Abstract
Opinion statement
Skeletal metastases threaten quality of life, functionality, and longevity in patients with metastatic castration-resistant prostate cancer (mCRPC). Therapeutic strategies for bone metastases in prostate cancer can palliate pain, delay/prevent skeletal complications, and prolong survival. Pharmacologic agents representing several drug classes have demonstrated the ability to achieve these treatment goals in men with mCRPC. Skeletal-related events such as fracture and the need for radiation can be delayed using drugs that target the osteoclast/osteoblast pathway. Cancer-related bone pain can be palliated using beta-emitting bone-seeking radiopharmaceuticals such as samarium-153 EDTMP and strontium-89. Also, prospective randomized studies have demonstrated that cytotoxic chemotherapy can palliate bone pain. For the first time, bone-directed therapy has been shown to prolong survival using the novel alpha-emitting radiopharmaceutical radium-223. Given these multifold clinical benefits, treatments targeting bone metabolism, tumor-bone stromal interactions, and bone metastases themselves are now central elements of routine clinical care. Decisions about which agents, alone or in combination, will best serve the patient’s and clinician’s clinical goals is contingent on the treatment history to date, present disease manifestations, and symptomatology. Clinical trials exploring novel agents such as those targeting c-Met and Src are under way, using endpoints that directly address how patients feel, function, and survive.
Keywords: Bone-directed therapy, Bone metastases, Skeletal-related events, Radiopharmaceuticals
Introduction
Prostate cancer has a distinct tropism for bone, making it the most common, and frequently the only, site of metastatic disease [1–4]. This pattern of disease distribution yields a unique set of symptoms and challenges for clinicians, patients, and researchers. Early in the natural history of the disease, bone metastases are generally asymptomatic, but ultimately at least 35–45 % will be affected by bone pain, 14–22 % of patients will endure pathologic fracture, and 3–7 % will experience spinal cord compression, as reported in contemporary clinical trials [5••, 6–8]. Collectively, skeletal metastases can lead to devastating neurologic compromise and decline in functionality.
Treatment of skeletal metastases, therefore, holds two potential benefits for patients: (1) reducing existing bone-related symptoms, and (2) prolonging the time to onset of a new bone-related insult to quality of life or patient survival. In order to ensure that these crucial treatment goals be a focus even in early clinical trials, a panel of experts in prostate cancer clinical trial design established the Prostate Cancer Working Group 2 (PCWG2) Consensus Criteria [9]. This consensus report defined clinical trial designs in terms of eligibility criteria, treatment assessments, and outcome measures to demonstrate that a drug either effectively controls/relieves existing symptoms or prevents/delays new disease manifestations.
Examples of control/relieve endpoints include palliation of bone pain, fatigue, and other cancer-related symptoms, while prevent/delay endpoints evaluate time to radiographic progression, new onset of bone pain, or skeletal-related events (SRE). The latter includes pathologic fracture, need for radiation or surgery to bone, or spinal cord compression [6]. These control/relieve and prevent/delay endpoints are useful in phase II and phase III trials, and speak to the often-quoted metric needed to secure approval by the Food and Drug Administration (FDA): demonstrating that a drug impacts how patients “feel, function, and survive.”
For example, delaying the onset of SREs has been recognized by the FDA as a clinically important reflection of preservation of quality of life and functionality. These criteria were used as the basis for approval of zoledronic acid and denosumab, as will be discussed below. Palliation of existing bone pain is the basis for FDA approval of the beta-emitting bone-seeking radiopharmaceuticals strontium-89 and samarium-153 and the cytotoxic chemotherapy drug mitoxantrone. As an even more direct measure of patient benefit, the FDA has proposed that trials should reflect not just the physician’s interpretation of a patient’s pain or quality of life, but the patient’s own unfiltered reports of such. These data can be captured through surveys of patient-reported outcomes, and the FDA has issued detailed guidelines on their use and incorporation into clinical trials as endpoints [10]. Lastly, prolonging survival is a critical time-to-event metric recognized by the FDA.
However, some time-to-event endpoints have not been accepted as sufficient evidence that a clinically relevant event has in fact been prevented. For example, a clinical trial of denosumab vs. placebo in 1432 patients with non-metastatic castration-resistant prostate cancer (CRPC) demonstrated that bone-metastasis-free survival was improved by 15 % (hazard ratio [HR] 0.85, 95 % CI 0.73 to 0.98, p=0.028), or 4.2 months [11]. However, the FDA did not endorse the argument that delaying time to first metastasis by 4.2 months represented a significant clinical benefit, and denosumab has not been approved for this indication [12, 13].
This review will focus on pharmacologic interventions that delay/prevent or control/relieve bone manifestations as framed by PCWG2, using the clinical sequelae that the FDA has defined as clinically relevant, such as SREs, pain, and survival. All drugs currently approved by the FDA for metastatic CRPC (mCRPC) are listed in Table 1, with a summary of their clinical benefits. Figure 1 highlights agents with mechanisms targeting the bone microenvironment and bone-tumor interactions.
Table 1.
