Abstract
To elucidate the cortical mechanisms of color vision, we recorded from individual primary visual cortex (V1) neurons in macaque monkeys performing a chromatic detection task. Roughly 30% of the neurons that we encountered were unresponsive at the monkeys' psychophysical detection threshold (PT). The other 70% were responsive at threshold but on average, were slightly less sensitive than the monkey. For these neurons, the relationship between neurometric threshold (NT) and PT was consistent across the four isoluminant color directions tested. A corollary of this result is that NTs were roughly four times lower for stimuli that modulated the long- and middle-wavelength sensitive cones out of phase. Nearly one-half of the neurons that responded to chromatic stimuli at the monkeys' detection threshold also responded to high-contrast luminance modulations, suggesting a role for neurons that are jointly tuned to color and luminance in chromatic detection. Analysis of neuronal contrast-response functions and signal-to-noise ratios yielded no evidence for a special set of “cardinal color directions,” for which V1 neurons are particularly sensitive. We conclude that at detection threshold—as shown previously with high-contrast stimuli—V1 neurons are tuned for a diverse set of color directions and do not segregate naturally into red–green and blue–yellow categories.
Keywords: color vision, visual cortex, detection psychophysics, electrophysiology
understanding vision requires understanding the signal processing that supports visual detection, which is often investigated with two complementary approaches: psychophysics and neurophysiology. Psychophysicists infer a set of theoretical visual “mechanisms” that parsimoniously explain a body of behavioral data, whereas neurophysiologists measure the neuronal responses that are presumably the biological basis of these mechanisms. Forging links between these two bodies of work has greatly improved our understanding of signal processing in the visual system. For example, many aspects of achromatic contrast detection can be explained on the basis of the spatiotemporal contrast sensitivity of neurons in the primary visual cortex (V1) (Boynton et al. 1999; Geisler and Albrecht 1997; Hawken and Parker 1990; Palmer et al. 2007; Tolhurst et al. 1983). Whether the same is true for chromatic contrast detection is unknown.
