Abstract
OBJECTIVE
This article reviews important complications of targeted drug therapies for solid malignancies that can be identified on diagnostic imaging. Wherever possible, known or proposed mechanistic explanations for drug complications are emphasized.
CONCLUSION
Familiarity with the toxicity profiles of different targeted cancer therapies is important for identifying drug-related complications and for differentiating drug effects from disease progression. A mechanistic understanding may be useful for associating individual drugs with their complications and for predicting the complications of emerging agents.
Keywords: bevacizumab, drug toxicities, imatinib, ipilimumab, targeted agents, temsirolimus, vemurafenib
Targeted cancer therapies are agents designed to interfere with specific cell signaling pathways necessary for tumor growth and progression. A primary goal of targeted therapies is to attack cancer cells with more precision than traditional chemotherapy, which typically affects rapidly dividing cells in the body indiscriminately. State-of-the-art cancer treatment has been shifting toward identifying specific mutations within a particular tumor and using this information to tailor therapy with targeted agents [1]. At present, there are thousands of active clinical trials evaluating different targeted agents for use alone and in combination across a wide spectrum of malignancies [2].
The increasing use of targeted agents has important implications for imaging, especially with regard to surveillance for drug toxicities and assessment of therapeutic response. Familiarity with the toxicity patterns associated with different targeted therapies is crucial both for identifying drug-related complications and for differentiating drug effects from disease progression.
This article reviews important complications of targeted agents that can be identified by conventional diagnostic imaging, focusing on agents currently approved by the U.S. Food and Drug Administration (FDA) for treatment of solid malignancies. We propose that a rational understanding of why certain drugs and drug classes produce certain complications should be more useful than a simple “laundry list” approach, both for associating the currently approved agents with their complications and for predicting the complications of newer agents as they come to market. We therefore emphasize, wherever possible, known or proposed mechanistic explanations for targeted drug complications.
Classification of Targeted Agents
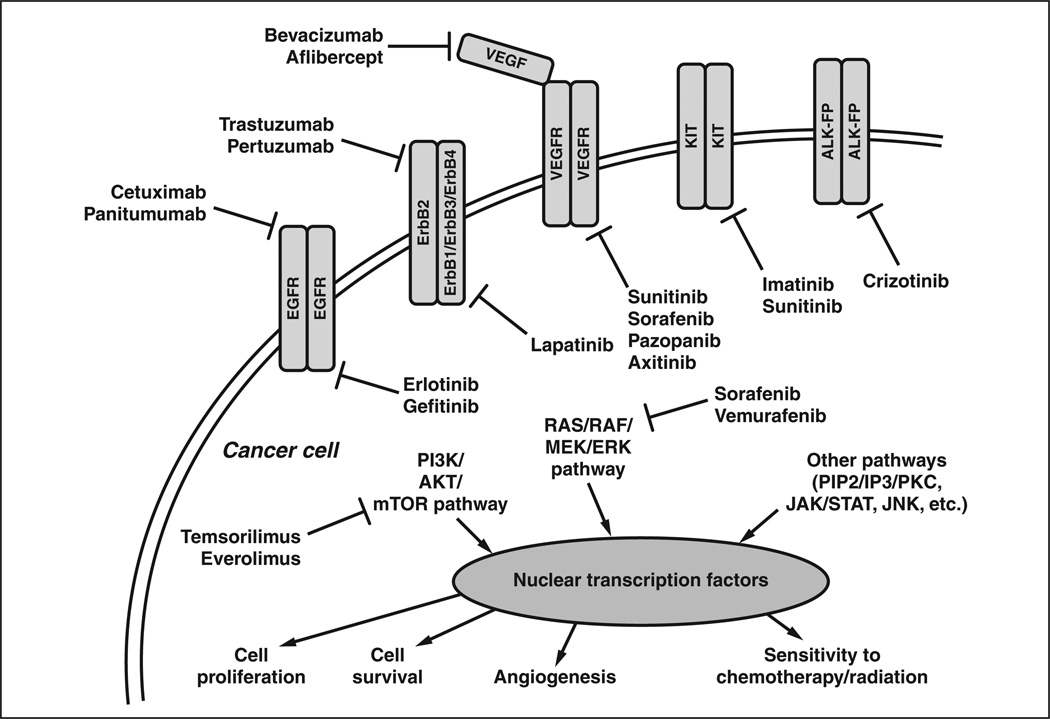
The currently approved targeted agents for solid malignancies exploit a handful of key signal transduction pathways (Fig. 1). Agents with primary molecular targets on the cell surface (Table 1) include the epidermal growth factor receptor (EGFR) inhibitors cetuximab, panitumumab, erlotinib, and gefitinib; the human ErbB2 (formerly HER2) receptor inhibitors trastuzumab, pertuzumab, and lapatinib; the vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) inhibitors bevacizumab, aflibercept, axitinib, pazopanib, sorafenib, and sunitinib; the KIT (or stem cell factor receptor) inhibitors imatinib and sunitinib; and the anaplastic lymphoma kinase (ALK) inhibitor crizotinib. Agents with molecular targets downstream of cell surface receptors (Table 2) include the RAF (in the RAS/RAF/MEK/ERK pathway) inhibitors sorafenib and vemurafenib and the mammalian target of rapamycin (mTOR, in the PI3K/ AKT/mTOR pathway) inhibitors temsirolimus and everolimus. Also included in many classification schemes are the targeted immune modulators (Table 3), of which ipilimumab is the prototype.
Fig. 1.
Schematic shows current targeted cancer therapies approved by the Food and Drug Administration and their interactions with their primary molecular targets. EGFR = epidermal growth factor receptor, ErbB2 (formerly, HER2) = human epidermal growth factor, VEGF = vascular endothelial growth factor, VEGFR = VEGF receptor, KIT = stem cell factor receptor, ALK-FP = anaplastic lymphoma kinase fusion protein.
TABLE 1.
Currently Approved Targeted Cancer Therapies With Molecular Targets on the Cell Surface
| Category | Agent | Class | Molecular Target(s) | FDA-Approved Indication(s) |
|---|---|---|---|---|
| Agents targeting EGFR | Cetuximab (Erbitux, ImClone) | mAb | EGFR | CRC (EGFR mutated, KRAS wildtype) SCCHN |
| Panitumumab (Vectibix, Amgen) | mAb | EGFR | CRC (KRAS wildtype) | |
| Erlotinib (Tarceva, Genentech) | TKI | EGFR | NSCLC Pancreatic cancer |
|
| Gefitinib (Iressa, AstraZeneca) | TKI | EGFR | NSCLC with known prior benefit from gefitinib (limited approval) | |
| Agents targeting ErbB2 | Trastuzumab (Herceptin, Genentech) | mAb | ErbB2 | Breast cancer (ErbB2 positive) Gastric/gastroesophageal junction cancer (ErbB2 positive) |
| Pertuzumab (Perjeta, Genentech) | mAb | ErbB2 | Breast cancer (ErbB2 positive) | |
| Lapatinib (Tykerb, GlaxoSmithKline) | TKI | ErbB2 EGFR(ErbB1) |
Breast cancer (ErbB2 positive) | |
| Agents targeting VEGF/VEGFR | Bevacizumab (Avastin, Genentech) | mAb | VEGFIigand | CRC NSCLC Glioblastoma RCC |
| Aflibercept (Zaltrap, Sanofi) | Other | VEGFIigand | CRC | |
| Pazopanib (Votrient, GlaxoSmithKline) | TKI | VEGFR PDGFR KIT |
RCC Soft-tissue sarcoma |
|
| Axitinib (Inlyta, Pfizer) | TKI | VEGFR PDGFR KIT |
RCC | |
| Sorafenib (Nexavar, Onyx Pharmaceuticals) | TKI/STKI | VEGFR PDGFR KIT RAF |
HCC RCC |
|
| Sunitinib (Sutent, Pfizer) | TKI | VEGFR PDGFR KIT RET |
GIST RCC Pancreatic neuroendocrine tumor |
|
| Agents targeting KIT | Imatinib (Gleevec, Novartis Pharmaceuticals) | TKI | KIT PDGFR ABL |
GIST DFSP Multiple hematologic malignancies including Philadelphia chromosome-positive ALL and CML |
| Sunitinib (Sutent) | TKI | VEGFR PDGFR KIT RET |
GIST RCC Pancreatic neuroendocrine tumor |
|
| Agents targeting ALK | Crizotinib (Xalkori, Pfizer) | TKI | ALK | NSCLC (with ALK fusion) |
Note—FDA = Food and Drug Administration, CRC = colorectal cancer, EGFR = epidermal growth factor receptor, SCCHN = squamous cell carcinoma of the head and neck, mAb = monoclonal antibody, TKI = tyrosine kinase inhibitor, NSCLC = non-small cell lung cancer, VEGF = vascular endothelial growth factor, VEGFR = VEGF receptor, PDGFR = platelet-derived growth factor receptor, KIT = stem cell factor receptor, STKI = serine/threonine kinase inhibitor, RCC = renal cell carcinoma, HCC = hepatocellular carcinoma, GIST = gastrointestinal stromal tumor, DFSP = dermatofibrosarcoma protuberans, ALL = acute lymphoblastic leukemia, CML = chronic myeloid leukemia, ALK = anaplastic lymphoma kinase.
