Abstract
Background: The Vitamin D Standardization Program (VDSP) has developed protocols for standardizing procedures of 25-hydroxyvitamin D [25(OH)D] measurement in National Health/Nutrition Surveys to promote 25(OH)D measurements that are accurate and comparable over time, location, and laboratory procedure to improve public health practice.
Objective: We applied VDSP protocols to existing ELISA-derived serum 25(OH)D data from the Irish National Adult Nutrition Survey (NANS) as a case-study survey and evaluated their effectiveness by comparison of the protocol-projected estimates with those from a reanalysis of survey serums by using liquid chromatography–tandem mass spectrometry (LC–tandem MS).
Design: The VDSP reference system and protocols were applied to ELISA-based serum 25(OH)D data from the representative NANS sample (n = 1118). A reanalysis of 99 stored serums by using standardized LC–tandem MS and resulting regression equations yielded predicted standardized serum 25(OH)D values, which were then compared with LC–tandem MS reanalyzed values for all serums.
Results: Year-round prevalence rates for serum 25(OH)D concentrations <30, <40, and <50 nmol/L were 6.5%, 21.9%, and 40.0%, respectively, via original ELISA measurements and 11.4%, 25.3%, and 43.7%, respectively, when VDSP protocols were applied. Differences in estimates at <30- and <40-nmol/L thresholds, but not at the <50-nmol/L threshold, were significant (P < 0.05). A reanalysis of all serums by using LC–tandem MS confirmed prevalence estimates as 11.2%, 27.2%, and 45.0%, respectively. Prevalences of serum 25(OH)D concentrations >125 nmol/L were 1.2%, 0.3%, and 0.6% by means of ELISA, VDSP protocols, and LC–tandem MS, respectively.
Conclusion: VDSP protocols hold a major potential for national nutrition and health surveys in terms of the standardization of serum 25(OH)D data.
INTRODUCTION
Knowledge of distributions of serum 25-hydroxyvitamin D [25(OH)D]6 concentrations in nationally representative populations is critical for the quantification of vitamin D deficiency as well as for devising Dietary Reference Values (DRVs) and food-based strategies for its prevention (1, 2). National Health/Nutrition Surveys are instituted to provide government ministries or agencies with nationally representative data to inform, evaluate, and update government policy and regulatory decisions and to plan public health programs (3). Although the NHANES in the United States (4) and the Canadian Health Measures Survey (5) have provided useful descriptions of serum 25(OH)D concentrations in North America, equivalent data for the European Union (EU) are of variable quality, making it difficult to estimate the prevalence of vitamin D deficiency across member states. Even within the 2 North American surveys (4, 5), the Institute of Medicine (IOM), although having examined distributions of total 25(OH)D as part of the development of Dietary Reference Intakes (DRIs) for vitamin D (6), showed that the mean serum 25(OH)D concentration was consistently higher across ages 16–79 y in the Canadian survey despite the higher latitudes. Although there are many likely contributory reasons for differences in prevalence estimates between populations, differences in the analytic methodology for serum total 25(OH)D are also likely contributing factors (1). Several reports have shown that available 25(OH)D assays can yield markedly differing results (7–11).
As a consequence of these widespread, method-related differences in results of total 25(OH)D, which have confounded international efforts to develop evidenced-based guidelines (8, 12), the Vitamin D Standardization Program (VDSP) was established in November 2010 with the goal of promoting a measurement of 25(OH)D that is accurate and comparable over time, location, and laboratory procedure to improve clinical and public health practice worldwide. An overview of the VDSP, which is an international effort coordinated by the Office of Dietary Supplements, NIH, in collaboration with several agencies, institutes, and universities, has been provided elsewhere (1). The initial focus of the VDSP is on standardizing the measurement of 25(OH)D in national health and nutrition surveys around the world, and there are 2 basic protocols, one protocol for standardizing current and future 25(OH)D measurement procedures and another protocol for standardizing 25(OH)D values from past surveys or studies, which have been described in detail elsewhere (1, 13).
The objective of the current study was to apply VDSP protocols to existing ELISA-derived serum 25(OH)D data from the Irish National Adult Nutrition Survey (NANS) conducted in 2008–2010 (14) as a case-study and evaluate their effectiveness by comparison of the protocol-projected estimates with those from a reanalysis of all NANS serums by using a liquid chromatography–tandem mass spectrometry (LC–tandem MS) method. Validation of VDSP protocols for standardizing 25(OH)D values from past surveys would facilitate international comparisons of serum 25(OH)D values and, thus, aid the development of guidelines to tackle vitamin D deficiency.
SUBJECTS AND METHODS
Subject sampling and recruitment procedures and methods of data collection
A detailed description of the methodology used in the NANS, including the sampling procedure as well as sample recruitment, has been reported elsewhere (14, 15). Briefly, the fieldwork phase of the NANS was carried out between October 2008 and April 2010, which provided a seasonal balance to the data and biological sample collection. To achieve a nationally representative sample of community-dwelling adults aged ≥18 y, a quota sampling approach was adopted by using data from the most recently published Census (16). A sample of 1500 free-living adults, which represented a population of ∼4.2 million people, participated in the dietary survey. There were few exclusion criteria other than pregnancy or lactation and an inability to complete the survey because of a disability. The study was approved by the Clinical Research Ethics Committee of the Cork Teaching Hospitals, University College Cork, and the Human Ethics Research Committee of University College Dublin. All eligible and willing participants gave their written consent according to the Helsinki declaration.