FDA-approved agents in mCRPC organized by results of clinical trial endpointa (primary, secondary, or exploratory endpoint)
| SRE prevention | Palliation of pain | Overall survival | |
|---|---|---|---|
| Mitoxantrone: Tannock et al., 1996 [46] | not examined | + primary | − secondary |
| Kantoff et al., 1999 [48] | not examined | + secondary | − primary |
| Docetaxel: Tannock et al., 2004 [7] | not examined | + secondary | + primary |
| Petrylak et al., 2004 [50] | not examined | − secondary | + primary |
| Cabazitaxel [8] | not examined | − secondary | + primary |
| Abiraterone [65] | + exploratory | + exploratory | + primary |
| Zometa [6, 15] | + primary | + secondary | − exploratory |
| Denosumab [6] | + primary | not examined | − exploratory |
| Strontium-89 [34, 38, 66] | not examined | + primary | not examinedb |
| Samarium-153 [40, 42] | not examined | + primary | not examined |
| Sipuleucel-T [67] | not examined | N/A; not examined | + primary |
The treatment of bone metastases in prostate cancer can be organized by clinical trial endpoints: prevention of skeletal-related events, palliation of pain, and extension of survival. With some exceptions, therapies serve non-overlapping goals and virtually all are aimed at metastatic castration-resistant disease
Beta-emitting radiopharmaceuticals have not been well studied to detect a survival advantage. As reviewed in the text, one trial explored a survival benefit in a randomized phase 2 setting
indicates benefit
indicates no benefit
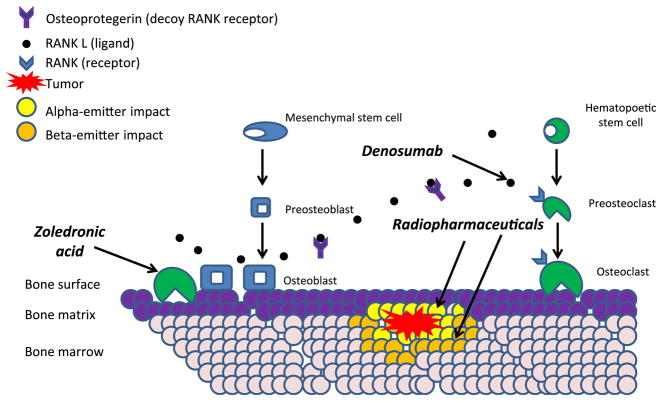
Figure 1.
Simplified schema of bone directed therapies and their primary targets. Osteoblasts produce receptor activator of nuclear factor kappa b ligand (RANKL) which binds to its receptor (RANK) on osteoclast precursors, stimulating osteoclast activity. Regulation of such stimulation is exerted by osteoblast production of osteoprotegerin, a soluble decoy receptor of RANKL. Available treatments utilize these pathways. Denosumab binds to RANKL, preventing it from binding to its receptor on osteoclast precursors. Bisphosphonates prevent osteoclast activity by binding to hydroxyapatite in bone. Radiopharmaceuticals deliver ionized radiation to bone metastases. The impact of alpha and beta emitters on surrounding bone marrow are not drawn to scale.
Delaying/preventing the skeletal complications of prostate cancer
Overview
Targeting the osteoclast-osteoblast pathway is a therapeutic strategy that focuses on the bone microenvironment to preserve bone health and reduce morbidity. Osteoclasts are the cells most responsible for bone resorption, while osteoblasts rebuild bone as part of normal homeostasis—a process that is disrupted in skeletal metastases [14]. The bisphosphonate zoledronic acid and the receptor activator of nuclear factor-kappa B ligand (RANKL) inhibitor denosumab are the only two agents that are FDA approved to prevent skeletal-related events in mCRPC. Although there has been suggestion of anti-tumor growth, but not survival benefit, in prostate cell lines, neither agent has been found to improve survival in patients with metastatic disease [15–18]. Both agents have been and currently are under investigation for other therapeutic indications in non-meta-static prostate cancer, namely the treatment of low bone mineral density and prevention of bone metastasis. Denosumab is FDA approved to mitigate loss of bone mineral density in men receiving androgen-deprivation therapy (ADT) who are at high risk for fracture. The use of zoledronic acid, while not specifically FDA approved to improve bone density in men with non-metastatic disease on ADT, is acceptable under National Comprehensive Cancer Network (NCCN) guidelines when the fracture risk from secondary osteoporosis warrants treatment.
Zoledronic acid
Preventing SREs
The primary goal of zoledronic acid is the prevention of SREs. It is a potent, third-generation nitrogen-containing bisphosphonate that binds to hydroxyapatite, preventing bone resorption mediated by the osteoclast. This intravenous agent was FDA approved for SRE prevention in 2002 based on results from a trial of 643 patients with mCRPC. This study began as a 3-arm trial comparing placebo, 4 mg IV, or 8 mg IV administered every 3 weeks. Approximately 19 % of patients had baseline serum creatinine >1.4 mg/dL. Increased renal impairment was seen in the 8 mg arm, and the Data Safety and Monitoring Board and Renal Advisory Board recommended changing the maximum dose to 4 mg [15, 19]. The statistical plan was changed prior to study completion or unblinding so that the primary efficacy endpoint would compare placebo to 4 mg. The primary efficacy endpoint was the proportion of men with at least one SRE, and secondary endpoints included time to SRE and bone pain. A greater proportion of the group receiving placebo experienced an SRE than the group who received 4 mg every 3 weeks for 15 months (44.2 % vs 33.2 %; 95 % CI −20.3 % to −1.8 %, p=0.021). With follow-up at 24 months, zoledronic acid 4 mg IV every 3 weeks decreased the risk of SREs by 36 % (relative risk [RR]=0.64, 95 % CI 0.485 to 0.845, p=0.002), increased the time to first on-study SRE (488 days vs 321 days, p=0.009), and decreased bone pain (−0.47 % difference on the Bone Pain Index at 24 months, p=0.024) in comparison to placebo [6]. It is worth noting that there are no other bisphosphonates that are FDA approved for the prevention of SREs in metastatic prostate cancer. Pamidronate has been used off-label; however, in a combined analysis (of 2 multicenter trials, INT 05 and CGP 032) of 350 men with mCRPC, 90 mg given intravenously every 3 weeks for 27 weeks did not result in an improvement in SREs or bone pain when compared to placebo [20].