TABLE 2.
Currently Approved Targeted Cancer Therapies With Molecular Targets Downstream From Cell Surface Receptors
| Category | Agent | Class | Molecular Target(s) | FDA-Approved Indication(s) |
|---|---|---|---|---|
| Agents targeting RAF | Sorafinib (Nexavar, Onyx Pharmaceuticals) | TKI/STKI | RAF VEGFR PDGFR KIT |
HCC RCC |
| Vemurafenib (Zelboraf, Genentech) | STKI | RAF | Melanoma (BRAFV600E) | |
| Agents targeting mTOR | Temsirolimus (Torisel, Pfizer) | STKI | mTOR | RCC |
| Everolimus (Afinitor, Novartis Pharmaceuticals) | STKI | mTOR | Breast cancer (ER positive, ErbB2 negative) Pancreatic neuroendocrine tumor RCC Angiomyolipoma associated with TSC Nonresectable SEGA associated with TSC |
|
Note—FDA = Food and Drug Administration, VEGFR = vascular endothelial growth factor receptor, PDGFR = platelet-derived growth factor receptor, KIT = stem cell factor receptor, TKI = tyrosine kinase inhibitor, STKI = serine/threonine kinase inhibitor, HCC = hepatocellular carcinoma, mTOR = mammalian target of rapamycin, RCC = renal cell carcinoma, ER = estrogen receptor, TSC = tuberous sclerosis complex, SEGA = subependymal giant cell astrocytoma.
TABLE 3.
Currently Approved Cancer Agents With Target Molecules on Host Immune Cells (Immune Modulators)
| Category | Agent | Class | Molecular Target | FDA-Approved Indication |
|---|---|---|---|---|
| Immune modulators | Ipilimumab (Yervoy, Bristol-Myers Squibb) | mAb | CTLA | Melanoma |
Note—FDA = Food and Drug Administration, mAb = monoclonal antibody, CTLA = cytotoxic T-lymphocyte antigen.
It should be noted that targeted cancer therapies include both monoclonal antibodies and small molecules. Monoclonal antibodies (typically ending in “-mab” by conventional nomenclature) target receptor molecules on the surface of cells, whereas small molecules (typically ending in “-ib”) can penetrate the cell membrane to interact either with the intracellular domain of a transmembrane receptor molecule or with deep targets inside a cell. Monoclonal antibodies tend to be fairly specific, targeting only one receptor or a narrow family of receptors, whereas small molecules can interact with multiple intracellular targets. Small molecules often have a wider toxicity profile than monoclonal antibodies because of their relative lack of target specificity.
Agents Targeting EGFR
The currently approved EGFR inhibitors include the monoclonal antibodies cetuximab (Erbitux, ImClone) and panitumumab (Vectibix, Amgen) as well as the small molecule receptor tyrosine kinase inhibitors erlotinib (Tarceva, Genentech) and gefitinib (Iressa, AstraZeneca). Cetuximab and panitumumab are indicated for treatment of colorectal cancer with absence of a downstream KRAS mutation, which if present renders EGFR inhibition ineffective [3]. Cetuximab is also indicated for squamous cell carcinoma of the head and neck. Erlotinib is indicated for treatment of non–small cell lung cancer (NSCLC) and metastatic pancreatic cancer. The FDA approval of Gefitinib has been largely revoked due to postmarketing studies showing lack of efficacy [4], but it retains limited approval for use in NSCLC, for which prior benefit has been documented clinically.
Interstitial Lung Disease
Interstitial lung disease (ILD) is the most important toxicity identifiable by imaging for the EGFR pathway agents. The mechanism is not fully understood, but the proposed pathophysiology involves disruption of alveolar epithelial maintenance and repair functions normally performed by EGFR-expressing type 2 pneumocytes, with resultant potentiation of lung injury due to other causes [5]. Concordant with this proposed mechanism, the risk for ILD is higher in smokers and in patients with preexisting lung disease [6–8]. ILD is more common with the small molecules erlotinib and gefitinib than with the monoclonal antibodies cetuximab and panitumumab, probably due to small molecule interactions with downstream intracellular targets that are yet to be elucidated.
A study by Endo et al. [9] found that gefitinib-related ILD has a wide range of imaging manifestations, including nonspecific ground-glass opacity (GGO) with and without interlobular septal thickening and multifocal consolidations with or without traction bronchiectasis. These patterns are fairly nonspecific and have a broad differential diagnosis, including pneumonia, bronchiolitis, diffuse alveolar damage, radiation or hypersensitivity pneumonitis, pulmonary fibrosis, and progressive multifocal bronchoalveolar carcinoma. Pulmonary toxicity from other chemotherapeutic agents, such as gemcitabine and paclitaxel, can also have similar findings [10].
Hepatic Dysfunction
Erlotinib and gefitinib are both associated with elevations in hepatic aminotransferases. Investigators have reported hepatorenal syndrome and hepatic failure, quite rare but occasionally fatal, particularly in patients with preexisting hepatic impairment [11]. Drug-related hepatitis can manifest on imaging as altered hepatic attenuation or echogenicity, reactive gallbladder wall thickening, and periportal edema [12].
Agents Targeting ErbB2
Agents targeting ErbB2 include the monoclonal antibodies trastuzumab (Herceptin, Genentech) and pertuzumab (Perjeta, Genentech) as well as the tyrosine kinase inhibitor lapatinib (Tykerb, GlaxoSmithKline). All three agents are indicated for treatment of breast cancers that overexpress ErbB2. Trastuzumab carries an additional FDA indication for treatment of ErbB2-positive gastric or gastroesophageal cancer.
Cardiotoxicity is the most relevant toxicity identifiable by imaging for ErbB2 pathway agents; it is most common and clinically most important with trastuzumab. The proposed mechanism involves direct effects on myocardial ErbB2 receptors that are coupled to cell signaling pathways involved in cardiac sarcomere stability [13]. Patients treated with trastuzumab typically undergo echocardiography or multigated acquisition radionuclide angiography before treatment initiation and are monitored both clinically and with serial imaging every 3 months while on therapy.
Agents Targeting VEGF/VEGFR
Agents targeting VEGF/VEGFR include the monoclonal antibody bevacizumab (Avastin, Genentech), the VEGF “trap” aflibercept (Zaltrap, Sanofi), and the small molecules axitinib (Inlyta, Pfizer), pazopanib (Votrient, GlaxoSmithKline), sorafenib (Nexavar, Onyx Pharmaceuticals), and sunitinib (Sutent, Pfizer). Bevacizumab has a long list of FDA-approved indications, including colorectal cancer, NSCLC, and renal cell carcinoma; in November 2011 the FDA rescinded its approval for use in breast cancer due to lack of a survival benefit [14]. Aflibercept was recently approved for metastatic colorectal cancer. Axitinib was recently approved for renal cell carcinoma. Pazopanib is indicated for treatment of renal cell carcinoma and advanced soft-tissue sarcomas. Sorafenib is indicated for renal cell carcinoma and hepatocellular carcinoma. Sunitinib is indicated for renal cell carcinoma, gastrointestinal stromal tumor (GIST), and pancreatic neuroendocrine tumor.