An analysis of the demographic features in this sample has shown it to be a representative sample of Irish adults with respect to age, sex, social class, and geographical location compared with Census data (16). Participation in the survey did not require the provision of a blood sample. Of subjects who completed the 4-d food diary, 75.5% of respondents (n = 1132) provided a blood sample. Demographic features of the group of participants who provided a blood sample and subjects in the entire sample have been described elsewhere (14). Seasonality was based on the date that respondents provided the blood sample [ie, from November to March (which represented the winter period) or from April to October (which represented the summer period)], which was consistent with studies based on the NHANES (4) and the recent analysis of the Canadian Health Measures Survey for vitamin D status (5).
Blood collection and analysis of serum 25(OH)D
Blood samples were collected by venipuncture into an evacuated tube by a qualified nurse at designated centers within the survey area or in the respondent's home if the respondent could not travel. Blood samples were transported to the laboratory for additional processing, and serum was stored at −80°C until required for analysis. Concentrations of 25(OH)D in serum samples were measured by the Vitamin D Research Group at University College Cork by using an ELISA [OCTEIA 25-Hydroxy Vitamin D; Immuno Diagnostic Systems Ltd (IDS)] approach, and the data has been described in detail elsewhere (14). The intraassay and interassay CVs for the ELISA method were 5.9% and 6.6%, respectively. This ELISA assay is used for the quantitative determination of serum or plasma 25(OH)D, additional details of which have been described previously (17). Of the original 1132 serums analyzed by using an ELISA (14), 1118 serums were available for reanalysis by using LC–tandem MS.
LC–tandem MS method
The LC–tandem MS method used by the Vitamin D Research Group at University College Cork measures the 3-epimer of 25-hydroxyvitamin D3, which is not chromatographically resolved from 25(OH)D3 by most routine LC–tandem MS methods (1), in addition to 25-hydroxyvitamin D2 [25(OH)D2] and 25(OH)D3 in serum. The presence of 3-epimers of 25(OH)D can pose problems for LC–tandem MS methods because the mass and fragmentation patterns are the same as 25(OH)D, and thus, a failure to account for these metabolites can result in the overestimation of 25(OH)D2 and 25(OH)D3 (18, 19). See supplemental methodological information under “Supplemental data” in the online issue for additional details of the LC–tandem MS method. The interassay CV of the method was <5% for all 25(OH)D metabolites, whereas the intraassay CV was <6%. The total serum 25(OH)D concentration was calculated as the sum of 25(OH)D2 and 25(OH)D3 concentrations. The quality and accuracy of serum 25(OH)D analyses by using both the IDS ELISA and LC–tandem MS in our laboratory are assured on an ongoing basis by participation in the Vitamin D External Quality Assessment Scheme (Charing Cross Hospital).
VDSP reference system and protocols
The VDSP reference system has been described elsewhere (1), but in brief, it included 2 reference measurement procedures (ie, definitive methods that have been developed to the highest analytic standards that current knowledge permits) for serum 25(OH)D, which have been reviewed by the Joint Committee for Traceability in Laboratory Medicine and were deemed to meet the requirements of International Organization for Standardization 15193 and, accordingly, were listed on its database [one at the National Institute of Standards and Technology (NIST) in the United States (20) and one at Ghent University (21); both procedures were based on isotope-dilution LC–tandem MS], the NIST standard reference material 2972 calibration solutions for 25(OH)D2 and 25(OH)D3, and the VDSP single donor serum set (which formed the backbone of the system). The standard reference material was used to calibrate the reference measurement procedures, which, in turn, assigned values for concentrations of total 25(OH)D, 25(OH)D2, 25(OH)D3, and 3-epimer of 25(OH)D3 to each of the 50 single donor serum samples for use in an interlaboratory comparison study. The VDSP interlaboratory comparison study was conducted over the winter 2011 and Spring 2012 period during which a set of 50 single donor serum samples (procured from Solomon Park by the CDC Vitamin D Coordinating Center) were collected and prepared for shipment according to Clinical and Laboratory Standards Institute C37-A guidelines (22). On the basis of a preliminary analysis by the CDC Fat-Soluble Vitamins and Nutrients Laboratory, the set had a total serum 25(OH)D range of ∼15–145 nmol/L. In October and November 2011, individual sets were sent out by the Coordinating Center to VDSP participating survey laboratories. A random run order was also prepared by the Coordinating Center and sent to each of the laboratories. The protocol for participating laboratories consisted of analysis of the 50 serum samples in duplicate on 3 d. It was recommended that a different calibrator lot be used on each of the 3 d.
The value assignment protocol consisted of the measurement by Ghent University of the 50 samples in triplicate on 3 separate occasions and also the measurement by the NIST of the 50 samples in duplicate on 2 separate occasions. The comparison of the survey and reference measurement-procedure laboratory results was used to develop a set of master regression equations that could be used to convert current survey results to the reference measurement procedure [protocol 1: standardizing current and future 25(OH)D measurement procedures]. Only data from Ghent University were available at the time that these analyses were conducted. The final master equation will include analyses compared with the jointly assigned values from the NIST and Ghent University. However, preliminary results of 25 of the single donor patient samples indicated that the correlation (ie, r2) between the NIST and Ghent was 0.998 (data not shown).
The VDSP protocol for the standardization of serum 25(OH)D data from past surveys, as used in this study, entails the following 3 steps: 1) as previously outlined, the use of results from the VDSP interlaboratory comparison study to develop a master regression equation to convert values on the basis of the current measurement procedure (LC–tandem MS in the current study) to the reference measurement procedure at Ghent University (protocol 1), 2) a reanalysis of a statistically defined subsample of the stored sera from the past survey and a regression equation developed to convert all past values to the current measurement procedure (protocol 2), and 3) the 2 sets of regression equations are used in union to convert past survey values to the reference measurement procedure.
