Abstract
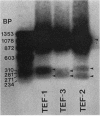
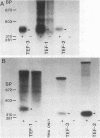
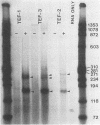
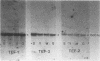
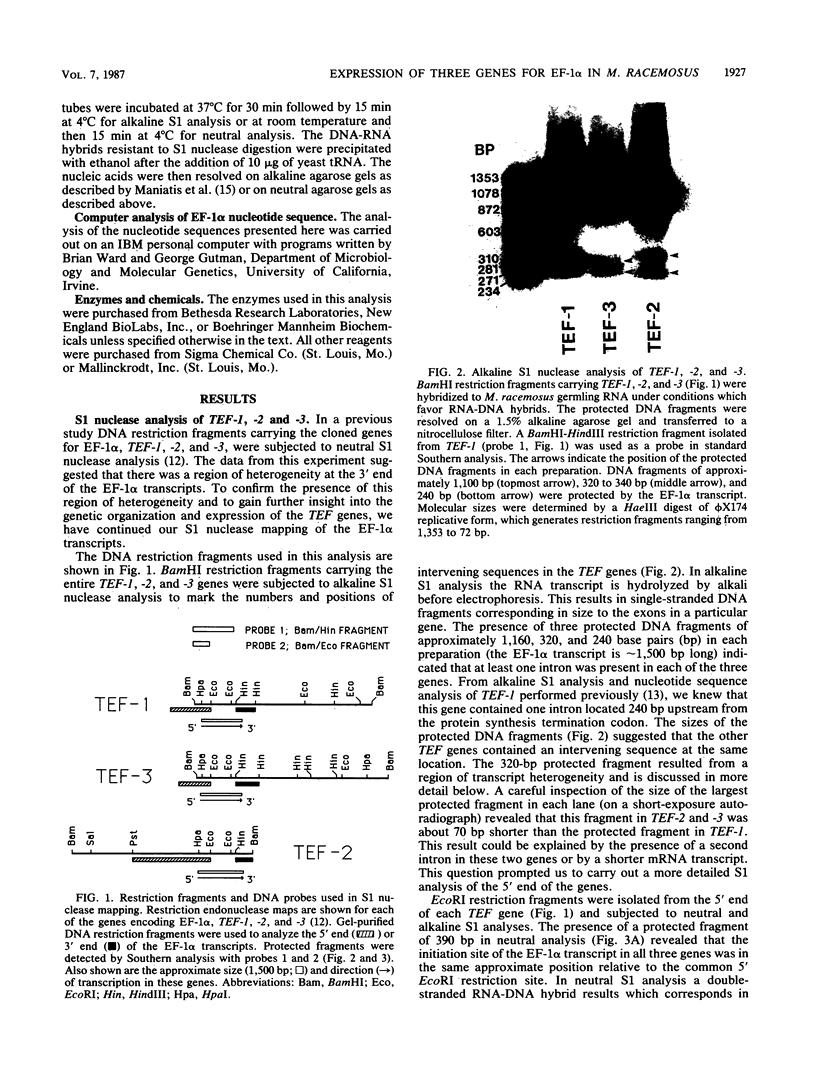
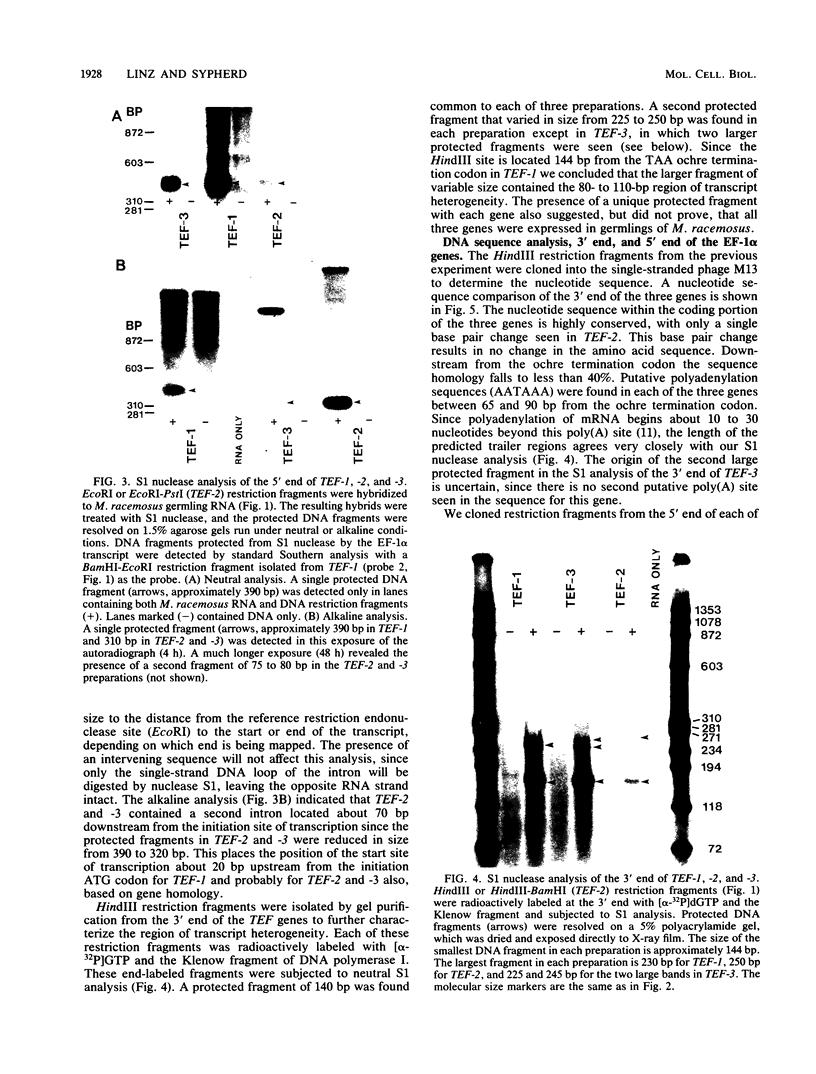
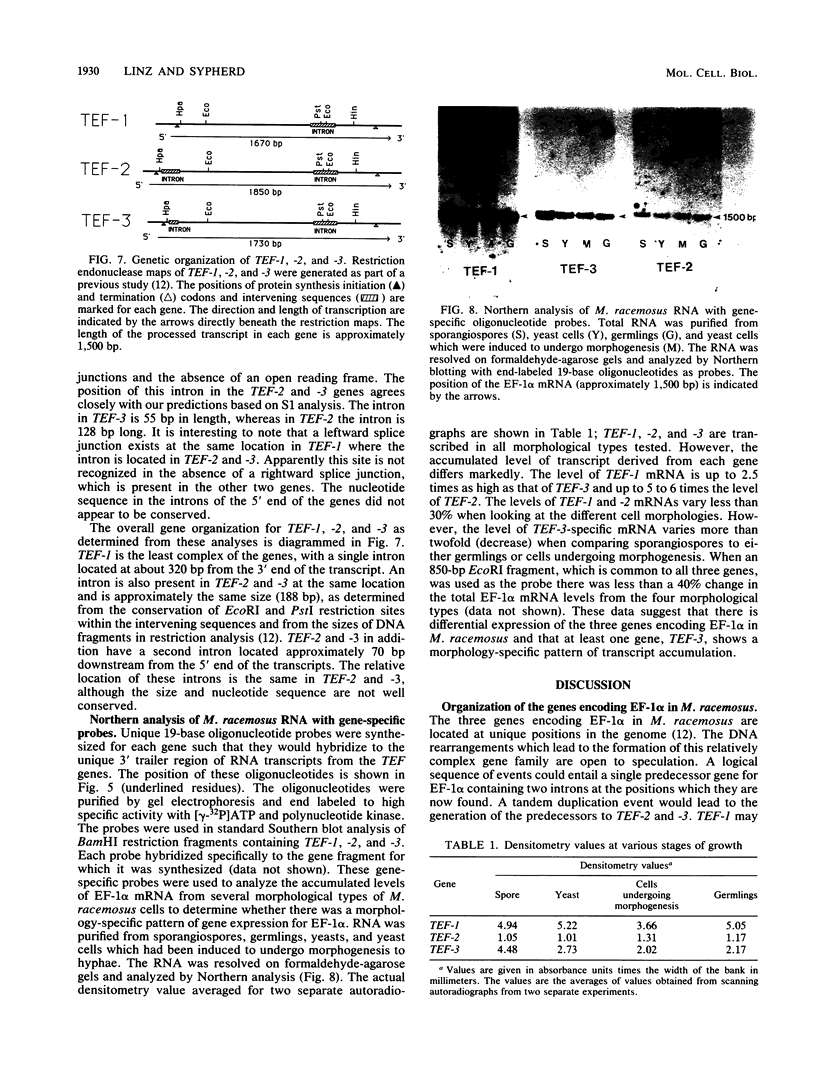
Three genes, TEF-1, -2, and -3, encode elongation factor 1 alpha in Mucor racemosus. Neutral and alkaline S1 nuclease analyses revealed that the genetic organization is unique for each of the genes. The number and size of the intervening sequences vary in these closely related genes, which suggests that complex genetic rearrangements gave rise to the elongation factor 1 alpha gene family. Nucleotide sequence data from restriction fragments isolated from the 5' and 3' ends of TEF-2 and -3 confirmed the presence of a second intervening sequence in these genes. These data along with S1 nuclease mapping revealed a region at the 3' end of the three genes which was predicted to be transcribed but untranslated. Unique oligonucleotides containing 19 bases were synthesized to hybridize to this unique trailer region in the elongation factor 1 alpha transcripts. These oligonucleotides were used as probes in standard Northern analysis of RNA purified from M. racemosus cells of several morphological types. It was determined that all three genes were expressed in the cell morphological types studied. However, the accumulated level of transcript derived from each gene varied considerably, with TEF-1 mRNA present in approximately twofold greater quantity than the TEF-3 transcript and up to sixfold greater quantity than TEF-2. The level of TEF-1 and -2 mRNA varied little among the cell morphological types studied, whereas TEF-3 mRNA was present in twofold greater quantity in sporangiospores than in either germlings or yeast cells which had been induced to undergo morphogenesis to hyphae. These data suggest that there is differential expression of the genes encoding elongation