Abstract
Purpose
This study evaluated the validity of the Patient Specific Functional Scale (PSFS) in patients with upper extremity nerve injury.
Methods
Following Research Ethics Boards (REB) approval, we included English-speaking adults, with greater than 6 months after an upper extremity nerve injury. Patient reported questionnaires included: PSFS, 36-item short-form health survey (SF-36), Disabilities of the Arm, Shoulder and Hand (DASH), McGill Pain Questionnaire, Pain Catastrophizing Scale (PCS) and Pain Disability Index (PDI). Statistical analyses evaluated the relationships among the outcome measures and the independent variables (age, gender, nerve injured, time since injury, work status, worker’s compensation/litigation). Linear regression was used to evaluate the variables that predicted the PSFS.
Results
There were 157 patients (53 women, 104 men); median time since injury of 14 months. The mean ± SD scores were: PSFS 3.1 ± 2.3, DASH 44 ± 22, PCS 16 ± 15, pain intensity 4.2 ± 3.0, pain rating index 13 ± 11, PDI 28.3 ± 17.6 and SF-36 component scores physical (41.8 ± 8.7) mental (45.9 ± 12.6). There were moderate correlations between the PSFS and the DASH, and the SF-36 physical role domain. The PSFS was significantly lower in brachial plexus injuries. The final model explained 20.7 % of the variance and independent variables were DASH, nerve injured and age.
Conclusion
This study provides evidence of construct validity of the PSFS for patients with upper extremity nerve injury. The PSFS is a valid method to assess functional limitations identified by the individual and can be completed in a shorter period of time than the DASH.
Keywords: Validity, Nerve injury, Upper extremity, Self-report function, Outcome
Introduction
Upper extremity nerve injury may result in motor and sensory dysfunction. Patient outcome is often assessed in terms of the physical impairment associated with loss of sensation and motor function and the impact of these impairments on the individual have been less frequently described. More recently, disease specific questionnaires such as the Disabilities of the Arm, Shoulder and Hand (DASH) have been included in the assessment of patients with upper extremity nerve injury and substantial levels of disability have been reported [1, 21, 22].
Self report questionnaires provide an indication of the individual’s estimation of their injury and/or recovery. Generic and disease specific self report questionnaires such as the SF-36 and DASH are often used to assess health status and disability. These types of questionnaires contain items that have been selected by the developers as items or tasks that are important to the construct or index being assessed. However, patients may differ in the importance they place on the specific items relative to their activities and lifestyle. Identification of items selected by the patient may provide a more accurate assessment of functional limitations specific to each individual.
The Patient Specific Functional Scale (PSFS) was developed to provide a measure that would assess functional impairment of patient selected items [29]. The PSFS provides the individual with the opportunity to identify specific tasks or activities that are difficult for them to perform and to rank the difficulty on a numeric scale. Good validity and responsiveness to change of this measure have been reported in patients with back pain, neck pain, knee pain and various hand pathologies [7, 10, 17, 25, 29, 36]. This scale has not been previously validated in patients with upper extremity nerve injury. The purpose of this study was to evaluate the validity of the PSFS in patients with traumatic upper extremity nerve injuries. We hypothesized that the PSFS scores would be strongly associated with pain and disability and would be lower (indicating more functional limitations) in patients with more complex brachial plexus nerve injuries.
Methods
Subjects
The study sample included adults who were between 6 months and 10 years following an upper extremity peripheral nerve injury. Patients with an amputation injury, a previous upper motor neuron lesion, or who were unable to understand the questionnaires were excluded from the study. Patient recruitment occurred when the clinical co-ordinator was present in the clinic and was from the University of Toronto Hand Program, Toronto, Ontario, Canada and the Division of Plastic & Reconstructive Surgery, Washington University School of Medicine, St. Louis, Missouri, USA. This study was approved by our institutional and university Research Ethics Boards.