The VEGF/VEGFR pathway is implicated in the proliferation and maintenance of tumor neovascularity. Many of the complications associated with VEGF/VEGFR-targeted agents are therefore proposed to derive from vascular endothelial cell disruption or its sequelae.
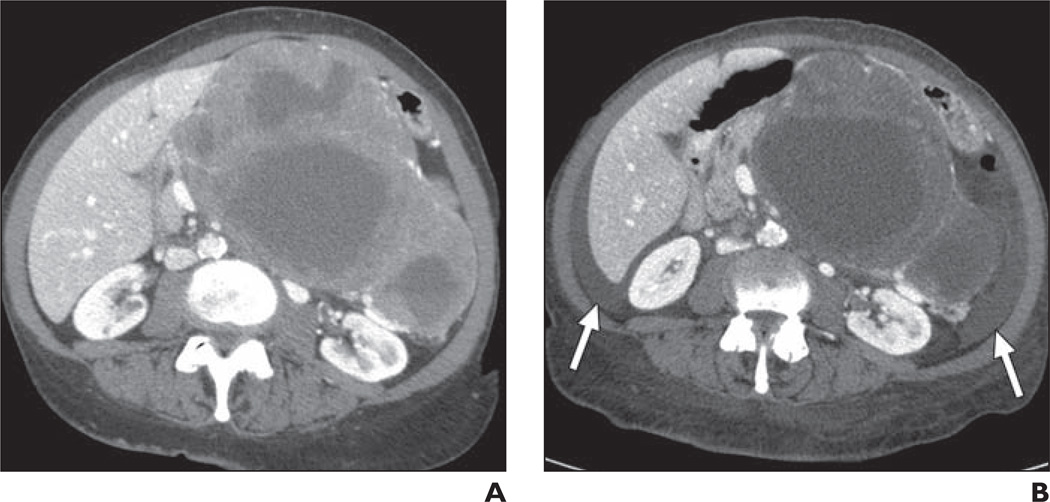
Gastrointestinal Pneumatosis and Perforation
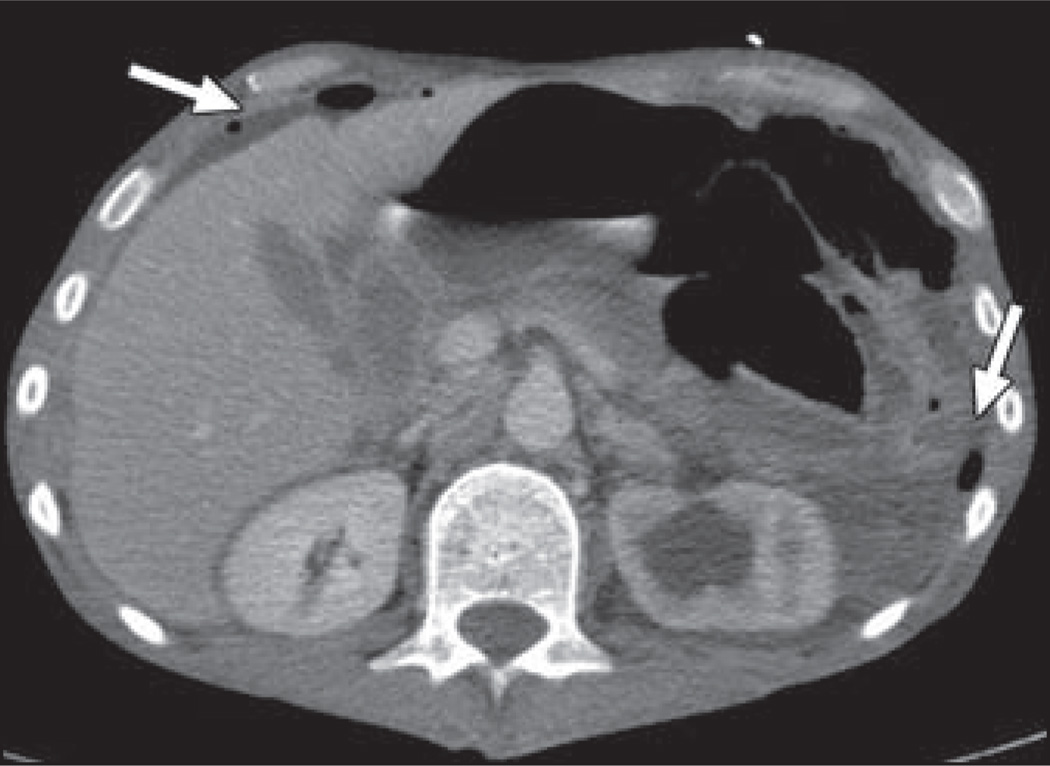
The FDA has required a “black box” warning concerning the risk of gastrointestinal tract perforation with bevacizumab (Fig. 2) because an 11% rate of gastrointestinal perforation was reported in a phase 2 clinical trial [15]. Since that time, there has been debate over the true additional contribution of bevacizumab to the risk of gastrointestinal perforation because patients in some clinical trials may have had an increased risk for perforation due to the presence of tumor along serosal surfaces, and no significant association between bevacizumab and gastrointestinal perforation has been shown in any randomized controlled clinical trials. However, a recent large meta-analysis reported that gastrointestinal perforation with bevacizumab occurs with an incidence of approximately 0.9% and that patients treated with bevacizumab have a significantly increased relative risk of gastrointestinal perforation over control subjects [16].
Fig. 2.
44-year-old man with metastatic rectal cancer being treated with bevacizumab who presented with signs of peritonitis. Contrast-enhanced CT scan shows pneumoperitoneum and free fluid (arrows). Tumor-related left-sided hydronephrosis is also present. Sigmoid colon perforation was found at surgery.
Proposed mechanisms for gastrointestinal perforation with bevacizumab include drug-induced alterations in the gastrointestinal microvasculature leading to local ischemia and inhibition of VEGF-mediated healing of gastrointestinal tract insults, including ulcers and diverticulitis [17]. Ongoing research is examining the association between bevacizumab-associated gastrointestinal perforation and possible risk factors, including past diverticulitis or ulcers, recent endoscopy, previous gastrointestinal surgery, gastrointestinal obstruction, presence of tumor along serosal surfaces, and varying primary malignancies. Imaging shows free air and free fluid with possible complications, including abscess and fistula formation. Pneumatosis intestinalis may be a harbinger of perforation and should be reported to the treating oncologist but is itself a nonspecific finding that can be seen with either ischemia or inflammation in the setting of a wide range of chemotherapeutic and immunosuppressive therapies [10].
Gastrointestinal and Nongastrointestinal Fistulas
Patients being treated with VEGF/VEGFR agents are also at increased risk for fistula formation, probably through the same mechanisms involved in gastrointestinal pneumatosis and perforation, including alterations in tissue microvasculature with local ischemia and impaired healing of inflammatory insults. Importantly, drug-associated fistulas may involve either gastrointestinal or nongastrointestinal locations (Fig. 3), with reported sites of involvement including tracheoesophageal, bronchopleural, biliary, vaginal, renal, and vesical.
Fig. 3.
46-year-old woman with breast cancer being treated with paclitaxel and bevacizumab who complained of 4 days of green stool leaking from her vagina. Patient had no history of inflammatory bowel disease or pelvic radiation. Axial CT scan after positive rectal contrast administration shows fistula tract arising along posterior rectum and extending anteriorly to vagina (arrow). Patient underwent examination under anesthesia with abscess drainage and placement of seton drain.