To facilitate protocol 2, a statistical algorithm for the estimation of the number of stored samples that needed to be reanalyzed was developed within the VDSP. The sample size of stored serum samples required for this protocol was calculated by using procedures for the estimation of the predicted LC–tandem MS–based 25(OH)D value for a given IDS-ELISA–based 25(OH)D value with a predefined precision of a 95% CI, which have been described elsewhere (23). The predicted LC–tandem MS serum 25(OH)D concentration at 30 nmol/L was of particular interest because of its use as the cutoff to define vitamin D deficiency (6). Thus, the desired precision of a 95% CI was set at 3 nmol/L, which resulted in a sample size of 97. The final sample size of stored survey serums used in the VDSP protocol was 100.
Serum samples were selected by dividing the range [serum 25(OH)D concentrations from 19.4 to 202.5 nmol/L] of the IDS-ELISA–based measurements into 4 quartiles (41.6, 57.2, and 74.1 nmol/L). Each quartile was sampled according to a uniform distribution. This method has been shown, via computer simulations, to be statistically more efficient than uniform random sampling in the entire range.
One value of the 100 samples chosen for remeasurement was >4 SDs away from the line and, thus, was defined as an outlier and eliminated from additional analyses so that our sample size for the VDSP protocol went from 100 to 99.
Serum 25(OH)D thresholds
Measured serum 25(OH)D concentrations were compared with cutoffs for 25(OH)D as per the IOM DRI committee's recent definitions as follows: persons are at risk of deficiency at serum 25(OH)D concentrations <30 nmol/L, whereas concentrations of 40 and 50 nmol/L are consistent with the Estimated Average Requirement–like and Recommended Dietary Allowance–like serum values, respectively (6). In addition, serum 25(OH)D concentrations >125 nmol/L have been suggested by the IOM DRI committee as being possibly of some reason for concern (6).
Data interpretation and statistical analysis
A data and statistical analysis was conducted with SPSS software (version 15.0 for Windows; SPSS Inc), Stata 11 software (StataCorp LP), and the Analyze-It software package (version 2.21 Excel 12+ Analyze-It). Descriptive statistics (frequencies and means) were used to estimate year-round as well as summer and winter serum 25(OH)D concentrations by threshold by using IDS-ELISA–measured, VDSP-protocol–predicted, and LC–tandem MS–measured values. All estimates were based on data weighted to represent the Irish adult population aged 18–84 y (16). Variance estimates (95% CIs) were also calculated. Regression models were used to examine the relation between serum 25(OH)D values derived from different methods and to generate the VDSP master regression equations as previously mentioned. Bland-Altman plots were also used as a graphical method to compare 2 measurement techniques and as a means to estimate bias. Bias referred to the mean percentage difference between our reported LC–tandem MS value and the true (or reference) value for each sample as derived by the higher-order reference measurement procedure at Ghent University (LC–tandem MS). The year-round estimates of serum 25(OH)D below IOM thresholds of 30, 40, and 50 nmol/L that arose from the VDSP predicted and IDS-ELISA–based values were compared by using one-sample Student's t tests.
RESULTS
VDSP protocol 1 [standardizing current and future 25(OH)D measurement procedures]
Linear regression analysis and Bland-Altman plots were used to examine the relation between serum 25(OH)D values for the VDSP 50 single donor serum samples as measured by the LC–tandem MS of the Vitamin D Research Group in Cork and that of the higher-order reference measurement procedure at Ghent University (LC–tandem MS). The regression equation that related the LC–tandem MS at Cork to that at Ghent was as follows:
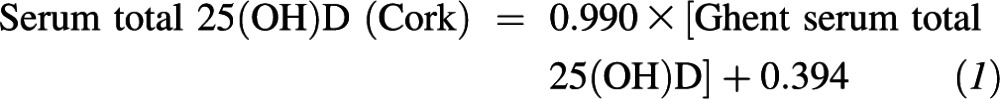 |
The 95% CIs on the slope (0.975, 1.004) and y intercept (−0.777, 1.583) indicated that the slope was not significantly different from 1, and the y intercept was not statistically different from zero. Regression analysis showed r2 = 0.997. The VDSP used the following analytic performance goals and limits, which were based on a proposed concept to derive specifications for the trueness and precision of the reference measurement system (24): imprecision (CV) ≤10% and bias ≤5%. One hundred percent of our samples were within the 10% imprecision limit, and a Bland-Altman plot showed that our mean bias was −0.2% relative to the reference measurement procedure (data not shown).
