Abstract
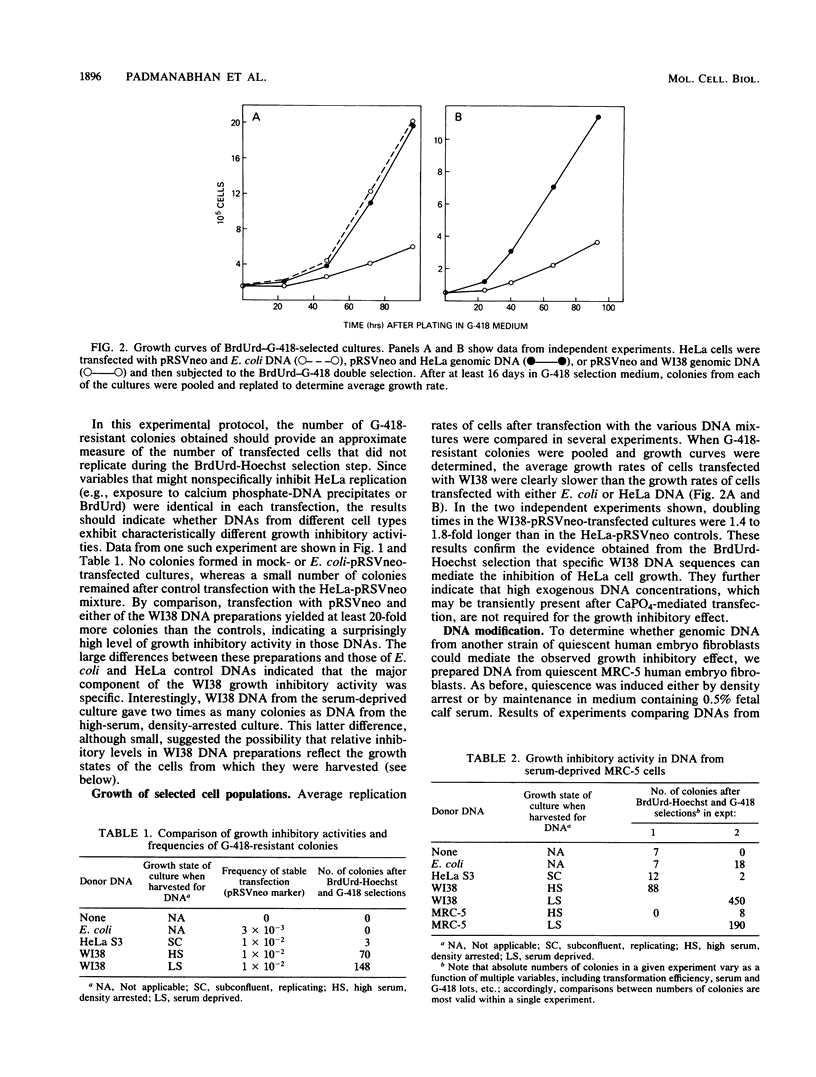
We used HeLa cells as recipients in a gene transfer assay to characterize DNA sequences that negatively regulate mammalian cell growth. In this assay, genomic DNA from quiescent human embryo fibroblasts was more inhibitory for HeLa replication than was DNA from either Escherichia coli or HeLa cells. Surprisingly, growth inhibitory activity depended on the growth state of the cells from which genomic DNA was prepared; it was strongest in DNA prepared from serum-deprived, quiescent embryo fibroblasts. This latter observation implies a role for DNA modification(s) in regulating the activity of the inhibitory sequences detected in our assay. The level of the observed growth inhibitory activity was sometimes high, suggesting that the relevant sequences may be abundantly represented in the mammalian genome. We speculate that these findings may provide new insights into the molecular mechanisms involved in cellular quiescence and in vitro senescence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augenlicht L. H., Baserga R. Changes in the G0 state of WI-38 fibroblasts at different times after confluence. Exp Cell Res. 1974 Dec;89(2):255–262. doi: 10.1016/0014-4827(74)90789-7. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Weissman B. E., Mark C., Stanbridge E. J. Tumorigenicity of human HT1080 fibrosarcoma X normal fibroblast hybrids: chromosome dosage dependency. Cancer Res. 1984 Aug;44(8):3471–3479. [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. L., Tarrant G. M. Limited lifespan in somatic cell hybrids and cybrids. Exp Cell Res. 1980 Jun;127(2):385–396. doi: 10.1016/0014-4827(80)90443-7. [DOI] [PubMed] [Google Scholar]
- Burmer G. C., Rabinovitch P. S., Norwood T. H. Evidence for differences in the mechanism of cell cycle arrest between senescent and serum-deprived human fibroblasts: heterokaryon and metabolic inhibitor studies. J Cell Physiol. 1984 Jan;118(1):97–103. doi: 10.1002/jcp.1041180116. [DOI] [PubMed] [Google Scholar]
- Craig R. W., Sager R. Suppression of tumorigenicity in hybrids of normal and oncogene-transformed CHEF cells. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2062–2066. doi: 10.1073/pnas.82.7.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Shmookler Reis R. J. Genetic modifications during cellular aging. Mol Cell Biochem. 1984 Sep;64(1):15–30. doi: 10.1007/BF00420924. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Gusella J., Geller R., Clarke B., Weeks V., Housman D. Commitment to erythroid differentiation by friend erythroleukemia cells: a stochastic analysis. Cell. 1976 Oct;9(2):221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Harris H., Miller O. J., Klein G., Worst P., Tachibana T. Suppression of malignancy by cell fusion. Nature. 1969 Jul 26;223(5204):363–368. doi: 10.1038/223363a0. [DOI] [PubMed] [Google Scholar]
- Holliday R., Huschtscha L. I., Tarrant G. M., Kirkwood T. B. Testing the commitment theory of cellular aging. Science. 1977 Oct 28;198(4315):366–372. doi: 10.1126/science.910134. [DOI] [PubMed] [Google Scholar]