There were 157 patients (53 women, 104 men) with a mean age of 41 ± 16 years, and the median time from injury was 14 months. The nerve injuries included brachial plexus (n = 62), single nerve in the shoulder region (n = 14), median, ulnar or radial nerve (n = 75) and digital nerves (n = 6). The dominant hand was injured in 95 cases and 50 patients reported involvement of workers’ compensation or litigation. At the time of the study, 81 patients were working, 55 were not working and 21 were students, retired or homemakers.
Testing Protocol
Following signed informed consent, all patients completed the questionnaires at one clinic appointment. Demographic data were obtained and each patient was asked to complete these questionnaires; PSFS, DASH, SF-36, McGill Pain Questionnaire Short-Form (MPQ-sf), Pain Catastrophizing Scale (PCS) and Pain Disability Index (PDI). The order of the questionnaires was randomized and computer software was used to generate the randomization schedule.
Patient Specific Functional Scale
The PSFS was developed to assess individual functional status with items that were specifically chosen by the patient [29]. Good validity, reliability and responsiveness have been shown in patients with neck, low back and various hand pathologies [7, 10, 17, 25, 26, 29, 36]. Patients were asked to identify three activities or tasks that they were unable to perform or had difficulty performing (Fig. 1). The degree of difficulty was indicated on a 10 cm visual analog scale (VAS) from 0 to 10; where 0 = unable to perform activity and 10 = able to perform the activity at a pre-injury level. The VAS was measured to indicate a score for each item and the mean value for the three items was recorded as the PSFS score. A lower score indicated more functional limitation.
Fig. 1.
Patient Specific Functional Scale. Each patient selects three items and ranks the difficulty of each item on a 10-cm 0–10 visual analog scale. The mean of the three items is the Patient Specific Functional Scale score
DASH
The DASH is a 30-item questionnaire to assess disability in patients with upper extremity musculoskeletal disorders [2, 13]. Each item is ranked on a 5-point Likert scale and a normalized score was calculated from these responses. A higher DASH score reflects a higher level of disability. Good psychometric properties of validity, reliability and responsiveness have been shown for the DASH [2, 11, 13]. As recommended by the developers of the DASH, missing single item values were replaced by the mean score for that item [2, 13]. In our study, no patient had more than two missing items.
SF-36
The SF-36 was used to assess health status [3, 8, 15, 16, 35]. There are eight domains (physical functioning, role–physical, bodily pain, general health, vitality, social functioning, role–emotional, mental health), and two summary scores for the physical and mental components. Good validity and reliability have been reported for the SF-36 [3, 15, 16, 35]. A higher SF-36 score indicates better health.
Pain Assessment — McGill Pain Questionnaire Short-Form
The MPQ-sf was used to assess pain [18, 19] The Pain Rating Index was calculated as the summation of scores from the selected pain descriptors and pain intensity was measured on a 10 cm VAS which ranged from 0 (no pain) to 10 (worst possible pain).
Pain Catastrophizing Scale
The PCS was designed to assess exaggerated negative thinking relative to the experience of pain [30]. Each item is ranked from 0 (not at all) to 4 (all the time) to indicate how the individual feels about each thought/feeling when in pain and higher scores indicate higher pain catastrophizing [30]. Good validity and reliability have been reported for this measure of pain catastrophizing [6, 9, 23, 24, 34].
Pain Disability Index
The PDI was used to evaluate the impact of pain on activities of daily living [27, 32, 33]. The patient ranks each item from 0 (no disability) to 10 (total disability) and a higher score is indicative of higher pain disability. Good reliability and validity have been reported with the PDI [5, 27, 31–33].
