Abstract
Brain corticotropin-releasing factor (CRF) acting on CRF receptor type 1 (CRF1) is a main signaling pathway in the stress response. CRF is also produced in a variety of peripheral sites and acts locally as a proinflammatory mediator. We investigated CRF1 mRNA expression in the human gastrointestinal tract, and localized CRF1 immunoreactive cells in the colonic mucosa of healthy subjects and patients with ulcerative colitis (UC). In 4 male healthy subjects (24-29 yrs), CRF1 transcript was detected by RT-PCR throughout the gastrointestinal tract with the highest levels in the ileum and rectum and the lowest level in the colon. Immunohistochemistry on whole thickness sigmoid colon sections showed that CRF1 was localized in the lamina propria and epithelial cells and enteric neurons. In sigmoid colonic biopsies, immunohistochemically double-labeled cells with CRF1 and CD163, a marker for macrophages, represent 79% of total CRF1 immunoreactive (IR) cells in healthy subjects. In 10 UC patients, the total number of CRF1 IR cells and CRF1/CD163 double-labeled macrophages was increased by 4.2 and 4.0 folds respectively compared to healthy subjects. These findings indicate that CRF1 is distributed throughout the GI tract of healthy human subjects. The increase of CRF1 IR cells prominently in macrophages of the sigmoid colonic mucosa of UC patients provides anatomical support for a role of CRF1 signaling in modulating the immune-inflammatory process of UC.
Keywords: Colon, CRF, CRF1 receptor, gut, sigmoid biopsy, macrophage, ulcerative colitis
1. Introduction
Activation of corticotropin releasing factor (CRF) signaling in the brain plays a key role in orchestrating the endocrine, behavioral and gastrointestinal (GI) responses to stress [3,39]. Convergent evidence indicates that CRF is also expressed in peripheral tissues where the peptide acts locally as a proinflammatory mediator [2,7,16,27,44]. In rodents, the increased expression of CRF has been found in inflamed tissues during carrageenin-induced granulomas [16], inflammatory arthritis [7], experimental autoimmune uveoretinitis [27], chronic granulomatous enterocolitis [44] and Clostridium difficile toxin A-mediated ileitis [2,20]. Our previous study also showed that CRF is upregulated at both mRNA and protein levels by peripheral injection of low dose of endotoxin in the rat colon [47]. Conversely, blockade of CRF signaling pathways by CRF immunoneutralization using anti-CRF antibody, pharmacological blockade of CRF receptors with antagonists or genetic approaches using CRF deficient mice or CRF RNA interference, dampened the local inflammatory response triggered in these tissues including in the intestine [2,10,16,20,27,44]. In humans, likewise, peripheral CRF is up-regulated at sites of rheumatoid arthritis [6], Hashimoto thyroiditis [36], endometriosis [18], psoriasis [19] as well as in the colon of patients with ulcerative colitis (UC) [17].
The biological actions of CRF and related peptides, urocortin (Ucn) 1, Ucn 2 and Ucn 3 are mediated via binding to two distinct receptor types, CRF1 and/or CRF2, both belonging to the class B of G-protein coupled receptor superfamily [12,22]. CRF1 has high affinity for CRF or Ucn 1 and no appreciable binding affinity for Ucn 2 and Ucn 3. In contrast, CRF2 displays high affinity for Ucn 1, Ucn 2 and Ucn 3 and low affinity for CRF [12]. Our functional studies in rodents indicate that the colonic secretory motor alterations and visceral hyperalgesia induced by peripheral administration of CRF and Ucn 1 are mediated by peripheral CRF1 receptor [21,26,35,41]. This is further supported by anatomical evidence that CRF1 receptors are expressed in colonic myenteric neurons, immune and endocrine cells in experimental animals [4,23,47]. By contrast, gene expression of CRF1 receptor in the human whole GI tract has been little investigated [31,43] particularly in the human intestinal mucosa which contains the majority of cells involved in immune reactions including macrophages, mast cells, granulocytes, T and B lymphocytes, and dentritic cells [14]. Among the immune cells, macrophages play a key role in orchestrating mucosal inflammatory responses [37]. Gaining insight to CRF1 receptor expression in the human GI tract, and in particular the cellular location in the colon and possibly altered expression under the conditions associated with inflammatory bowel disease (IBD) would provide anatomical support for potential sites of CRF/Ucn 1 action in clinical setting. This will be particularly relevant in the context of UC based on clinical reports that CRF, a preferential CRF1 endogenous agonist [12], and Ucn 1 are upregulated in the colonic mucosal of UC patients [17,34].
Therefore in the present study, we first mapped the CRF1 mRNA distribution in the different segments of GI tract in healthy human subjects using reverse transcription polymerase chain reaction (RT-PCR). Then we assessed the expression and localization of CRF1 at the protein level by immunohistochemistry on whole thickness sigmoid colonic sections and in sigmoid colonic mucosal biopsies of healthy subjects. Lastly, in sigmoid colonic biopsies of healthy control and UC patients, we compared the numbers of cells immunostained with CRF1 antibody and double labeled with CRF1 and CD163, a marker of resident macrophages [8].
2. Materials and methods
2.1. Human tissue specimens
2.1.1. Whole thickness gastrointestinal tissue samples from healthy subjects
Total RNA samples extracted from whole thickness GI segments including the esophagus, gastric fundus, pylorus, duodenum, jejunum, ileum, cecum, ascending colon, descending colon, transverse colon, sigmoid colon and rectum and paraffin tissue slides of sigmoid colon were purchased from BioChain Institute, Inc. (Hayward, CA, USA). These tissues were collected from 4 male healthy subject donors (24-29 years) who died from sudden death due to accidents. Tissue collection was done at the time of death with a post mortem delay between 4-6 h (BioChain Institute, Inc). These specimens were used to perform the mapping of CRF1 mRNA distribution along the GI tract and cellular localization of CRF1 receptor in the sigmoid colon. Approval for the use of human tissue samples and all procedures to process them was obtained from the Committee for Medical Research Ethics (VA Greater Los Angeles Health System, VA project#: 0016).