- Klinger H. P., Shows T. B. Suppression of tumorigenicity in somatic cell hybrids. II. Human chromosomes implicated as suppressors of tumorigenicity in hybrids with Chinese hamster ovary cells. J Natl Cancer Inst. 1983 Sep;71(3):559–569. [PubMed] [Google Scholar]
- Levenson R., Housman D. Commitment: how do cells make the decision to differentiate? Cell. 1981 Jul;25(1):5–6. doi: 10.1016/0092-8674(81)90225-7. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Macieira-Coelho A. Genome reorganization during cellular senescence. Mech Ageing Dev. 1984 Oct 15;27(2):257–262. doi: 10.1016/0047-6374(84)90051-4. [DOI] [PubMed] [Google Scholar]
- Norwood T. H., Pendergrass W. R., Martin G. M. Reinitiation of DNA synthesis in senescent human fibroblasts upon fusion with cells of unlimited growth potential. J Cell Biol. 1975 Mar;64(3):551–556. doi: 10.1083/jcb.64.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood T. H., Pendergrass W. R., Sprague C. A., Martin G. M. Dominance of the senescent phenotype in heterokaryons between replicative and post-replicative human fibroblast-like cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2231–2235. doi: 10.1073/pnas.71.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Nagai Y. Genes in multiple copies as the primary cause of aging. Birth Defects Orig Artic Ser. 1978;14(1):501–514. [PubMed] [Google Scholar]
- Paul D. Growth control in HeLa cells by serum and anchorage. Exp Cell Res. 1978 Jul;114(2):435–438. doi: 10.1016/0014-4827(78)90503-7. [DOI] [PubMed] [Google Scholar]
- Pendergrass W. R., Saulewicz A. C., Burmer G. C., Rabinovitch P. S., Norwood T. H., Martin G. M. Evidence that a critical threshold of DNA polymerase-alpha activity may be required for the initiation of DNA synthesis in mammalian cell heterokaryons. J Cell Physiol. 1982 Oct;113(1):141–151. doi: 10.1002/jcp.1041130123. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Evidence for the recessive nature of cellular immortality. Science. 1983 Sep 2;221(4614):964–966. doi: 10.1126/science.6879195. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Expression of SV40 T antigen in finite life-span hybrids of normal and SV40-transformed fibroblasts. Somatic Cell Genet. 1981 Jul;7(4):411–421. doi: 10.1007/BF01542986. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S., Norwood T. H. Comparative heterokaryon study of cellular senescence and the serum-deprived state. Exp Cell Res. 1980 Nov;130(1):101–109. doi: 10.1016/0014-4827(80)90046-4. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A., Wilson G. Escherichia coli K-12 restricts DNA containing 5-methylcytosine. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9070–9074. doi: 10.1073/pnas.83.23.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini M., Lin J. C., Baserga R. Effects of prolonged quiescence on neuclei and chromatin of WI-38 fibroblasts. J Cell Physiol. 