Data Analysis
Data were summarized as follows; for continuous data with means and standard deviations and for categorical data with frequency counts. Correlations were used to assess the relationship between the PSFS and the questionnaire scores (DASH, pain rating index, pain intensity, PCS, PDI, SF-36), patient age and time since injury. The PSFS was compared using t-tests for these independent variables; gender (male vs. female) and workers’ compensation or litigation involvement (yes vs. no). A one-way analysis of variance (ANOVA) was used to compare the PSFS scores between the nerve injured categories (brachial plexus, single shoulder nerve, median/ulnar/radial nerve or digital nerve injuries) and between the work status categories (employed, unemployed or retired/student/homemaker). If a significant main effect was found (p < 0.05), a Tukey’s post hoc analysis was used.
Linear regression analysis (backward manual elimination) was used to evaluate the variables that were associated with the PSFS scores. Using manual backward elimination, removal of variables was based upon the beta coefficient p-value and the final model included those variables with a p-value of 0.1 or less. The sample size of 157 patients provided sufficient power (0.8) for the regression analysis; one main dependent variable (PSFS) and ten patients per predictor variable (fewer than 15 variables in the preliminary model) [20]. Statistical analyses were performed with SPSS (Version 15.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results
Relationship Between PSFS and Disablity, Pain and Patient Factors
As indicated by the mean values, high levels of functional limitations, disability and pain were reported (Table 1). The specific items identified in the PSFS were variable and ranged from fine dexterity items (such as typing, writing, tying shoes, etc.) to overhead activities to sports, work and housekeeping items. The correlation between the PSFS and the DASH scores was moderate (r = −0. 37) indicating that patients with impaired function as assessed by the PSFS had high levels of disability (Table 2). There was a weaker correlational relationship between the PSFS and the PDI, pain intensity and MPQ-sf pain rating index (Table 2). There were significant correlational relationships between the PSFS and the SF-36 role physical domain (r = 0.33) and SF-36 physical component score (r = 0.27) and no statistical relationship with the SF-36 mental component score (r = 0.08, p = 0.35), age (r = −0.09, p = 0.27) or time since injury (r = 0.11, p = 0.18).
Table 1.
Summary of questionnaire scores
| Mean score ± SD | Scale range | |
|---|---|---|
| Patient Specific Functional Scale | 3.1 ± 2.3 | 0–10 |
| DASH Score | 44.1 ± 21.5 | 0–100 |
| McGill Pain Questionnaire | ||
| Pain Rating Index | 13.0 ± 10.8 | 0–45 |
| Pain Intensity | 4.2 ± 3.0 | 0–10 |
| Pain Disability Index | 28.1 ± 17.6 | 0–70 |
| Pain Catastrophizing Scale | 15.3 ± 14.5 | 0–52 |
| SF-36 | ||
| Physical component score | 41.8 ± 8.8 | 0–100 |
| Mental component score | 46.0 ± 12.7 | 0–100 |
Table 2.
Correlational relationship between the PSFS scores and other questionnaires: correlation coefficient (p-value)
| PSFS | DASH | Pain Intensity | McGill PRI | PDI | PCS | SF-36 Role physical | SF-36 physical | SF-36 mental | |
| PSFS | 1 | ||||||||
| DASH | −0.37 (<0.001) | 1 | |||||||
| Pain Intensity | −0.18 (0.01) | 0.51 (<0.001) | 1 | ||||||
| McGill PRI | −0.16 (0.03) | 0.52 (<0.001) | 0.74 (<0.001) | 1 | |||||
| PDI | −0.27 (<0.001) | 0.77 (<0.001) | 0.54 (<0.001) | 0.62 (<0.001) | 1 | ||||
| PCS | −0.11 (0.08) | 0.46 (<0.001) | 0.66 (<0.001) | 0.69 (<0.001) | 0.55 (<0.001) | 1 | |||
| SF-36 Role Physical | 0.33 (<0.001) | −0.69 (<0.001) | −0.43 (<0.001) | −0.41 (<0.001) | −0.67 (<0.001) | −0.38 (<0.001) | 1 | ||
| SF-36 Physical Composite Score | 0.27 (<0.001) | −0.67 (<0.001) | −0.50 (<0.001) | −0.48 (<0.001) | −0.66 (<0.001) | −0.36 (<0.001) | 0.77 (<0.001) | 1 | |
| SF-36 Mental Composite Score | 0.08 (0.17) | −0.35 (<0.001) | −0.37 (<0.001) | −0.44 (<0.001) | −0.48 (<0.001) | −0.53 (<0.001) | 0.44 (<0.001) | −0.09 (0.12) | 1 |
PSFS Patient Specific Functional Scale; DASH Disabilities of the Arm, Shoulder and Hand; PRI Pain Rating Index; PDI Pain Disability Index; PCS Pain Catastrophizing Scale
Analysis of Patient Factors Associated with PSFS
There was no statistically significant difference in the PSFS between genders (male vs. female) or between patients with workers’ compensation or litigation and those with no compensation or litigation (Table 3).
