is less consistent with previous data (36), perhaps explained by more recent cultivation of green leaves in home gardens throughout the year.
The variability in dietary intake when measured on 2 consecutive days was very low, which suggested that the women tend to eat similar foods from one day to another within a short time period. However, the dietary intake changed from one month to another, after adjustment for seasonality (Figure 3A), which indicates a low reliability in the measure of habitual intake. Many factors could contribute to this, including cash availability for purchasing foods, independent of season. Regardless, it is clear that dietary intake data should be interpreted with caution and that plasma biomarkers may offer a more robust assessment of one-carbon–nutrient status than dietary intake.
Blood biomarkers
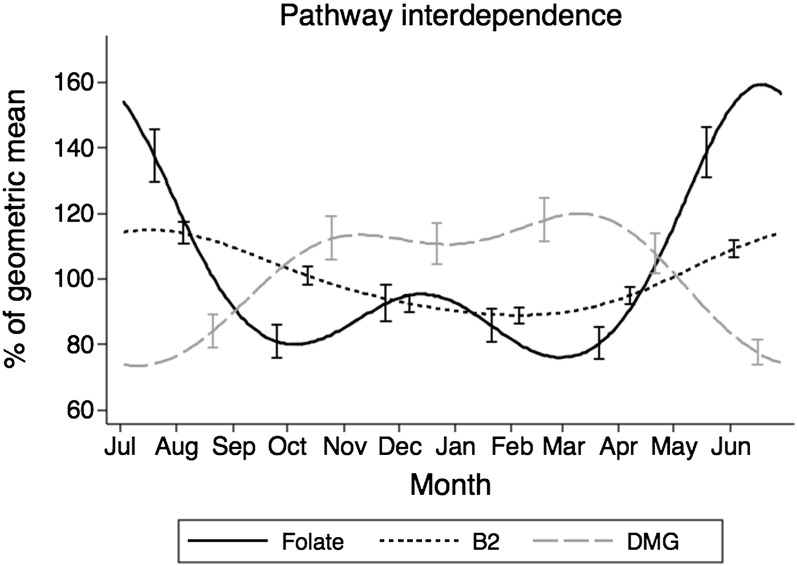
All plasma biomarkers measured showed significant annual variation (Figure 4), although the timing of the peaks and troughs differed between the different biomarkers. Experimental studies have shown a coordinated reciprocal regulation of the folate-dependent and betaine-dependent remethylation of homocysteine to methionine, such that deficiency of either folate or choline and betaine leads to increased utilization of the alternate pathway (37). Notably, the increase in dimethylglycine and in the ratio of dimethylglycine to betaine occurred inversely to the decrease in folate and riboflavin (Figure 4F, Figure 5), consistent with a reciprocal switch from the folate-dependent to the betaine-dependent remethylation of homocysteine by betaine-homocysteine methyltransferase (Figure 1). Riboflavin deficiency is highly prevalent in this population, which raises the possibility that the seasonal nadirs in riboflavin status may impair the folate remethylation pathway and lead to an activation of the betaine pathway. This effect of riboflavin would be due to its role as cofactor for methylenetetrahydrofolate reductase, which catalyzes the formation of 5-methyltetrahydrofolate needed for homocysteine remethylation (Figure 1) (38). Whereas vitamin B-12, which is a cofactor for methionine synthase (Figure 1), could have a similar effect, blood concentrations of vitamin B-12 do not seem to be limiting in this population. Plasma tHcy decreased when vitamin B-6 increased (Figure 4H), which suggests that homocysteine entry into the transsulfuration pathway, leading to cysteine (Figure 1), is a major determinant of homocysteine flux.
FIGURE 5.
Seasonal trends of folate, B2, and DMG blood concentrations, expressed as a percentage of the geometric mean of all measurements (between July 2009 and June 2010). Interdependence of folate-dependent and betaine-dependent pathways. Number of biomarker measurements = 316 (293 for riboflavin). Bars indicate 95% CIs at 2-mo intervals (different by substance to avoid excessive overlap and for visual clarity). Generalized least-squares regression with 2 pairs of Fourier terms was used. Riboflavin status is shown as the inverse of EGRAC assay results. B2, riboflavin; DMG, dimethylglicine; EGRAC, erythrocyte glutathione reductase activation coefficient.