Substantial psychophysical evidence supports the idea that chromatic detection is mediated by two cardinal mechanisms: a red–green mechanism that receives antagonistic signals from the long- and middle-wavelength sensitive cones (i.e., L−M) and a blue–yellow mechanism that receives strong input from the short-wavelength-sensitive cones in opposition to inputs from the L- and M-cones (Cole et al. 1993; Krauskopf et al. 1982; LeGrand 1949; Nagy et al. 1987; Sankeralli and Mullen 1996, 1997). Under the cardinal mechanisms model (Fig. 1), stimulus modulations in the two mechanism-isolating directions are detected by distinct populations of neurons. Stimuli in intermediate color directions are detected by both populations and could be detectable via probability summation, even if neither population reaches detection threshold individually (Graham 1977; Sachs et al. 1971).
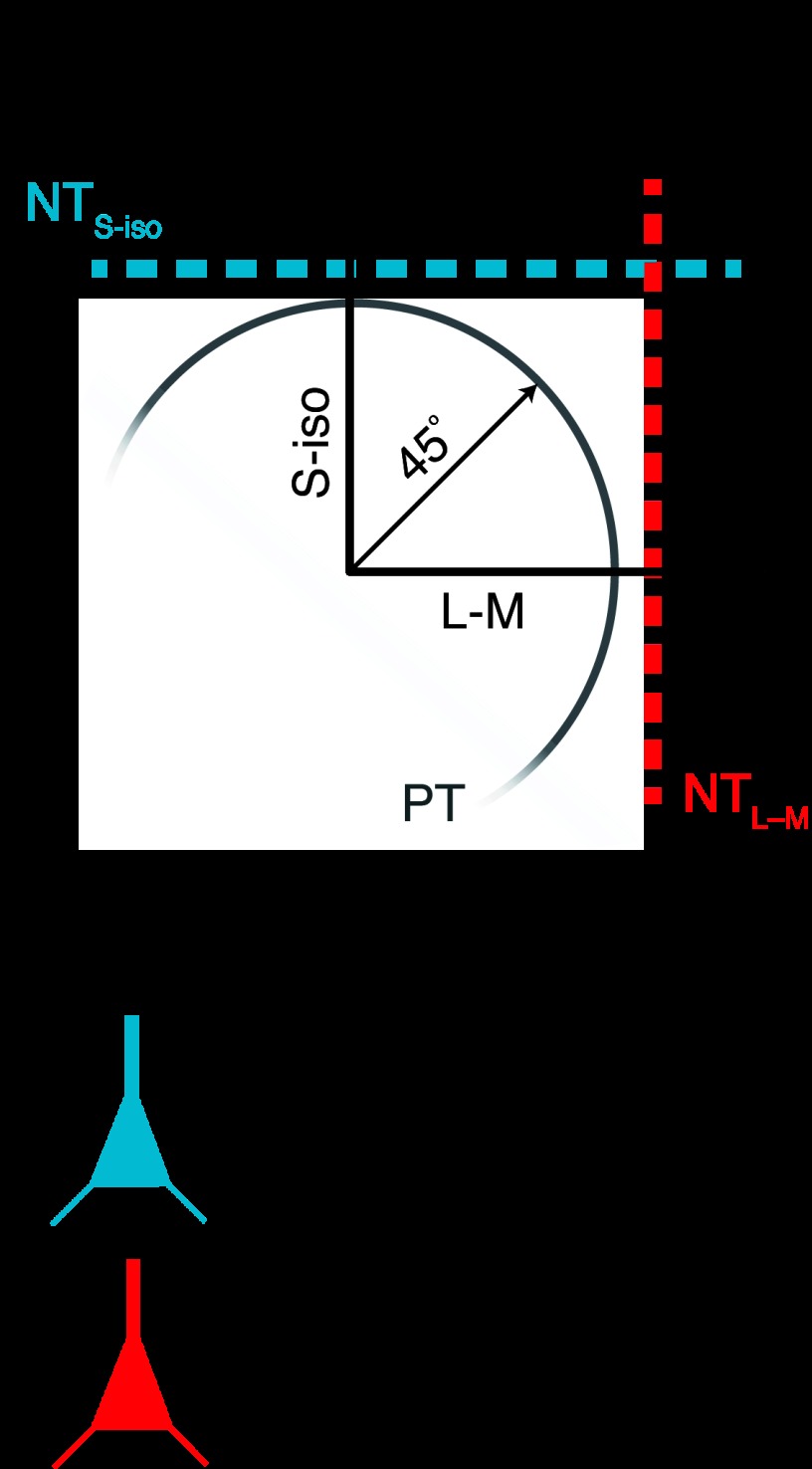
Fig. 1.
Relationship between neuronal and psychophysical detection thresholds (NT and PT, respectively) under the cardinal mechanisms model. A: under the cardinal mechanisms model, primary visual cortex (V1) neurons are tuned to out-of-phase modulations of the long- and middle-wavelength sensitive cones (i.e., L−M) or to modulations of the short-wavelength sensitive cones (S-iso). The response of a neuron to intermediate stimuli is determined by the projection of the stimulus onto its preferred cardinal color direction. Thus NTs can be represented by lines in the isoluminant plane (red and blue dashed lines for a L−M and S-cone tuned neuron, respectively). PT is assumed to result from pooling across all responding neurons and is lower than the NT in either of the cardinal color directions. Due to probability summation, PTs trace out an arc in the interior of the square formed by the cardinal NTs. B: under the cardinal model, individual V1 neurons respond robustly to 1 of the 2 cardinal color directions but weakly to the intermediate color directions.
We asked whether signals measured in V1 at a psychophysical detection threshold (PT) are consistent with the cardinal mechanisms model. Although V1 neurons are not tuned to the cardinal color directions when tested with high-contrast stimuli (Horwitz et al. 2007; Johnson et al. 2004; Lennie et al. 1990; Solomon and Lennie 2005), the responses of V1 neurons have not been measured previously at chromatic detection threshold, and nonlinearities in neuronal responses to high-contrast stimuli complicate extrapolations to a low-contrast regime (Conway and Livingstone 2006; De Valois et al. 2000; Hanazawa et al. 2000; Horwitz et al. 2005; Horwitz and Hass 2012; Solomon and Lennie 2005). The possibility remains that near detection threshold, individual V1 neurons are preferentially sensitive to modulations in the cardinal color directions, as would be the case if only L−M or S-cone-dominated lateral geniculate nucleus (LGN) afferents were active at threshold. Such a result would indicate that chromatic detection is mediated by distinct populations of red–green and blue–yellow V1 neurons.