Hemorrhage
Two distinct patterns of hemorrhage are recognized in association with VEGF/VEGFR agents: minor bleeding (most commonly mild epistaxis) and serious hemorrhagic events (including hemoptysis, gastrointestinal bleeding, CNS hemorrhage, major epistaxis, and vaginal bleeding). The proposed mechanism involves disruption in VEGF-mediated vascular endothelial cell renewal [18]. In non–small cell lung cancer, pulmonary hemorrhage has been associated with squamous cell histology and with baseline tumor cavitation or necrosis [19].
Arterial Thrombotic Events
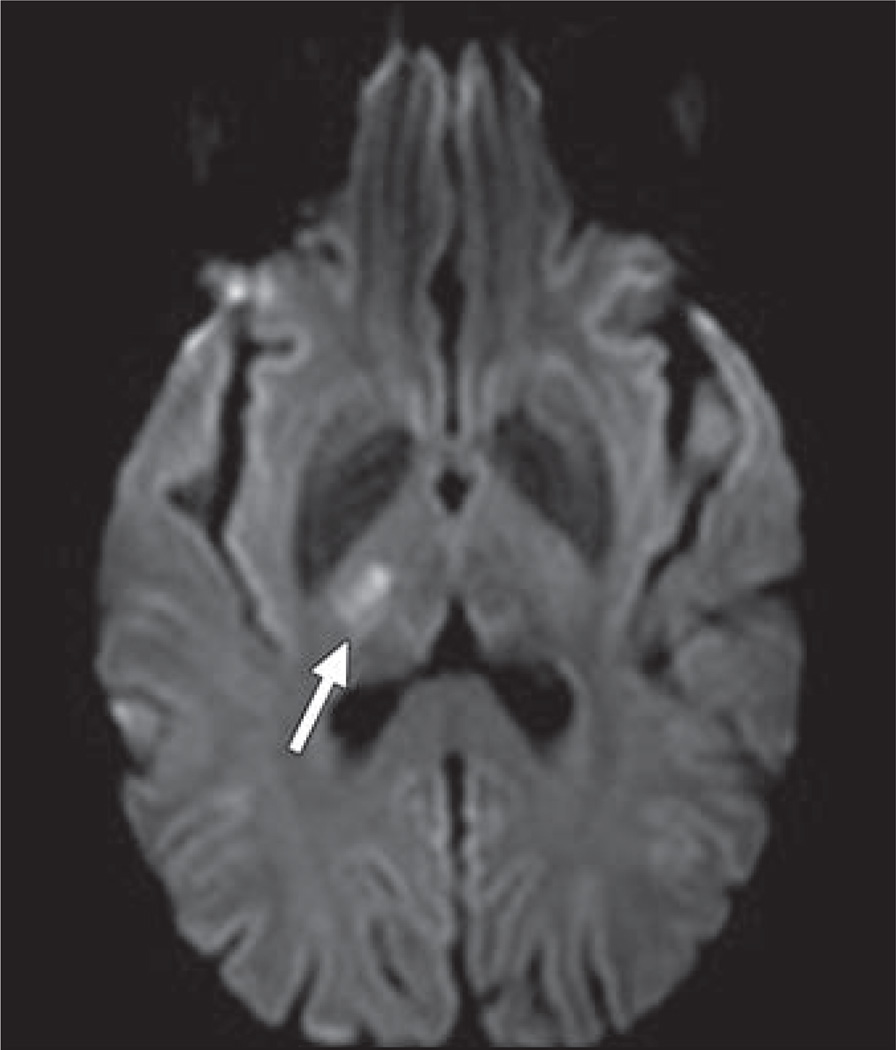
All of the VEGF/VEGFR pathway agents carry an increased risk for thromboembolic events beyond the increased risk attributable to the malignancy itself (Fig. 4), thought to be related to drug-induced prothrombotic endothelial dysfunction [18]. In a meta-analysis, increased risk was only observed for arterial thromboembolism and not for venous [20]. Arterial thromboembolic complications are mostly cardiac and cerebrovascular, but thrombosis has also been reported in the aorta, mesenteric vessels, and extremities [10].
Fig. 4.
52-year-old woman with metastatic ovarian carcinoma who developed acute-onset left-sided numbness and weakness 5 days after initiation of combination chemotherapy with paclitaxel, cisplatin, and bevacizumab. MR image shows focus of restricted diffusion in right thalamus (arrow), consistent with acute infarct.
Progressive Reversible Encephalopathy Syndrome
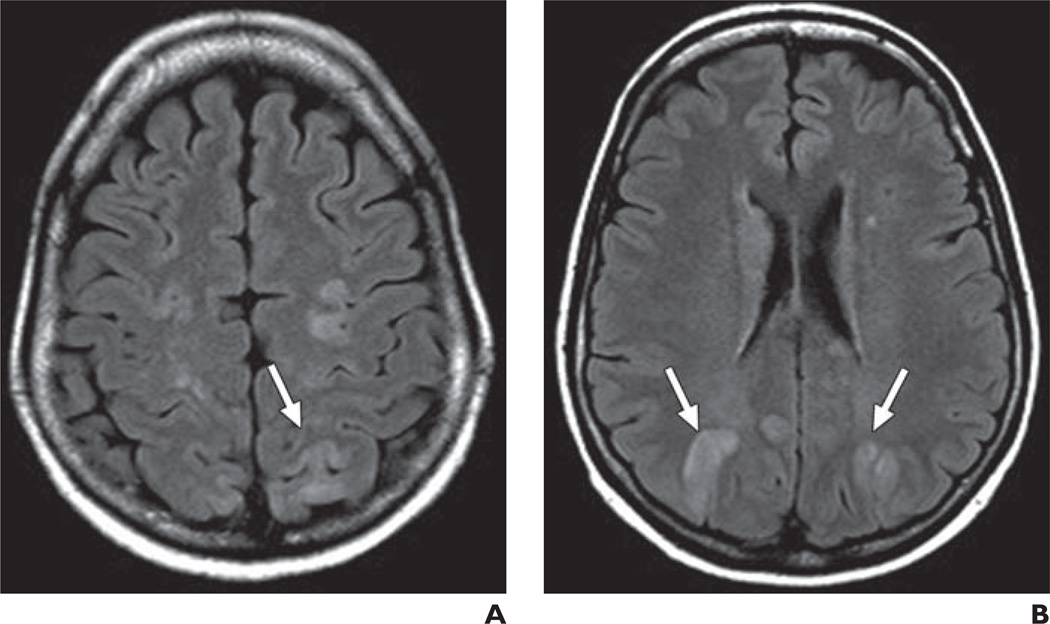
Progressive reversible encephalopathy syndrome (PRES) (Fig. 5) is an uncommon complication (incidence < 0.1%) of VEGF/VEGFR pathway therapy that has been reported with bevacizumab, sorafenib, and sunitinib [21–23]. It is thought to arise from disruption in VEGF signaling within cerebrovascular endothelial cells, leading to impaired cerebrovascular auto-regulation [23]. PRES has a preference for the posterior circulation, presumably because cerebrovascular autoregulation in the posterior circulation is not as robust as in the anterior circulation. On MRI, affected patients manifest T2-hyperintense lesions in the posterior circulation, mostly involving the subcortical white matter but with occasional involvement of the overlying cortex. Lesions can show enhancement after contrast administration because of breakdown of the blood-brain barrier.
Fig. 5.
57-year-old woman with non-small cell lung cancer who presented with seizure after two cycles of carboplatin, paclitaxel, and bevacizumab.
A and B, MR images through centrum semiovale (A) and at level of lateral ventricles (B)show bilateral subcortical white matter FLAIR hyperintensity, primarily located within posterior circulation (arrows). Lesions resolved after discontinuation of bevacizumab.
Osteonecrosis of the Jaw
Osteonecrosis of the jaw is an established complication of bisphosphonates and other bone modifying agents often used in patients with solid malignancies [24]. Some retrospective studies have suggested that agents targeting VEGF/VEGFR, including bevacizumab and sunitinib, may increase the risk for osteonecrosis of the jaw when administered in combination with bone modifying agents [25, 26]. The proposed mechanism involves impaired vascular repair superimposed on suppressed osteoclast activity leading to avascular necrosis [27, 28]. Osteonecrosis of the jaw manifests at imaging as osseous sclerosis with loss of corticomedullary differentiation followed by periosteal reaction, cortical erosion, and bony sequestra in advanced stages of the disease [29].