A higher availability of methyl groups and cofactors—as seen with increased concentrations of folate, betaine, and riboflavin over the rainy season—may explain why SAM is also higher at this time. Transfer of methyl groups from SAM to a methyl acceptor also generates SAH, which is further metabolized to homocysteine in a reversible reaction. Efficient removal of homocysteine by remethylation or transsulfuration maintains the forward reaction and a high SAM/SAH ratio, which in the current study was 17.6% higher in the rainy season. In contrast, higher SAH with a decrease in the SAM/SAH ratio inhibits many cell methyltransferases (20–22), consistent with lower DNA methylation at specific loci in Gambian children conceived during the dry season (12).
The stability and reliability of our biomarker measurements are generally better than those of dietary intakes measurements. The comparatively lower reliability of SAH and dimethylglycine measures could be due to strong intraindividual fluctuations or to sample processing. SAM may convert to SAH during sample processing; however, every effort was made to minimize delays in processing, and we regarded this to exert minimal effects on our measurements.
Dietary intake and blood biomarkers
Dietary intake was directly associated with the blood biomarkers only for betaine, riboflavin, vitamin B-6, and active vitamin B-12. The weak associations between the monthly trends in biomarker concentrations and their estimated dietary intake may involve complex effects of balance between supply and demand, mutual interdependence of these nutrients in metabolic pathways, and unavoidable inherent errors associated with estimating dietary intake. The reciprocal interactions between the folate- and betaine-dependent pathways for remethylation of homocysteine (39) and the potential for both diet and endogenous synthesis to contribute to plasma choline and betaine are examples of this complexity (40).
Limitations of the study
As is true of most studies that attempt to assess nutrient intakes in free-living conditions, this study had several limitations. Although the weighed food record is the preferred method for quantitative dietary assessment (24), behavioral changes from the daily intakes could have taken place (41). As in any dietary assessment, errors in food-composition data can also introduce error to the accuracy of estimates of nutrient intakes. The random errors inherent to any dietary assessment method could have attenuated correlations between the estimated intakes and blood nutrient concentrations (42), as suggested by the reliability curves shown in Figure 3. Last, the need to replace study participants as a result of withdrawal throughout the year may have introduced an additional layer of variation.
Conclusions
This study showed a natural climate-dependent seasonality in dietary intake and nutrition status. This provided an excellent opportunity to examine the links between diet and the metabolome and their implications for DNA methylation in humans, particularly periconceptional and gestational effects, in a “natural experiment.” This study allowed us to reach the following conclusions: 1) because of the biases and imprecision inherent in estimating dietary intake and the complex relations between intake and nutrient utilization in metabolic pathways, plasma biomarkers are preferable, more accurate, and more proximal indicators of methyl donor status; 2) the reciprocal compensation between the folate- and betaine-dependent remethylation pathways (39) may be driven by a moderate-to-severe riboflavin deficiency that is paradoxically worst in the dry/harvest season; 3) contrary to our initial assumption that the rainy/hungry season would put women at greatest risk of methyl donor deficiency, the reverse was clearly apparent as evidenced by a lower homocysteine concentration and higher SAM/SAH ratio; and 4) these findings are consistent with our previously reported findings of elevated DNA methylation at metastable epialleles in individuals conceived during the rainy season (12).
Supplementary Material
Acknowledgments
We thank the women of West Kiang who patiently participated in the study and acknowledge the enthusiastic work of the fieldworkers, laboratory technicians, and nurses who collected the data and samples and all the staff at MRC Keneba in general. We also thank Dorothea Nitsch (London School of Hygiene & Tropical Medicine) and Chris Bates, Lorna Cox, Gail Goldberg, and Celia J Prynne (MRC Human Nutrition Research) for their valuable advice and input to the study and Tongwen Wang (University of North Carolina) and Jannette King (University of British Columbia) for their assistance with the laboratory analyses.
The authors’ responsibilities were as follows: PD-S, SEM, SEC, AJCF, RAW, AMP, and BJH: conceived and designed the study; PD-S, SEM, and BJH: conducted the research; PD-S: was involved in all hands-on experiments, conducted the sample and data collection, and drafted the manuscript; PD-S and AJCF: performed the statistical analyses; DC: provided technical support for dietary intake data processing; and K-AdC, RAD, SMI, and SHZ: conducted the biochemical analyses. All authors critically revised and approved the manuscript. No conflicts of interest were declared.