VDSP protocol 2 [standardizing 25(OH)D values from past surveys]
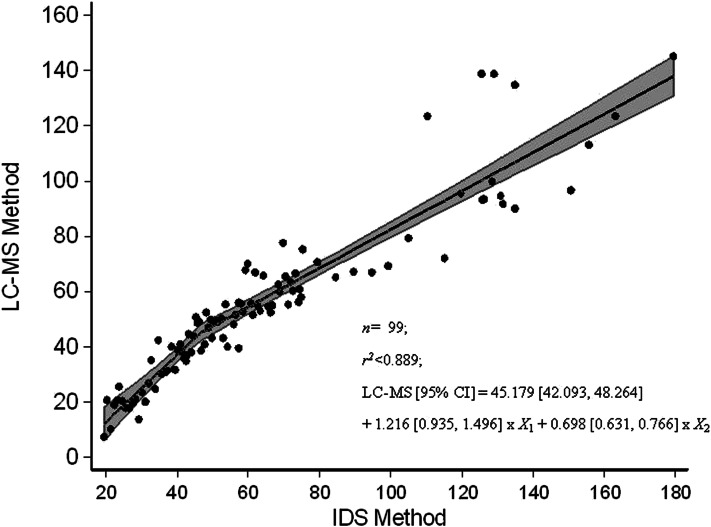
The relation between serum 25(OH)D in the statistical algorithm–defined subset (n = 99) of NANS serum samples, as measured by using the IDS ELISA and reanalyzed by using our traceable LC–tandem MS method, is shown in Figure 1. Several best-fit regression lines were evaluated and included linear, quadratic, fractional polynominal relations (r2 < 0.884 in all cases; data not shown). However, the best fit was the piecewise linear fit
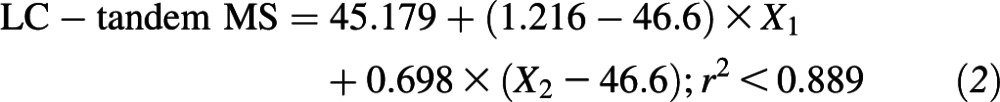 |
which took account of the following 2 linear relations: one relation at concentrations <46.6 nmol/L and another relation at concentrations of serum 25(OH)D greater than or equal to this cutoff. The 95% confidence limits on regression variables were as follows: intercept, 42.093, 48.264; X1, 0.935, 1.496; and X2, 0.631, 0.766. In addition, 95% CIs for intercepts of the first (more-steep) and second (less-steep) lines within the piecewise linear fit in Figure 1 were −22.935, −0.038 and 6.925, 18.339, respectively. There was a much greater deviation between serum 25(OH)D values by using the 2 methods at concentrations above ∼100 nmol/L, with a positive bias for IDS-ELISA values overall.
FIGURE 1.
Relation between serum 25(OH)D (nmol/L) in a subsample (n = 99) of National Adult Nutrition Survey samples measured by using an IDS ELISA and standardized LC-MS at University College Cork as per Vitamin D Standardization Program protocol 2. The figure shows the piecewise linear fit regression line; the shaded area represents 95% confidence bands. IDS, Immuno Diagnostic Systems Ltd; LC-MS, liquid chromatography–tandem mass spectrometry; 25(OH)D, 25-hydroxyvitamin D.
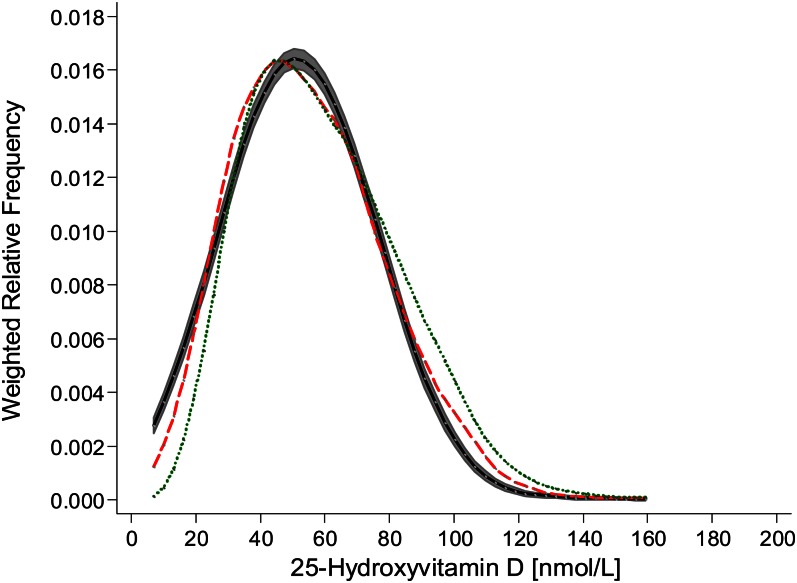
As per VDSP protocol 2, the resulting regression equation was applied to the 1132 previously measured IDS-ELISA–based NANS serum 25(OH)D values (14). The weighted relative frequency for serum 25(OH)D as measured by using the IDS ELISA and predicted by using the VDSP protocol as well as measured by using LC–tandem MS (n = 1118; there was an insufficient volume available for a LC–tandem MS reanalysis in 14 of the original 1132 values) are shown in Figure 2. Compared with that from the IDS-ELISA analysis, the distribution for serum 25(OH)D as predicted by using the VDSP protocol was shifted to the left, and although the 95% confidence bands did not overlap at all parts of the distribution, the overall distribution appeared to align more closely with that of the standardized LC–tandem MS–measured values than with that of the IDS-ELISA–measured values.
FIGURE 2.
Weighted relative frequency for serum 25-hydroxyvitamin D of National Adult Nutrition Survey samples (n = 1118) measured by using the Immuno Diagnostic Systems Ltd enzyme-linked immunosorbent assay (dotted green line) and standardized liquid chromatography–tandem mass spectrometry at University College Cork (dashed red line) and predicted as per Vitamin D Standardization Program protocol 2 [the black line with shaded area represents 95% confidence bands as estimated by using the method of Fiorio (25)].
A Bland-Altman comparison was also performed to compare LC–tandem MS–measured and VDSP-predicted serum 25(OH)D in the NANS sample. Of the 1118 samples, 4 samples were deemed outliers by using an outlier limit of 4 SDs (Figure 3).
FIGURE 3.
