Abstract
Different diagnostic procedures exist for the detection of bile duct lesions in clinical practice. However, neither retrograde contrast imaging of the bile duct endoscopic retrograde cholangiopancreatogram nor other imaging procedures allow a safe diagnosis of the lesions. Therefore choledochoscopy may be a useful diagnostic procedure in macroscopic assessing lesions of the bile duct. Even if the diagnostic sensitivity and specificity is not sufficient, first studies suggest an enhanced diagnostic accuracy for choledochoscopy. Since the progress of choledochoscopy has started in the 1970 different improvements were achieved. Meanwhile, the examination can be performed by an examiner and samples can be taken. Image and Resolution quality has improved over the past years, also. The SpyGlass system is a technically advanced cholangioscopic device to provide endoscopic diagnosis in case of inconclusive bile duct findings. Further more, two more lumina allow specific biopsy forceps and optical fibers for electrohydraulic or laser lithotripsy. The most frequent useful insert of SpyGlass in clinical practice are in complex gallstones and bile duct lesions of unclear dignity.
Keywords: Endoscopic retrograde cholangiography, Endoscopic choledochoscopy, SpyGlass Direct Visualization SystemTM, “Mother-baby” endoscope technique, Gallstones, Bile duct lesions
Core tip: To date, technical restrictions of endoscopic retrograde cholangiopan-creatogram may explain the insufficient sensitivity of diagnostics when biliary changes are suspected. Therefore choledochoscopy may be a direct diagnostic procedure to help. SpyGlassTM is a technically advanced cholangioscopy system facilitating diagnostics in the bile duct due to its single-operator feature. Different studies reported a clearly enhance diagnostic accuracy for this technique. However, the visualization of bile duct lesions itself is of great value since it offers precise dignity evaluation based on macroscopic criteria.
INTRODUCTION
To date, the prediction of dignity for indistinct bile duct lesions in clinical practice are a difficult endeavour and mean a true diagnostic challenge to all disciplines involved. Neither retrograde contrast imaging of the bile duct endoscopic retrograde cholangiopancreatogram (ERCP) nor other imaging procedures allow for a safe diagnosis of the type if biliary duct findings are inconclusive like the ones experienced with strictures or intraluminal defects[1]. Even with steadily improved endosonography and the use of microprobes enhancing bile duct lesion imaging, a number of limitations set by these investigation methods are still to overcome[2]. Choledochoscopy may be a direct diagnostic procedure to help in macroscopically assessing inconclusive lesions inside the biliary duct system. However, technical means were limited so far as the “mother-baby” system had to be operated by two interventionalists, while confirming the results of malignity-suspicious findings remained a true histological challenge[3-5]. Technical restrictions of the above mentioned procedures may explain the insufficient sensitivity of diagnostics when it comes to biliary changes[6].
SpyGlass is a technically advanced cholangioscopy system facilitating diagnostics in the bile duct due to its single-operator feature. First studies show that the use of SpyGlass may clearly enhance diagnostic accuracy. First of all, cholangioscopy-guided tissue acquisition in the biliary duct is much easier to perform even though diagnostic sensitivity and specificity require further improvement[7].
PROGRESS IN CHOLEDOCHOSCOPY
Since the 1970s, choledochoscopy is used mainly in centers focussing on hepatobiliary diagnostics to macroscopically diagnose bile duct lesions[8]. This procedure directly investigates the biliary tract endoscopically and benefits from directly assessing the mucous membrane so as to help evaluate the dignity of inconclusive lesions in the bile duct[9]. For the first time, this offered diagnostic options superior to other imaging procedures in this region[8,9].
In choledochoscopy, a general distinction is made between percutaneous transhepatic and retrograde endoscopic access using the so-called “mother-baby” endoscope technique[10,11].
With the frequently used and less invasive “mother-baby“ endoscope technique, a thin choledochoscope (“baby scope”) is introduced in the bile duct for ERCP via instrument channel of a duodenoscope (“mother scope”) (Figure 1).
Figure 1.
