Abstract
The adult heart has been recently recognized as a self-renewing organ that contains a pool of committed resident cardiac stem cells (CSCs) and cardiac progenitor cells (CPCs). These adult CSCs and CPCs can be induced by cytokines and growth factors to migrate, differentiate, and proliferate in situ and potentially replace lost cardiomyocytes. Ligand-receptor systems, such as the tyrosine kinase receptor mesenchymal–epithelial transition factor (Met) and its ligand hepatocyte growth factor (HGF), are potential candidates for boosting migration, engraftment and commitment of CSCs. Here, we discuss the possible application of HGF/Met gene therapy to enhance the ability of CSCs to promote myocardial regeneration.
Keywords: Cardiac stem cells, Cardiac progenitor cells, Cell-based therapy, Hepatocyte growth factor, Gene therapy, Cardiac repair
Introduction
Injury to the myocardium, such as that occurring as a consequence of myocardial infarction, is a major cause of death, as the human heart has a limited capacity to regenerate. Possible approaches to treat the late consequences of myocardial damage, i.e., heart failure, include transplantation of stem cells into the heart. Several types of stem cells have been used so far to repopulate functional myocytes or vascular cells in the damaged myocardium, including cardiac stem cells (CSCs) and cardiac progenitor cells (CPCs) [12, 13], endothelial progenitor cells (EPCs) [26, 35, 55, 57], bone marrow-derived stromal/stem cells [1, 22, 38, 46, 59] and adipose tissue-derived stromal/stem cells [10, 31, 34, 36, 39, 56, 63, 70, 72]. In most studies, stem cell therapy has proven to provide beneficial increases in function, although mechanisms underlying this improvement are unclear. Besides differentiation into cardiomyocytes, these approaches likely involve altered remodeling of the vasculature, scar and extracellular matrix and other poorly defined paracrine effects. Few studies have indeed clearly demonstrated stem cell differentiation into functional adult cardiac myocytes [20, 25, 53, 54, 74]. In vivo and in vitro studies employing bone marrow-derived stem cells have actually shown that newly differentiated myocytes from such undifferentiated cells do not acquire the adult phenotype of cardiomyocytes, but rather resemble neonatal cells, which die over time by apoptosis [20].
Recently, the seminal observation that the adult heart possesses a pool of resident CSCs (which are self-renewing, clonogenic and multipotent stem cells) and CPCs has suggested a different approach to myocardial repair, i.e., to facilitate myocardial regeneration by inducing endogenous cardiac cells to migrate, differentiate, and proliferate in situ to replace lost cardiomyocytes [6]. Access to tissue for the isolation of CSCs and CPCs can be obtained from patient myocardium via atrial appendage harvest during bypass surgery (SCIPIO Trial) or by endomyocardial biopsy making use of these cell populations feasible for cell therapy. Despite this, there is still significant controversy as to whether or not CSCs differentiate into new blood vessels or new myocytes.
While the best cell type, optimal timing, and route for transplantation still need to be determined, there has been increasing attention toward novel pharmacological and genetic strategies to enhance the implantation of genetically modified stem cells or for boosting endogenous regeneration of myocardial tissue. Pharmacological agents or genetic modification may enhance the efficacy of cell therapy in several ways: mobilizing endogenous stem/progenitor cells in vivo; promoting cell proliferation and differentiation; and augmenting cell engraftment and survival in the injured myocardium. In this context, a major challenge is presented by the identification of growth factors (GFs) and signaling pathways that selectively promote resident CSC proliferation, migration, engraftment, and differentiation [2, 29], the understanding of which might open new prospects for stem cells in cardiovascular repair and regeneration.
Recent studies [32, 62] have reported the in vivo and in vitro abilities of cytokines and GFs, such as insulin growth factor (IGF) and hepatocyte growth factor (HGF), to promote the translocation of CSCs into the injured area and to activate their growth and differentiation, resulting in the formation of functionally competent myocardium. Indeed, Anversa and colleagues [32, 62] have recently reported that the intramyocardial injection of HGF and IGF stimulates the translocation of resident CSCs into the infarct area from the surrounding myocardium and promotes the formation of new myocardium, leading to improvement in local wall motion and overall ventricular function in mice and dogs. The property of HGF to induce translocation of CSCs has been attributed to the expression of matrix metalloproteinases (MMPs) [23], which digest collagen and other extracellular components of the interstitium, facilitating cell migration and homing in the heart [62]. Indeed, the inhibition of MMP-2/MMP-9 significantly abrogated the invasive properties of CSCs stimulated by HGF [32]. Based on these observations, the authors suggested that local administration of GFs, such as HGF, may become a novel powerful therapeutic strategy for repairing the infarcted heart [62].
Here, we will review key concepts and mechanisms of signal transduction that support a role for the HGF/Met system in myocardial regeneration. Here, we will discuss the potential therapeutic use of HGF and Met via a gene therapy approach to enhance CSC function and to target the tissue environment during cell transplantation.