We recorded the responses of V1 neurons to near-threshold chromatic stimuli in awake, behaving monkeys to test a prediction of the cardinal mechanisms model: responses of individual neurons to intermediate colors should be determined by the magnitude of the stimulus component in the preferred cardinal color direction. This was the case for only the minority of the V1 neurons that we tested. Instead, we found a close relationship between the sensitivity of individual V1 neurons and the monkeys' behavioral sensitivity that generalized across cardinal and intermediate color directions. Additionally, we identified a population of neurons that responded preferentially to chromatic modulations and a population that was equally responsive to chromatic and luminance modulations. These populations were similarly sensitive to near-threshold chromatic modulations—a result that supports a role for neurons that are jointly tuned to color and luminance in chromatic detection. We conclude that the chromatic contrast sensitivity of individual V1 neurons is well matched to that of the monkey and that even at low contrasts, a privileged status for a set of cardinal color directions is not evident.
MATERIALS AND METHODS
Animal preparation.
Two female Macaca mullata participated in this study. Each monkey was surgically implanted with a stabilization head-post and scleral search coil. Neuronal recordings were obtained via surgically implanted recording chambers (Crist Instrument, Hagerstown, MD), which were centered over the posterior occipital cortex, adjacent to the longitudinal fissure. Surgical procedures were performed under sterile conditions using isoflurane or sevoflurane anesthesia. Following surgery, monkeys were administered the following analgesics: buprenorphine (0.01–0.03 mg/kg BID for 2 days) and ketoprofen (5 mg/kg BID for 3 days). All animal procedures, including those related to surgery, housing, and behavioral training, were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the University of Washington's Institutional Animal Care and Use Committee.
Behavioral task and stimuli.
Monkeys were trained to perform the spatial two alternative forced choice (2AFC) detection task, illustrated in Fig. 2A. Monkeys viewed a computer monitor at a distance of 100 cm while seated in a primate chair in an otherwise dark room. Each trial began when the monkey fixed its gaze on a 0.1° black square located at the center of the monitor. Five hundred milliseconds later, a Gabor stimulus appeared in one of two mirror-symmetric locations about the fixation point and disappeared after 666 ms. Following a brief delay (100–600 ms), the fixation point disappeared, and two choice targets appeared. Choice targets were 0.2° black squares positioned between the fixation point and the two possible stimulus locations. Monkeys were given juice rewards for making an eye movement to the choice target located in the direction of the Gabor stimulus. No feedback was given for incorrect choices. All stimuli were presented binocularly. Trials were aborted if at any time before the onset of the choice targets, the monkey's gaze deviated from an electronically defined 1° square window centered on the fixation point. Event-timing and eye-position monitoring was controlled by routines written in REX (Laboratory of Sensorimotor Research, National Eye Institute, Bethesda, MD). Stimuli were generated using custom software written in Matlab (MathWorks, Natick, MA) that used routines from The Psychophysics Toolbox (Brainard 1997).
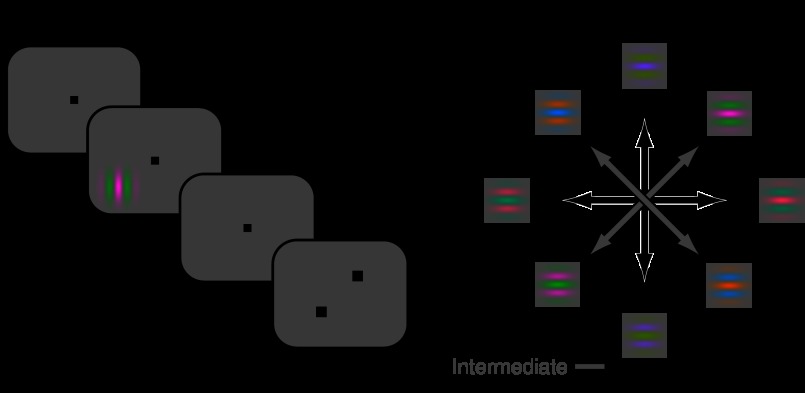
Fig. 2.