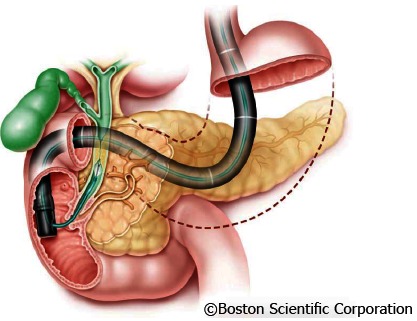
Principle of “Mother-Baby” endoscope technique.
However, a number of limitations using the mother-baby choledochoscopy technique are still to cope with: The first fiber optic choledochoscopies provided a poor image quality with low resolution and poor illumination of the bile duct. The steerability of the microendoscope in only two planes considerably limited the maneuverability in the bile duct. Another clear disadvantage of the mother-baby endoscope technique was the need of two operators required to perform the procedure. However, the greatest detriment of all for a great many years was the fact that tissue acquisition was impossible which limited the use to diagnostic indications.
In the 1980s, a second generation of choledochoscopies was introduced providing a working channel and offering improved maneuverability.
In the late 1990s, first prototypes of video choledochoscopies were tested and first images of staining or virtual chromoendoscopy in the bile duct were presented, yet more to provide evidence of the possible and feasible than to introduce a serious means of routine endoscopy. To date, all available choledochoscopies on the market are fiber optic systems and all reports of high-resolution video choledochoscopies are based on a few prototype case reports only[12-14].
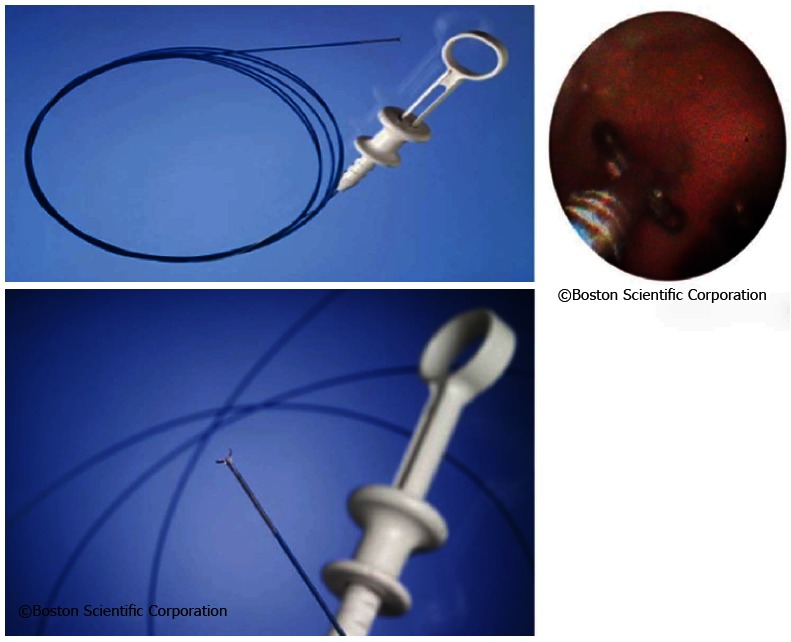
The first single-operator choledochoscopy system was presented in 2005 by Boston Scientific under the name “SpyGlass Direct Visualization System®”. The system is a technically advanced cholangioscopic device to provide endoscopic diagnosis in case of inconclusive bile duct findings[15].
The system does not only without the need of a second operator but also visualizes the bile duct lesions in a way to allow for effective assessment of their dignity (Figure 2). The targeted tissue acquisition performed by the same operator represented another novelty and allowed for further investigation of abnormalities[15].
Figure 2.
Single-operator choledochoscopy system: “SpyGlass Direct Visualization System®”.
SPYGLASS DIRECT VISUALIZATION SYSTEM
The SpyGlass system consists of an integrated platform with a light source, camera, and monitor (Figure 3). Proven fiber optic technique is still used to illuminate the bile duct, yet with improved resolution to optimize bile duct visualization. An advanced steering system of the 10 Fr cholangioscopy catheter to be attached to the duodenoscope has been re-designed and eliminates the need for a second operator to handle the choledochoscope[15,16]. This steering unit with its two steering wheels provides steering options in four planes, comparable to standard endoscopes (Figure 4).