The HGF/Met system: structural features
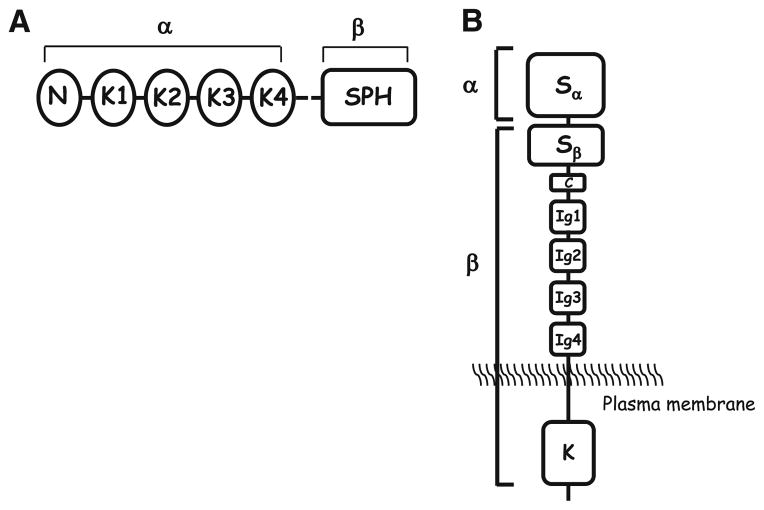
HGF, also named scatter factor (SF), was first discovered for the independent properties of HGF and SF, through its ability to induce hepatocyte growth in culture [9] and scatter epithelial cells, i.e., of inducing the dissociation of epithelial cells in culture, letting them assume mesenchymal cell morphology and motility [67]. Subsequently, both these properties were associated with the same factor [66]. HGF/SF (subsequently referred to as HGF) is a large, multidomain protein similar to plasminogen, consisting of six domains: an amino-terminal domain (N), four kringle domains (K1–K4), and a serine proteinase homology (SPH) domain, which lacks enzymatic activity as a result of mutations in essential amino acid residues. Like plasminogen, HGF is synthesized as an 82-kDa single-chain, largely inactive precursor (pro-HGF/SF), and is converted proteolytically into a two-chain, active heterodimer. Cleavage of pro-HGF occurs at a trypsin-like site that is located after K4, and this produces a disulphide-linked α- and β-chain heterodimer [7].
The HGF receptor Met is a disulphide-linked heterodimer, which results from cleavage of a precursor (170 kDa) at a site located between amino acid residues 307 and 308. The mature form of Met consists of an extracellular α-chain (50 kDa) and a longer β-chain (140 kDa). The β-chain encompasses the remainder of the Met ectodomain, the transmembrane helix and the cytoplasmic portion. The latter contains the juxtamembrane and kinase domains, as well as a carboxy-terminal tail that is essential for downstream signaling. The juxtamembrane region contains several negative regulatory sites, which are involved in the downregulation and/or degradation of the receptor, corresponding to the site of caspase-mediated cleavage of Met. The α-chain and the first 212 amino acid residues of the β-chain are sufficient for HGF binding [7] (Fig. 1).
Fig. 1.
The structure of hepatocyte growth factor/scatter factor (HGF/SF) (a) and Met tyrosine kinase receptor (b). HGF consists of six domains: an amino-terminal domain (N), four kringle domains (K1–K4) and a serine proteinase homology (SPH) domain. The mature form of Met consists of an extracellular α-chain and a longer β-chain. The β-chain encompasses the remainder of the Met ectodomain, the transmembrane helix and the cytoplasmic portion. The latter contains the juxtamembrane and kinase domains as well as a carboxy-terminal tail that is essential for downstream signaling. S semaphorin domain, C cysteine-rich domain, Ig immunoglobulin domain, K kinase domain, α and β refer to the subunits of the receptor that are present after proteolytic cleavage
Cell targets and signaling pathways of the HGF/Met system
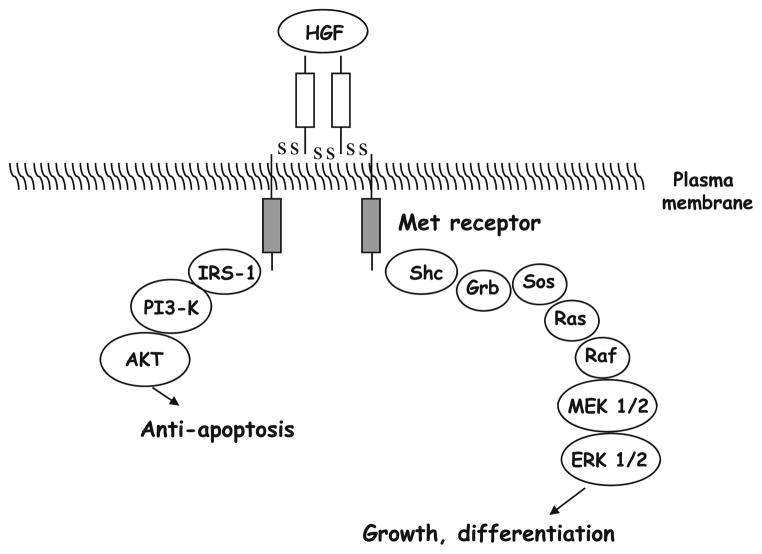
Upon HGF binding, Met dimerizes, and its tyrosine kinase activity is stimulated, resulting in the autophosphorylation of the receptor at tyrosine residues Y1349 and Y1356 [9]. Phosphorylated Met binds intracellular substrates, such as growth factor receptor-bound protein (Grb)-associated binder (Gab)1, Grb2, and phosphatidylinositol-3-kinase (PI3K). Steric hindrance prevents two substrates from binding simultaneously to one Met molecule. However, Gab1 can bind to phosphorylated (p)Y1349 on one, and Grb2 can bind to pY1356 on a second Met molecule. Therefore, an interaction between Gab1 and Grb2 may occur on Met dimers or multimers. Most of these Gab1 and Grb2 molecules contain binding sites for Src homology-2 (SH2)-domain, which mediate binding to SH2-domain-containing substrates. Substrates for phosphorylated Gab1 are protein tyrosine phosphatase 2 (Shp2), phosphoinositide 3-kinases (PI3K), and phospholipase C. Phosphorylated Grb2 binds to ras and raf, which in turn activates the mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase (ERK) [9]. In response to Met signaling, proliferative and anti-apoptotic responses are evoked. Typical cell targets for HGF are hepatocytes, renal tubule cells, and endothelial cells, where HGF induces proliferation [18]. In addition, HGF/Met signaling can induce several different epithelial and mesenchymal cell types to undergo a differentiation program termed “branching morphogenesis”. This results in the creation of branching patterns leading to the formation of many essential organs—the lung, vascular system, and most glands—composed of ramifying networks of epithelial tubes that transport fluids [8, 24]. In vivo, Met expression is predominantly found in cells of epithelial origin, while HGF expression is usually restricted to fibroblasts and stromal cells in the surrounding mesenchyma [27]. Paracrine signaling between HGF and Met is believed to play an important role in regulating these epithelial–mesenchymal cell interactions.