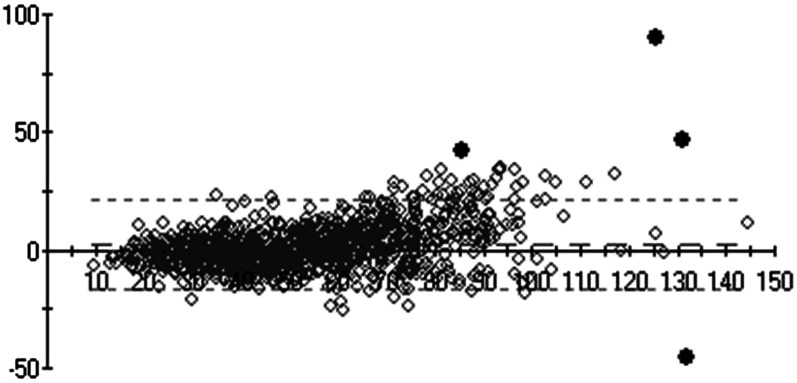
Bland-Altman comparison plot (y axis: standardized LC–tandem MS–measured minus LC–tandem MS–predicted differences; x axis: mean of LC–tandem MS–predicted and LC–tandem MS–measured values) of serum 25(OH)D of National Adult Nutrition Survey samples (n = 1118) measured by using standardized LC–tandem MS at University College Cork and predicted as per Vitamin D Standardization Program protocol 2. The diamonds indicate individual data points. Four black data points represent those that were deemed outliers by using an outlier limit of 4 SDs. LC–tandem MS; liquid chromatography–tandem mass spectrometry; 25(OH)D, 25-hydroxyvitamin D.
Effect of the VDSP protocols on prevalence estimates of vitamin D deficiency or inadequacy and validation of protocols
Measured and VDSP-protocol–predicted prevalence rates for serum 25(OH)D concentrations below the thresholds of the IOM DRI committee and stratified by season are shown in Table 1. Year-round estimates for serum 25(OH)D concentrations <30 nmol/L by using the VDSP-protocol–predicted approach were almost twice those by using the IDS-ELISA–measured approach (P < 0.05). The estimate of serum 25(OH)D concentrations <40 nmol/L was also higher by using the VDSP-protocol–predicted compared with IDS-ELISA–measured methods (P < 0.05), whereas there was no significant difference (P > 0.1) in estimates of serum 25(OH)D <50 nmol/L by using the 2 approaches.
TABLE 1.
An evaluation of the VDSP protocol for standardization of serum 25(OH)D data from a past NANS of adults aged 18–84 y in Ireland, 2008–2010: VDSP-projected compared with LC–tandem MS measured values1
| Serum 25(OH)D concentrations | Year round (n = 1118) | Winter (n = 489) | Summer (n = 629) |
| <30 nmol/L | |||
| Measured by using IDS ELISA | 6.5 (5.0, 8.1); 73 | 10.6 (8.2, 13.1); 53 | 3.4 (2.1, 4.7); 20 |
| VDSP projected2 | 11.4 (9.1, 13.6); 127* | 17.5 (14.3, 20.8); 88 | 6.6 (4.4, 8.7); 39 |
| Measured by using LC–tandem MS3 | 11.2 (9.1, 13.4); 125 | 18.2 (14.9, 21.5); 88 | 5.9 (4.0, 7.9); 37 |
| <40 nmol/L | |||
| Measured by using IDS ELISA | 21.9 (18.2, 25.5); 244 | 30.9 (26.5, 35.3); 151 | 15.6 (12.5, 18.7); 93 |
| VDSP projected2 | 25.3 (22.8, 29.2); 281* | 36.2 (31.5, 41.0); 176 | 17.4 (14.1, 20.7); 105 |
| Measured by using LC–tandem MS3 | 27.2 (23.2, 31.3); 304 | 39.7 (34.9, 44.6); 193 | 17.8 (14.3, 21.2); 111 |
| <50 nmol/L | |||
| Measured by using IDS ELISA | 40.0 (35.2, 44.8); 446 | 55.2 (49.4, 61.0); 267 | 29.0 (24.7, 33.3); 179 |
| VDSP projected2 | 43.7 (38.7, 48.7); 488 | 59.5 (53.5, 65.5); 290 | 31.9 (27.4, 36.4); 198 |
| Measured by using LC–tandem MS3 | 45.0 (40.0, 50.0); 501 | 59.9 (54.0, 65.9); 291 | 34.3 (29.7, 38.9); 210 |
All values are percentages; 95% CIs in parentheses; n. All estimates and 95% CIs were based on data weighted to represent the Irish adult population. *VDSP-predicted year-round estimates were significantly higher than IDS-ELISA–based estimates, P < 0.05 (one-sample Student's t tests). IDS, Immuno Diagnostic Systems Ltd; LC–tandem MS, liquid chromatography–tandem mass spectrometry; NANS, Irish National Adult Nutrition Survey; VDSP, Vitamin D Standardization Program; 25(OH)D, 25-hydroxyvitamin D.
On the basis of serum 25(OH)D values derived by VDSP-protocol application to the existing IDS-ELISA values and projected to those of LC–tandem MS.
Measured by using LC–tandem MS, which was traceable and standardized to the reference measurement procedure at Ghent University.
To test the effectiveness of the VDSP protocol, serum samples (n = 1118) were reanalyzed by using standardized LC–tandem MS, and resulting prevalence estimates of serum 25(OH)D concentrations <30, <40, and <50 nmol/L were compared. There was close agreement between predicted and LC–tandem MS–measured year-round prevalence estimates at all 3 thresholds, which were also evident when stratified by winter and summer (Table 1).