A: display geometry. B: event timing of the chromatic contrast detection task. C: the 4 color directions represented as vectors in the isoluminant plane. For each neuron, only 2 color directions were tested during the detection task: the neuron's preferred cardinal and intermediate colors, as identified during an initial characterization procedure.
To avoid high temporal-frequency cues, which might favor detection via luminance-tuned mechanisms, stimulus contrast ramped up and down smoothly (Wandell 1985). Contrast increased linearly for 167 ms, remained constant for 332 ms, and then decreased linearly for 167 ms (Fig. 2B). The sinusoidal component of the Gabor stimulus drifted at 3 Hz, and the Gaussian envelope had a SD of 0.4°. The orientation and spatial frequency of the Gabor stimulus were optimized for each neuron, as described previously (Horwitz and Hass 2012). The time course, Gaussian envelope, and drift rate of the Gabor stimulus were held constant across all psychophysical and neurophysiological experiments.
Stimuli were displayed on a cathode ray tube computer monitor (Sony Trinitron; 760 × 1400 pixels; 75 Hz refresh rate), whose phosphor emission spectra were characterized with a spectroradiometer (PR-650; Photo Research, Chatsworth, CA). The color depth of each channel of the monitor was increased from eight to 14 bits using a digital video signal processor (Bits++; Cambridge Research Systems, Kent, UK) at the expense of spatial resolution; each pixel was twice as wide as it was tall. Gamma correction was performed in software. All stimuli were presented on a uniform gray background (International Commission on Illumination coordinates: x = 0.33, y = 0.33, Y = 100 cd/m2).
Stimuli were generated using the method of silent substitution (Estévez and Spekreijse 1982) and were based on the Stockman et al. (1993) cone fundamentals. For 69 neurons, stimuli were based on the 2° fundamentals, and for 27 neurons, stimuli were based on the 10° fundamentals. Results from these two sets of experiments were similar and thus have been pooled together in this report. Most importantly, the main conclusion of this study—that the signal-to-noise ratio of individual V1 neurons at PT does not depend on color direction—was unaffected by this manipulation. A more thorough analysis of the differences between the two stimulus sets and their impact on our results can be found in discussion. Unless otherwise stated, contrast was defined as the vector norm of the stimulus in L-, M-, and S-cone contrast space
| (1) |
Recording procedures.
Recordings from individual V1 neurons were attained via glass-tipped transdural tungsten microelectrodes (FHC, Bowdoin, ME) with impedances of 1–2 MΩ, measured at 1 kHz. The raw-voltage signal was amplified, band-pass filtered (100 Hz–8 kHz), and digitized at 40 kHz using the Multichannel Acquisition Processor (Plexon, Dallas, TX). Single-unit isolation was assessed by stability in the action potential waveform over the duration of the recording and the absence of interspike intervals < 1 ms.
During an initial characterization procedure, we estimated each neuron's color tuning using circularly apertured, drifting sinusoidal gratings of a preferred orientation, size, and spatial frequency. Chromaticities were selected from in an isoluminant plane defined by two axes (Fig. 2C): one in which L- and M-cones modulated out of phase (L−M or 0°) and one in which only the S-cones were modulated (S-cone isolating or 90°). The plane spanned by L−M and S-cone axes consists of stimuli that are approximately isoluminant for the Stockman et al. (1993) standard observer. Stimuli in this plane are expected to be approximately isoluminant for the monkeys. During the initial characterization procedure, stimuli were presented in four color directions (two cardinal and two intermediate), and contrasts in the L−M and S-cone color directions were roughly matched for detectability (∼13 x threshold). Thus modulations in the intermediate color directions (45° and 135°) produced roughly equal changes in the detectability of their S and L−M components. Had we equated the S and L−M components for cone contrast, the S-cone component would have been subthreshold when the L−M component was clearly visible. The suprathreshold appearance of intermediate colors was distinct from that of the cardinal colors. The 45°/225° intermediate appeared lime/magenta, and the 135°/315° intermediate appeared orange/cyan.