factor 1 alpha in M. racemosus. At least one gene, TEF-3, shows a morphology-specific pattern of transcript accumulation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrelle P., Thiele D., Price V. L., Memet S., Micouin J. Y., Marck C., Buhler J. M., Sentenac A., Fromageot P. Cloning, nucleotide sequence, and expression of one of two genes coding for yeast elongation factor 1 alpha. J Biol Chem. 1985 Mar 10;260(5):3090–3096. [PubMed] [Google Scholar]
- Daum H. A., 3rd, Bragg P. W., Sittman D. B., Dholakia J. N., Woodley C. L., Wahba A. J. The expression of a gene for eukaryotic elongation factor Tu in Artemia during development. Translation of poly(A)+ RNA and the use of a synthetic oligonucleotide to detect the presence of eukaryotic elongation factor Tu-specific mRNA. J Biol Chem. 1985 Dec 25;260(30):16347–16353. [PubMed] [Google Scholar]
- Fonzi W. A., Katayama C., Leathers T., Sypherd P. S. Regulation of protein synthesis factor EF-1 alpha in Mucor racemosus. Mol Cell Biol. 1985 May;5(5):1100–1103. doi: 10.1128/mcb.5.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi W. A., Sypherd P. S. Expression of the gene for ornithine decarboxylase of Saccharomyces cerevisiae in Escherichia coli. Mol Cell Biol. 1985 Jan;5(1):161–166. doi: 10.1128/mcb.5.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt W. R., Garcia R., Merrick W. C., Sypherd P. S. Methylation of elongation factor 1 alpha from the fungus Mucor. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3433–3437. doi: 10.1073/pnas.79.11.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz J. E., Katayama C., Sypherd P. S. Three genes for the elongation factor EF-1 alpha in Mucor racemosus. Mol Cell Biol. 1986 Feb;6(2):593–600. doi: 10.1128/mcb.6.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz J. E., Lira L. M., Sypherd P. S. The primary structure and the functional domains of an elongation factor-1 alpha from Mucor racemosus. J Biol Chem. 1986 Nov 15;261(32):15022–15029. [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Muramatsu M. Preparation of RNA from animal cells. Methods Cell Biol. 1973;7:23–51. doi: 10.1016/s0091-679x(08)61770-7. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Orlowski M. Growth-rate-dependent adjustment of ribosome function in the fungus Mucor racemosus. Biochem J. 1981 May 15;196(2):403–410. doi: 10.1042/bj1960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. Regulation of translation rate during morphogenesis in the fungus Mucor. Biochemistry. 1978 Feb 21;17(4):569–575. doi: 10.1021/bi00597a002. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Schirmaier F., Philippsen P. Identification of two genes coding for the translation elongation factor EF-1 alpha of S. cerevisiae. EMBO J. 1984 Dec 20;3(13):3311–3315. doi: 10.1002/j.1460-2075.1984.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Toledo H., Jerez C. A. In vitro methylation of the elongation factor EF-Tu from Escherichia coli. FEBS Lett. 1985 Nov 25;193(1):17–21. doi: 10.1016/0014-5793(85)80070-3. [DOI] [PubMed] [Google Scholar]
- Travers A. Control of ribosomal RNA synthesis in vitro. Nature. 1973 Jul 6;244(5410):15–18. doi: 10.1038/244015a0. [DOI] [PubMed] [Google Scholar]
- Tuhácková Z., Ullrichová J., Hradec J. Regulation of the activity of eukaryotic peptide elongation factor 1 by autocatalytic phosphorylation. Eur J Biochem. 1985 Jan 2;146(1):161–166. doi: 10.1111/j.1432-1033.1985.tb08633.x. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]
- van Hemert F. J., Amons R., Pluijms W. J., van Ormondt H., Möller W. The primary structure of elongation factor EF-1 alpha from the brine shrimp Artemia. EMBO J. 1984 May;3(5):1109–1113. doi: 10.1002/j.1460-2075.1984.tb01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert F. J., Lenstra J. A., Möller W. Genes for elongation factor EF-1 alpha in the brine shrimp Artemia. FEBS Lett. 1983 Jul 4;157(2):295–299. doi: 10.1016/0014-5793(83)80564-x. [DOI] [PubMed] [Google Scholar]