In vivo, HGF and Met likely play a key role in regulating many aspects of embryonic development, including kidney and mammary gland formation, migration and development of muscle and neuronal precursors, as well as liver and placenta organogenesis [24]. HGF/Met signaling also promotes angiogenesis [5, 42], and has been described to facilitate wound healing and tissue regeneration [43]. The PI3K pathway is involved in HGF/Met-mediated morphogenesis and survival. It has indeed been shown that Gab1 regulates the orientation of PI3K to these different responses [19]. The overexpression of Gab1 inhibits HGF-mediated survival, likely as a consequence of inhibition of the sustained activation of Akt, whereas Gab1 overexpression promotes HGF-induced morphogenesis. The survival responses triggered by HGF are favored by the direct binding of PI3K to Met, leading to sustained activation of Akt, whereas recruitment of Gab1 directs HGF signaling toward morphogenesis. In response to HGF, Akt phosphorylates the proapoptotic protein Bcl-2-associated death promoter (BAD), leading to its inactivation and thereby preventing mitochondrial-dependent apoptosis [69]. Furthermore, survival responses induced by HGF are correlated with an increased expression of the anti-apoptotic Bcl-xL and Bcl-2 proteins, which again prevents mitochondrial-dependent apoptosis. Mitochondrial-dependent apoptosis depends on the release of cytochrome c from the mitochondria and the formation of the apoptosome, leading to caspase-9 activation and the subsequent activation, by cleavage, of caspase-3 [69].
Proliferation and survival responses to HGF also involve the ras-ERK pathway, through the binding of Met to Grb2 and the subsequent activation of the transcription factor nuclear factor-κB (NF-κB) [8]. Epithelial cells respond to HGF/Met signaling by ‘scattering’, i.e., undergoing colony dispersal and an increase in motility. Such dissociated cells thus invade collagen matrices. This phenomenon is used in an assay to estimate the invasive and metastatic capacity of cells. Moreover, when epithelial cells are cultured within a collagen matrix and treated with HGF, they form branched tubules. Tubular branching is a complex morphogenic process that is observed in culture, and requires a tight coordination of cell growth, cell–cell contacts, polarity, and movement. It has indeed been shown that HGF modulates cell–cell contacts and therefore tubular branching through a reorganization of the cytoskeleton [52]. For example, cadherin proteins form the core of adherens junctions, but become relocalized and randomly distributed in the cell membrane during stimulation with HGF [47]. β-Catenin, another adherens junction component, associates with E-cadherin, and then binds a third protein, α-catenin, to the cytoskeleton. HGF stimulation results in the tyrosine phosphorylation of β-catenin, which induces its dissociation from the cadherin complex [41].
Initial experiments showed that inhibitors of PI3K or ERK prevent the scattering of epithelial cells and cell–cell contacts, and this indicates that both the ERK and the PI3K pathways are important for the disassembly of adherens junctions, cell spreading and motility. The Gab1-Shp2-ERK cascade regulates transformation-specific sequence/activator protein 1 (ETS/AP1) transcription factors, as well as adhesion molecules, which control cell proliferation, junctional competence and motility [7] (Fig. 2).
Fig. 2.
Signaling pathway of HGF and Met. PI3-K phosphatidylinositol(PI)3-kinase, MEK MAPK (mitogen-activated protein kinase)/ERK (extracellular receptor kinase)-kinase, shc src homology-2 (SH2) and collagen homology; PKB Akt or protein kinase B; Grb growth factor receptor-bound protein
Effects of HGF/Met on the commitment of CSCs: in vitro studies
HGF has been shown to be a potent differentiating factor for human embryonic stem cells (ESC), as well as for rat bone marrow mesenchymal stem cells (MSC) [44]. HGF and its receptor Met are expressed not only in fully differentiated cardiac cells, but also in myocytes during early cardiogenesis. Based on this observation, it has been speculated that HGF might be involved in cardiac development [49]. Forte et al. [17] have recently demonstrated the involvement of HGF in the in vitro cardiac commitment of murine MSC. After 2 days of treatment with HGF (20 ng/mL), MSC started to express transcription factors for muscle differentiation and early cardiac development, such as myocyte enhancer factor (MEF)-2C, transcription enhancer factor (TEF)-1 and guanine/adenine/thymine/adenine (GATA)-4 binding protein, as well as cardiac contractile proteins, such as α-myosin heavy chain (MHC) and β-MHC, while losing stem cell markers such as nucleostemin, CD105, and c-kit [17]. Roggia et al. [50] have provided evidence that exogenous HGF can improve cardiac commitment of differentiating ESC through the upregulation of transcription factors for early cardiac development such as Nkx 2.5 and GATA-4. After HGF treatment of ESC, these authors showed an upregulation of markers for differentiated cardiomyocytes, such as tropo-nin I, α-MHC and β-MHC. These effects were mediated by the PI3K-Akt anti-apoptotic and survival pathways, as they were blocked by the PI3K inhibitor, wortmannin [50].