Figure 3.
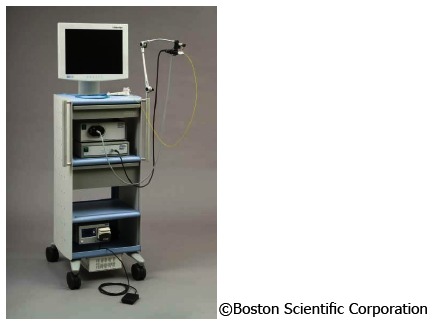
SpyGlass system as integrated platform with light source, camera and monitor.
Figure 4.

Components of the SpyGlass system.
The steering unit is positioned on top of the so-called 10-Fr guiding catheter or SpyScope equipped with four lumina (Figure 5). One lumen is intended for use of the fiber optic system to be advanced to the SpyScope’s end. The fiber optic system consists of a coherent bundle of optical fibers surrounded by light fibers representing the system’s most fragile component. Two more lumina are used for irrigation and a fourth one serves as the working channel for the specific biopsy forceps.
Figure 5.
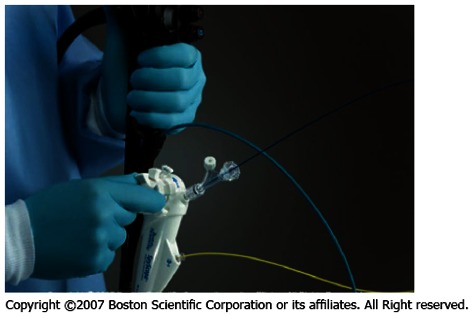
SpyScope.
The SpyScope itself is advanced into the bile duct similar to the mother-baby technique via duodenoscope working channel. Due to the particular stability of the SpyScope offering optimum protection to the optical glass fibers the sometimes unavoidable angulation may be achieved during introduction into the bile duct when fully activating the Albarran lever. When in the bile duct, mucus or tough bile may be removed via the SpyScope’s two dedicated irrigation channels by foot-activating the irrigation device. The most important access offers a 1.2 mm working channel. A specifically designed biopsy forceps (SpyBite) and also optical fibers for electrohydraulic or laser lithotripsy may thus be introduced into the biliary tract via working channel (Figure 6).
Figure 6.
Re-designed biopsy forceps.
SpyGlass technique
First, the steering unit is attached to the duodenoscope handle. Normally, the guidewire already positioned in the bile duct at the distal end of the guiding catheter is now threaded in to the SpyScope via working channel to ease bile duct intubation using the guiding catheter (SpyScope) and the guidewire as a guide rail. Before advancing the guiding catheter (SpyScope) via duodenoscope working channel to intubate the bile duct the optical fiber should be advanced to the tip with care through a suitable working channel. Self-explanatory symbols pointing to the correct access support the process.
Having reached the papilla the Albarran lever is easily used to achieve the required angulation facilitating intubation of the bile duct. With the SpyScope in the bile duct the optical fiber may be carefully advanced via catheter tip to directly inspect the bile duct lumen. Obstructive mucus or tough bile may be removed using the SpyScope’s foot-activated dedicated irrigation device.
INDICATIONS
Among the most frequent clinical uses of the SpyGlass choledochoscopy are complex gallstones and bile duct lesions of unclear dignity (Figure 7).
Figure 7.