1976 May;88(1):1–11. doi: 10.1002/jcp.1040880102. [DOI] [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation. Adv Cancer Res. 1985;44:43–68. doi: 10.1016/s0065-230x(08)60025-1. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: I. Expression of tumor-forming ability in hamster hybrid cell lines. Somatic Cell Genet. 1978 May;4(3):375–392. doi: 10.1007/BF01542849. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Sherwood S. W., Hill A. B., Johnston R. N. Overreplication and recombination of DNA in higher eukaryotes: potential consequences and biological implications. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2157–2161. doi: 10.1073/pnas.83.7.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmookler Reis R. J., Goldstein S. Loss of reiterated DNA sequences during serial passage of human diploid fibroblasts. Cell. 1980 Oct;21(3):739–749. doi: 10.1016/0092-8674(80)90437-7. [DOI] [PubMed] [Google Scholar]
- Smith J. R., Whitney R. G. Intraclonal variation in proliferative potential of human diploid fibroblasts: stochastic mechanism for cellular aging. Science. 1980 Jan 4;207(4426):82–84. doi: 10.1126/science.7350644. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stanbridge E. J., Der C. J., Doersen C. J., Nishimi R. Y., Peehl D. M., Weissman B. E., Wilkinson J. E. Human cell hybrids: analysis of transformation and tumorigenicity. Science. 1982 Jan 15;215(4530):252–259. doi: 10.1126/science.7053574. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Flandermeyer R. R., Daniels D. W., Nelson-Rees W. A. Specific chromosome loss associated with the expression of tumorigenicity in human cell hybrids. Somatic Cell Genet. 1981 Nov;7(6):699–712. doi: 10.1007/BF01538758. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J. Suppression of malignancy in human cells. Nature. 1976 Mar 4;260(5546):17–20. doi: 10.1038/260017a0. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. M., Gordon L., Beeson M. Carcinogen-transformed human cells are inhibited from entry into S phase by fusion to senescent cells but cells transformed by DNA tumor viruses overcome the inhibition. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5287–5291. doi: 10.1073/pnas.79.17.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. M. Quiescent human diploid cells can inhibit entry into S phase in replicative nuclei in heterodikaryons. Proc Natl Acad Sci U S A. 1981 May;78(5):3025–3029. doi: 10.1073/pnas.78.5.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetten G., Davidson R. L., Latt S. A. 33258 Hoechst enhances the selectivity of the bromodeoxyuridine--light method of isolating conditional lethal mutants. Exp Cell Res. 1977 Sep;108(2):447–452. doi: 10.1016/s0014-4827(77)80055-4. [DOI] [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Yanishevsky R. M., Stein G. H. Ongoing DNA synthesis continues in young human diploid cells (HDC) fused to senescent HDC, but entry into S phase is inhibited. Exp Cell Res. 1980 Apr;126(2):469–472. doi: 10.1016/0014-4827(80)90290-6. [DOI] [PubMed] [Google Scholar]