HGF/Met and stem cell therapy for cardiac repair: rationale and in vivo studies
The proposal to combine autologous adult progenitor/stem cell therapy with ex vivo genetic manipulation of these cells might be an innovative approach for the therapeutic delivery of target molecules instead of the standard delivery of plasmid DNA or recombinant proteins. In this context, one possible strategy, among several, would be to combine the regenerative capacity of the HGF/Met signaling pathway with that of CSCs, by creating HGF- or Met-overexpressing CSCs, which are then able to increase the level and duration of HGF and/or Met expression after CSC transplantation in the infarcted myocardium.
Although expressed only at low levels, CSCs already express both HGF and MET [50]. Despite the fact that many growth factors, including HGF, are released in the infarcted myocardium, it is possible that the endogenous levels of the ligand or receptor (HGF and Met) are insufficient to stimulate resident stem cells to take part in regenerating heart tissue, or to protect them from apoptosis. Thus, transplantation of CSCs overexpressing Met in the infarcted myocardium would sensitize the transplanted CSCs to HGF. On the other hand, resident CSCs may exit from quiescence when challenged with high doses of HGF after transplantation of HGF-overexpressing CSCs, and the activated state of CSCs may be maintained by the positive feedback loop created by HGF signaling. Nevertheless, the in vivo use of CSCs for transplantation in infarcted cardiac tissue may be limited by the increased level of effort required to isolate these stem cells compared with other stem cell sources (the adipose tissue, for example). So far, studies employing adult stem cells for HGF gene transfer into the heart have employed the human bone marrow- and adipose tissue-derived stem cells [21, 73, 75]. Overall, these studies indicate the possibility to blunt ischemic injury with transplantation of adult stem cells combined with HGF gene transfer. Zhu and colleagues [75] investigated the effects of endogenous transplantation of human adipose tissue-derived stem cells overexpressing human HGF (hHGF) into a rat model of acute myocardial infarction. The infarction protocol in this study consisted of a 30-min coronary artery occlusion by ligating the left anterior descending coronary artery. Twenty-four hours after the infarction, adipose tissue-derived stem cells (AMI/ADSC group) or adipose tissue-derived stem cells over-expressing HGF (AMI/ADSChHGF group), or phosphate-buffered saline (AMI/PBS group, as control) were injected into the infarcted rats via the vena caudalis (1 × 108 cells in 1 mL of PBS, in the case of cells). The authors demonstrated that cardiac function parameters, including ejection fraction (EF), fractional shortening (FS), left ventricular end-diastolic (LVEDd) and end-systolic diameters (LVESd) were improved at 7, 14, and 28 days after cell transplantation in contrast with the AMI/PBS group. Furthermore, the authors found that transplantation of adipose tissue-derived stem cells induced angiogenesis by suppressing fibrosis and increasing capillary density in the ischemic myocardium. More importantly, compared to the AMI/ADSC group, these effects were enhanced in the AMI/ADSChHGF group. In the study by Guo et al. [21], the authors investigated the effects of bone marrow-derived MSC overexpressing HGF (MSC-HGF) on post-ischemic heart failure. In this study, 4 weeks after myocardial infarction (induced by coronary artery occlusion by ligation of the left anterior descending coronary artery) in rats, MSC-HGF or MSC or PBS were injected into the infarcted area by multiple intramuscular injections. After 8 weeks from coronary occlusion, the MSC-HGF groups featured better left ventricular systolic and diastolic function compared with the other groups. The authors observed enhanced angiogenesis and reduced apoptosis. Using an innovative approach consisting of the delivery of bone marrow mononuclear cells (BMNC) by an injectable poly-ethylene-glycol-treated (PEGylated) fibrin biomatrix that covalently binds HGF, Zhang et al. [73] showed improved cardiac function in a mouse model of myocardial infarction again after left anterior descending coronary artery ligation. Mice were randomized to receive an intramyocardial injection of saline or an HGF-loaded injectable biomatrix with or without entrapped BMNC. At days 14 and 28 after the infarction, the authors observed an increase in EF and improvements of LVEDd and LVESd, together with lower levels of apoptosis in HGF-treated groups. Finally, Shabbir et al. [58] have recently shown the possibility of facilitating the recruitment of CSCs to the site of myocardial injury by overexpressing various growth factors, including HGF. The study investigated the effects of intramuscularly injected bone marrow MSC or MSC-conditioned medium containing high levels of HGF, vascular endothelial growth factor (VEGF) and IGF into TO2 hamsters, a model of dilated cardiomyopathy harboring a genetic defect in the δ-sarco-glycan gene. Both the MSC and the MSC-conditioned medium groups showed improved ventricular function 1 month after the injection, and this was associated with mobilization of c-kit-positive CPCs.