Footnotes
Abbreviations used: CCV, coefficient of cyclic variation; CFRI/UBC, Child and Family Research Institute at the University of British Columbia; CVi, coefficient of interbatch variation; EAR, Estimated Average Requirement; EGRAC, erythrocyte glutathione reductase activation coefficient; MRC, Medical Research Council; RBC, red blood cell; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; tHcy, total homocysteine.
REFERENCES
- 1.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002;31:1235–9 [DOI] [PubMed] [Google Scholar]
- 2.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 2009;10:295–304 [DOI] [PubMed] [Google Scholar]
- 3.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr 2007;27:363–88 [DOI] [PubMed] [Google Scholar]
- 4.Dominguez-Salas P, Cox SE, Prentice AM, Hennig BJ, Moore SE. Maternal nutritional status, C(1) metabolism and offspring DNA methylation: a review of current evidence in human subjects. Proc Nutr Soc 2012;71:154–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr 2006;136(suppl):1636S–40S [DOI] [PubMed] [Google Scholar]
- 6.Kalhan SC, Marczewski SE. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev Endocr Metab Disord 2012;13:109–19 [DOI] [PubMed] [Google Scholar]
- 7.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol 2003;23:5293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA 2007;104:19351–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 2008;105:17046–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper WN, Khulan B, Owens S, Elks CE, Seidel V, Prentice AM, Belteki G, Ong KK, Affara NA, Constancia M, et al. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: results of a pilot randomized controlled trial. FASEB J 2012;26:1782–90 [DOI] [PubMed] [Google Scholar]
- 11.Khulan B, Cooper WN, Skinner BM, Bauer J, Owens S, Prentice AM, Belteki G, Constancia M, Dunger D, Affara NA. Periconceptional maternal micronutrient supplementation is associated with widespread gender related changes in the epigenome: a study of a unique resource in the Gambia. Hum Mol Genet 2012;21:2086–101 [DOI] [PubMed] [Google Scholar]
- 12.Waterland RA, Kellermayer R, Laritsky E, Rayco-Solon P, Harris RA, Travisano M, Zhang W, Torskaya MS, Zhang J, Shen L, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet 2010;6:e1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prentice A, Laskey MA, Shaw J, Hudson GJ, Day KC, Jarjou LM, Dibba B, Paul AA. The calcium and phosphorus intakes of rural Gambian women during pregnancy and lactation. Br J Nutr 1993;69:885–96 [DOI] [PubMed] [Google Scholar]
- 14.McCrae JE, Paul AA. 2nd ed. Cambridge, United Kingdom: Centre MRC Dunn Nutrition, 1996. [Google Scholar]
- 15.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem 2002;74:4734–40 [DOI] [PubMed] [Google Scholar]
- 16.Innis SM, Davidson AG, Melynk S, James SJ. Choline-related supplements improve abnormal plasma methionine-homocysteine metabolites and glutathione status in children with cystic fibrosis. Am J Clin Nutr 2007;85:702–8 [DOI] [PubMed] [Google Scholar]
- 17.Innis SM, Hasman D. Evidence of choline depletion and reduced betaine and dimethylglycine with increased homocysteine in plasma of children with cystic fibrosis. J Nutr 2006;136:2226–31 [DOI] [PubMed] [Google Scholar]
- 18.Midttun O, Hustad S, Solheim E, Schneede J, Ueland PM. Multianalyte quantification of vitamin B6 and B2 species in the nanomolar range in human plasma by liquid chromatography-tandem mass spectrometry. Clin Chem 2005;51:1206–16 [DOI] [PubMed] [Google Scholar]
- 19.Vuilleumier JP, Keller HE, Keck E. Clinical chemical methods for the routine assessment of the vitamin status in human populations. Part III: The apoenzyme stimulation tests for vitamin B1, B2 and B6 adapted to the Cobas-Bio analyzer. Int J Vitam Nutr Res 1990;60(2):126–35. [PubMed] [Google Scholar]
- 20.James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J Nutr 2002;132(suppl):2361S–6S [DOI] [PubMed] [Google Scholar]
- 21.Caudill MA, Wang JC, Melnyk S, Pogribny IP, Jernigan S, Collins MD, Santos-Guzman J, Swendseid ME, Cogger EA, James SJ. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J Nutr 2001;131:2811–8 [DOI] [PubMed] [Google Scholar]