During the detection task, behavioral performance and neuronal responses were measured simultaneously. Stimuli were modulated in the neuron's preferred cardinal and intermediate color directions, and psychophysical thresholds were estimated using the method of constant stimuli. We identified each neuron's preferred cardinal and intermediate color direction on the basis of mean firing rates to the suprathreshold sinusoidal gratings used during the initial characterization procedure. Gabor stimuli were presented at seven contrasts spanning the monkeys' PT. Presentations of 15 stimuli were interleaved randomly across trials: two color directions × seven contrasts + one zero contrast (blank).
Contrast-selection procedure.
Measuring psychometric functions via the method of constant stimuli required a judiciously selected range of contrasts. If contrasts were too high or too low or the contrast range too wide, then the informative (steep) portion of the psychometric function would have been poorly sampled. This problem was exacerbated by the fact that we tailored the spatial frequency and color direction of the stimuli to maximize each neuron's response and thus presumably, its relevance to behavioral performance. Because PTs vary by as much as two orders of magnitude across the color directions and spatial frequencies that we tested (Burr et al. 1994; Mullen 1985), using the same contrast range for each neuron would have undoubtedly led to poor sampling of the psychometric function.
To facilitate selecting appropriate contrast ranges for the detection task, we first measured the behavioral detection threshold for 32 Gabor stimuli (each combination of four color directions and eight spatial frequencies). Thresholds were determined using the QUEST adaptive procedure (Watson and Pelli 1983). Contrast-sensitivity functions in each color direction were fit independently with a second-order polynomial (Fig. 3). The fits were then used to determine the contrasts used during neuronal recordings. Contrasts were typically chosen to span a range from 0.25 to two times the detection threshold estimated by the fit but in rare cases were adjusted manually to ensure adequate sampling.
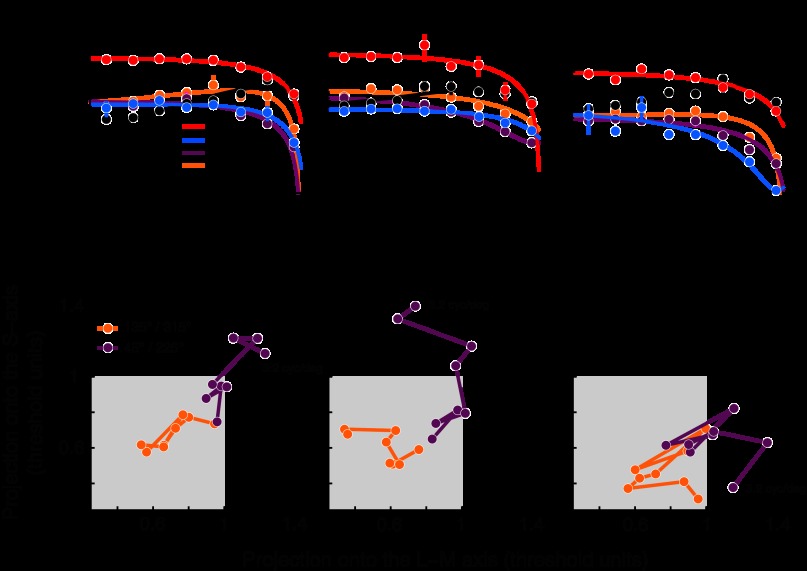
Fig. 3.
A–C: spatial contrast-sensitivity functions for 2 monkeys and 1 human subject in the 4 isoluminant color directions shown in Fig. 2C and the achromatic color direction. Data points represent the average sensitivity (1/threshold) estimated with the QUEST adaptive procedure. Curves are the best-fitting 2nd-order polynomials. Error bars represent ±1 SE. D–F: data from A–C replotted in threshold units along the L−M (abscissa) or S-cone-isolating (ordinate) cardinal color directions. Points that lie on the interior of the shaded squares are qualitatively consistent with the cardinal mechanisms model (i.e., probability summation among the cardinal mechanisms). Individual points are color coded according to color direction and are joined together in ascending order of spatial frequency. The point corresponding to the highest spatial-frequency stimulus in the 45°/225° color direction is labeled in each panel. Cycles/degree (cyc/deg).