The majority of clinical trials published to date have used bone marrow-derived mononuclear cells (BMMNC), which include EPC (CD34+, CD133+) and MSC. CSCs, such as MSC, have several features such as the capability to contribute to angiogenesis or vasculogenesis, to home to injured myocardium, and to survive long term after engraftment in newly injured myocardium that make them suitable for cardiovascular cell therapy. However, compared with CSCs, MSC [4] or other types of progenitor cells such as multipotent adult progenitor cells (MAPC) [64] have the advantage that they do not require immunosuppressive therapy and therefore they have been used in allogeneic transplantation. EPCs, on the contrary, have the disadvantage of poor long-term survival after engraftment in infarcted myocardium. Unlike MSC, MAPC and EPCs, CSCs are naturally predisposed to differentiate into all three cardiac cell lineages (endothelial cells, cardiomyocytes and smooth muscle cells). All these features have provided a solid rationale for using CSCs as a vehicle for HGF and/or Met, and justified a move toward clinical applications. The first human study of CSCs “Myocardial Regeneration Using Cardiac Stem Cells (SCIPIO)” (registered at www.clinicaltrials.gov) started in 2009 and is currently in the recruiting phase.
Lentiviral and non-lentiviral vectors to deliver HGF/Met genes into CPCs
The introduction of exogenous genes such as HGF and Met in CSCs might be a useful strategy to specifically tailor CSC for cardiovascular repair. Therefore, it is of utmost importance to develop efficient gene delivery systems that achieve high levels of expression of potentially therapeutic genes in CSCs. Important experimental variables that should be taken into account for a successful gene therapy include multiplicity of infection, length of time or viral incubation and medium used for viral incubation. Optimal assessment of such experimental conditions would increase gene transfer efficiency and obviate the need for selective antibiotic-based enrichment and long-term culture, which may contribute to senescence or compromise their long-term engraftment efficiency and/or multipotency [51]. In addition, by increasing gene transfer efficiency, fewer cells may be required to achieve a therapeutic effect. This justifies the use of lentiviral vectors for transducing adult stem cells, by virtue of their ability to transduce both dividing and non-dividing cells and their relative ease of use and comparable nature to adeno-associated virus (AAV) which is clinically preferred. Lentivirus-based systems would be ideal vectors for the transduction of adult stem cells since they overcome some problems, such as the short duration of gene expression and the occurrence of significant inflammatory responses, which plaque other types of gene vectors (such as adenoviruses) for the transduction of adult stem cells. Lentiviruses are a retrovirus subgroup that includes the human type 1 immunodeficiency virus (HIV). While retroviral systems are inefficient in transducing non-dividing or slowly dividing cells, lentivirus-based vectors, after being pseudotyped with vesicular stomatitis virus glycoprotein G (VSV-G) (i.e., using the glycoprotein envelope from the vesicular stomatitis virus to package recombinant retroviruses) [15], have the capacity to mediate genome integration into both non-dividing and dividing cells (Fig. 3). There is evidence that lentiviral vectors can also transduce more primitive, quiescent progenitors with stable transgene integration [11]. In comparison with retroviral vectors, lentiviral systems allow the immediate transduction without prior expansion, or with growth factor stimulation for only short exposure times. Compared with adenoviral vectors, lentiviral vectors offer also the major advantages of causing little or no disruption of the target cells and of not promoting any inflammatory response [30]. Studies employing lentivirus for HGF gene transfer into human adult stem cell have been limited so far to human bone marrow- and adipose tissue-derived stem cells [21, 75]. AAV vectors represent an alternate type of vector that may also be used for long-term transgene expression in the heart through cell-based therapy [60]. Like lentivirus, AAV can stably integrate into the host genome providing long-term transgene expression, with a minimal inflammatory response. However, AAV can cause insertional mutagenesis and can only carry genes which are less than 5 kb [14].
Fig. 3.
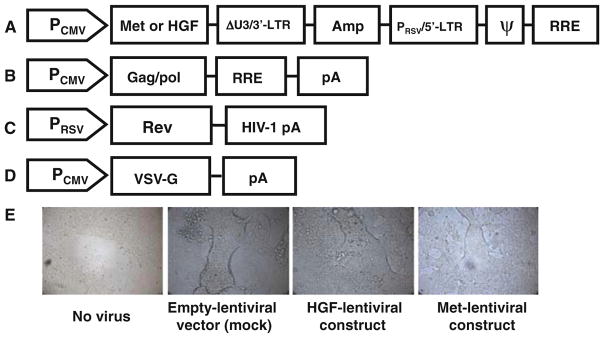
Schematic representation of the transducing vector construct (a), packaging construct (b, c) and VSV-G Env expressing construct (d). Amp ampicillin resistance gene, ΔU3 deleted region of the 3′LTR, which allows for biosafety of the vector; Gag, pol, env genes codifying for envelope proteins, HGF gene construct encoding HGF, Met gene construct encoding the Met receptor, PCMV cytomegalovirus promoter, 3′-LTR 3′-long terminal repeats for viral packaging, PRSV Rous Sarcoma Virus (RSV) enhancer/promoter, 5′-LTR 5′-long terminal repeats for viral packaging, ψ psi packaging sequence for viral packaging, pA polyadenylation signal, RRE HIV-1 Rev response element, VSV-G G glycoprotein gene from Vesicular Stomatitis Virus (VSV-G) as a pseudotyping envelope. e Photographs representing the experimental steps of production of the lentiviral stock (containing the packaged expression construct) by cotransfecting the packaging construct (b, c), the VSV-G Env expressing construct (d) and transducing vector construct (a) into the 293FT virus producer cell line. The expression of the VSV-G glycoprotein causes 293FT cells to fuse, resulting in the appearance of large, multinucleated cells known as syncytia
A possible drawback of the use of lentiviral and AAV vectors for delivering genes that codify growth factors might be that they can cause a chronic overexpression of the protein, with an uncertain therapeutic effect. Short-term gene expression of the growth factor gene would be desirable if the goal is to deliver a secreted protein such as growth factors like IGF-1, vascular endothelial growth factor (VEGF) and HGF, while long-term expression would be preferable if the goal is to express membrane proteins such as receptors for growth factors which require stable expression. Possible strategies to induce short-term gene expression of the transgene include plasmid transfection or adenoviral vectors [48]. Limitations of these strategies are the low transfection efficiency with plasmids and the immunogenic response of the host with adenovirus. We are currently verifying these hypotheses in our laboratory and at the same time testing the appropriate type of vector for gene transfer of HGF and its receptor Met in CSCs.