2.1.2. Sigmoid colonic mucosa biopsies from healthy subjects and UC patients
All sigmoid colonic mucosal biopsy specimens were obtained from the Mucosal Immunology Core (UCLA AIDS Institute Center for AIDS Research). Approval to conduct the study was obtained from the UCLA Human Subjects Protection Committee. All participants provided written consent. Sigmoid colonic biopsies were collected by flexible sigmoidoscopy, 10-20 cm from the anal verge, from 6 healthy controls (33-65 yrs; 1 male, 5 females) and 10 UC patients (32-83 yrs; 6 males and 4 females). Patients had UC for >10 years, based on history, endoscopic and pathology reports over the years. Four UC patients had active clinical disease presentation and met the criteria for mild-moderate active disease (graded as 2-3) and the 6 UC patients met criteria for minimal to no active inflammation with markers of quiescent disease (graded as 1-2). Grades were based on Matts’ UC classification [28] as used in other studies [34]. No participants were taking steroids and one was taking a low dose immunosuppressive (6-mercaptopurine at 25mg/day). All were treated with 5-aminosalicylate except one patient who was taking only omega-3 fatty acid gel caps.
2.2. RT-PCR
cDNA was synthesized using total RNA extracted from whole thickness segments of healthy subject GI tract (BioChain Institute, Inc.) in a total volume of 20 μl reaction by ThermoScript™ RT-PCR system (Invitrogen, CA) as reported previously [46]. Briefly, total RNA (5 μg) was denatured at 65°C for 5 min and then followed by the procedures of the reverse transcription which was stopped by incubation at 85°C for 5 min. Then the reaction mixture was incubated with RNase H at 37 °C for 30 min to remove total RNA template. The sequences of oligonucleotide primers specific for human CRF1 used in RT-PCR are as follows. Forward primer: 5′-ACAAACAATGGCTACCGGGA-3′, reverse primer: 5’-TCGCAGGCACCGGATGCTC-3′, which cover human CRF1 Exon 4 and Exon 5, thus amplify the functional wild type form of CRF1a. PCR reactions were performed in a final volume of 30 μl using RedTaq System (Sigma-Aldrich, Saint Louis, MO). The reaction was pre-denatured at 95°C for 2 min, and then amplified 34 times (94°C, 40 sec; 59°C, 40 sec; 72°C, 2 min), followed by a 5 min extension at 72°C in Thermal Cycler (MyCycler™ Thermal Cycler, BIO-RAD Laboratories, Hercules, CA). The house keeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control to assure cDNA quality and equal loading. The sequences of the primers for human GAPDH are as follows. Forward primer: 5′-GGTCGGAGTCAACGGATTTG -3′, reverse primer: 5′-ATGAGGTCCACCACCCTGTT -3′. Negative control contained all reagents, except that 1 μl H2O was substituted for reverse transcriptase in RT reaction to exclude the possibility of genomic or other DNA contamination. PCR products were separated by 1% agarose gel electrophoresis and visualized with ethidium bromide. Gel images were acquired by Kodak EDAS 290 system and the band density from tissues was quantified by NIH Image system (Scion Corporation, Frederick, MD) and standardized by taking the ratio to that of GAPDH in each sample respectively.
2.3. Immunohistochemistry
2.3.1. CRF1 receptor immunostaining
Paraffin sections (5 μ m) of whole thickness sigmoid colon from healthy male subjects (BioChain Institute Inc., n=3) and sigmoid colonic biopsies mostly containing the mucosa from healthy controls (n=6) and patients with UC (n=10) were processed for immunohistochemistry. Tissue sections were deparaffinized in xylene and hydrated in descending grades of ethanol. After washing 2 times (5 min each) in phosphate buffered saline (PBS), slides were placed in a plastic Coplin jar filled with 10 mM citrate buffer (pH 6.0), boiled for 8 min, and followed by cooling to room temperature. Endogenous peroxidase activity was blocked by incubation for 30 min with 0.3% hydrogen peroxide in PBS and nonspecific reaction was blocked by incubation in 3% normal donkey serum for 30 min at room temperature. Slides were incubated overnight at 4°C with goat anti-CRF1 antibody (1:500; C-20, Santa Cruz Biotechnology, CA, USA) diluted in PBS containing 0.3% Triton X-100, followed by incubation with biotinylated donkey anti-goat IgG (1:1000; Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature. Sections were subsequently processed for avidin-biotin-peroxidase procedure using diaminobenzidine as a chromogen, and then counterstained with hematoxylin. Sections of human cerebral cortex (BioChain Institute, Inc.), well known to express CRF1 [13] as in rodents [42], were taken as a positive control. Immunohistochemical negative control was routinely performed following the same procedures, except that the primary antibody was replaced by PBS.
2.3.2. CRF1 receptor and macrophage marker double immunostaining
Paraffin sections (5 μm) of sigmoid colonic biopsies from healthy controls (n=6) and patients with UC (n=10) were deparaffinized in xylene and hydrated in descending grades of ethanol, followed by an antigen retrieval procedure as described above, tissue slides from biopsy specimens were washed three times at 10-min intervals with PBS, then were incubated in 10% normal donkey serum (Jackson ImmunoResearch Laboratories) in 0.3% Triton-X 100/PBS for 30 min at room temperature followed by 48-h incubation with the mixture of goat anti-CRF1 antibody (1:50, C-20, Santa Cruz Biotechnology) and mouse anti-rat/human CD163 (1:200, Serotec, Inc. Raleigh, NC, USA), a marker of macrophages [8]. Sections were washed with PBS three times at 10-min intervals and incubated for 2 h at room temperature with the mixture of Rhodamine Red™-X-conjugated donkey anti-goat IgG and FITC-conjugated donkey anti-mouse IgG (1:100. Jackson ImmunoResearch Laboratories). After washing three times in PBS, sections were subsequently mounted on slides with anti-fade mounting media (Vector Laboratory Inc., Burlingame, CA) and visualized by standard fluorescence microscopy.