To confirm that the monkeys were performing the task near threshold difficulty levels for humans, the first author performed the QUEST version of the 2AFC detection task using the same display that was used in the monkey experiments. Eye position was not measured in these experiments, and psychophysical reports were indicated with button presses. Written, informed consent was acquired from the human observer, and the experimental procedures conformed to the policies of the University of Washington Human Subjects Division, from which Institutional Review Board approval was obtained.
Fitting contrast-response functions.
A linear neuron tuned to a cardinal color direction will respond to modulations in intermediate color directions simply by virtue of the fact that intermediate colors have a component in the preferred cardinal direction. Expressed rigorously, the response of such a neuron depends on the projection of a stimulus onto a vector pointing in the neuron's preferred cardinal color direction. To ask whether V1 neurons behave this way, we measured contrast-response functions (CRFs) in each neuron's preferred cardinal and intermediate color directions and fit them simultaneously with the following model
| (2) |
| (3) |
In this model, CRFs in the two color directions are modeled as horizontally scaled versions of each other. β0, β1, and β2 are fitted parameters that represent the background discharge, contrast gain, and response threshold, respectively. β3 is a fitted parameter that determines the scaling between the cardinal and intermediate CRFs. If the CRF in the cardinal direction is steeper than in the intermediate direction, β3 is <1. If the CRF in the intermediate color direction is steeper than in the cardinal color direction, β3 is >1. Parameters were fit using maximum likelihood estimation, assuming Poisson error. Contrast was defined as the projection of each stimulus onto the preferred cardinal axis for Fig. 4, C and D, and defined as multiples of PT in Fig. 4, F and G.
Fig. 4.
Analysis of contrast-response functions (CRFs) measured during the detection task. A and B: CRFs for 2 example neurons in response to stimulus modulations along their preferred cardinal (black) and intermediate (gray) color directions. Contrast was quantified as the vector norm of the stimulus (Eq. 1). C and D: CRFs for the same neurons in A and B but recalculating contrast as the stimulus projection onto the preferred cardinal axis. Fits are half-squaring functions (Eqs. 2 and 3). Under the cardinal mechanisms model, the 2 CRFs should be identical. E: histogram of scale factors (β3 in Eq. 3) across the population of neurons tested (n = 67). F and G: CRFs for the neurons in A and B but quantifying contrast in units of PT. H: histogram of scale factors (β3 in Eq. 3) after redefining contrast in units of PT. Error bars represent ±1 SE. Triangles in E and F represent geometric means.
The basis of the fit, half-squaring, has been used to describe the CRFs of V1 neurons before (Anzai et al. 1999; Heeger 1992), but we found that the addition of a non-zero baseline firing rate (β0) and a response threshold (β2) improved the quality of many fits. The β3 parameter was included to allow us to test the hypothesis that CRFs in the cardinal and intermediate directions were horizontally scaled versions of each other (i.e., β3 = 1). The model does not include response saturation, because none of the neurons showed signs of saturation over the contrasts that we tested.
We considered quantifying neuronal activity differently for simple and complex V1 neurons (i.e., F1 and F0 components of the response, respectively), but results obtained this way were nearly identical to those obtained using raw spike counts. Although we encountered simple cells with robust F1 modulation during our initial characterization procedure, this F1 modulation in the 2AFC task was obscured by the temporal envelope of the Gabor stimulus (see Fig 2B). We thus quantified neuronal activity as spike counts during the stimulus interval for all analyses.
Quantification of psychometric and neurometric sensitivity.
A primary objective of this study was to quantify chromatic responses in V1 and to relate these signals to behavioral sensitivity. We quantified behavioral sensitivity by fitting a cumulative Weibull function to the psychophysical data
| (4) |
where P(correct) is the probability of a correct detection, and x is the stimulus contrast. The fitted parameters, α and β, correspond to the threshold (i.e., the contrast necessary to support 82% correct detection) and slope of the psychometric function, respectively.
To quantify the reliability of neuronal signals in a way that is directly comparable with behavioral thresholds, we used an ideal observer analysis based on the responses of each neuron (Britten et al. 1992; Palmer et al. 2007; Tolhurst et al. 1983). For each color direction and contrast, we calculated a receiver-operating characteristic (ROC) curve based on the distribution of spike rates in response to a stimulus inside the receptive field (RF; signal) and no stimulus presented (noise). The performance of the ideal observer was calculated as the area under the ROC curve, which gave rise to a single point on a neurometric function (e.g., Fig. 5, A and B).