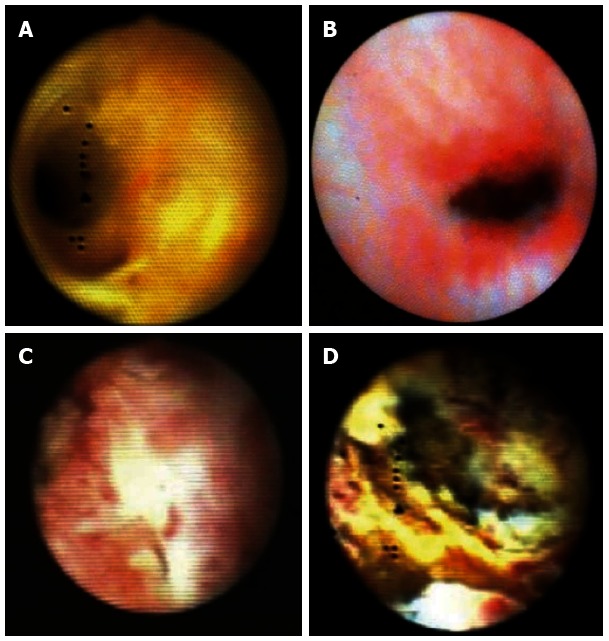
Typical cholangioscopic findings (source: A Hoffman). A: Normale bile duct; B: Inflammation with stricture; C: Cholangiocellular carcinoma with villous like appearance; D: Cholangiocellular carcinoma with ulcers and intraluminal growth.
Use of SpyGlass in bile duct lesions
Diseased bile ducts often are a clinical challenge since diagnostics have their limitations; on the other hand, quick and therapeutically relevant decisions for the patient may urgently be required[17,18]. Sound assessment of the dignity is essential for therapy planning, however often difficult. Especially histological confirmation of malignity-suspicious findings is a key issue gastroenterologists have to cope with[19-21]. Cholangiocarcinoma portend a dismal prognosis which makes an early decision for surgery based on timely diagnosis desirable[17]. Limited diagnostic approaches hardly offer any solution, and patients may not be diagnosed properly until symptomatic with the tumor being in an advanced stage beyond any curative therapy[19]. Brush cytology and endosonographically guided fine needle aspiration biopsy may be the preferred investigation methods to date, yet in almost all the studies the low sensitivity of the method is a serious issue[21-26].
Cytology may provide good specificity which is why false positive cases are rarely found in literature but the low sensitivity of about 50% remains a key problem of this method[21-26].
The golden standard when diagnosing bile duct diseases remains to be ERCP[27]. Using ERCP provides good imaging of the bile duct anatomy including any pathological changes such as strictures ad intraluminal filling defects. However, they might be insufficient, especially in early stages, to make definitive therapy decisions.
Special risk populations e.g., patients with a primary sclerosing cholangitis (PSC), have an increased carcinoma risk due to years of chronic bile duct inflammation[28].
Checks on a regular basis are supposed to detect in a timely manner carcinomatous prestages especially in such patient collective with multiple bile duct changes, yet the problem of safe differentiation between inflammatory/benign and dysplastic, potentially malign lesions remains unsolved.
Peroral choledochoscopy as the direct visualization of the bile duct therefore represents an important and interesting enhancement of ERCP[7,29].
Since the introduction of the SpyGlass Direct Visualization System several studies and a number of publications describe a variety of clinical experiences[15] (Table 1). A center point of the publications was the accessibility and macroscopic imaging of suspicious lesions. A current study documents that the sensitivity of macroscopic evaluation using SpyGlass is significantly higher than with ERCP (81% vs 53%)[29]. Another multi-center prospective study with nearly 300 enrolled patients investigated as a primary study endpoint whether there was success in reaching the suspicious lesion and acquiring tissue[7].
Table 1.
Overview about the sensitivity and specificity of SpyGlass
| First author, publication, year | Study design | Patient (n) | Sensitivity for visual diagnosisual diagnosis/biopsy | Specificity for visual diagnosis/biopsy | PPV for visual diagnosis/biopsy | NPV for visual diagnosis/biopsy | Accuracy for biopsy/visual diagnosis |
| Chen[15], 2007 | Prospective study | 35 | 100%/71% | 77%/100% | 70%/100% | 100%/87% | |
| Ramchandani et al, 2012 | Prospective study | 36 | 95%/82% | 79%/82% | 88%/100% | 92%/100% | 89%/82% |
| Hartmann et al, 2012 | Retrospective analysis | 89 | /57% | /100% | /100% | /68% | /78% |
| Chen et al, 2011 | Prospective study | 297 | 77.8%/48.9% | 82%/98% | 80%/100% | 80%/72% | 80%/75% |
| Kalaitzakis et al, 2012 | Retrospective analysis | 141 | 72% | 97% | 93% | 86% | 88% |
PPV: Positive predictive value; NPV: Negative predictive value.