The standard dose of zoledronic acid for mCRPC is 4 mg given intravenously every 4 weeks. Renal insufficiency with a glomerular filtration rate (GFR) of <30 mL/min/1.7 m2 or evidence of acute renal impairment are contraindications, and if the GFR is 30–60 ml/min/1.7 m2, the dose should be adjusted. Principal side effects include flu-like reactions such as myalgias, fatigue, or fever in the first 3 days (up to 44 %), hypocalcemia (6 %), and osteonecrosis of the jaw (ONJ) (1 %) [5••]. Risk factors for ONJ with bisphosphonate use include duration and dose frequency of zoledronic acid, and dental intervention (e.g., tooth extraction) [21]. Importantly, the optimal duration of therapy using zoledronic acid is unknown, but it is an important question, as treatment has associated side effects, costs, and inconvenience, and duration of efficacy with long-term use in this population is unknown.
The Alliance for Clinical Trials in Oncology cooperative group is also evaluating zoledronic acid with ADT versus ADT alone in castration-sensitive metastatic disease with a primary endpoint of time to first SRE. This trial has nearly completed accrual. At this time, the use of zoledronic acid to prevent SRE in castration-sensitive metastatic disease is considered off-label and without any known clinical benefit.
Reducing bone fragility
Investigations into whether introducing a bisphosphonate earlier in the treatment history of prostate cancer could convey a clinical benefit are ongoing. Zoledronic acid has been studied in small, randomized trials in men with non-metastatic disease beginning ADT, which found improvements in bone mineral density with both single dosing and dosing every 3 months [22, 23]. The use of zoledronic acid for androgen-deprivation therapy induced bone loss in non-metastatic disease, however, is considered off label.
Delaying metastases
The ongoing ZEUS trial is investigating the use of zoledronic acid 4 mg IV every 3 months for metastasis prevention, and results are anticipated soon. This is an open, randomized trial being conducted in Europe and Scandinavia for men at high risk for metastatic disease. The primary endpoint is to determine the proportion of men that develop metastatic disease, with secondary endpoints including overall survival, bone mineral density, and prostate-specific antigen (PSA) doubling time. It is unclear at this time, as discussed above, whether delaying metastatic disease is an approvable clinical trial endpoint in prostate cancer from a regulatory standpoint. At present, zoledronic acid is not recommended for metastasis prevention outside of a clinical trial.
Denosumab
Preventing SREs
Denosumab is a fully human monoclonal antibody against the receptor activator nuclear kappa B (RANK) ligand. This ligand is present on osteoblasts and bone marrow stromal cells, while its receptor is on the osteoclast. Binding of RANKL to RANK stimulates bone resorption and is highly implicated in both osteolytic and osteoblastic metastases [14]. Denosumab was approved in November 2010 for the prevention of SREs in all patients with solid tumors and bone metastases, based on data in metastatic breast cancer (n=2046) and solid tumors (other than breast and prostate) which demonstrated superiority/non-inferiority over zoledronic acid in delaying time to first on-study SRE [24, 25].
Research into the prevention of SREs in prostate cancer has been limited to mCRPC, with castration resistance defined as failure of at least one hormonal intervention. The large, multinational Study “103” phase III trial comparing denosumab to zoledronic acid established the role of denosumab 120 mg given subcutaneously every 4 weeks for mCRPC [5••]. This noninferiority trial of 1904 men with mCRPC found time to first SRE was delayed by 3.6 months (20.7 months for denosumab and 17.1 months for zoledronic acid) (HR 0.82, 95 % CI 0.71 to 0.95; p=0.0002 for non-inferiority; p=0.008 for superiority). There was no survival difference (19.4 months vs 19.8 months) between these agents in mCRPC (HR 1.06, 95 % CI 0.95 to 1.18, p=0.30). The leading SREs were the need for radiation to bone (19–21 % of patients) and pathologic fracture (14–15 % of patients). Pathologic fracture included those radiographically detected, not those that were clinically symptomatic per se. As such, delaying the need for a course of radiation or delaying the radiographic detection of a new fracture by 3.6 months is a decision that must be considered in the context of an agent’s toxicity profile.
The risk of ONJ with denosumab in prostate cancer has been reported to be between 2–5 % [5••, 26•]. Significant caution is advised in treating patients with preexisting hypocalcemia, given that 58 % of patients will experience some degree of hypocalcemia [5••]. Patients should take calcium and vitamin D supplementation while on therapy, with monitoring of these levels. Denosumab is not renally cleared and as a monoclonal antibody was believed to be safe in patients with renal dysfunction. However, eligible mCRPC patients in the randomized “103” trial were required to have a creatinine clearance (CrCl) rate of >50 mL/min, as they may have been randomized to zoledronic acid. For this reason it has not been as rigorously studied in patients with renal insufficiency. Patients with CrCl <30 mL/min are at higher risk of hypocalcemia and hypophosphatemia, and at this time the appropriate dose, safety, and frequency is unknown in this population.