2.3.3. Cell counting
The number of CRF1 immunoreactive (IR) and CRF1/CD163 double labelled cells in the lamina propria of colonic biopsies were counted and quantified in an average number of 5 fields (340 μm×260 μm/field) from each specimen in a blinded fashion such that the information on the clinical, endoscopic and pathological findings were unknown until all counting was completed.
2.4. Statistic analysis
All data are expressed as mean ± SE. Comparison of CRF1 mRNA levels among GI segments was performed with one-way ANOVA followed by Duncan's test. The numbers of CRF1, and CRF1/CD163 double-labeled cells in the lamina propria of the sigmoid biopsies of healthy subjects and UC patients were compared and analyzed by Student t-test. A P value <0.05 was considered statistically significant.
3. Results
3.1. Distribution of CRF1 mRNA expression in the various gastrointestinal tract segments of healthy subjects
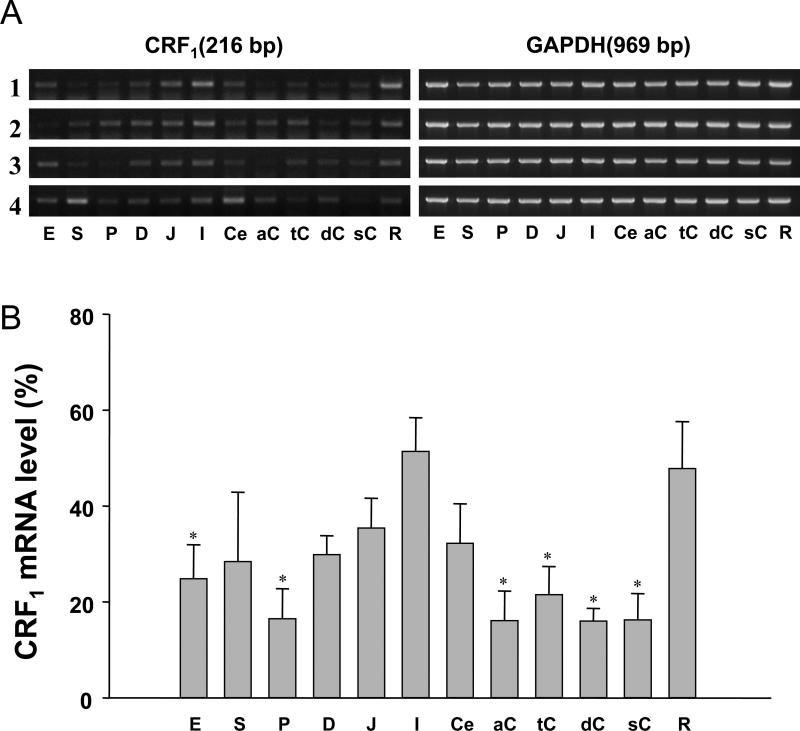
CRF1 mRNA expression was detected in all GI segments examined from the esophagus to the rectum and the distribution pattern was consistent among 4 healthy subjects (Fig.1A). CRF1 mRNA was expressed predominantly in the small intestine and the rectum with the highest levels found in the ileum and rectum followed by the esophagus, gastric fundus, duodenum, jejunum, cecum, with lower levels in the pylorus, and colon (ascending, transverse, descending, sigmoid). In small intestinal segments CRF1 mRNA expression is linearly increased from the duodenum to the ileum. In colonic segments, however, CRF1 mRNA levels in the ascending, transverse, descending and sigmoid colon were 31.4%, 41.8%, 31.2%, and 31.7% respectively significantly lower than in the ileum (Fig. 1B).
Fig. 1.
RT-PCR of CRF1 in the human gastrointestinal tract from four male healthy subjects. A. Gel images of PCR products. PCR products were separated by 1% agarose gel electrophoresis and visualized with ethidium bromide. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control to assure cDNA quality and equal loading. E: esophagus, S: stomach (fundus), P: stomach pylorus, D: duodenum, J: Jejunum, I: ileum, Ce: cecum, aC: ascending colon, tC: transverse colon, dC: descending colon, sC: sigmoid colon, R: rectum. B. Quantitative analysis of PCR products. The densitometric analysis of PCR products was performed by using NIH Image system (Scion Corporation, Frederick, MD) and standardized by taking the ratio to that of GAPDH in each sample respectively. * p<0.05 vs ileum (I). n=4.