Secondary study endpoints were the sensitivity and specificity of the cholangioscopically guided biopsies. A total of 96% of the biliary strictures were reached endoscopically using the cholangioscopic catheter and provided sufficient visualization. Additional tissue acquisition was possible in 88% of the cases[7].
In his pilot study, researchers was able to clearly show in 35 patients that SpyGlass not only ensures reaching the lesions but also allows for sufficient macroscopic evaluation of findings with a sensitivity of 100% and specificity of 77%. In an additional SpyGlass-guided biopsy a sensitivity of 71% and specificity of 100% were achieved, both significantly superior to brush cytology results[30].
The most frequently expressed criticism with this method is that sensitivity of the cholangioscopically guided tissue acquisition is low; in some papers it even had to be adjusted downwards. To be stressed are quantity and quality of the acquired tissue frequently considered insufficient by pathologists. Grounds may be the too small a size of the tissue samples acquired using the SpyBite forceps offering no bigger option. To ensure sufficient amount of tissue for pathological investigation multiple tissue acquisitions (3 to 4 biopsies) from the lesion in question are recommended[15,31]. But apart from the already mentioned histological criteria, macroscopic aspects should not be ignored. For effective differentiation of lesions, their macroscopic appearance in the bile duct is of great importance. It is in fact known that almost all malign changes in the hepatobiliary system are characterized by significant vascularization including tortuous and dilated vessels. In addition, exophytic growth, ulcerations, and being raised are considered further aspects of malignity suspicion allowing for correct diagnosis[32].
In a retrospective study including 129 patients, the initial working diagnosis was modified in 68% of the patients with biliary strictures based on the SpyGlass investigation[33]. The significance of this result cannot be overestimated considering that in as many as 45% of the patients an initial tumor suspicion of the lesion was not confirmed when SpyGlass was used for diagnosis meaning for the individual patient a completely different therapeutic proceeding.
Use of SpyGlass in the treatment of gallstones
Cholecysto- and choledocholithiasis are an important issues in the Western industrialized countries and a main reason for hospitalization due to gastrointestinal complaints.
An estimated 15%-20% of the Caucasian population is supposed to suffer from some sort of gallbladder disease, 15%-20% of which also have stones in their biliary tracts. Normally, ERCP succeeds in removing these stones from the biliary duct system avoiding potential complications such as pancreatitis or cholangitis[34,35]. In some cases, however, stones cannot be removed via traditional ERCP due to the large size of the calculi or their specific anatomy. Unfortunately, the success rate of extracorporeal shock wave lithotripsy with subsequent endoscopic extraction is also very low in these special cases[33-35]. Using the SpyGlass system with its option of a full-fledged working channel in addition to dedicated irrigation, a probe may be advanced under direct visual control until it reaches the stone to perform lithotripsy using short-pulsed laser waves (Nd-YAG-2 laser or Holmium laser) or electrohydraulic waves[36-38]. Direct advancement of the probe to the stone reduces the risk of bleeding or perforation of the bile duct and significantly increases the success rate of stone extraction versus extracorporeal shock wave lithotripsy[39,40] (Figure 8).
Figure 8.
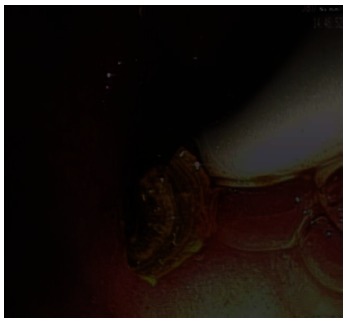
Stone after direct probe-targeted fragmentation via short-pulsed laser waves (holmium laser) (Source: A Hoffman).
Another important aspect is stones overlooked during ERCP. In two studies-one particularly with PSC patients, the other after routine ERCPs-an immediately following SpyGlass procedure diagnosed an initially overlooked 29% and 30% of stones[41,42].