Reducing bone fragility
Similar to zoledronic acid, denosumab has been explored for other indications in earlier clinical states such as the treatment of bone fragility. A double blind, placebo-controlled trial of men (n=1468) with non-metastatic castration-sensitive prostate cancer at risk for fracture on ADT [26•] evaluated the utility of denosumab in bone fragility. This trial demonstrated improved bone mineral density and reduced radiographically detected vertebral skeletal fractures (1.5 % vs 3.9 % with placebo) when 60 mg of denosumab was administered subcutaneously every 6 months (RR 0.38, 95 % CI 0.19 to 0.78, p=0.006). The FDA approved denosumab for this second indication in September 2011.
Delaying metastases
In a population of 1432 men with non-metastatic, castration resistant disease considered at high risk for metastases (PSA ≥8 μg/L, doubling time ≤10 months, or both), denosumab 120 mg given subcutaneously every 4 weeks was compared to placebo to determine if this agent could delay the time to the first skeletal metastasis [11]. As mentioned previously, denosumab improved bone-metastasis-free survival by 4.2 months (HR=0.85, 95 % CI 0.73 to 0.98, p=0.028) and delayed time to first skeletal metastasis by approximately 3.7 months (33.2 vs 29.5) when compared to placebo (HR=0.84, 95 % CI 0.71 to 0.98, p=0.032), with no survival advantage (HR=1.01, 95 % CI 0.85 to 1.20, p=0.91). The ultimate clinical benefit is unclear and, as discussed previously, these results did not meet the FDA criteria of evidence of patient benefit and denosumab has not been approved for this indication. While denosumab is generally well tolerated, there are potential toxicities such as ONJ and therefore introducing its use in earlier disease states without a survival benefit comes with risk and certainly cost.
Controlling/relieving existing bone pain
Overview
The hallmark agents known to target and improve painful bone metastases in CRPC are radiopharmaceuticals. These are radioactive isotopes that are preferentially incorporated into osteoblastic bone metastases when administered intravenously. There are two general varieties: predominantly beta-emitting radiopharmaceuticals (strontium, samarium) and a newer generation of alpha-emitting radiopharmaceuticals. The alpha-emitting radiopharmaceutical radium-223 has been shown to prolong survival (as discussed later in this review); the beta emitters have been shown to control and relieve bone pain in patients with osteoblastic disease. The principal difference between these two types of emitters relates to the higher amount of energy delivered by alphas than betas, but with significantly shorter tissue penetration, resulting in less damage to the surrounding marrow and less myelosuppression. Other characteristics that distinguish each radiopharmaceutical include its half-life, gamma energy, route of excretion, and anticipated onset and duration of clinical response. In addition to beta emitters, cytotoxic chemotherapy using mitoxantrone has a well-documented role in the palliation of pain in this disease that makes tumor-directed strategies worthy of discussion.
Radiopharmaceuticals
As a drug class, radiopharmaceuticals have a long history of palliating skeletal metastases by targeting tumor cells in the bone. They nonetheless are frequently underutilized, given clinicians’ concern for cytopenias that can be associated with these agents. Lack of awareness of the different properties between beta-emitting radiopharmaceuticals, lack of collaboration with nuclear medicine physicians who administer treatment, and concern for hematologic suppression have all contributed to their lack of widespread use. Furthermore, until recently there has not been data supporting a survival advantage with radiopharmaceuticals.
Strontium-89
Strontium-89 chloride was FDA approved in 1993 as the first beta-emitting radiopharmaceutical for metastatic prostate cancer. It has a half-life of 50.5 days, beta energy of 1.5 MeV, virtually no gamma energy, and is rapidly excreted by the kidneys and incorporated into bone mineral [27–29]. It is administered at a standard dose of 4.0 mCi. There is a well-established body of literature demonstrating its efficacy for pain palliation through 11 randomized trials, the majority of which included or were exclusive to prostate cancer [28, 30–35]. A recent systematic review reported mean overall response rates of 76 %, and pain relief typically began within 2 weeks [27, 35, 36].
While palliation of pain is established, the data for a survival advantage is sparse. One study suggested a survival advantage: 103 patients with mCRPC who responded to induction chemotherapy with the KAVE regimen were randomized to doxorubicin alone or doxorubicin with strontium-89, with a median overall survival of 16.8 months and 27.7 months, respectively [33]. Neither the KAVE regimen nor doxorubicin has been proven to prolong survival in mCRPC. This study was small, and it is noteworthy that only responders were randomized to consolidation with strontium-89/doxorubicin or doxorubicin alone. Conceptually, however the use of combined chemotherapy and radio-pharmaceuticals provided valuable evidence for this therapeutic strategy as well as associated toxicity. Of note, no patients receiving combination therapy in this trial developed bone marrow failure within 6 months of receiving strontium-89 [37]. The principal toxicity of strontium-89 is hematologic in nature, with an average reduction in white blood cells (WBC) of 15 % and platelet count of 25–45 % in patients receiving the recommended dose of 4.0 m Ci or 150 MBq [34, 38]. Nadirs occur around 6 weeks, and count recovery can take up to 3–6 months [34, 38].