Table 3.
Analyses between patient specific functional scale, DASH and independent variables
| Patient Specific Functional Scale | |
|---|---|
| Mean ± SD | |
| (p value) | |
| Gender | |
| Female | 2.9 ± 2.2 |
| Male | 3.2 ± 2.3 |
| (p = 0.54) | |
| Workers’ compensation or litigation | |
| Yes | 2.6 ± 1.9 |
| No | 3.3 ± 2.4 |
| (p = 0.07) | |
| Dominant Hand Injured | |
| Yes | 2.9 ± 2.1 |
| No | 3.4 ± 2.5 |
| (p = 0.22) | |
| Work status | |
| Working (full or part time) | 3.5 ± 2.2 |
| Unemployed | 2.4 ± 2.2 |
| Homemaker, retired or student | 3.4 ± 2.5 |
| (p = 0.02) | |
| Nerve Injured | |
| Brachial plexus | 2.3 ± 1.8 |
| Single nerve shoulder region | 2.9 ± 2.2 |
| Median/ulnar/radial | 3.5 ± 2.3 |
| Digital | 6.4 ± 3.1 |
| (p < 0.001) | |
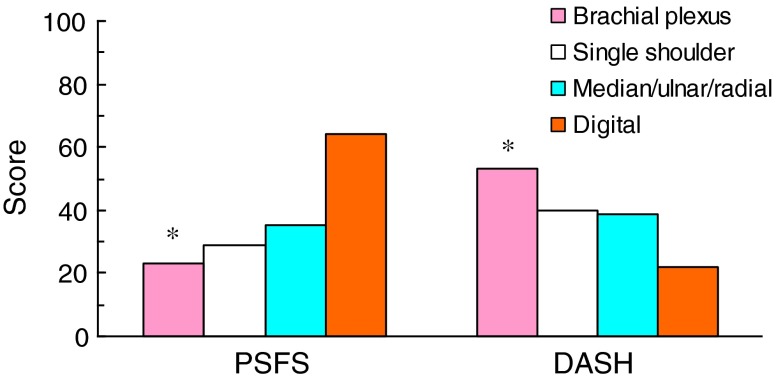
The one-way ANOVA main effect comparing the PSFS between four nerve groups (brachial plexus, single shoulder, median/ulnar/radial, digital) was statistically significant (F = 8.9, df = 3, p < 0.001; Fig. 2). The post hoc analysis revealed that patients with brachial plexus nerve injuries had significantly lower PSFS scores than patients with median/ulnar/radial nerve injuries and digital nerve injuries which indicated more functional impairment in the patients with brachial plexus nerve injuries (p = 0.001). Similarly, for the DASH scores, the one-way ANOVA main effect between four nerve groups was statistically significant (F = 7.9, df = 3, p < 0.001; Fig. 2); brachial plexus nerve injuries reported higher disability than patients with median/ulnar/radial nerve injuries and digital nerve injuries.
Fig. 2.