COMPLICATIONS
Based on published data for SpyGlass to date, only a few but not severe procedure related complications are to be assumed. But currently published complications do not differ from those of therapeutic ERCP without accompanying cholangioscopy. Apart from the complications associated with ERCP a complication rate of only 0.3% is assumed whereas it is difficult to differentiate whether the complication was caused by ERCP itself or by the cholangioscopy[33].
The most common complication reported is cholangitis (3%). In some reports ascending cholangitis or cholangitis with intrahepatic abscess, especially after taking biopsies are reported. Some cases of ascending cholangitis, which were only marked by jaundice without fever, but white blood cell elevation or positive blood cultures, developed even some days after SpyGlass examination.
Irrigation should not be excessive when proximal of a stenosis especially with already existing cholangitis since it may significantly increase the risk of bacteremia. But all of the published studies are done by experts in ERCP with a low complication rate in all ERCP related therapeutic procedures. There is no published data about the complication rate during the learning curve of choledochoscopy or the complication rate of trainees in ERCP using SpyGlass.
Among other complications are: drop in blood pressure, abdominal pain, pancreatitis, and bile duct perforation caused by the guidewire.
CONCLUSION
The SpyGlass Direct Visualization System introduces a new type of cholangioscope for endoscopic use. Not only can cholangioscopy now be performed by a single operator but the optimized steering unit enables the user to exactly fix the biliary target lesion and acquire tissue providing true diagnostic benefit. The visualization of bile duct lesions itself is of great value since it offers precise dignity evaluation based on macroscopic criteria. Literature includes more and more reports on the safe and efficient use of the unit in clinical practice. Sceptics of the method mostly criticize the low sensitivity of cholangioscopically guided tissue acquisition. Standardization of the number of biopsies and further development of biopsy forceps may result in the desired enhancement of sensitivity.
Even if histological confirmation of the visual findings may remain difficult using SpyGlass-acquired tissue this new investigation method represents a valuable complement in the diagnostic algorithm of inconclusive bile duct findings in terms of staged diagnostics.
Footnotes
P- Reviewers Tham TCK, Robles-Medranda C S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Harewood GC. Endoscopic tissue diagnosis of cholangiocarcinoma. Curr Opin Gastroenterol. 2008;24:627–630. doi: 10.1097/MOG.0b013e32830bf7e1. [DOI] [PubMed] [Google Scholar]
- 2.Mohamadnejad M, DeWitt JM, Sherman S, LeBlanc JK, Pitt HA, House MG, Jones KJ, Fogel EL, McHenry L, Watkins JL, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71–78. doi: 10.1016/j.gie.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 3.Siddique I, Galati J, Ankoma-Sey V, Wood RP, Ozaki C, Monsour H, Raijman I. The role of choledochoscopy in the diagnosis and management of biliary tract diseases. Gastrointest Endosc. 1999;50:67–73. doi: 10.1016/s0016-5107(99)70347-0. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda Y, Tsuyuguchi T, Sakai Y, Tsuchiya S, Saisyo H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest Endosc. 2005;62:374–382. doi: 10.1016/j.gie.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Seo DW, Lee SK, Yoo KS, Kang GH, Kim MH, Suh DJ, Min YI. Cholangioscopic findings in bile duct tumors. Gastrointest Endosc. 2000;52:630–634. doi: 10.1067/mge.2000.108667. [DOI] [PubMed] [Google Scholar]
- 6.de Bellis M, Sherman S, Fogel EL, Cramer H, Chappo J, McHenry L, Watkins JL, Lehman GA. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2) Gastrointest Endosc. 2002;56:720–730. doi: 10.1067/mge.2002.129219. [DOI] [PubMed] [Google Scholar]