Samarium-153
Samarium-153 conjugated to ethylene-diamine-tetra-methylene-phosphonic acid (EDTMP) is another beta-emitting radiopharmaceutical that was FDA approved in 1997. Samarium-153 maintains several advantages over strontium-89 and therefore is the preferred beta-emitting radiopharmaceutical of many clinicians. The half-life is 1.9 days and pain relief is rapid—generally between 2 and 7 days [39, 40]. Gamma emission is 103 keV, allowing for scintigraphic imaging, and indeed images strongly correlate with conventional technium-99 bone scans. Marrow toxicity is the principal side effect. Platelet and WBC counts nadir between 3 and 6 weeks and generally recover by 8 weeks [39, 41]. Across three randomized trials using a single administration of samarium-153 1.0 mCi/kg, grade 3+ thrombocytopenia was 3–15 % and grade 3+ neutropenia was 5–14 % [40, 42, 43]. At standard dose, mean platelet reductions were 43–45 % and mean WBC declines were 49–51 % of baseline [40, 43]. As such, most clinical trials have used the following hematologic parameters at trial entry: WBC <2500 mm3, platelet count 100,000 mm3. Other contraindications to use of beta-emitting radiopharmaceuticals include radiotherapy within the previous 2 months, impending cord compression or pathologic fracture, significant renal insufficiency, Karnofsky Performance Status <50 %, and disseminated intra-vascular coagulation.
As single dosing samarium-153 has demonstrated palliative responses, the tolerability of repeated dosing has also been explored. Samarium-153 can be administered with repeat dosing of 1.0 mCi/kg both safely and effectively [41]. In patients receiving two or more doses of samarium-153, time to platelet or WBC nadir did not change after the first dose. 12 % experienced grade 3+ thrombocytopenia and recovery to a platelet count >75,000/mm3 occurred by week 8 in 90.4 % of patients.
In combining a radiopharmaceutical with chemotherapy to enhance anti-tumor effects, several phase I/II trials have explored the use of repeated doses of samarium-153 in combination with increasing doses of docetaxel. These trials did not reach doselimiting toxicity [44, 45]. Thus, can one reap the benefits of one agent known to increase survival (docetaxel) and use this concurrently with a radiopharmaceutical known to improve bone metastasis pain, thereby extending life and improving pain. There are no trials comparing samarium-153 with docetaxel versus docetaxel alone; however, as a concept, it has further laid the foundation for combination chemotherapy with a radiopharmaceutical.
Cytotoxics
Mitoxantrone
Mitoxantrone serves an historic role as one of the earliest chemotherapies approved for mCRPC. An anthracenedione, mitoxantrone was FDA approved in 1996 on the basis of improving pain relief in patients with mCRPC. A randomized trial of 161 patients with symptomatic mCRPC compared mitoxantrone 12 mg/m2 every 3 weeks with prednisone versus prednisone alone and demonstrated improvements in pain relief with combination mitoxantrone and prednisone. Palliative response, defined by the protocol as the primary endpoint, was observed in 23 % versus 12 % of patients, respectively (p=0.01). Response was considered a 2-point reduction in the 6-point “present pain intensity scale” of the McGill-Melzack Pain Questionnaire. The duration of response (43 weeks vs 18 weeks) was also longer in the mitoxantrone arm [46]. Improvements in global quality of life (p=0.09) and functionality (p<.01) were also seen [47]. Another randomized trial in mCRPC, CALGB 9182, found isolated quality-of-life issues such as pain severity to be improved with mitoxantrone/hydrocortisone when compared to hydrocortisone alone (p=0.03)[48].
While mitoxantrone is generally well tolerated and neutropenic fever is uncommon (0–1 %) [46, 48], no randomized study has demonstrated a survival benefit when compared to prednisone or hydrocortisone alone [46, 48]. As such, it is less commonly used, given the availability of agents known to improve survival that have been developed over the past decade for mCRPC. Mitoxantrone has not, however, been completely phased out of the therapeutic armamentarium and is still being studied in clinical trials. For example, there is a phase I/II trial of AMG102, a hepatocyte growth factor inhibitor, in combination with mitoxantrone versus mitoxantrone alone as well as a phase III trial comparing XL184, a cMet/VEGFR2 inhibitor, versus mitoxantrone with a primary endpoint of pain using patient-reported outcomes.
Docetaxel
The question arises for the role of contemporary chemotherapy in the palliation of bone pain in mCRPC. Unlike the mitoxantrone trial that used pain palliation as a primary endpoint, trials of docetaxel were constructed to evaluate pain as secondary endpoints, and ultimately docetaxel does not have an indication for the palliation of pain. Docetaxel gained FDA approval in 2004 for mCRPC on the basis of a survival benefit seen in two large randomized trials, TAX 327 and SWOG 99-16. In TAX 327, men who received docetaxel 75 mg/m2 every 3 weeks had a 24 % reduction in the risk of death when compared to men who received mitoxantrone (HR 0.76, 95 % CI 0.62 to 0.94, p=0.0009), corresponding to an overall survival advantage of 2.4 months (18.9 vs 16.5). Pain response was also improved (35 % vs 22 %, p=0.01) [7]. Docetaxel 30 mg/m2 weekly for 5 of 6 weeks was not associated with a statistically significant survival benefit or pain response in comparison to placebo in the TAX 327 trial. SWOG 99-16 randomized patients to docetaxel 60 mg/m2 every 3 weeks with 280 mg oral estramustine 3 times a day on days 1–5 versus mitoxantrone 12 mg/m2 every 3 weeks. The overall survival benefit was 1.9 months (17.5 vs 15.6, p=0.02); however, this study, which like TAX 327 used the Present Pain Index, failed to demonstrate a difference in pain response [49, 50]. On the spectrum of cytotoxic therapy, docetaxel is relatively well tolerated. The recommended dosing is docetaxel 75 mg/m2 every 3 weeks with oral prednisone 5 mg twice a day. Grade 3+ neutropenia is approximately 32 %, with less than 3 % neutropenic fever. Granulocyte colony-stimulating factor should be used in patients at high risk for complications of neutropenic fever. Sensory neuropathy of any grade occurred in 30 % of patients.