- 22.Yi P, Melnyk S, Pogribna M, Pogribny IP, Hine RJ, James SJ. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J Biol Chem 2000;275:29318–23 [DOI] [PubMed] [Google Scholar]
- 23.Fulford AJ, Rayco-Solon P, Prentice AM. Statistical modelling of the seasonality of preterm delivery and intrauterine growth restriction in rural Gambia. Paediatr Perinat Epidemiol 2006;20:251–9 [DOI] [PubMed] [Google Scholar]
- 24.Gibson R. Principles of Nutritional Assessment, 2nd ed. New York: Oxford University Press, Inc., 2005 [Google Scholar]
- 25.WHO/FAO Vitamin and mineral requirements in human nutrition. 2nd ed. Rome, Italy: WHO/FAO, 2004 [Google Scholar]
- 26. Institute of Medicine, National Academy of Sciences. Dietary reference intakes for folate, thiamine, riboflavin, niacin, vitamin B12, panthothenic acid, biotine, and choline. Washington, DC: IOM, 1998. [PubMed]
- 27.Dhonukshe-Rutten RA, de Vries JH, de Bree A, van der Put N, van Staveren WA, de Groot LC. Dietary intake and status of folate and vitamin B12 and their association with homocysteine and cardiovascular disease in European populations. Eur J Clin Nutr 2009;63:18–30 [DOI] [PubMed] [Google Scholar]
- 28.Hill MH, Bradley A, Mushtaq S, Williams EA, Powers HJ. Effects of methodological variation on assessment of riboflavin status using the erythrocyte glutathione reductase activation coefficient assay. Br J Nutr 2009;102:273–8 [DOI] [PubMed] [Google Scholar]
- 29.Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr 2011;94:666S–72S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loikas S, Lopponen M, Suominen P, Moller J, Irjala K, Isoaho R, Kivela SL, Koskinen P, Pelliniemi TT. RIA for serum holo-transcobalamin: method evaluation in the clinical laboratory and reference interval. Clin Chem 2003;49:455–62 [DOI] [PubMed] [Google Scholar]
- 31.Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 2004;50:3–32 [DOI] [PubMed] [Google Scholar]
- 32.Prentice AM, Whitehead RG, Roberts SB, Paul AA. Long-term energy balance in child-bearing Gambian women. Am J Clin Nutr 1981;34:2790–9 [DOI] [PubMed] [Google Scholar]
- 33.Roberts SB, Paul AA, Cole TJ, Whitehead RG. Seasonal changes in activity, birth weight and lactational performance in rural Gambian women. Trans R Soc Trop Med Hyg 1982;76:668–78 [DOI] [PubMed] [Google Scholar]
- 34.Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. Is it time to reevaluate methyl balance in humans? Am J Clin Nutr 2006;83:5–10 [DOI] [PubMed] [Google Scholar]
- 35.Mudd SH, Poole JR. Labile methyl balances for normal humans on various dietary regimens. Metabolism 1975;24:721–35 [DOI] [PubMed] [Google Scholar]
- 36.Bates CJ, Prentice AM, Paul AA. Seasonal variations in vitamins A, C, riboflavin and folate intakes and status of pregnant and lactating women in a rural Gambian community: some possible implications. Eur J Clin Nutr 1994;48:660–8 [PubMed] [Google Scholar]
- 37.Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME. Folate nutriture alters choline status of women and men fed low choline diets. J Nutr 1999;129:712–7 [DOI] [PubMed] [Google Scholar]
- 38.Moat SJ, Ashfield-Watt PA, Powers HJ, Newcombe RG, McDowell IF. Effect of riboflavin status on the homocysteine-lowering effect of folate in relation to the MTHFR (C677T) genotype. Clin Chem 2003;49:295–302 [DOI] [PubMed] [Google Scholar]
- 39.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr 2002;132(suppl):2333S–5S [DOI] [PubMed] [Google Scholar]
- 40.Fischer LM, da Costa KA, Kwock L, Galanko J, Zeisel SH. Dietary choline requirements of women: effects of estrogen and genetic variation. Am J Clin Nutr 2010;92:1113–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudson GJ. Food intake in a west African village. Estimation of food intake from a shared bowl. Br J Nutr 1995;73:551–69 [DOI] [PubMed] [Google Scholar]
- 42.Block G, Hartman AM. Issues in reproducibility and validity of dietary studies. Am J Clin Nutr 1989;50(Suppl):1133–8, discussion 231–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.