- 7.Neuhaus H, Parsi MA, Binmoeller K, Chen YK. Peroral cholangioscopy for biliary strictures and bile duct stones-an international registry using Spyglass. London: United european Gastroenterology Federation; 2009. [Google Scholar]
- 8.Urakami Y, Seifert E, Butke H. Peroral direct cholangioscopy (PDCS) using routine straight-view endoscope: first report. Endoscopy. 1977;9:27–30. doi: 10.1055/s-0028-1098481. [DOI] [PubMed] [Google Scholar]
- 9.Neuhaus H. Cholangioscopy. Endoscopy. 1994;26:120–125. doi: 10.1055/s-2007-1008938. [DOI] [PubMed] [Google Scholar]
- 10.Neuhaus H, Schumacher B. Miniscopes. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:33–48. doi: 10.1053/bega.1999.0006. [DOI] [PubMed] [Google Scholar]
- 11.Nimura Y, Shionoya S, Hayakawa N, Kamiya J, Kondo S, Yasui A. Value of percutaneous transhepatic cholangioscopy (PTCS) Surg Endosc. 1988;2:213–219. doi: 10.1007/BF00705323. [DOI] [PubMed] [Google Scholar]
- 12.Meenan J, Schoeman M, Rauws E, Huibregtse K. A video baby cholangioscope. Gastrointest Endosc. 1995;42:584–585. doi: 10.1016/s0016-5107(95)70015-3. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman A, Kiesslich R, Bittinger F, Galle PR, Neurath MF. Methylene blue-aided cholangioscopy in patients with biliary strictures: feasibility and outcome analysis. Endoscopy. 2008;40:563–571. doi: 10.1055/s-2007-995688. [DOI] [PubMed] [Google Scholar]
- 14.Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Moriyasu F, Gotoda T. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos) Gastrointest Endosc. 2007;66:730–736. doi: 10.1016/j.gie.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 15.Chen YK. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest Endosc. 2007;65:303–311. doi: 10.1016/j.gie.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Kodama T, Sato H, Horii Y, Tatsumi Y, Uehira H, Imamura Y, Kato K, Koshitani T, Yamane Y, Kashima K. Pancreatoscopy for the next generation: development of the peroral electronic pancreatoscope system. Gastrointest Endosc. 1999;49:366–371. doi: 10.1016/s0016-5107(99)70015-5. [DOI] [PubMed] [Google Scholar]
- 17.Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33–42. doi: 10.1038/ncpgasthep0389. [DOI] [PubMed] [Google Scholar]
- 18.Slattery JM, Sahani DV. What is the current state-of-the-art imaging for detection and staging of cholangiocarcinoma? Oncologist. 2006;11:913–922. doi: 10.1634/theoncologist.11-8-913. [DOI] [PubMed] [Google Scholar]
- 19.Singh P, Patel T. Advances in the diagnosis, evaluation and management of cholangiocarcinoma. Curr Opin Gastroenterol. 2006;22:294–299. doi: 10.1097/01.mog.0000218967.60633.64. [DOI] [PubMed] [Google Scholar]
- 20.Zajaczek JE, Keberle M. [Value of radiological methods in the diagnosis of biliary diseases] Radiologe. 2005;45:976–98, 976-98. doi: 10.1007/s00117-005-1285-z. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad NA, Shah JN, Kochman ML. Endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography imaging for pancreaticobiliary pathology: the gastroenterologist’s perspective. Radiol Clin North Am. 2002;40:1377–1395. doi: 10.1016/s0033-8389(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 22.Mansfield JC, Griffin SM, Wadehra V, Matthewson K. A prospective evaluation of cytology from biliary strictures. Gut. 1997;40:671–677. doi: 10.1136/gut.40.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvaggi SM. Biliary brushing cytology. Cytopathology. 2004;15:74–79. doi: 10.1111/j.1365-2303.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 24.Govil H, Reddy V, Kluskens L, Treaba D, Massarani-Wafai R, Selvaggi S, Gattuso P. Brush cytology of the biliary tract: retrospective study of 278 cases with histopathologic correlation. Diagn Cytopathol. 2002;26:273–277. doi: 10.1002/dc.10098. [DOI] [PubMed] [Google Scholar]