Perspectives and open questions
The use of CSCs in patients with myocardial infarction and heart failure requires the establishment of a large, expanded bank of CSCs. The use of expanded CSCs has inherent caveats associated with the difficult accessibility, a heterogenous cell population and the stability of the desired CSC phenotype in in vitro culture. However, their capability to home and to survive into injured myocardium, together with their natural predisposition to differentiate into all three cardiac cell lineages, makes these cells attractive for in vivo trials. The introduction of therapeutic genes into CSCs by modifying them ex vivo (by transfection before implantation) to allow them to express one or more selected genes that might selectively promote resident CSC proliferation, migration, engraftment, and differentiation would represent an interesting additional potential currently being explored to enhance therapeutic effects of transplanted CSCs.
An important question in the context of gene delivery into CSCs relates to the immunogenicity of the transduced cells. Indeed, one of the major barriers to stable gene transfer by lentiviruses is the development of innate and adaptive immune responses to the delivery vector, even less than for adenoviral vectors, and the transferred therapeutic transgene (i.e., HGF/SF and Met). However, several strategies can be used to overcome the host immune response to the transfer vector, including the injection of vector doses below the threshold that determines an increased inflammatory gene expression or the recruitment of inflammatory cells [16, 33]. Therefore, the only immunologically relevant protein here would remain the HGF or Met proteins. Recent studies have shown the role of HGF in promoting immune-mediated disorders. HGF promotes B- and T-lymphocyte migration [3, 65, 68], counteracts the immunosuppressive effects of tumor growth factor (TGF)-α, a potent immunosuppressive cytokine [28, 40, 61], suppresses dendritic cell function [45] and stimulates mast cells in response to Listeria infection [37]. However, other studies conducted in the mouse model of allogeneic heart transplantation have shown that HGF reduces acute and chronic rejection of the allograft, indicating that HGF might induce allograft tolerance and reduce acute and chronic rejection [71]. Thus, the role of HGF and Met in immunity is not well defined and whether Met-transduced CSCs can trigger Met-specific immune response in vivo after transplantation remains to be investigated.
Conclusions
CSCs can be genetically manipulated ex vivo with the purpose of reintroducing them in vivo, where they could provide therapeutically relevant levels of HGF and Met. The high probability that this therapy may be long-lasting represents a substantial theoretical advantage over treatments with recombinant proteins. In addition, the expanded population of autologous CSCs can be reliably stored for future repeated use, if and when needed. Therefore, expanded CSCs can be used to provide a source of autologous cells for the expression and secretion of any of a wide variety of proteins that might facilitate local intra-cardiac, regenerative effects.
Acknowledgments
The original studies here reported were supported by National Institutes of Health grants R01 HL55757, HL-70897, HL-76794, and HL78825 (to R. Bolli); and grants by the Italian Ministry of Research and Scientific Research and the Istituto Italiano Ricerche Cardiovascolari (to Prof. R. De Caterina).
Contributor Information
Rosalinda Madonna, Email: rmadonna@unich.it, rmadonna@heart.thi.tmc.edu, Institute of Cardiology, Center of Excellence on Aging, “G. d’Annunzio” University Chieti, Via dei Vestini, 66013 Chieti, Italy.
Gregg Rokosh, Institute of Molecular Cardiology, University of Louisville, Louisville, KY, USA.
Raffaele De Caterina, Institute of Cardiology, Center of Excellence on Aging, “G. d’Annunzio” University Chieti, Via dei Vestini, 66013 Chieti, Italy.
Roberto Bolli, Institute of Molecular Cardiology, University of Louisville, Louisville, KY, USA.