Delaying death/improving survival by targeting bone
Overview
While prolonging survival by targeting bone has been and remains a viable goal, only one agent, radium-223, truly occupies this space. Many agents have been shown to prolong survival in mCRPC; however, these either target the tumor itself or alter immune surveillance and are not specific to bone. As such, the alpha-emitter radium-223 is the sole agent that has definitively been shown to prolong life via a bone-targeted mechanism.
Radium-223
Radium-223 is an alpha-emitting bone-seeking radiopharmaceutical that is excreted through the gastrointestinal tract with a half-life of 11.4 days and low gamma irradiation [51, 52]. Moreover, it is unique in comparison to beta emitters in that it delivers high linear energy with very small track length (<0.1 mm in tissue) or scatter and subsequently far less myelosuppression to the bone marrow. Bruland et al. found neutrophils were affected more than platelets, both with reduced toxicity in comparison to beta emitters [53]. The most mature data for radium-223 comes from the large, randomized ALSYMPCA trial in mCRPC patients with symptomatic bone metastases. Patients were randomized 2:1 to receive 50 kBq/kg IV every 4 weeks for 6 cycles, or placebo. The primary endpoint of this trial was overall survival, while quality of life, although not pain per se, was a secondary endpoint. Radium-223 improved overall survival when compared to placebo by 2.8 months (11.2 vs 14; HR=0.695, 95 % CI 0.552 to 0.875, p=0.00185) [54]. Interim results reported myelosuppression was very low, with 1.8 % of patients treated with radium-223 experiencing grade 3+ neutropenia [54]. Similar to the strategy pursued with beta emitters and docetaxel, a dose-escalation study of radium-223 in combination with docetaxel (NCT01106352) is under way under the auspices of the Prostate Cancer Clinical Trials Consortium.
Novel agents/future directions
There are many agents under investigation in prostate cancer, and those with specific effects on bone are particularly exciting. Despite the failure of some recent clinical trials examining the endothelin receptor and VEGF pathways using zibotentan and bevacizumab, respectively [55, 56], strategies targeting bone remain an important area of investigation as our understanding of the bone microenvironment grows. Among these novel therapeutics are dasatinib and the tyrosine kinase inhibitor XL184.
Cabozantinib (XL184)
c-Met is overexpressed in prostate cancer cells, particularly those within bone metastases, and is associated with tumor progression and metastatic invasion in bone [57–59]. The c-Met ligand is a hepatocyte growth factor secreted by stromal cells [60]. XL184 is a novel receptor tyrosine kinase inhibitor that inhibits c-Met and VEGFR2, among other pathways. Results from a phase II randomized trial presented at ASCO 2011 highlighted remarkable findings in bone: 86 % (56 of 65 patients evaluable by bone scan) had complete or partial resolution of lesions on bone scan as early as week 6. Complete resolutions, in particular, are virtually unheard of in mCRPC and were seen dramatically on cabozantinib; 55 % of patients with mCRPC had declines of ≥50 % in plasma C-telopeptide, and 56 % of patients with elevated total alkaline phosphatase had declines of ≥50 % [61]. Whether the radiographic bone responses translate into a survival benefit and durable clinical response will be determined in upcoming phase III trials. Improvement in bone pain is noteworthy with this agent. In phase II published data, of the 28 patients receiving narcotics for bone pain, 64 % had improvement in pain intensity and 46 % were able to decrease or discontinue narcotics [61]. The palliative effects have prompted the phase III study known as COMET-2 (CabOzantinib MET Inhibition CRPC Efficacy Trial) of cabozantinib versus mitoxantrone and prednisone to demonstrate a primary endpoint of pain reduction. A separate phase III trial, COMET-1, will assess for a survival advantage.
Dasatinib
Dasatinib is a tyrosine kinase inhibitor that inhibits Src, a mediator of osteoclastic activity, tumor growth, and metastases [62]. In a phase I/II trial of dasatinib combined with docetaxel, 30 % (n=14) of patients had disappearance of a lesion on bone scan and another 41 % (n=19) had stable bone scans. Bone markers also declined in >75 % of patients (87 % experienced urine N-telopeptide declines and 76 % had decreases in bone-specific alkaline phosphatase levels) [63]. Although there is evidence of effect in bone, antitumor activity (as measured by serum PSA levels and Response Evaluation Criteria in Solid Tumors [RECIST]) with monotherapy is less impressive [64]. Results detected in bone prompted a phase III trial with a primary endpoint of overall survival, and secondary endpoints of SRE and pain. The preliminary results of this phase III trial of dasatinib with docetaxel versus docetaxel alone are expected soon.
These novel therapies leverage the biology of the bone microenvironment and bone-tumor pathways in a highly targeted and sophisticated fashion; however, the litmus test by which a drug’s efficacy is assessed has been (and remains, until a surrogate biomarker is available) its ability to improve how a patient feels, functions, and survives.