Relationship between PSFS, DASH and Level of Nerve Injury. The PSFS scores were measured on a 10 cm visual analog scale and these values were converted to millimeters to present the data comparison with the DASH scores (0–100). There was a statistically significant main effect difference with both the DASH and PSFS (p < 0.001). Post hoc analyses revealed that brachial plexus injuries had significantly higher DASH scores and lower PSFS scores compared to digital and median/ulnar/radial injuries
The preliminary linear regression model to investigate the variables that predicted the PSFS included the following independent variables; gender, age, employment status, workers’ compensation/litigation, dominant hand affected, time since injury, nerve injured, pain intensity, MPQ-sf pain rating index, SF-36 physical component score, SF-36 role physical score, DASH, PCS and PDI. The final model accounted for 20.7 % of the variance and independent variables were the DASH (Beta = −0.317, p < 0.001), nerve injured (Beta = 0.223, p = 0.005) and age (Beta = 0.125, p = 0.098).
Discussion
This study provides evidence for the construct validity of the PSFS in patients with upper extremity nerve injury. The PSFS was moderately correlated with the DASH which is a validated measure of upper extremity disability. The DASH scores indicated significantly higher levels of disability in patients with brachial plexus injuries compared to distal nerve injuries. As we hypothesized, the PSFS scores were significantly lower in patients with brachial plexus injuries which indicated a lower level of function in these patients compared to patients with digital nerve injuries and median, ulnar or radial nerve injuries. Because the PSFS is easily completed and scored, it was our impression that the PSFS presented less burden to the patients to complete and examiners to score compared to other self report questionnaires.
Standardized questionnaires such as the DASH provide the opportunity to assess the construct of disability and because standard items are completed by all patients, comparison of scores between patients is meaningful. These types of questionnaires therefore are excellent outcome measures to represent group results and comparisons. Because standard questionnaires contain items that have been carefully selected by the developers, all items may not be relevant to every patient and the importance of items may differ between patients. The PSFS allows the selection of items that are relevant on an individual basis. In our study, the mean PSFS was 3.1 which was lower than previously reported in a group of patients with multiple upper extremity pathologies (mean PSFS 4.4). In our study, the mean DASH score was 44.1, which indicates a high level of upper extremity disability compared to the US normative value (10.1 ± 14.9) previously reported [14]. Our study of patients with nerve injury supports previous studies which have provided evidence of validity of this measure in patients with upper extremity pathologies [12, 17, 28].
In patients with upper extremity nerve injury we found high levels of disability as measured by self-report particularly in patients with brachial plexus nerve injury. There were moderate correlations between the PSFS and the DASH and the SF-36 role physical score and a weaker correlation with the PDI. Gross et al. evaluated the PSFS in workers’ compensation claimants who were diagnosed with musculoskeletal disorders [10]. A moderate correlation was reported between the PSFS and the PDI and SF-36 physical role scale. In patients with a variety of upper extremity pathologies, McMillan et al. [17] reported a mean PSFS 4.4 and in our study of patients with nerve injury, we found higher levels of functional limitations (mean PSFS 3.1). Chatman et al. [4] evaluated patients with lower extremity dysfunction related to the knee and reported moderate correlations with the PSFS and SF-36 domains related to physical function. These relationships were similar in our study of patients with upper extremity nerve injury; moderate correlations with the SF-36 role physical domain and physical component score.
The limitations of this study include a cross-sectional study design and small sample sizes in each group of upper extremity nerves injured. The cross-sectional study design assessed a single point in time at various durations following nerve injury. Because only a single time point was assessed, we were unable to investigate the responsiveness of the PSFS. While our study did show good construct validity for this measure, establishment of responsiveness is an important psychometric construct and future investigation is necessary to assess the responsiveness of the PSFS after upper extremity nerve injury. In this study, we included patients with injuries to the brachial plexus, ulnar, median, radial and digital nerves and single nerve in the shoulder region. Statistical analysis with the ANOVA revealed a significant difference between groups which provided evidence of the validity of the PSFS. However, the small sample sizes in the specific nerve groups limited the sub-analyses that could be performed. Further investigation is necessary to evaluate differences in the PSFS that may exist in specific nerve injuries.