- 25.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–1072. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh V, Bhasin S, Nain CK, Gupta SK, Singh G, Bose SM. Brush cytology in malignant biliary obstruction. Indian J Pathol Microbiol. 2003;46:197–200. [PubMed] [Google Scholar]
- 27.Cohen S, Bacon BR, Berlin JA, Fleischer D, Hecht GA, Loehrer PJ, McNair AE, Mulholland M, Norton NJ, Rabeneck L, et al. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, January 14-16, 2002. Gastrointest Endosc. 2002;56:803–809. doi: 10.1067/mge.2002.129875. [DOI] [PubMed] [Google Scholar]
- 28.Awadallah NS, Chen YK, Piraka C, Antillon MR, Shah RJ. Is there a role for cholangioscopy in patients with primary sclerosing cholangitis? Am J Gastroenterol. 2006;101:284–291. doi: 10.1111/j.1572-0241.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 29.Abstracts of Digestive Disease Week, May 17-22, 2008 and the ASGE (American Society for Gastrointestinal Endoscopy) Postgraduate Course, May 21-22, 2008. San Diego, California, USA. Gastrointest Endosc. 2008;67:AB57–A349. [PubMed] [Google Scholar]
- 30.Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video) Gastrointest Endosc. 2007;65:832–841. doi: 10.1016/j.gie.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 31.Abstracts of Digestive Disease Week and ASGE (American Society for Gastrointestinal Endoscopy) Annual Postgraduate Course, May 2007, Washington, DC, USA. Gastrointest Endosc. 2007;65:AB90–A371. [PubMed] [Google Scholar]
- 32.Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000;52:635–638. doi: 10.1067/mge.2000.108969. [DOI] [PubMed] [Google Scholar]
- 33.Fishman DS, Tarnasky PR, Patel SN, Raijman I. Management of pancreaticobiliary disease using a new intra-ductal endoscope: the Texas experience. World J Gastroenterol. 2009;15:1353–1358. doi: 10.3748/wjg.15.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 35.Everhart JE, Yeh F, Lee ET, Hill MC, Fabsitz R, Howard BV, Welty TK. Prevalence of gallbladder disease in American Indian populations: findings from the Strong Heart Study. Hepatology. 2002;35:1507–1512. doi: 10.1053/jhep.2002.33336. [DOI] [PubMed] [Google Scholar]
- 36.Van Dam J, Sivak MV. Mechanical lithotripsy of large common bile duct stones. Cleve Clin J Med. 1993;60:38–42. doi: 10.3949/ccjm.60.1.38. [DOI] [PubMed] [Google Scholar]
- 37.Cho YD, Cheon YK, Moon JH, Jeong SW, Jang JY, Lee JS, Shim CS. Clinical role of frequency-doubled double-pulsed yttrium aluminum garnet laser technology for removing difficult bile duct stones (with videos) Gastrointest Endosc. 2009;70:684–689. doi: 10.1016/j.gie.2009.03.1170. [DOI] [PubMed] [Google Scholar]
- 38.Moon JH, Cha SW, Ryu CB, Kim YS, Hong SJ, Cheon YK, Cho YD, Kim YS, Lee JS, Lee MS, et al. Endoscopic treatment of retained bile-duct stones by using a balloon catheter for electrohydraulic lithotripsy without cholangioscopy. Gastrointest Endosc. 2004;60:562–566. doi: 10.1016/s0016-5107(04)02012-7. [DOI] [PubMed] [Google Scholar]
- 39.Bratcher J, Kasmin F. Choledochoscopy-assisted intraductal shock wave lithotripsy. Gastrointest Endosc Clin N Am. 2009;19:587–595. doi: 10.1016/j.giec.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Piraka C, Shah RJ, Awadallah NS, Langer DA, Chen YK. Transpapillary cholangioscopy-directed lithotripsy in patients with difficult bile duct stones. Clin Gastroenterol Hepatol. 2007;5:1333–1338. doi: 10.1016/j.cgh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Park MS, Kim TK, Kim KW, Park SW, Lee JK, Kim JS, Lee JH, Kim KA, Kim AY, Kim PN, et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233:234–240. doi: 10.1148/radiol.2331031446. [DOI] [PubMed] [Google Scholar]
- 42.Chen YK, Parsi MA, Binmoeller KF. Peroral cholangioscopy (PC) using a disposable steerable single operator catheter for biliary stone therapy and assess-ment of indeterminate strictures-A multicenter experience using Spyglass. Gastrointest Endosc. 2009;69:AB264–AB265. [Google Scholar]