Footnotes
Disclosure
KA Autio: none; HI Scher: Consultancy for Amgen, Bristol-Myers Squibb, Dendreon, Endo Pharmaceuticals, Millennium, Novartis, Ortho Biotech Oncology Research, Sanofi-Aventis, and Senior Scientific LLC; MJ Morris: consultancy for Millennium Pharmaceuticals.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Coleman R. Management of bone metastases. Cancer Treat Rev. 1997;23 (Suppl 1):S69–75. doi: 10.1016/s0305-7372(97)90009-8. [DOI] [PubMed] [Google Scholar]
- 2.Saylor PJ, Lee RJ, Smith MR. Emerging therapies to prevent skeletal morbidity in men with prostate cancer. J Clin Oncol. 2011;29(27):3705–14. doi: 10.1200/JCO.2010.34.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fizazi K, Beuzeboc P, Lumbroso J, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2009;27 (15):2429–35. doi: 10.1200/JCO.2008.18.9811. [DOI] [PubMed] [Google Scholar]
- 4.Saad F, Olsson C, Schulman CC. Skeletal morbidity in men with prostate cancer: quality-of-life considerations throughout the continuum of care. Eur Urol. 2004;46(6):731–39. doi: 10.1016/j.eururo.2004.08.016. discussion 739–40. [DOI] [PubMed] [Google Scholar]
- 5••.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–22. doi: 10.1016/S0140-6736(10)62344-6. This large, randomized, non-inferiority trial demonstrated that denosumab delayed skeletal-related events by 3.6 months relative to zoledronic acid in men with mCRPC. Both agents are FDA approved to delay skeletal events in metastatic castration-resistant disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879–82. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 7.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351 (15):1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 8.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 9.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Dept of Health and Human Services, Food and Drug Administration. [Accessed 1 Mar 2012.];Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. 2010 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf.
- 11.Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furlow B. US FDA advisory panel rejects early denosumab therapy. Lancet Oncol. 2012;13(3):e94. [Google Scholar]
- 13.Pradhan SM. FDA Presentation sBLA 125320/28 Xgeva™ (denosumab) [Accessed 1 Mar 2012.];Food and Drug Administration, Oncology Drugs Advisory Committee Briefing Document. 2012 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM290400.pdf.
- 14.Guise T. Examining the metastatic niche: targeting the microenvironment. Semin Oncol. 2010;37 (Suppl 2):S2–14. doi: 10.1053/j.seminoncol.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458–68. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 16.Corey E, Brown LG, Quinn JE, et al. Zoledronic acid exhibits inhibitory effects on osteoblastic and osteolytic metastases of prostate cancer. Clin Cancer Res. 2003;9(1):295–306. [PubMed] [Google Scholar]
- 17.Brubaker KD, Brown LG, Vessella RL, et al. Administration of zoledronic acid enhances the effects of docetaxel on growth of prostate cancer in the bone environment. BMC Cancer. 2006;6:15. doi: 10.1186/1471-2407-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung TT, Chan J, Russell PJ, et al. Zoledronic acid preserves bone structure and increases survival but does not limit tumour incidence in a prostate cancer bone metastasis model. PLoS One. 2011;6(5):e19389. doi: 10.1371/journal.pone.0019389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. N Engl J Med. 2003;349(17):1676–9. doi: 10.1056/NEJM200310233491721. discussion 1676–9. [DOI] [PubMed] [Google Scholar]
- 20.Small EJ, Smith MR, Seaman JJ, et al. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21(23):4277–84. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 21.Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2011 doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 22.Smith MR, Eastham J, Gleason DM, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169(6):2008–12. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 23.Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25(9):1038–42. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 25.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–32. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 26•.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361 (8):745–55. doi: 10.1056/NEJMoa0809003. This placebo-controlled randomized phase III study established that denosumab can mitigate the bone-wasting effects of androgen-deprivation therapy in prostate cancer patients. Bone density improved and fracture rates were reduced with the use of denosumab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith HS. Painful osseous metastases. Pain Physician. 2011;14(4):E373–403. [PubMed] [Google Scholar]
- 28.Taylor AJ., Jr Strontium-89 for the palliation of bone pain due to metastatic disease. J Nucl Med. 1994;35 (12):2054. [PubMed] [Google Scholar]
- 29.Kasalicky J, Krajska V. The effect of repeated strontium-89 chloride therapy on bone pain palliation in patients with skeletal cancer metastases. Eur J Nucl Med. 1998;25(10):1362–7. doi: 10.1007/s002590050309. [DOI] [PubMed] [Google Scholar]
- 30.Turner SL, Gruenewald S, Spry N, et al. Less pain does equal better quality of life following strontium-89 therapy for metastatic prostate cancer. Br J Cancer. 2001;84(3):297–302. doi: 10.1054/bjoc.2000.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baczyk M, Milecki P, Baczyk E, et al. The effectivness of strontium 89 in palliative therapy of painful prostate cancer bone metastases. Ortop Traumatol Rehabil. 2003;5(3):364–8. [PubMed] [Google Scholar]
- 32.Gunawardana DH, Lichtenstein M, Better N, et al. Results of strontium-89 therapy in patients with prostate cancer resistant to chemotherapy. Clin Nucl Med. 2004;29(2):81–5. doi: 10.1097/01.rlu.0000109721.58471.44. [DOI] [PubMed] [Google Scholar]
- 33.Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357:326–7. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 34.Pons F, Herranz R, Garcia A, et al. Strontium-89 for palliation of pain from bone metastases in patients with prostate and breast cancer. Eur J Nucl Med. 1997;24(10):1210–4. doi: 10.1007/s002590050143. [DOI] [PubMed] [Google Scholar]