Pancreatitis
Acute pancreatitis has been reported as a rare complication of sorafenib, sunitinib, and pazopanib, although asymptomatic elevations of serum amylase and lipase levels are more common and may occur in up to 50% of patients treated with VEGF/VEGFR pathway tyrosine kinase inhibitors [12, 30, 31]. Investigators have postulated that drug-induced microvascular ischemia may predispose to pancreatic inflammation, either directly or via sphincter of Oddi dysfunction [32].
Hepatic Steatosis and Hepatitis
Case reports have described hepatic steatosis and hepatitis occurring in patients receiving bevacizumab and pazopanib. Given that hepatic steatosis may progress to steatohepatitis, recognition of progressing fatty liver should be correlated with serum liver function test levels [12].
Acalculous Cholecystitis
Case reports have identified sporadic instances of acalculous cholecystitis occurring in the setting of sunitinib treatment [33, 34]. Investigators have postulated a possible mechanism involving drug-induced local endothelial injury leading to gallbladder ischemia [12].
Agents Targeting KIT
Agents targeting KIT (stem cell factor receptor) include the tyrosine kinase inhibitors imatinib (Gleevec, Novartis Pharmaceuticals) and sunitinib (Sutent). Both agents are indicated for treatment of GIST. Imatinib is also indicated for dermatofibrosarcoma protuberans and for multiple hematologic malignancies.
Fluid Retention
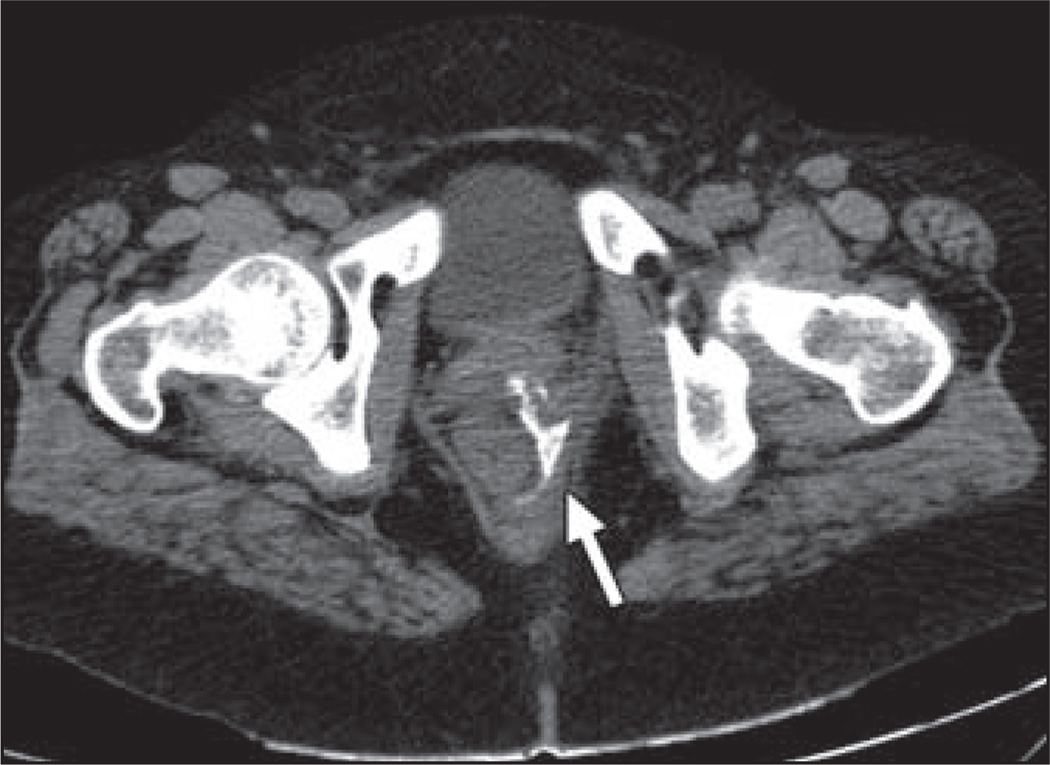
Fluid retention and systemic body edema have been identified as important complications of KIT pathway agents. The mechanism is unclear but may involve cross-inhibition of the platelet-derived growth factor receptor with resultant impaired regulation of interstitial fluid pressure [35]. Mild peripheral and periorbital edema is the most common pattern, but central fluid retention may also occur in 1–3% of patients, with imaging manifestations including pleural or pericardial effusions, pulmonary edema, ascites, and anasarca. A key point for diagnostic imaging is that new ascites or pleural effusions should not necessarily be interpreted as a sign of disease progression when tumor burden is otherwise stable or decreasing on KIT-targeting agents [36] (Fig. 6).
Fig. 6.
71-year-old woman with gastric gastrointestinal stromal tumor being treated with imatinib.
A, Baseline CT scan shows large heterogeneous soft-tissue mass in upper abdomen.
B, Follow-up CT scan 5 months after treatment initiation shows that mass is slightly smaller and lower in attenuation but there is new ascites (arrows) attributable to treatment effect rather than disease progression. Patient also had periorbital edema on clinical examination at this time. Imatinib was continued, and subsequent CT (not shown) showed continued shrinkage of mass with resolution of ascites.
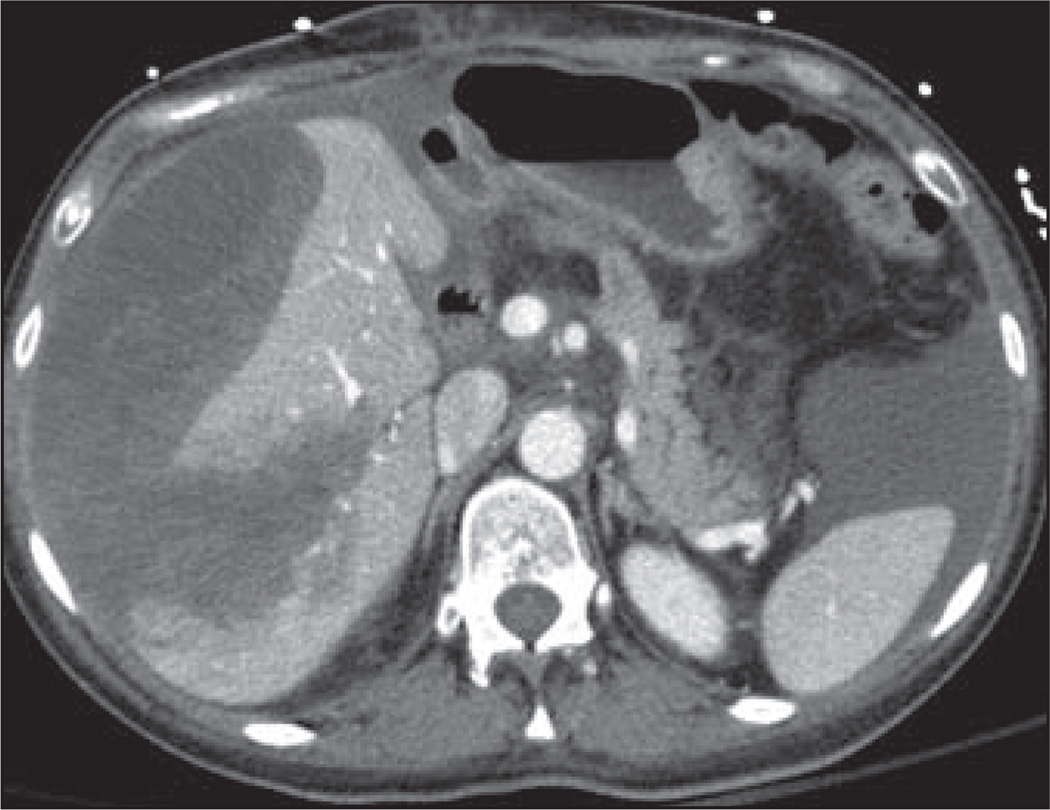
Hemorrhage
Hemorrhage with imatinib is especially prevalent in patients with bulky GISTs, occurring with a frequency of up to 5% [36]. Hemorrhage is predominantly intratumoral and peritumoral, suggesting a mechanism related to treatment-induced tumor necrosis (Fig. 7). Hemoperitoneum can also be observed, probably secondary to hemorrhagic necrosis of exophytic lesions along the bowel wall.