The PSFS provides a method to assess individual functional limitations, provides the opportunity for selection of items that are patient specific and in the future may be used to guide specific therapy bridging the outcomes measure with clinical care. This measure can be completed and scored in a shorter period of time than the DASH and overall patients preferred the PSFS compared to the DASH. For individual assessment, the PSFS will capture the patient specific performance because the patient identifies items specific to their function and for group comparisons, the DASH may be preferable because standard items are assessed in a composite normalized score. We believe that the PSFS may be used in conjunction with the DASH and both questionnaires are useful for assessment of upper extremity functional limitations and disability.
Acknowledgments
Christine Novak was supported by a Canadian Institutes of Health Research (CIHR) Doctoral Fellowship Award and is supported in part through IAMGOLD Fellowship. Joel Katz is supported by a CIHR Canada Research Chair in Health Psychology at York University. This research study was supported in part by a Research Award from the American Association for Hand Surgery. This study was presented at the American Society for Surgery of the Hand Annual meeting, Chicago, IL, September 8, 2012.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ahmed-Labib M, Golan JD, Jacques L. Functional outcome of brachial plexus reconstruction after trauma. Neurosurgery. 2007;61(5):1016–23. doi: 10.1227/01.neu.0000303197.87672.31. [DOI] [PubMed] [Google Scholar]
- 2.Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2):128–46. doi: 10.1016/S0894-1130(01)80043-0. [DOI] [PubMed] [Google Scholar]
- 3.Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatman A, Hyams S, Neel J, Binkley J, Stratford P, Schomberg A, et al. The Patient-Specific Functional Scale: measurement properties in patients with knee dysfunction. Phys Ther. 1997;77:820–9. doi: 10.1093/ptj/77.8.820. [DOI] [PubMed] [Google Scholar]
- 5.Chibnall JT, Tait RC. The Pain Disability Index: factor structure and normative data. Arch Phys Med Rehabil. 1994;75(10):1082–6. doi: 10.1016/0003-9993(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 6.Chibnall JT, Tait RC. Confirmatory factor analysis of the Pain Catastrophizing Scale in African American and Caucasian workers' compensation claimants with low back injuries. Pain. 2005;113(3):369–75. doi: 10.1016/j.pain.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JA, Fritz JM, Whitman JM, Palmer JA. The reliability and construct validity of the Neck Disability Index and Patient Specific Functional Scale in patients with cervical radiculopathy. Spine. 2006;31(5):598–602. doi: 10.1097/01.brs.0000201241.90914.22. [DOI] [PubMed] [Google Scholar]
- 8.Garratt AM, Ruta DA, Abdalla MI, Buckingham JK, Russell IT. The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? BMJ. 1993;306(6890):1440–4. doi: 10.1136/bmj.306.6890.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127(4):835–43. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 10.Gross DP, Mattie MC, Asante AK. The Patient-Specific Functional Scale: validity in workers' compensation claimants. Arch Phys Med Rehabil. 2008;89(7):1294–9. doi: 10.1016/j.apmr.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 11.Gummesson C, Atroshi I, Ekdahl C. The Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11–6. doi: 10.1186/1471-2474-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hefford C, Abbot JH, Arnold R, Baxter GD. The patient-specific functional scale: validity, reliability, and responsiveness in patients with upper extremity musculoskeletal problems. J Orthop Sports Phys Ther. 2012;42:56–65. doi: 10.2519/jospt.2012.3953. [DOI] [PubMed] [Google Scholar]