Serum 25(OH)D concentrations >125 nmol/L are regarded by the DRI committee as being of some reason for concern (6).With the use of data from the IDS-ELISA analysis, 1.2% of the NANS sample had concentrations greater than this cutoff throughout the year, but this percentage fell to a predicted 0.3% (95% CI: 0.2%, 0.4%) by using the VDSP protocol and 0.6% when measured by using LC–tandem MS. However, these estimates need to be interpreted with caution because the n values were quite small (n= 3–13).
Deciles of serum 25(OH)D concentrations within the total sample arising from the use of IDS-ELISA, VDSP-predicted, and LC–tandem MS–measured methods are shown in Table 2. The 95% CIs of VDSP-predicted values bracketed the LC–tandem MS–measured values over the first 5 deciles but began to increasingly fall below the LC–tandem MS–measured values with incremental deciles above the fifth. None of the measured IDS-ELISA values were within the 95% CIs of the VDSP-predicted values; instead, all values were above the 95% CIs.
TABLE 2.
Deciles of serum 25(OH)D concentrations from the NANS of adults aged 18–84 y in Ireland, 2008–2010, as measured by using the IDS ELISA, VDSP-protocol–projected estimates, and LC–tandem MS1
| Serum 25(OH)D |
|||
| Deciles of serum 25(OH)D | Via IDS ELISA | VDSP-protocol predicted (95% CI) | Via LC–tandem MS2 |
| nmol/L | |||
| 10 | 32.4 | 28.0 (26.6, 29.8) | 27.8 |
| 20 | 38.7 | 35.6 (34.1, 37.7) | 34.9 |
| 30 | 43.5 | 41.5 (40.1, 43.1) | 40.4 |
| 40 | 50.1 | 47.6 (46.0, 48.7) | 46.3 |
| 50 | 57.1 | 52.5 (51.1, 53.4) | 53.4 |
| 60 | 63.2 | 56.8 (55.3, 58.2) | 58.9 |
| 70 | 70.5 | 61.9 (60.3, 63.5) | 65.9 |
| 80 | 79.9 | 68.4 (66.4, 70.1) | 74.1 |
| 90 | 91.8 | 76.8 (75.4, 78.6) | 86.3 |
IDS, Immuno Diagnostic Systems Ltd; LC–tandem MS, liquid chromatography–tandem mass spectrometry; NANS, Irish National Adult Nutrition Survey; VDSP, Vitamin D Standardization Program; 25(OH)D, 25-hydroxyvitamin D.
Measured by using LC–tandem MS, which was traceable and standardized to the reference measurement procedure at Ghent University.
DISCUSSION
Widespread, method-related differences in results of total serum and plasma 25(OH)D from national surveys have confounded international efforts to develop evidence-based guidelines for the evaluation of vitamin D status, including what constitutes deficiency or insufficiency (8, 12). It has been suggested that new high-performance liquid chromatographic and mass spectrometric methods may offer more-robust and -reliable measurements of circulating 25(OH)D than commercial methods do (26). However, even with these newer methods, international quality-assurance schemes have shown that results were highly operator and laboratory dependent (8–10). In the current study, the year-round prevalence of serum 25(OH)D concentration <30 nmol/L [ie, the IOM threshold for vitamin D deficiency (6)] in Irish adults aged 18–84 y in the NANS significantly increased from 6.5% to 11.2% when the analytic platform was switched from the IDS-ELISA to a LC–tandem MS method, respectively. Importantly, the LC–tandem MS method used was shown via the VDSP to be traceable to a higher-order reference measurement procedure and provided a very high degree of confidence in its serum 25(OH)D values. These, to our knowledge, new data on prevalence of vitamin D deficiency and inadequacy for Irish adults can be validly compared with data of other VDSP-participating international national health and nutrition surveys in Europe, North America, and elsewhere once they are likewise standardized.
Although the standardization of current and, indeed, future 25(OH)D measurements is critically important to the field, the standardization of serum 25(OH)D values from past surveys could also provide more clearly defined prevalence estimates of vitamin D deficiency within and across continents as well as temporal changes within a country. In this regard, the validation in the current study of the VDSP protocol for the standardization of 25(OH)D values from past surveys is an important contribution toward international standardization. A relatively modest reanalysis by using LC–tandem MS of only 99 specifically selected biobanked serums from the NANS allowed us to predict LC–tandem MS–like serum 25(OH)D values for the entire NANS sample (n = 1118). When prevalence estimates for deficiency or inadequacy from these predicted serum values were compared with actual measured LC–tandem MS values for the entire sample, there was a very good concordance between estimates for all 3 IOM-suggested thresholds [serum 25(OH)D concentrations <30, <40, and <50 nmol/L). In particular, the agreement at the <30-nmol/L serum 25(OH)D threshold, which is a clinically significant one because it defines deficiency (6), was striking at 11.4% and 11.2% for the VDSP protocol-predicted and standardized LC–tandem MS–measured methods, respectively. Although there is a strong public health emphasis on serum 25(OH)D concentrations <50 nmol/L, VDSP-protocol–predicted estimates tended to be lower than those from the LC–tandem MS–measured values at concentrations >60 nmol/L. However, even so, the VDSP-protocol–predicted estimates for these higher concentrations were still closer to the LC–tandem MS–measured values than to the IDS-ELISA–based values.