Fig. 7.
42-year-old woman with gastrointestinal stromal tumor metastatic to liver being treated with combined sunitinib/imatinib who presented with abdominal pain, mental status change, and hypotension. Axial contrast-enhanced CT scan shows necrotic hepatic metastases with subcapsular liver hemorrhage and hemoperitoneum that were new from prior imaging performed 2 days earlier. Patient required ICU admission and multiple transfusions.
Hepatic Toxicity
Imatinib causes elevations in serum liver function tests in 15–20% of patients. Although extremely rare, fatal hepatic necrosis has been reported [37]. To our knowledge, there has been no description of imatinib-associated liver necrosis in the radiology literature.
Agents Targeting Anaplastic Lymphoma Kinase
The recently approved tyrosine kinase inhibitor crizotinib (Xalkori, Pfizer) is indicated for treatment of the subset of NSCLC containing a fusion oncogene rearrangement between the echinoderm microtubule-associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) genes.
Severe crizotinib-associated pneumonitis was reported in phase 2 clinical trials with a frequency of 1–2%. All patients presented within 2 months of treatment initiation with dyspnea with or without cough [38] (package insert, Pfizer). The mechanism is unknown, and, to our knowledge, there has been no published description of the imaging findings of crizotinib-associated pneumonitis in the imaging literature.
Agents Targeting RAF
Agents targeting RAF include sorafenib (Nexavar) and vemurafenib (Zelboraf, Genentech), which both interact with RAF via serine-threonine kinase inhibition. Sorafenib indications and toxicities were described earlier under VEGF/VEGFR agents. Vemurafenib is indicated for treatment of melanoma with a BRAF V600E mutation.
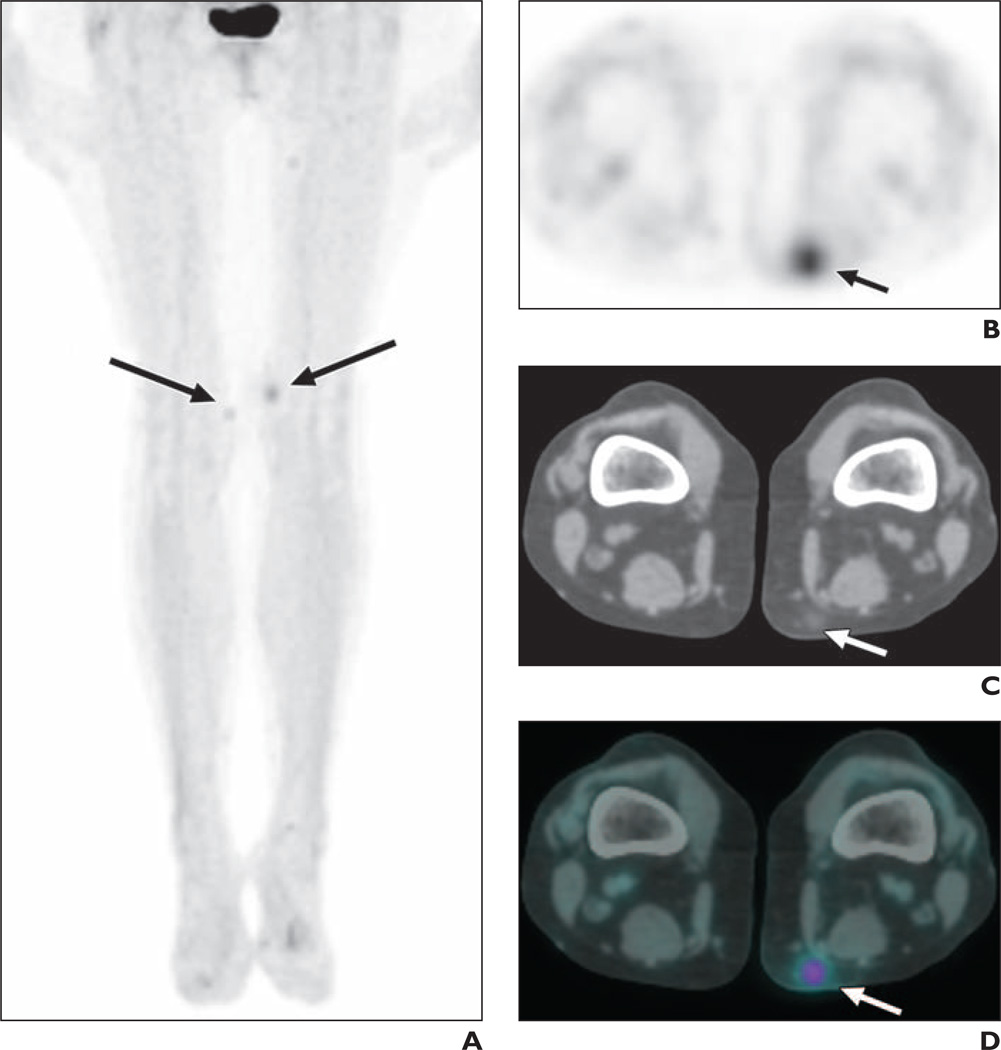
Nodular Panniculitis
Vemurafenib may induce a nodular subcutaneous panniculitis associated with focal radiotracer uptake on 18F-FDG PET (Fig. 8). Given the use of FDG PET in the setting of metastatic melanoma, it is important to include nodular panniculitis as a differential diagnostic consideration when subcutaneous FDG-avid foci in the extremities are observed in patients taking vemurafenib.
Fig. 8.
55-year-old woman with metastatic melanoma being treated with vemurafenib. Patient had developed painful nodular rash on medial thighs.
A, Coronal maximum-intensity-projection image from 18F-FDG PET shows foci of increased radiotracer avidity in both medial thighs (arrows).
B–D, Axial PET (B), axial CT(C), and axial fused PET/CT(D) images show subcutaneous lesion in medial left thigh manifesting as focal FDG avidity in region of subcutaneous soft-tissue nodule with surrounding fat stranding (arrows). Biopsy of this lesion showed perivascular inflammatory infiltrate and occasional neutrophils, compatible with suppurative panniculitis.
Cutaneous Squamous Cell Carcinoma
Vemurafenib is also associated with development of cutaneous squamous cell carcinomas in up to 24% of patients [39] (package insert, Genentech). These lesions are in the differential diagnosis for focal soft-tissue nodularity or FDG avidity on the skin surface. Recent evidence points to a mechanism involving accelerated growth of preexisting but quiescent skin lesions with mutated RAS (upstream of RAF) through paradoxical activation of mitogen-activated protein kinase signaling [40].
Agents Targeting Mammalian Target of Rapamycin
Agents targeting mTOR include the serine-threonine kinases temsirolimus (Torisel, Pfizer) and everolimus (Afinitor, Novartis Pharmaceuticals). Both are indicated for treatment of renal cell carcinoma. Everolimus is also indicated for treatment of hormone receptor-positive breast cancer, pancreatic neuroendocrine tumor, and angiomyolipoma and subependymal giant cell astrocytoma associated with tuberous sclerosis complex.