References
- 1.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Latif A, Zuba-Surma EK, Case J, Tiwari S, Hunt G, Ranjan S, Vincent RJ, Srour EF, Bolli R, Dawn B. TGF-beta1 enhances cardiomyogenic differentiation of skeletal muscle-derived adult primitive cells. Basic Res Cardiol. 2008;103(6):514–524. doi: 10.1007/s00395-008-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams DH, Harvath L, Bottaro DP, Interrante R, Catalano G, Tanaka Y, Strain A, Hubscher SG, Shaw S. Hepatocyte growth factor and macrophage inflammatory protein 1 beta: structurally distinct cytokines that induce rapid cytoskeletal changes and subset-preferential migration in T cells. Proc Natl Acad Sci USA. 1994;91:7144–7148. doi: 10.1073/pnas.91.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell LN, Cai L, Johnstone BH, Traktuev DO, March KL, Considine RV. A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am J Physiol Endocrinol Metab. 2008;294:E336–E344. doi: 10.1152/ajpendo.00272.2007. [DOI] [PubMed] [Google Scholar]
- 6.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 8.Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 9.Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 10.Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL. IFATS series: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27(1):230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Case SS, Price MA, Jordan CT, Yu XJ, Wang L, Bauer G, Haas DL, Xu D, Stripecke R, Naldini L, Kohn DB, Crooks GM. Stable transduction of quiescent CD34(+)CD38(−) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci USA. 1999;96:2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawn B, Bolli R. Cardiac progenitor cells: the revolution continues. Circ Res. 2005;97:1080–1082. doi: 10.1161/01.RES.0000195610.71671.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, Sands MS. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 15.Emi N, Friedmann T, Yee JK. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991;65:1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Follenzi A, Santambrogio L, Annoni A. Immune responses to lentiviral vectors. Curr Gene Ther. 2007;7:306–315. doi: 10.2174/156652307782151515. [DOI] [PubMed] [Google Scholar]
- 17.Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, Prat M, Di Nardo P. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 18.Gherardi E, Stoker M. Hepatocyte growth factor–scatter factor: mitogen, motogen, and met. Cancer Cells. 1991;3:227–232. [PubMed] [Google Scholar]
- 19.Graziani A, Gramaglia D, Cantley LC, Comoglio PM. The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J Biol Chem. 1991;266:22087–22090. [PubMed] [Google Scholar]
- 20.Guan K, Hasenfuss G. Do stem cells in the heart truly differentiate into cardiomyocytes? J Mol Cell Cardiol. 2007;43:377–387. doi: 10.1016/j.yjmcc.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, He J, Wu J, Yang L, Dai S, Tan X, Liang L. Locally overexpressing hepatocyte growth factor prevents post-ischemic heart failure by inhibition of apoptosis via calcineurin-mediated pathway and angiogenesis. Arch Med Res. 2008;39:179–188. doi: 10.1016/j.arcmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103(6):525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 23.Hamasuna R, Kataoka H, Moriyama T, Itoh H, Seiki M, Koono M. Regulation of matrix metalloproteinase-2 (MMP-2) by hepatocyte growth factor/scatter factor (HGF/SF) in human glioma cells: HGF/SF enhances MMP-2 expression and activation accompanying up-regulation of membrane type-1 MMP. Int J Cancer. 1999;82:274–281. doi: 10.1002/(sici)1097-0215(19990719)82:2<274::aid-ijc19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Hogan BL, Kolodziej PA. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- 25.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 26.Kaur S, Kumar TR, Uruno A, Sugawara A, Jayakumar K, Kartha CC. Genetic engineering with endothelial nitric oxide synthase improves functional properties of endothelial progenitor cells from patients with coronary artery disease: an in vitro study. Basic Res Cardiol. 2009;104(6):739–749. doi: 10.1007/s00395-009-0039-x. [DOI] [PubMed] [Google Scholar]
- 27.Kopp JB. Hepatocyte growth factor: mesenchymal signal for epithelial homeostasis. Kidney Int. 1998;54:1392–1393. doi: 10.1046/j.1523-1755.1998.00126.x. [DOI] [PubMed] [Google Scholar]
- 28.Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 29.Kuang D, Zhao X, Xiao G, Ni J, Feng Y, Wu R, Wang G. Stem cell factor/c-kit signaling mediated cardiac stem cell migration via activation of p38 MAPK. Basic Res Cardiol. 2008;103(3):265–273. doi: 10.1007/s00395-007-0690-z. [DOI] [PubMed] [Google Scholar]
- 30.Lever AM. HIV and other lentivirus-based vectors. Gene Ther. 1996;3:470–471. [PubMed] [Google Scholar]
- 31.Li B, Zeng Q, Wang H, Shao S, Mao X, Zhang F, Li S, Guo Z. Adipose tissue stromal cells transplantation in rats of acute myocardial infarction. Coron Artery Dis. 2007;18(3):221–227. doi: 10.1097/MCA.0b013e32801235da. [DOI] [PubMed] [Google Scholar]
- 32.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K, Castro MG. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther. 2007;7:347–360. doi: 10.2174/156652307782151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madonna R, Geng YJ, De Caterina R. Adipose tissue-derived stem cells: characterization and potential for cardiovascular repair. Arterioscler Thromb Vasc Biol. 2009;29(11):1723–1729. doi: 10.1161/ATVBAHA.109.187179. [DOI] [PubMed] [Google Scholar]
- 35.Marsboom G, Janssens S. Endothelial progenitor cells: new perspectives and applications in cardiovascular therapies. Expert Rev Cardiovasc Ther. 2008;6:687–701. doi: 10.1586/14779072.6.5.687. [DOI] [PubMed] [Google Scholar]
- 36.Mazo M, Planat-Benard V, Abizanda G, Pelacho B, Leobon B, Gavira JJ, Penuelas I, Cemborain A, Penicaud L, Laharrague P, Joffre C, Boisson M, Ecay M, Collantes M, Barba J, Casteilla L, Prosper F. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur J Heart Fail. 2008;10:454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 37.McCall-Culbreath KD, Li Z, Zutter MM. Crosstalk between the alpha2beta1 integrin and c-met/HGF-R regulates innate immunity. Blood. 2008;111:3562–3570. doi: 10.1182/blood-2007-08-107664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 39.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno S, Kurosawa T, Matsumoto K, Mizuno-Horikawa Y, Okamoto M, Nakamura T. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J Clin Invest. 1998;101:1827–1834. doi: 10.1172/JCI1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, Wang X, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–2071. [PubMed] [Google Scholar]