3.2 Cellular localization of CRF1 immunoreactivity in the whole thickness of sigmoid colon from healthy subjects
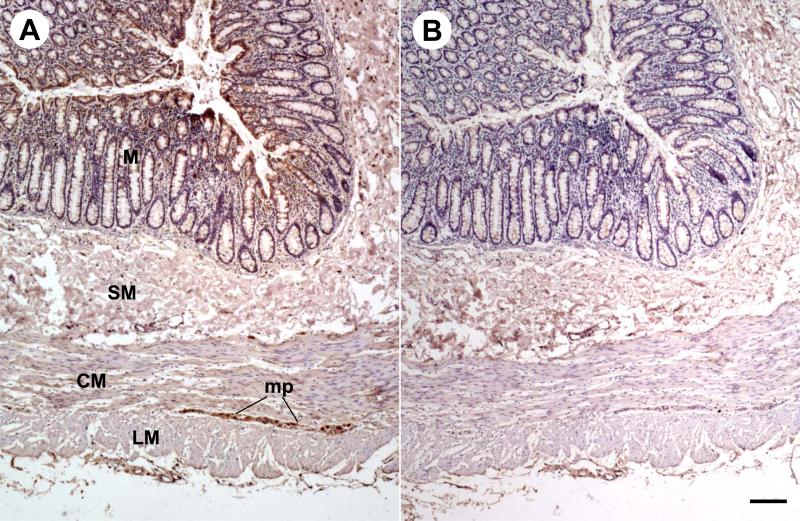
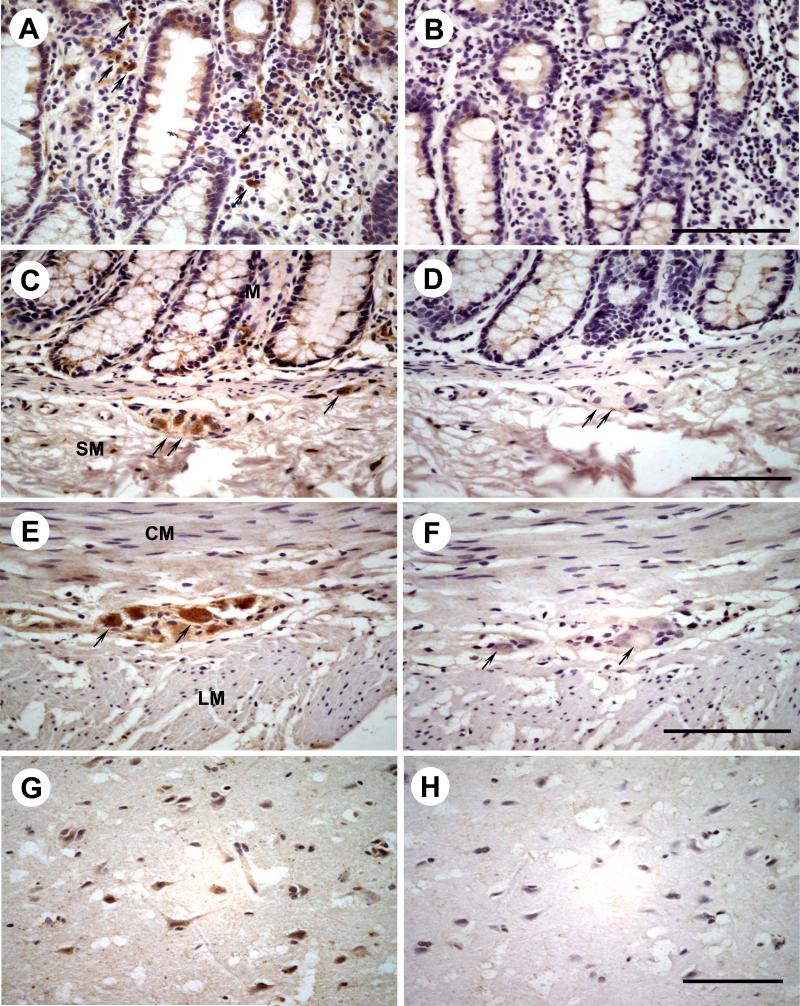
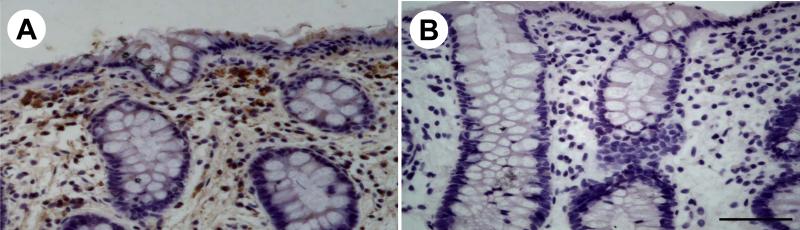
In the whole thickness tissue sections of the sigmoid colon from healthy subjects, CRF1 immunoreactivity was located in cells scattered within the lamina propria (Figs. 2A, 3A), submucosal (Fig. 3C) and myenteric plexus (Fig. 2A, 3E). A weak staining was also found at the base of the absorptive surface epithelial cells (Fig. 2A). In biopsy sections of sigmoid colon from healthy subjects, CRF1 immunoreactivity was mainly located in cells of the lamina propria underneath the epithelia (Fig. 4A). In sections of human cerebral cortex taken as a positive control [13], CRF1 immunoreactivity was located in neurons (Fig. 3G). The immunostaining was not observed when the CRF1 antibody was replaced by PBS (Figs. 2B, 3B, 3D, 3F, 3H, and 4B).
Fig. 2.
CRF1 immunohistochemistry in a pair of serial whole thickness tissue sections of a representative adult human sigmoid colon (n=3). Immunoreactivity appears brown as a result of the DAB colorimetric reaction. Hematoxylin was used as counterstaining. A, Marked CRF1 immunoreactivity was detected in mucosa (M) and myenteric plexus (MP). Submucosa (SM), circle muscle layer (CM), and longitudinal muscle layer (LM) are negative. B, No immunostaining was seen in all layers of an adjacent whole thickness tissue slide after the CRF1 antibody was replaced by PBS. Bar: 100 μm.
Fig. 3.
The high magnification images of immunohistochemistry for CRF1 in the different layers of a representative adult human sigmoid colon (n=3) and in the adult human brain cortex as a positive control. CRF1 immunoreactivity was detected in the cells scattered in lamina propria (A), submucosal neurons (C), myenteric neurons (E) and cerebral neurons (G). No immunoreactivity was seen in the lamina propria (B), submucosal plexus (D), myenteric plexus (F) and cerebral cortex (H) after the CRF1 antibody was replaced by PBS. The arrows point to CRF1 immunoreactive positive cells and the corresponding cells in negative controls. Bar: 100 μm.