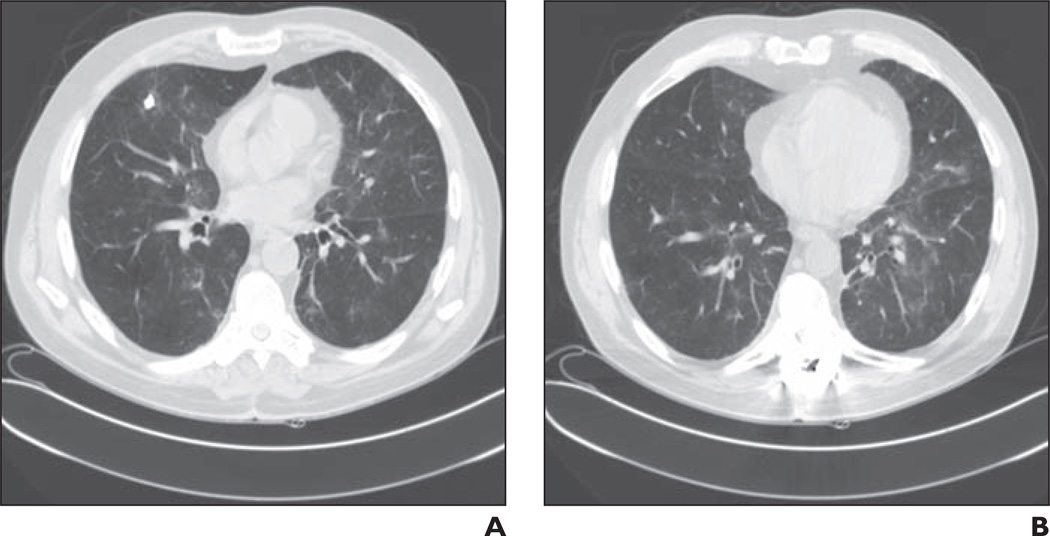
Both temsirolimus and everolimus are associated with interstitial pneumonitis (Fig. 9), with 36% of patients taking temsirolimus having developed pulmonary abnormalities in one series [41]. Drugs in the mTOR inhibitor class possess immunosuppressive properties; the proposed mechanism for interstitial pneumonitis involves either a cell–mediated autoimmune response or a T cell–mediated delayed-type hypersensitivity reaction [42]. Two radiologic patterns have been described: GGO with or without diffuse interstitial disease and parenchymal consolidation [41]. As noted previously, this imaging pattern is nonspecific and can be seen in a variety of pulmonary disease processes as well as with other targeted and conventional chemotherapeutic agents.
Fig. 9.
61-year-old man with metastatic papillary renal cell carcinoma who presented with progressive shortness of breath after six cycles of temsirolimus.
A and B, Chest CT images show patchy bilateral ground-glass opacities. Temsirolimus was discontinued and patient was treated with steroids.
Targeted Immune Modulators
Ipilimumab (Yervoy, Bristol-Myers Squibb) is the prototype agent in a class of immune modulators that function by boosting the host antitumor immune response. Ipilimumab interacts with the cytotoxic T-lymphocyte antigen-4 (CTLA-4) receptor on the surface of host T cells and prevents ligand binding that would otherwise downregulate T cell function; this absence of an inhibitory signal is thought to promote T cell–mediated antitumor activity. Ipilimumab is indicated for the treatment of metastatic melanoma.
Ipilimumab removes an immune system checkpoint, thus enhancing T cell activation and proliferation and predisposing patients to a variety of autoimmune reactions. Reactions may involve any organ system, with the most common clinical manifestations including enterocolitis, hepatitis, dermatitis, neuropathy, and endocrinopathy. Because the presence of autoimmune reactions is a marker for drug activity, it has been proposed that these reactions may correlate with a survival benefit [43, 44].
Ipilimumab-associated grade 3 or 4 enterocolitis occurs in 10–20% of patients. The imaging findings mimic those of inflammatory bowel disease and colitis associated with other chemotherapeutic agents, including colonic wall thickening and pericolonic inflammatory changes [45]. Ipilimumab-associated enterocolitis can result in bowel perforation.
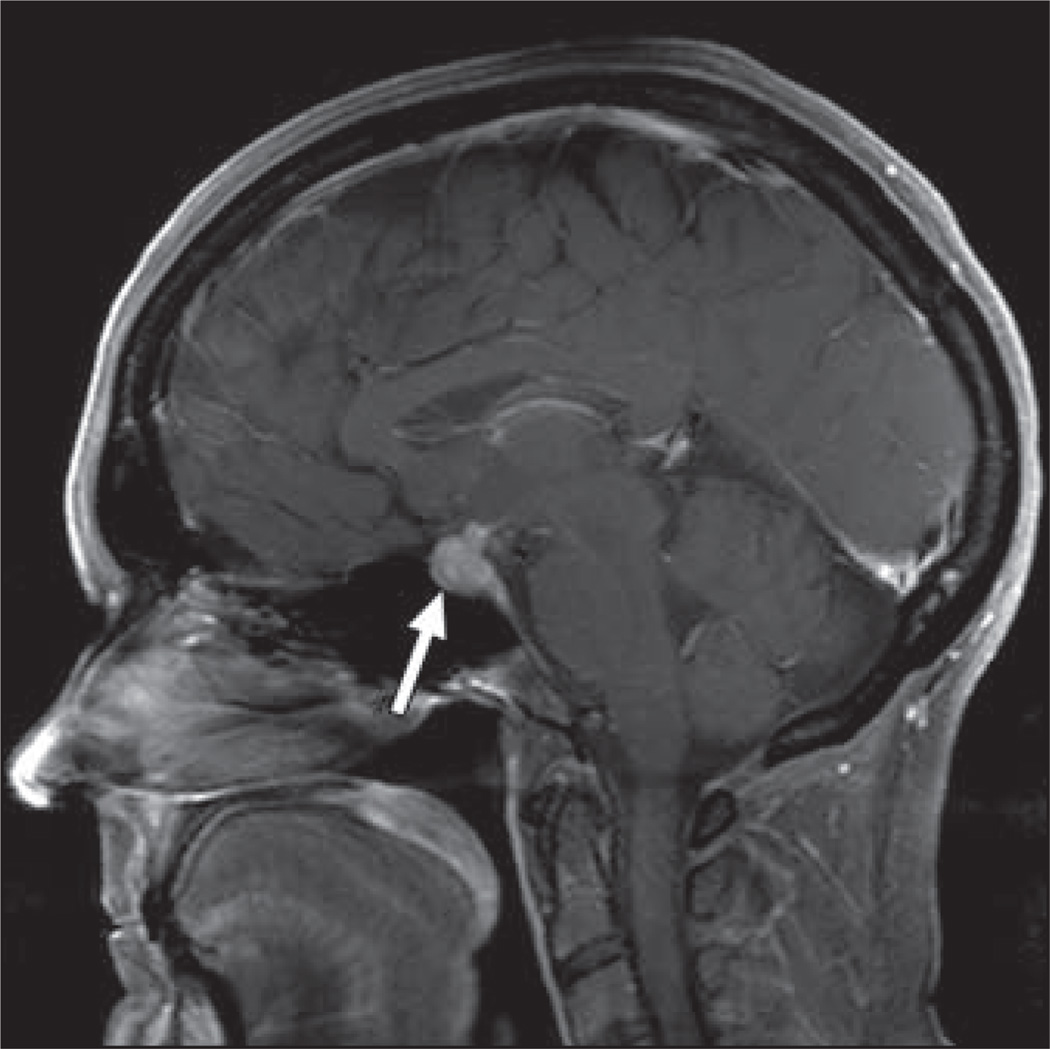
Ipilimumab-associated hypophysitis (Fig. 10) has been reported in up to 5% of patients [44]. Imaging findings include pituitary gland enlargement with homogeneous or heterogeneous enhancement [46]. Findings often resolve on follow-up imaging after discontinuation of the drug.
Fig. 10.
54-year-old woman with metastatic melanoma who presented with headache and visual field defects 3 months after initiation of ipilimumab. Sagittal T1-weighted gadolinium-enhanced MR image shows enlargement and avid enhancement of pituitary gland and stalk, compatible with hypophysitis (arrow). Ipilimumab was discontinued, and findings resolved on follow-up imaging.