- 35.Finlay IG, Mason MD, Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncol. 2005;6(6):392–400. doi: 10.1016/S1470-2045(05)70206-0. [DOI] [PubMed] [Google Scholar]
- 36.Robinson RG, Preston DF, Baxter KG, et al. Clinical experience with strontium-89 in prostatic and breast cancer patients. Semin Oncol. 1993;20:44–8. [PubMed] [Google Scholar]
- 37.Tu SM, Kim J, Pagliaro LC, et al. Therapy tolerance in selected patients with androgen-independent prostate cancer following strontium-89 combined with chemotherapy. J Clin Oncol. 2005;23(31):7904–10. doi: 10.1200/JCO.2005.01.2310. [DOI] [PubMed] [Google Scholar]
- 38.Laing AH, Ackery DM, Bayly RJ, et al. Strontium-89 chloride for pain palliation in prostatic skeletal malignancy. Br J Radiol. 1991;64:816–22. doi: 10.1259/0007-1285-64-765-816. [DOI] [PubMed] [Google Scholar]
- 39.Turner JH, Claringbold PG, Hetherington EL, et al. A phase I study of samarium-153 ethylenediaminete-tramethylene phosphate therapy for disseminated skeletal metastases. J Clin Oncol. 1989;7:1926–31. doi: 10.1200/JCO.1989.7.12.1926. [DOI] [PubMed] [Google Scholar]
- 40.Serafini AN, Houston SJ, Resche I, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol. 1998;16 (4):1574–81. doi: 10.1200/JCO.1998.16.4.1574. [DOI] [PubMed] [Google Scholar]
- 41.Sartor O, Reid RH, Bushnell DL, et al. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer. 2007;109(3):637–43. doi: 10.1002/cncr.22431. [DOI] [PubMed] [Google Scholar]
- 42.Sartor O, Reid RH, Hoskin PJ, et al. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63(5):940–5. doi: 10.1016/j.urology.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 43.Resche I, Chatal J-F, Pecking A, et al. A dose-controlled study of 153-Sm-ethylenediaminetetrame-thylenephosphonate (EDTMP) in the treatment of patients with painful bone metastases. Eur J Cancer. 1997;33:1583–91. doi: 10.1016/s0959-8049(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 44.Tu SM, Mathew P, Wong FC, et al. Phase I study of concurrent weekly docetaxel and repeated samarium-153 lexidronam in patients with castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27 (20):3319–24. doi: 10.1200/JCO.2008.20.5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris MJ, Pandit-Taskar N, Carrasquillo J, et al. Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27(15):2436–42. doi: 10.1200/JCO.2008.20.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–64. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 47.Osoba D, Tannock IF, Ernst S, et al. Health related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol. 1999;17(6):1654–63. doi: 10.1200/JCO.1999.17.6.1654. [DOI] [PubMed] [Google Scholar]
- 48.Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;18(8):2506–13. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 49.Berry DL, Moinpour CM, Jiang CS, et al. Quality of life and pain in advanced stage prostate cancer: results of a Southwest Oncology Group randomized trial comparing docetaxel and estramustine to mitoxantrone and prednisone. J Clin Oncol. 2006;24 (18):2828–35. doi: 10.1200/JCO.2005.04.8207. [DOI] [PubMed] [Google Scholar]
- 50.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 51.Nilsson S, Franzen L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8 (7):587–94. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson S, Larsen RH, Fossa SD, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res. 2005;11(12):4451–9. doi: 10.1158/1078-0432.CCR-04-2244. [DOI] [PubMed] [Google Scholar]
- 53.Bruland OS, Nilsson S, Fisher DR, et al. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006;12(20 Pt 2):6250s–7s. doi: 10.1158/1078-0432.CCR-06-0841. [DOI] [PubMed] [Google Scholar]
- 54.Parker C, Heinrich D, Sullivan JM, et al. Overall survival benefit of radium 223 chloride (Alpharadin) in the treatment of patients with symptomatic bone metastases in castrate resistant prostate cancer: a phase III randomized trial. European Multidisciplinary Cancer Congress; Stockholm. 2011. [Google Scholar]
- 55.James ND, Caty A, Payne H, et al. Final safety and efficacy analysis of the specific endothelin A receptor antagonist zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain: a double-blind, placebo-controlled, randomized Phase II trial. BJU Int. 2010;106 (7):966–73. doi: 10.1111/j.1464-410X.2010.09638.x. [DOI] [PubMed] [Google Scholar]
- 56.Kelly WKHS, Carducci MA, et al. A randomized, double-blind, placebo-controlled phase III trial comparing docetaxel, prednisone, and placebo with docetaxel, prednisone, and bevacizumab in men with metastatic castration-resistant prostate cancer (mCRPC): Survival results of CALGB 90401. J Clin Oncol. 2010;28(18s suppl):abstr LBA4511. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knudsen BS, Gmyrek GA, Inra J, et al. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002;60(6):1113–7. doi: 10.1016/s0090-4295(02)01954-4. [DOI] [PubMed] [Google Scholar]
- 58.Humphrey PA, Zhu X, Zarnegar R, et al. Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am J Pathol. 1995;147(2):386–96. [PMC free article] [PubMed] [Google Scholar]
- 59.Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 60.Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251 (4995):802–4. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 61.Hussain MSM, Sweeney C, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): Results from a phase II randomized discontinuation trial. J Clin Oncol. 2011;29(suppl):Abstr 4516. [Google Scholar]
- 62.Saad F, Lipton A. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev. 2010;36(2):177–84. doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Araujo JC, Mathew P, Armstrong AJ, et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1–2 study. Cancer. 2011 doi: 10.1002/cncr.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu EY, Massard C, Gross ME, et al. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology. 2011;77 (5):1166–71. doi: 10.1016/j.urology.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tu SM, Delpassand ES, Jones D, et al. Strontium-89 combined with doxorubicin in the treatment of patients with androgen-independent prostate cancer. Urol Oncol. 1996;2(6):191–7. doi: 10.1016/s1078-1439(97)00013-6. [DOI] [PubMed] [Google Scholar]
- 67.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]