Fig. 4.
Immunohistochemistry for CRF1 in a representative section of sigmoid colonic biopsies in healthy subjects (n=6). A, CRF1 immunoreactivity is mainly located in the cells in the lamina propria underneath the epithelia. B, No immnostaining was visualized after the CRF1 antibody was replaced by PBS. Bar: 100 μm.
3.3. CRF1 immunoreactivity in sigmoid colonic mucosa from healthy subjects and UC patients
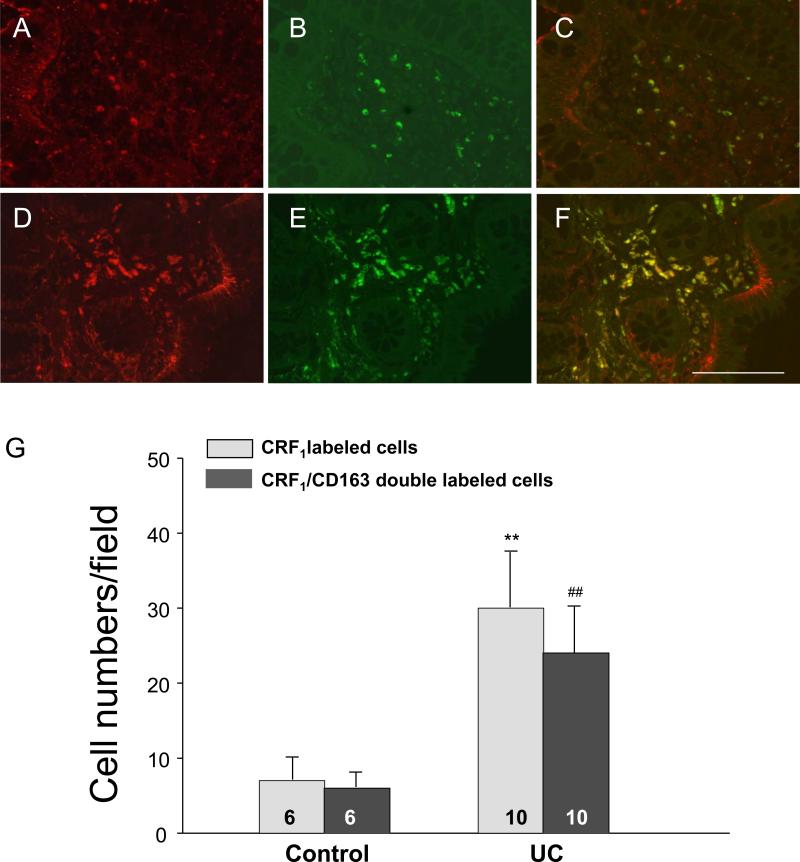
To characterize CRF1 immunoreactive (IR) positive cells in the lamina propria, double staining of CRF1 and CD163 using a marker antibody for macrophages, was conducted in tissue sections of sigmoid colonic biopsies from healthy and UC subjects. All cells labeled by monoclonal antibody against CD163 also showed CRF1 immunoreactivity. CRF1/CD163 double-labeled cells represented 79% of total CRF1 IR positive cells in the lamina propria while only 21% of CRF1 IR positive cells was not labeled by CD163 indicating that CRF1 receptor is mainly located in the colonic lamina propria macrophages (Fig. 5A, B, C, G). The number of CRF1 IR positive cells in the mucosal lamina propria was significantly increased in sigmoid biopsies of UC patients (30±8 vs. 7±3/field in healthy subjects, n=10 and 6 respectively p<0.01). The increase occurred irrespectively of these chronic UC patients being in remission with minimal inflammation (31±10/field n=6) or active with mild to moderate inflammation (29±5 /field, n= 4). Similarly, CRF1/CD163 double-labeled cells (macrophages) in the mucosa were also significantly elevated in the UC patients (24±6 vs 6±2/field in healthy subjects, n=10 and 6 respectively, p<0.01) (Fig. 5D, E, F, G) with similar increase in UC in remission and active (25±6, n=6 and 23±5/field, n=4, respectively).
Fig. 5.
Double immunostaining of CRF1/CD163, a marker of macrophages in the representative sections of sigmoid colonic biopsies from healthy subjects (A-C, n=6) and UC patients (D-F, n=10). A, D: CRF1 immunostaining (red color); B, E: CD163 immunostaining (green color); C, F: Merged images showing the colocalization of CRF1 and CD163. Bar: 100 μm. G: The number of CRF1 immunoreactive (IR) positive and CRF1/CD163 double labeled cells in the lamina propria were counted and quantified as the average number of 5 fields (340 μm×260 μm/field) from each specimen. CRF1 IR positive cells and CRF1/CD163 double-labeled cells (macrophages) in the mucosa were significantly increased in UC patients. Each column represents the mean ± SE of 6-10 subjects indicated in the figure. ** p<0.01 vs CRF1 IR positive cells in the control group; ## p<0.01 vs CRF1/CD163 double-labeled cells (macrophages) in the control group.