It should be noted that ipilimumab treatment can induce benign lymph node enlargement as well as inflammatory soft-tissue changes, including myositis, fasciitis, and retroperitoneal fat haziness [43]. These findings can be problematic when assessing treatment response. A dedicated set of immune-related response criteria—beyond the scope of this article—has been proposed for use with ipilimumab and other similar agents in development [47].
Summary
Targeted therapies are becoming a more important component of medical oncology, and familiarity with targeted drug toxicities will become increasingly important for oncologic imaging. A mechanistic understanding of drug complications can facilitate surveillance for drug toxicities and can prevent the misinterpretation of drug effect as disease progression. A mechanistic framework should also be useful as additional agents under development are gradually integrated into routine care.
References
- 1.Vanderbilt-Ingram Cancer Center (VICC) website. [Accessed January 15, 2012];My cancer genome. www.mycancergenome.org.
- 2.National Cancer Institute (NCI) website. [Accessed December 13, 2011];Active clinical trials for targeted therapies. www.cancer.gov/cancertopics/understandingcancer/targeted-therapies/activeclinicaltrials.
- 3.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:el7. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Aoshiba K, Yokohori N, Nagai A. Epidermal growth factor receptor tyrosine kinase inhibition augments a murine model of pulmonary fibrosis. Cancer Res. 2003;63:5054–5059. [PubMed] [Google Scholar]
- 6.Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24:2549–2556. doi: 10.1200/JCO.2005.04.9866. [DOI] [PubMed] [Google Scholar]
- 7.Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177:1348–1357. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 8.Takano T, Ohe Y, Kusumoto M, et al. Risk factors for interstitial lung disease and predictive factors for tumor response in patients with advanced non-small cell lung cancer treated with gefitinib. Lung Cancer. 2004;45:93–104. doi: 10.1016/j.lungcan.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Endo M, Johkoh T, Kimura K, Yamamoto N. Imaging of gefitinib-related interstitial lung disease: multi-institutional analysis by the West Japan Thoracic Oncology Group. Lung Cancer. 2006;52:135–140. doi: 10.1016/j.lungcan.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Torrisi JM, Schwartz LH, Gollub MJ, Ginsberg MS, Bosl GJ, Hricak H. CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology. 2011;258:41–56. doi: 10.1148/radiol.10092129. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Makrauer FL, Qamar AA, Janne PA, Odze RD. Fulminant hepatic failure secondary to erlotinib. Clin Gastroenterol Hepatol. 2007;5:917–920. doi: 10.1016/j.cgh.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Howard SA, Krajewski KM, Thornton E, et al. Decade of molecular targeted therapy: abdominal manifestations of drug toxicities—what radiologists should know. AJR. 2012;199:58–64. doi: 10.2214/AJR.11.7432. [DOI] [PubMed] [Google Scholar]
- 13.Widakowich C, de Castro G, Jr., de Azambuja E, Dinh P, Awada A. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist. 2007;12:1443–1455. doi: 10.1634/theoncologist.12-12-1443. [DOI] [PubMed] [Google Scholar]
- 14.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 15.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 16.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559–568. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 17.Choi YI, Lee SH, Ahn BK, et al. Intestinal perforation in colorectal cancers treated with bevacizumab (Avastin) Cancer Res Treat. 2008;40:33–35. doi: 10.4143/crt.2008.40.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandler AB, Schiller JH, Gray R, et al. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with carboplatin and paclitaxel plus bevacizumab. J Clin Oncol. 2009;27:1405–1412. doi: 10.1200/JCO.2008.16.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 21.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–982. doi: 10.1056/NEJMc052954. discussion, 982. [DOI] [PubMed] [Google Scholar]
- 22.Govindarajan R, Adusumilli J, Baxter DL, El-Khoueiry A, Harik SI. Reversible posterior leukoencephalopathy syndrome induced by RAF kinase inhibitor BAY 43–9006. J Clin Oncol. 2006;24:e48. doi: 10.1200/JCO.2006.08.4608. [DOI] [PubMed] [Google Scholar]
- 23.Kapiteijn E, Brand A, Kroep J, Gelderblom H. Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukoencephalopathy syndrome. Ann Oncol. 2007;18:1745–1747. doi: 10.1093/annonc/mdm454. [DOI] [PubMed] [Google Scholar]
- 24.Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65:369–376. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Brunello A, Saia G, Bedogni A, Scaglione D, Basso U. Worsening of osteonecrosis of the jaw during treatment with sunitinib in a patient with metastatic renal cell carcinoma. Bone. 2009;44:173–175. doi: 10.1016/j.bone.2008.08.132. [DOI] [PubMed] [Google Scholar]
- 26.Christodoulou C, Pervena A, Klouvas G, et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology. 2009;76:209–211. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 27.Allegra A, Oteri G, Nastro E, et al. Patients with bisphosphonates-associated osteonecrosis of the jaw have reduced circulating endothelial cells. Hematol Oncol. 2007;25:164–169. doi: 10.1002/hon.819. [DOI] [PubMed] [Google Scholar]
- 28.Bertoldo F, Santini D, Lo Cascio V. Bisphosphonates and osteomyelitis of the jaw: a pathogenic puzzle. Nat Clin Pract Oncol. 2007;4:711–721. doi: 10.1038/ncponc1000. [DOI] [PubMed] [Google Scholar]
- 29.Morag Y, Morag-Hezroni M, Jamadar DA, et al. Bisphosphonate-related osteonecrosis of the jaw: a pictorial review. RadioGraphics. 2009;29:1971–1984. doi: 10.1148/rg.297095050. [DOI] [PubMed] [Google Scholar]
- 30.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 32.Pezzilli R, Corinaldesi R, Morselli-Labate AM. Tyrosine kinase inhibitors and acute pancreatitis. JOP. 2010;11:291–293. [PubMed] [Google Scholar]
- 33.de Lima Lopes G, Jr, Rocha Lima CM. Emphysematous cholecystitis in a patient with gastrointestinal stromal tumor treated with sunitinib. Pharmacotherapy. 2007;27:775–777. doi: 10.1592/phco.27.5.775. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Abuin G, Karam AA, Mezzadri NA, Bas CA. Acalculous cholecystitis in a patient with metastatic renal cell carcinoma treated with sunitinib. Clin Genitourin Cancer. 2009;7:62–63. doi: 10.3816/CGC.2009.n.011. [DOI] [PubMed] [Google Scholar]
- 35.Pietras K, Ostman A, Sjoquist M, et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases trans-capillary transport in tumors. Cancer Res. 2001;61:2929–2934. [PubMed] [Google Scholar]
- 36.Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. RadioGraphics. 2006;26:481–495. doi: 10.1148/rg.262055097. [DOI] [PubMed] [Google Scholar]
- 37.Cross TJ, Bagot C, Portmann B, Wendon J, Gillett D. Imatinib mesylate as a cause of acute liver failure. Am J Hematol. 2006;81:189–192. doi: 10.1002/ajh.20486. [DOI] [PubMed] [Google Scholar]
- 38.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duran I, Siu LL, Oza AM, et al. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer. 2006;42:1875–1880. doi: 10.1016/j.ejca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Pham PT, Pham PC, Danovitch GM, et al. Sirolimus-associated pulmonary toxicity. Transplantation. 2004;77:1215–1220. doi: 10.1097/01.tp.0000118413.92211.b6. [DOI] [PubMed] [Google Scholar]
- 43.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR. 2011;197:1293. doi: 10.2214/AJR.10.6198. [web]W992-W1000. [DOI] [PubMed] [Google Scholar]
- 44.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–830. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Regan KN, Jagannathan JP, Ramaiya N, Hodi FS. Radiologic aspects of immune-related tumor response criteria and patterns of immune-related adverse events in patients undergoing ipilimumab therapy. AJR. 2011;197:283. doi: 10.2214/AJR.10.6032. [web]W241-W246. [DOI] [PubMed] [Google Scholar]
- 46.Carpenter KJ, Murtagh RD, Lilienfeld H, Weber J, Murtagh FR. Ipilimumab-induced hypophysitis: MR imaging findings. AJNR. 2009;30:1751–1753. doi: 10.3174/ajnr.A1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]