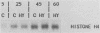Abstract
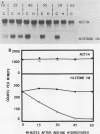
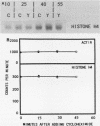
During the S phase of the cell cycle, histone gene expression and DNA replication are tightly coupled. In mitotically synchronous plasmodia of the myxomycete Physarum polycephalum, which has no G1 phase, histone mRNA synthesis begins in mid-G2 phase. Although histone gene transcription is activated in the absence of significant DNA synthesis, our data demonstrate that histone gene expression became tightly coupled to DNA replication once the S phase began. There was a transition from the replication-independent phase to the replication-dependent phase of histone gene expression. During the first phase, histone mRNA synthesis appears to be under direct cell cycle control; it was not coupled to DNA replication. This allowed a pool of histone mRNA to accumulate in late G2 phase, in anticipation of future demand. The second phase began at the end of mitosis, when the S phase began, and expression became homeostatically coupled to DNA replication. This homeostatic control required continuing protein synthesis, since cycloheximide uncoupled transcription from DNA synthesis. Nuclear run-on assays suggest that in P. polycephalum this coupling occurs at the level of transcription. While histone gene transcription appears to be directly switched on in mid-G2 phase and off at the end of the S phase by cell cycle regulators, only during the S phase was the level of transcription balanced with the rate of DNA synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. B., Mueller G. C. Control of histone synthesis in HeLa cells. Biochim Biophys Acta. 1973 Feb 4;294(1):481–496. doi: 10.1016/0005-2787(73)90104-4. [DOI] [PubMed] [Google Scholar]
- Carrino J. J., Laffler T. G. Transcription of alpha-tubulin and histone H4 genes begins at the same point in the Physarum cell cycle. J Cell Biol. 1986 May;102(5):1666–1670. doi: 10.1083/jcb.102.5.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L., Bromley S., Osley M. A. Periodic transcription of yeast histone genes. Cell. 1982 Aug;30(1):305–310. doi: 10.1016/0092-8674(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Kedes L. H. Histone genes and histone messengers. Annu Rev Biochem. 1979;48:837–870. doi: 10.1146/annurev.bi.48.070179.004201. [DOI] [PubMed] [Google Scholar]
- Kressmann A., Hofstetter H., Di Capua E., Grosschedl R., Birnstiel M. L. A tRNA gene of Xenopus laevis contains at least two sites promoting transcription. Nucleic Acids Res. 1979 Dec 11;7(7):1749–1763. doi: 10.1093/nar/7.7.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffler T. G., Carrino J. J. The tubulin and histone genes of Physarum polycephalum: models for cell cycle-regulated gene expression. Bioessays. 1986 Aug;5(2):62–65. doi: 10.1002/bies.950050205. [DOI] [PubMed] [Google Scholar]
- Laffler T. G., Carrino J. Cell-cycle-regulated translation of histone mRNA in Physarum plasmodia. J Bacteriol. 1987 May;169(5):2291–2293. doi: 10.1128/jb.169.5.2291-2293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffler T. G., Chang M. T., Dove W. F. Periodic synthesis of microtubular proteins in the cell cycle of Physarum. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5000–5004. doi: 10.1073/pnas.78.8.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson R., Cohn R., Kedes L., Mohun T. Expression and organization of histone genes. Annu Rev Genet. 1983;17:239–277. doi: 10.1146/annurev.ge.17.120183.001323. [DOI] [PubMed] [Google Scholar]
- Nader W. F., Edlind T. D., Huettermann A., Sauer H. W. Cloning of Physarum actin sequences in an exonuclease-deficient bacterial host. Proc Natl Acad Sci U S A. 1985 May;82(9):2698–2702. doi: 10.1073/pnas.82.9.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old R. W., Sheikh S. A., Chambers A., Newton C. A., Mohammed A., Aldridge T. C. Individual Xenopus histone genes are replication-independent in oocytes and replication-dependent in Xenopus or mouse somatic cells. Nucleic Acids Res. 1985 Oct 25;13(20):7341–7358. doi: 10.1093/nar/13.20.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H. L., Heintz N., Roeder R. G. Regulation of human histone gene expression during the HeLa cell cycle requires protein synthesis. Mol Cell Biol. 1984 Dec;4(12):2723–2734. doi: 10.1128/mcb.4.12.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Coffino P. Increased histone mRNA levels during inhibition of protein synthesis. Biochem Biophys Res Commun. 1983 Jul 18;114(1):131–137. doi: 10.1016/0006-291x(83)91604-2. [DOI] [PubMed] [Google Scholar]
- Wilhelm M. L., Wilhelm F. X. A transposon-like DNA fragment interrupts a Physarum polycephalum histone H4 gene. FEBS Lett. 1984 Mar 26;168(2):249–254. doi: 10.1016/0014-5793(84)80256-2. [DOI] [PubMed] [Google Scholar]