4. Discussion
In the present study we provided the first comprehensive description of CRF1 gene expression in the different segments throughout the GI tract of healthy human subjects based on levels of CRF1 mRNA. There was a very consistent pattern in CRF1 mRNA expression in the GI tract among the 4 subjects investigated with the highest levels found in the ileum and rectum, followed by the esophagus, gastric fundus, duodenum, jejunum, cecum, and lesser levels in the pylorus, and colon (ascending, transverse, descending and sigmoid). A previous study detected only CRF2 mRNA in the whole stomach of healthy human subjects but not CRF1 [5] which may be related to the difference in the primer efficiency between the two studies. In small intestinal segments, CRF1 mRNA expression is linearly increased from the duodenum to the ileum while in the large bowel segments, it is decreased from the cecum to the colon and then abruptly elevated in the rectum. Such a CRF1 transcriptional expression pattern in small intestine in humans differs from that reported in the rat which has a greater expression in the duodenum than in the ileum [33]. Previous functional studies in healthy human subjects showed that systemic injection of the preferential CRF1 agonist, CRF [12] stimulates pyloric and duodenal pressure waves switching postprandial duodenal motor activity to non-propagated high frequency contractions [29,40]. The present demonstration of CRF1 expression provides anatomic support for a local CRF1 mediated action within the human gut wall to influence GI motor function. Immunohistochemical cellular detection of CRF1 in whole thickness tissue sections of the sigmoid colon from healthy subjects showed CRF1 immunoreactivity with strong intensity in submucosal and myenteric neurons. The specificity of CRF1 antibody to detect human CRF1 receptor was characterized in our previous study by Western blot and immunofluorescence in HEK-293 cells transected with human CRF1a and in human BON-1B cells [45]. These data extend to humans reports so far only in experimental animals demonstrating CRF1 expression at gene and protein levels within colonic myenteric neurons [4,11,30,32,46]. Functional studies in healthy subjects showing that systemic injection of CRF induces segmental contractions in the descending and sigmoid colon [9] support this contention. In experimental animals, CRF and selective CRF1 agonist exert a direct CRF1 mediated excitatory action on colonic myenteric neurons to stimulate propulsive colonic motility [24,25,46].
In addition to neurons in the enteric plexi, CRF1 immunoreactivity in humans was detected in cells scattered in the lamina propria with a similar staining pattern regardless of whether the samples were whole thickness colonic tissues (post mortem) or freshly-acquired colonic biopsies. These data are consistent with previous studies showing CRF1 mRNA expression in lamina propria cells isolated from the mucosa-submucosal layer of normal human colorectal tissues [31] and CRF1 immunoreactivity in the lamina propria of human sigmoid biopsies [43]. In addition, we found that all of CD163 labeled cells were CRF1 IR and 79% of total CRF1 IR cells in the lamina propria were double-labeled by CRF1/CD163, providing the first evidence that CRF1 receptor is prominently located in human colonic lamina propria macrophages. The GI mucosa contains the largest reservoir of resident macrophages in the body and intestinal macrophages are the first phagocytic cells of the innate immune system to interact with microorganisms and microbial products that have breached the epithelium [37]. Located in the subepithelial lamina propria, mucosal macrophages have a dual role of protecting the host against foreign pathogens and regulating mucosal responses to commensal bacteria by the production of proinflammatory cytokines during inflammation [37,38]. The expression of CRF1 on macrophages in the lamina propria provides anatomical evidence that macrophages may represent an immune target for CRF1 cognate agonists, CRF and Ucn 1 also located in macrophage of the human colon [31]. Besides macrophages, there is a remaining 21% of CRF1 IR positive cells which were not labeled by CD163 in the lamina propria. A previous report showed that CRF1 IR cells were double labeled with mast cell tryptase in sigmoid biopsy of healthy subjects [43]. This may account for the other cell components in the lamina propria also expressing CRF1.
Related to the immunoinflammatory influences of CRF1 and the tissue macrophage immunocyte as well, the modulation of mucosal macrophages (and associated CRF1 expression) might be significantly altered in certain pathological conditions [15]. In the present study, the quantification of CRF1 IR positive cells and CRF1/CD163 double labeled macrophages in the distal colonic (sigmoid) mucosa lamina propia showed that CRF1 IR positive cells were significantly increased by 4.2 fold in non glucocorticoid treated UC patients compared to healthy subjects. Likewise, a previous report indicates the accumulation of CRF1 mRNA hybridization signal in colonic lamina propria cells of non glucocorticoid-treated UC patients although no quantification was performed comparative to control subjects [34]. Given the small number of patients investigated in this pilot study, we did not find difference between the UC samples from those patients with a moderate active inflammation or in a clinical state of remission. It is well known that in UC, a variety of inflammatory cells infiltrate the colon but an increase in the population of activated macrophages has been demonstrated to be a central characteristic of the inflammatory process involved in the disease [14]. Thus, the increased CRF1 IR positive cells most likely originate from the increased resident population of macrophages in UC patients (based on the near equivalency in active and remission disease). This was ascertained by our double immunostaining showing that all CD163 labeled cells are CRF1 IR positive and CRF1/CD163 double-labeled cells (macrophages) represent 79% of total CRF1 IR positive cells. CRF1/CD163 double-labeled macrophages were also significantly elevated in the UC patients by 4-fold compared to tissues of healthy controls. In addition other reports showed that CRF and Ucn 1 in the colonic lamina propria inflammatory (plasma) cells are upregulated in UC patients [17,34]. Taken together, these data support the hypothesis of autocrine/paracrine mechanisms whereby CRF/Ucn 1 act on CRF1 expressing macrophages of the lamina propria in UC patients which may have a bearing with the pro-inflammatory state. Indeed, there is in vitro evidence that CRF exerts a CRF1 mediated enhancing effect on endotoxin-induced cytokine production on primary culture of mouse macrophages or macrophage cell line [1,16].
In summary, the present study delineated a widespread and regionally different pattern of CRF1 mRNA expression along the GI tract of healthy subjects with a prominent expression in the ileum and rectum. As established in experimental animals [4,23,46], CRF1 IR was present in submucosal and myenteric neurons of the human colon, supporting that CRF/Ucn 1 can exert a direct action on colonic enteric nervous system. In addition, all macrophages in sigmoid colonic biopsies express CRF1 representing 79% of total CRF1 IR positive cells in the colonic lamina propria. Significant to the UC pathophysiology, total CRF1 IR cells and CRF1 IR present on macrophages in the mucosal lamina propria were significantly increased by 4.2 and 4.0 folds in UC patients respectively. This up regulation was maintained independent of UC being active or in remission. The enhanced CRF1 expression observed in the healthy human ileum raises the testable and consistent hypothesis that ileal samples from Crohn's disease patients may also show a similar alteration in total CRF1 IR cells and CRF1 IR located on macrophages. The present results along with reports that CRF and Ucn 1 are upregulated in the lamina propria cells of colonic mucosa in UC patients [17,34], are indicative that enhanced CRF/Ucn1/CRF1 signaling might be an important process in the mediation and/or exacerbation of inflammatory process in UC and may provide a new therapeutic target for UC.