The data from this study would suggest that, if the VDSP protocol for past surveys was applied to a limited number of biobanked NHANES and Canadian Health Measures Survey serum and plasma samples (which have been analyzed by using 2 different DiaSorin assays), the findings that the mean serum 25(OH)D concentration was consistently higher across ages 16–79 y in the Canadian survey (4, 5) could be substantiated or refuted because the values would be standardized and free of method-related differences. Within Europe, which is a continent that spans a latitude range from 34° to 70°N, past national surveys by various EU member states have used a range of commercially available methods for measuring 25(OH)D (14, 27–29), which have likely contributed to some as yet unmeasured extent, to differences in prevalence estimates of vitamin D deficiency in these free-living adult populations [typically in the range from 2% to 17% by using a serum 25(OH)D threshold of 25 nmol/L]. Such standardization within and across continents would not only allow for more-meaningful international comparisons but could also help identify contributory reasons for differences in prevalence estimates between populations, such as racial and ethnic compositions, ambient UVB sunlight and sun-exposure practices, and vitamin D food-fortification and -supplementation practices. Also of importance, such standardization would greatly benefit the careful monitoring of changes in vitamin D status in the population on the instigation of a policy such as mandatory vitamin D fortification of food.
The VDSP protocols also hold great promise for the additional development of vitamin D dietary reference intervals, such as DRIs in North America and DRVs in Europe, in a number of ways. First, the standardization of serum 25(OH)D values would greatly aid the assessment of the vitamin D status of a population on a transborder basis in North America and also in the EU and elsewhere; such an assessment is an important step in both DRIs and DRVs. Second, in terms of the evidence base to underpin the establishment of a DRI and DRV for vitamin D, the IOM recently suggested that “a single individual might be deemed deficient or sufficient, depending in the laboratory where the blood is tested” (6). This suggestion was strongly reemphasized in the current study, which showed significant differences between the assay performance on the same serum samples. Thus, these laboratory- and method-related differences in serum 25(OH)D results contribute to uncertainty in the comparison of data in studies and are likely to have been intrinsic across the various randomized controlled trial (RCT) and cohort studies used in the 2 North American systematic evidence-based reviews (30, 31) as well as in the meta-regression analysis of serum 25(OH)D compared with total vitamin D intake used by the IOM DRI panel to define Estimated Average Requirement and Recommended Dietary Allowance values for vitamin D (6). The current study showed that, when VDSP protocols were applied, the discriminatory power to correctly categorize an individual's serum 25(OH)D in the lower concentration range from <30 to 50 nmol/L was greatly improved. The VDSP protocols, although applied initially by design to national health and nutrition surveys for reasons outlined elsewhere (1), could be applied to other types of studies including RCTs and cohort studies in the area of vitamin D and health. Last, although the VDSP protocols described in the current work will likely be refined further to try and reduce the variation between predicted and LC–tandem MS–measured values at high serum 25(OH)D concentrations, even now, the protocols could also benefit DRI development in terms of safety. For example, the current study showed that the prevalence of serum 25(OH)D concentrations >125 nmol/L [regarded by the DRI committee as being of some reason for concern (6)] was much reduced when VDSP protocols were applied to nationally representative data. This result is also likely to be the case in cohort and RCT studies that report U- and reverse J-shaped relations between serum 25(OH)D and adverse consequences, including all-cause mortality, cancer, cardiovascular disease, parathyroid hormone suppression, and intrauterine growth restriction, among others (2, 6, 32). This outcome may have implications for the derivation of the Tolerable Upper Level for vitamin D. Thus, such standardization could hugely benefit the future interpretation of the vitamin D status and health as well as vitamin D intake and status relations, all of which are integral to DRI and DRV processes.
In conclusion, our evaluation of VDSP protocols for the standardization of serum 25(OH)D data in the NANS for Ireland, as a case study, shows the major potential the protocols hold for other national nutrition and health surveys and for other studies of vitamin D and health in terms of the standardization of 25(OH)D measurements.
Acknowledgments
We express our sincere thanks to Linda Thienpont, Ghent University, whose laboratory performed the reference measurement procedure for serum 25(OH)D in the 50 single-donor serum samples and for her comments on the manuscript.
The authors’ responsibilities were as follows—AF and MJG: were grant holders for the NANS; KDC: coordinated the ELISA analysis of serum 25(OH)D in the NANS; KDC, M Kiely, RAD-A, HWV, KWP, PMC, MFP, and CTS: are members of the VDSP and developed the concepts behind the work; M Kinsella, YZ, and AL: contributed to the sample analysis; KDC, RAD-A, and CTS: contributed to the data analysis; KDC and CTS: prepared an initial draft of the manuscript; and all authors: contributed to finalizing the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: DRI, Dietary Reference Intake; DRV, Dietary Reference Value; EU, European Union; IDS, Immuno Diagnostic Systems Ltd; IOM, Institute of Medicine; LC–tandem MS, liquid chromatography–tandem mass spectrometry; NANS, Irish National Adult Nutrition Survey; NIST, National Institute of Standards and Technology; RCT, randomized controlled trial; VDSP, Vitamin D Standardization Program; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyvitamin D2; 25(OH)D3, 25-hydroxyvitamin D3.