- 42.Morishita R, Aoki M, Hashiya N, Yamasaki K, Kurinami H, Shimizu S, Makino H, Takesya Y, Azuma J, Ogihara T. Therapeutic angiogenesis using hepatocyte growth factor (HGF) Curr Gene Ther. 2004;4:199–206. doi: 10.2174/1566523043346453. [DOI] [PubMed] [Google Scholar]
- 43.Neuss S, Becher E, Woltje M, Tietze L, Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405–414. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- 44.Oh SH, Miyazaki M, Kouchi H, Inoue Y, Sakaguchi M, Tsuji T, Shima N, Higashio K, Namba M. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500–504. doi: 10.1006/bbrc.2000.3985. [DOI] [PubMed] [Google Scholar]
- 45.Okunishi K, Dohi M, Nakagome K, Tanaka R, Mizuno S, Matsumoto K, Miyazaki J, Nakamura T, Yamamoto K. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol. 2005;175:4745–4753. doi: 10.4049/jimmunol.175.7.4745. [DOI] [PubMed] [Google Scholar]
- 46.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 47.Pollack AL, Runyan RB, Mostov KE. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- 48.Rabbany SY, Pastore J, Yamamoto M, Miller T, Rafii S, Aras R, Penn M. Continuous delivery of stromal cell-derived factor-1 from alginate scaffolds accelerates wound healing. Cell Transpl. 2009 doi: 10.3727/096368909X481782. [DOI] [PubMed] [Google Scholar]
- 49.Rappolee DA, Iyer A, Patel Y. Hepatocyte growth factor and its receptor are expressed in cardiac myocytes during early cardiogenesis. Circ Res. 1996;78:1028–1036. doi: 10.1161/01.res.78.6.1028. [DOI] [PubMed] [Google Scholar]
- 50.Roggia C, Ukena C, Bohm M, Kilter H. Hepatocyte growth factor (HGF) enhances cardiac commitment of differentiating embryonic stem cells by activating PI3 kinase. Exp Cell Res. 2007;313:921–930. doi: 10.1016/j.yexcr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 52.Rosario M, Birchmeier W. How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 2003;13:328–335. doi: 10.1016/s0962-8924(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 53.Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, Keating SC, Parker TG, Backx PH, Keating A. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884–2892. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 54.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Schenke-Layland K, Strem B, Jordan M, Deemedio M, Hedrick M, Roos K, Fraser J, Maclellan W. Adipose tissue derived cells improve cardiac function following myocardial infarction. J Surg Res. 2009;153(2):217–223. doi: 10.1016/j.jss.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuh A, Liehn EA, Sasse A, Hristov M, Sobota R, Kelm M, Merx MW, Weber C. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol. 2008;103(1):69–77. doi: 10.1007/s00395-007-0685-9. [DOI] [PubMed] [Google Scholar]
- 58.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296:H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schumichen C, Nienaber CA, Freund M, Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 60.Svensson EC, Marshall DJ, Woodard K, Lin H, Jiang F, Chu L, Leiden JM. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation. 1999;99:201–205. doi: 10.1161/01.cir.99.2.201. [DOI] [PubMed] [Google Scholar]
- 61.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, Fujimoto J. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 62.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 63.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 64.Van’t Hof W, Mal N, Huang Y, Zhang M, Popovic Z, Forudi F, Deans R, Penn MS. Direct delivery of syngeneic and allogeneic large-scale expanded multipotent adult progenitor cells improves cardiac function after myocardial infarct. Cytotherapy. 2007;9:477–487. doi: 10.1080/14653240701452065. [DOI] [PubMed] [Google Scholar]
- 65.van der Voort R, Taher TE, Keehnen RM, Smit L, Groenink M, Pals ST. Paracrine regulation of germinal center B cell adhesion through the c-methepatocyte growth factor/scatter factor pathway. J Exp Med. 1997;185:2121–2131. doi: 10.1084/jem.185.12.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weidner KM, Arakaki N, Hartmann G, Vandekerckhove J, Weingart S, Rieder H, Fonatsch C, Tsubouchi H, Hishida T, Daikuhara Y, et al. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci USA. 1991;88:7001–7005. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990;111:2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weimar IS, de Jong D, Muller EJ, Nakamura T, van Gorp JM, de Gast GC, Gerritsen WR. Hepatocyte growth factor/scatter factor promotes adhesion of lymphoma cells to extracellular matrix molecules via alpha 4 beta 1 and alpha 5 beta 1 integrins. Blood. 1997;89:990–1000. [PubMed] [Google Scholar]
- 69.Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci USA. 2001;98:247–252. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamada Y, Wang X, Yokoyama S, Fukuda N, Takakura N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem Biophys Res Commun. 2006;342(2):662–670. doi: 10.1016/j.bbrc.2006.01.181. [DOI] [PubMed] [Google Scholar]
- 71.Yamaura K, Ito K, Tsukioka K, Wada Y, Makiuchi A, Sakaguchi M, Akashima T, Fujimori M, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Suzuki J, Amano J, Isobe M. Suppression of acute and chronic rejection by hepatocyte growth factor in a murine model of cardiac transplantation: induction of tolerance and prevention of cardiac allograft vasculopathy. Circulation. 2004;110:1650–1657. doi: 10.1161/01.CIR.0000143052.45956.71. [DOI] [PubMed] [Google Scholar]
- 72.Zhang D-Z, Gai L-Y, Liu H-W, Jin Q-H, Huang J-H, Zhu X-Y. Transplantation of autologous adipose-derived stem cells ameliorates cardiac function in rabbits with myocardial infarction. Chin Med J. 2007;120(4):300–307. [PubMed] [Google Scholar]
- 73.Zhang G, Hu Q, Braunlin EA, Suggs LJ, Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A. 2008;14:1025–1036. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu XY, Zhang XZ, Xu L, Zhong XY, Ding Q, Chen YX. Transplantation of adipose-derived stem cells overexpressing hHGF into cardiac tissue. Biochem Biophys Res Commun. 2009;379:1084–1090. doi: 10.1016/j.bbrc.2009.01.019. [DOI] [PubMed] [Google Scholar]