Acknowledgements
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases R01 grant DK-57238, Center Grant DK-41301 (Clinical core), the NIAID Center Grant AI28697 (UCLA CFAR; Mucosal Immunology Core), Veteran Administration Research Career Scientist Award and Novartis Institutes for BioMedical Research (YT), and NIH DK-78676 (MM). We thank Dr. Eckhard Weber (Novartis Institutes for BioMedical Research, Basel) for insightful discussion.
Abbreviations
- CRF
corticotropin releasing factor
- CRF1
CRF receptor subtype 1
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IR
immunoreactive
- UC
ulcerative colitis
Footnotes
Contributors: Yuan designed, performed all experiments and data analysis and wrote the manuscript; Wu helped in molecular biology component of the study; Elliot and Anton contributed to the colonic biopsies, their characterization, experimental design and reviewed the manuscript; Chatzaki and Million contributed experimental design, discussion and reviewed the manuscript; Taché had input in experimental design, review of data and wrote the manuscript. All authors have read and approved the final article.
Conflict of interest
No conflict of interest from all authors.
Submission declaration: The article entitled “Expression of corticotropin releasing factor receptor type 1 (CRF1) in the human gastrointestinal tract and upregulation in the colonic mucosa in patients with ulcerative colitis” has not been published previously and is not under consideration for publication elsewhere. The article will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
References
- 1.Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect. Immun. 2002;70:6068–6074. doi: 10.1128/IAI.70.11.6068-6074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton PM, Gay J, Mykoniatis A, Pan A, O'Brien M, Brown D, Karalis K, Pothoulakis C. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8503–8508. doi: 10.1073/pnas.0402693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bale TL, Vale WW. CRF and CRF receptor: Role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 4.Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis D. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J. Neurochem. 2004;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- 5.Chatzaki E, Lambropoulou M, Constantinidis TC, Papadopoulos N, Taché Y, Minopoulos G, Grigoriadis DE. Corticotropin-releasing factor (CRF) receptor type 2 in the human stomach: protective biological role by inhibition of apoptosis. J. Cell Physiol. 2006;209:905–911. doi: 10.1002/jcp.20792. [DOI] [PubMed] [Google Scholar]
- 6.Crofford LJ, Sano H, Karalis K, Friedman TC, Epps HR, Remmers EF, Mathern P, Chrousos GP, Wilder RL. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J. Immunol. 1993;151:1587–1596. [PubMed] [Google Scholar]
- 7.Crofford LJ, Sano H, Karalis K, Webster EL, Goldmuntz EA, Chrousos GP, Wilder RL. Local secretion of corticotropin-releasing hormone in the joints of Lewis rats with inflammatory arthritis. J. Clin. Invest. 1992;90:2555–2564. doi: 10.1172/JCI116150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rat recognized by monoclonal antibodies ED1, ED2 and ED3. Adv. Exp. Med. Biol. 1985;186:409–419. doi: 10.1007/978-1-4613-2463-8_50. [DOI] [PubMed] [Google Scholar]
- 9.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gay J, Kokkotou E, O'Brien M, Pothoulakis C, Karalis KP. Corticotropin-releasing hormone deficiency is associated with reduced local inflammation in a mouse model of experimental colitis. Endocrinology. 2008;149:3403–3409. doi: 10.1210/en.2007-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gourcerol G, Wu SV, Yuan PQ, Pham H, Miampamba M, Larauche M, Sanders P, Amano T, Mulak A, Im E, Pothoulakis C, Rivier J, Taché Y, Million M. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology. 2011;140:1586–1596. doi: 10.1053/j.gastro.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current Status of the Nomenclature for Receptors for Corticotropin-Releasing Factor and Their Ligands. Pharmacol. Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Hiroi N, Wong ML, Licinio J, Park C, Young M, Gold PW, Chrousos GP, Bornstein SR. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol. Psychiatry. 2001;6:540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- 14.Hunyady B, Mezey E, Palkovits M. Gastrointestinal immunology: cell types in the lamina propria--a morphological review. Acta Physiol Hung. 2000;87:305–328. [PubMed] [Google Scholar]
- 15.Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, Akagawa KS, Hibi T. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin. Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254:421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- 17.Kawahito Y, Sano H, Mukai S, Asai K, Kimura S, Yamamura Y, Kato H, Chrousos GP, Wilder RL, Kondo M. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut. 1995;37:544–551. doi: 10.1136/gut.37.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kempuraj D, Papadopoulou N, Stanford EJ, Christodoulou S, Madhappan B, Sant GR, Solage K, Adams T, Theoharides TC. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am. J. Reprod. Immunol. 2004;52:267–275. doi: 10.1111/j.1600-0897.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim JE, Cho DH, Kim HS, Kim HJ, Lee JY, Cho BK, Park HJ. Expression of the corticotropin-releasing hormone-proopiomelanocortin axis in the various clinical types of psoriasis. Exp. Dermatol. 2007;16:104–109. doi: 10.1111/j.1600-0625.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 20.la Fleur SE, Wick EC, Idumalla PS, Grady EF, Bhargava A. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7647–7652. doi: 10.1073/pnas.0408531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larauche M, Gourcerol G, Wang L, Pambukchian K, Brunnhuber S, Adelson DW, Rivier J, Million M, Taché Y. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am. J. Physiol Gastrointest. Liver Physiol. 2009;297:G215–G227. doi: 10.1152/ajpgi.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liapakis G, Venihaki M, Margioris A, Grigoriadis D, Gkountelias K. Members of CRF family and their receptors: from past to future. Curr. Med. Chem. 2011;18:2583–2600. doi: 10.2174/092986711795933704. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Gao X, Gao N, Wang X, Fang X, Hu HZ, Wang GD, Xia Y, Wood JD. Expression of type 1 corticotropin-releasing factor receptor in the guinea pig enteric nervous system. J. Comp Neurol. 2005;481:284–298. doi: 10.1002/cne.20370. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Ren W, Qu MH, Bishop GA, Wang GD, Wang XY, Xia Y, Wood JD. Differential actions of urocortins on neurons of the myenteric division of the enteric nervous system in guinea pig distal colon. Br. J. Pharmacol. 2010;159:222–236. doi: 10.1111/j.1476-5381.2009.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569–1579. doi: 10.1053/gast.2000.20251. [DOI] [PubMed] [Google Scholar]
- 26.Martinez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J. Pharmacol. Exp. Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 27.Mastorakos G, Bouzas EA, Silver PB, Sartani G, Friedman TC, Chan CC, Caspi RR, Chrousos GP. Immune corticotropin-releasing hormone is present in the eyes of and promotes experimental autoimmune uveoretinitis in rodents. Endocrinology. 1995;136:4650–4658. doi: 10.1210/endo.136.10.7664685. [DOI] [PubMed] [Google Scholar]
- 28.Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q. J. Med. 1961;30:393–407. [PubMed] [Google Scholar]
- 29.Mayer EA, Sytnik B, Reddy NS, Van Deventer G, Taché Y. Corticotropin releasing factor (CRF) increases post-prandial duodenal motor activity in humans. J. Gastrointest. Motil. 1992;4:53–60. [Google Scholar]
- 30.Moeser AJ, Klok CV, Ryan KA, Wooten JG, Little D, Cook VL, Blikslager AT. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J Physiol Gastrointest. Liver Physiol. 2007;292:G173–G181. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- 31.Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799–1809. doi: 10.1016/s0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 32.o'malley D, Julio-Pieper M, Gibney SM, Gosselin RD, Dinan TG, Cryan JF. Differential stress-induced alterations of colonic corticotropin-releasing factor receptors in the Wistar Kyoto rat. Neurogastroenterol. Motil. 2010;22:301–311. doi: 10.1111/j.1365-2982.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- 33.Porcher C, Juhem A, Peinnequin A, Sinniger V, Bonaz B. Expression and effects of metabotropic CRF1 and CRF2 receptors in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1091–G1103. doi: 10.1152/ajpgi.00302.2004. [DOI] [PubMed] [Google Scholar]
- 34.Saruta M, Takahashi K, Suzuki T, Torii A, Kawakami M, Sasano H. Urocortin 1 in colonic mucosa in patients with ulcerative colitis. J. Clin. Endocrinol. Metab. 2004;89:5352–5361. doi: 10.1210/jc.2004-0195. [DOI] [PubMed] [Google Scholar]
- 35.Saunders PR, Maillot C, Million M, Taché Y. Peripheral corticotropin-releasing factor induces diarrhea in rats: role of CRF1 receptor in fecal watery excretion. Eur J Pharmacol. 2002;435:231–235. doi: 10.1016/s0014-2999(01)01574-6. [DOI] [PubMed] [Google Scholar]
- 36.Scopa CD, Mastorakos G, Friedman TC, Melachrinou M, Merino MJ, Chrousos GP. Presence of immunoreactive corticotropin releasing hormone in thyroid lesions. Am. J. Pathol. 1994;145:1159–1167. [PMC free article] [PubMed] [Google Scholar]
- 37.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol. Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 38.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stengel A, Taché Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp. Biol. Med. (Maywood. ) 2010;235:1168–1178. doi: 10.1258/ebm.2010.009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su YC, Doran S, Wittert G, Chapman IM, Jones KL, Smout AJ, Horowitz M. Effects of exogenous corticotropin-releasing factor on antropyloroduodenal motility and appetite in humans. Am. J. Gastroenterol. 2002;97:49–57. doi: 10.1111/j.1572-0241.2002.05422.x. [DOI] [PubMed] [Google Scholar]
- 41.Taché Y, Perdue MH. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol. Mot. 2004;16(Suppl. 1):1–6. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 42.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 43.Wallon C, Soderholm JD. Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann. N. Y. Acad. Sci. 2009;1165:206–210. doi: 10.1111/j.1749-6632.2009.04030.x. [DOI] [PubMed] [Google Scholar]
- 44.Wlk M, Wang CC, Venihaki M, Liu J, Zhao D, Anton PM, Mykoniatis A, Pan A, Zacks J, Karalis K, Pothoulakis C. Corticotropin-releasing hormone antagonists possess anti-inflammatory effects in the mouse ileum. Gastroenterology. 2002;123:505–515. doi: 10.1053/gast.2002.34783. [DOI] [PubMed] [Google Scholar]
- 45.Wu SV, Yuan PQ, Lai J, Wong K, Chen MC, Ohning GV, Taché Y. Activation of Type 1 CRH receptor isoforms induces serotonin release from human carcinoid BON-1N cells: an enterochromaffin cell model. Endocrinology. 2011;152:126–137. doi: 10.1210/en.2010-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan PQ, Million M, Wu SV, Rivier J, Taché Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol. Motil. 2007;19:923–936. doi: 10.1111/j.1365-2982.2007.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan PQ, Wu SV, Wang L, Taché Y. Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides. 2010;31:322–331. doi: 10.1016/j.peptides.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]