REFERENCES
- 1.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 2012;243:32–40 [DOI] [PubMed] [Google Scholar]
- 2.Cashman KD, Kiely M. Towards prevention of vitamin D deficiency and beyond - knowledge gaps and research needs in vitamin D nutrition and public health. Br J Nutr 2011;106:1617–27 [DOI] [PubMed] [Google Scholar]
- 3.Sempos CT, Briefel RR, Flegal KM, Johnson CL, Murphy RS, Woteki CE. Factors involved in selecting a dietary survey methodology for national nutrition surveys. Australian J Nutr Dietet 1992;49:96–101 [Google Scholar]
- 4.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001-2006. NCHS Data Brief 2011;59:1–8 [PubMed] [Google Scholar]
- 5.Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the 2011 Dietary Reference Intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr 2011;94:128–35 [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine Food and Nutrition Board Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academy Press, 2011 [Google Scholar]
- 7.Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 2004;89:3152–7 [DOI] [PubMed] [Google Scholar]
- 8.Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets 2011;12:19–28 [DOI] [PubMed] [Google Scholar]
- 9.Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem 2004;50:2195–7 [DOI] [PubMed] [Google Scholar]
- 10.Carter GD. 25-Hydroxyvitamin D: a difficult analyte. Clin Chem 2012;58:486–8 [DOI] [PubMed] [Google Scholar]
- 11.Lai JK, Lucas RM, Banks E, Posonby AL; Ausimmune Investigator Group Variability in vitamin D assays impairs clinical assessment of vitamin D status. Intern Med J 2012;42:43–50 [DOI] [PubMed] [Google Scholar]
- 12.Wallace AM, Gibson S, de la Hunty A. Lamberg-Allardt, Ashwell M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids 2010;75:477–88 [DOI] [PubMed] [Google Scholar]
- 13.Thienpont LM, Stepman HCM, Vesper HW. Standardization of measurements of 25-hydroxyvitamin D3 and D2. Scand J Clin Lab Invest Suppl 2012;243:41–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cashman KD, Muldowney S, McNulty B, Nugent A, FitzGerald AP, Kiely M, Walton J, Gibney MJ, Flynn A. Vitamin D status of Irish adults: findings from the National Adult Nutrition Survey. Br J Nutr 2013;109:1248–56 [DOI] [PubMed] [Google Scholar]
- 15.Irish Universities Nutrition Alliance (2011). National Adult Nutrition Survey: summary report. March 2011. Available at: http://www.iuna.net/wp-content/uploads/2010/12/National-Adult-Nutrition-Survey-Summary-Report-March-2011.pdf (cited 1 May 2012).
- 16.Central Statistics Office (CSO) Census 2006 principal demographic results. Dublin, Ireland: The Stationery Office, 2007 [Google Scholar]
- 17.Cashman KD, Hill TR, Cotter AA, Boreham CA, Dubitzky W, Murray L, Strain J, Flynn A, Robson PJ, Wallace JM, et al. Low vitamin D status adversely affects bone health parameters in adolescents. Am J Clin Nutr 2008;87:1039–44 [DOI] [PubMed] [Google Scholar]
- 18.Hollis BW. Editorial: The determination of circulating 25-hydroxyvitamin D: no easy task. J Clin Endocrinol Metab 2004;89:3149–51 [DOI] [PubMed] [Google Scholar]
- 19.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 2006;91:3055–61 [DOI] [PubMed] [Google Scholar]
- 20.Tai SSC, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 2010;82:1942–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stepman HCM, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography–tandem mass spectrometry. Clin Chem 2011;57:441–8 [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards (NCCLS) Preparation and validation of commutable frozen human serum pools as secondary reference materials for cholesterol measurement procedures; approved guideline. NCCLS document C37-A (ISBN 1-56238-392-2). Wayne, PA: NCCLS, Clinical and Laboratory Standards Institute, 1999.; [Google Scholar]
- 23.Tian L, Durazo-Arvizu RA, Myers G, Brooks S, Sarafin K, Sempos CT. The estimation of calibration equations for variables with heteroscedastic measurement error. Stat Med. [DOI] [PubMed] [Google Scholar]
- 24.Stöckl D, Sluss PM, Thienpont LM. Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin Chim Acta 2009;408:8–13 [DOI] [PubMed] [Google Scholar]
- 25.Fiorio CV. Confidence intervals for kernel density estimation. Stata J 2004;4:168–79 [Google Scholar]
- 26.Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays. Clin Chem 2006;52:1120–6 [DOI] [PubMed] [Google Scholar]
- 27.Ruston D, Hoare J, Henderson L, Gregory J, Bates CJ, Prentice A, Birch M, Swan G, Farron M. The National Diet and Nutrition Survey: adults aged 19-64 years. Volume 4: nutritional status (anthropometry and blood analytes), blood pressure and physical activity. London, United Kingdom: The Stationery Office, 2004. [Google Scholar]
- 28.Finch S, Doyle W, Lowe C, Bates CJ, Prentice A, Smithers G, Clarke PC. National Diet and Nutrition Survey: people aged 65 years and over., Vol 1: London, United Kingdom: The Stationery Office, 1998 [Google Scholar]
- 29.Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr 2008;62:1079–89 [DOI] [PubMed] [Google Scholar]
- 30.Agency for Healthcare Research and Quality (AHRQ) report: Vitamin D and calcium: a systematic review of health outcomes. AHRQ Publication No. 09-E015, August 2009. Available at: http://www.ahrq.gov/clinic/tp/vitadcaltp.htm (cited 28 October 2010).
- 31. Cranney A, Horsley T, OaposDonnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evidence Report/Technology Assessment No. 158 (Prepared by the University of Ottawa Evidence-based Practice Center (UO-EPC) under contract No. 290-02-0021. AHRQ Publication No. 07-E013. Rockville, MD: Agency for Healthcare Research and Quality, 2007.
- 32.Brannon PM. Key questions in vitamin D research. Scand J Clin Lab Invest Suppl 2012;243:154–62 [DOI] [PubMed] [Google Scholar]