- 13.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) Am J Ind Med. 1996;29(6):602–8. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Hunsaker FG, Cioffi DA, Amadio PC, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments. J Bone Joint Surg. 2002;84-A:208–15. doi: 10.2106/00004623-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res. 1994;3(1):7–12. doi: 10.1007/BF00647843. [DOI] [PubMed] [Google Scholar]
- 16.McHorney CA, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 17.McMillan CR, Binhammer PA. Which outcome measure is the best? Evaluating responsiveness of the Disabilities of the Arm, Shoulder and Hand questionnaire, the Michigan Hand Questionnaire and the Patient-Specific Functional Scale following hand and wrist surgery. Hand. 2009;4(3):311–8. doi: 10.1007/s11552-009-9167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–99. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 19.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 20.Norman GR, Streiner DL. Biostatistics: The bare essentials. 2. Hamilton, B.C.: Decker Inc.; 2000. [Google Scholar]
- 21.Novak CB, Anastakis DJ, Beaton DE, Katz J. Patient reported outcome following peripheral nerve injury. J Hand Surg. 2009;34A(2):281–7. doi: 10.1016/j.jhsa.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Novak CB, Anastakis DJ, Beaton DE, Mackinnon SE, Katz J. Biomedical and psychosocial factors associated with disability after peripheral nerve injury. J Bone Joint Surg. 2011;93A(10):929–36. doi: 10.2106/JBJS.J.00110. [DOI] [PubMed] [Google Scholar]
- 23.Osman A, Barrios FX, Gituerrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–65. doi: 10.1023/A:1005548801037. [DOI] [PubMed] [Google Scholar]
- 24.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589–605. doi: 10.1023/A:1025570508954. [DOI] [PubMed] [Google Scholar]
- 25.Pengel LHM, Refshauge KM, Maher CG. Responsiveness for pain, disability and physical impairment outcomes in patients with low back pain. Spine. 2004;29(8):879–83. doi: 10.1097/00007632-200404150-00011. [DOI] [PubMed] [Google Scholar]
- 26.Pietrobon R, Coeytaux RR, Carey TS, Richardson WJ, DeVellis RF. Standard scales for measurement of functional outcome for cervical pain or dysfunction. Spine. 2002;27(5):515–22. doi: 10.1097/00007632-200203010-00012. [DOI] [PubMed] [Google Scholar]
- 27.Pollard CA. Preliminary validity study of the Pain Disability Index. Percept Mot Skills. 1984;59(3):974. doi: 10.2466/pms.1984.59.3.974. [DOI] [PubMed] [Google Scholar]
- 28.Rosengren J, Brodin N. Validity and reliability of the Swedish version of the Patient Specific Functional Scale in patients with treated surgically for carpometacarpal joint osteoarthritis. J Hand Ther. 2012;26(1):53–61. doi: 10.1016/j.jht.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Stratford P, Gill C, Westaway M, Binkley J. Assessing disability and change on individual patients: a report of a patient specific measure. Physiother Can. 1995;47(4):258–62. doi: 10.3138/ptc.47.4.258. [DOI] [Google Scholar]
- 30.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–32. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- 31.Tait RC, Chibnall JT. Factor structure of the Pain Disability Index in Workers' Compensation claimants with low back injuries. Arch Phys Med Rehabil. 2005;86(6):1141–6. doi: 10.1016/j.apmr.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 32.Tait RC, Chibnall JT, Krause SJ. The Pain Disability Index: psychometric properties. Pain. 1990;40(2):171–82. doi: 10.1016/0304-3959(90)90068-O. [DOI] [PubMed] [Google Scholar]
- 33.Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehabil. 1987;68(7):438–41. [PubMed] [Google Scholar]
- 34.Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96(3):319–24. doi: 10.1016/S0304-3959(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 35.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Westaway M, Stratford P, Binkley J. The Patient-Specific Functional Scale: validation of its use in persons with neck dysfunction. J Orthop Sports Phys Ther. 1998;27(5):331–8. doi: 10.2519/jospt.1998.27.5.331. [DOI] [PubMed] [Google Scholar]