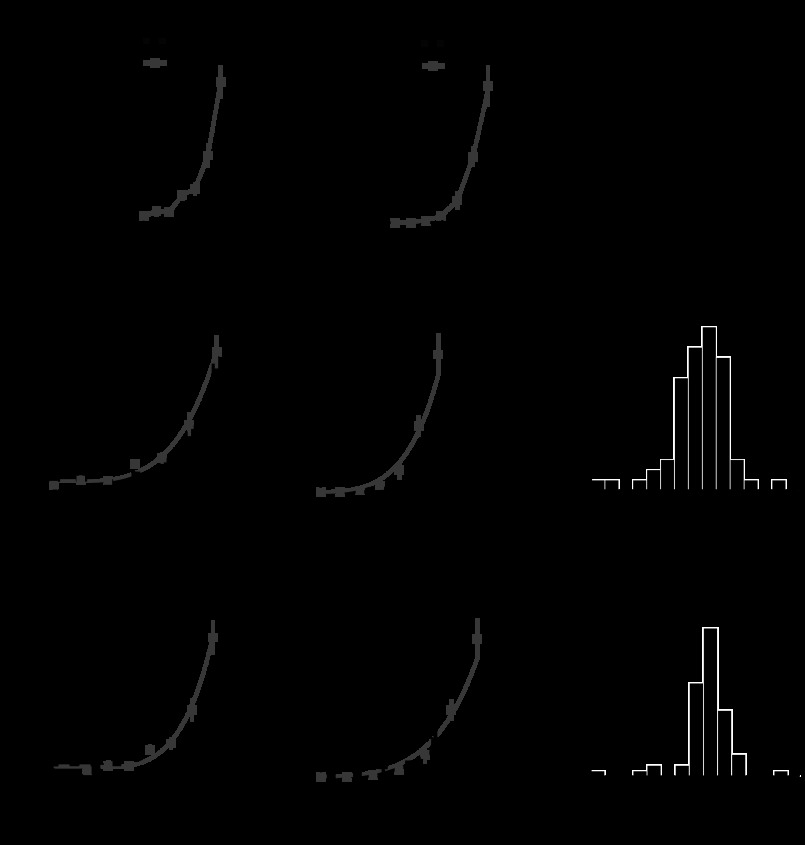
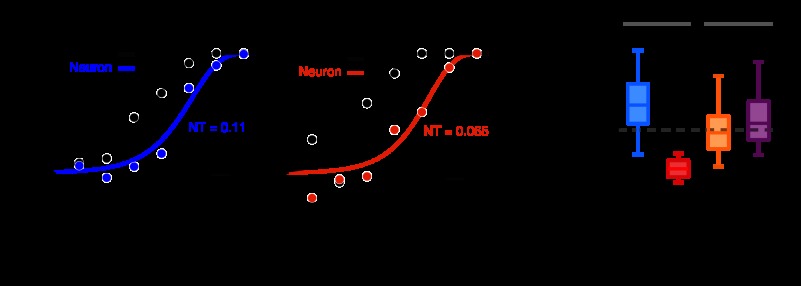
Fig. 5.
Ideal observer analysis of neuronal responses. A and B: example psychometric (black) and neurometric (blue and red) functions from a single neuron in response to modulations in the preferred cardinal (A) and intermediate (B) color directions. Black points represent the raw psychophysical data. Blue and red points represent the performance of an ideal observer with access to the neuronal responses (i.e., area under the receiver-operating characteristic curve). NTs and PTs were obtained from the best-fitting cumulative Weibull distribution (curves). p(Correct), probability of a correct detection. C: distributions of NTs for each color direction. The top and bottom of each box represent upper and lower quartiles, respectively, and the horizontal line inside each box represents the median. Vertical lines indicate the range. The dashed, horizontal line represents the median NT calculated across cells and color directions. The number of neurons tested in each color direction is indicated in parentheses. The total number of cells included in the analysis was 67 [each neuron contributes a NT to 1 cardinal and/or 1 intermediate color direction].
This method assumes a model of detection, in which, on each trial, the ideal observer receives a draw from a signal distribution (which represents the information available from the neuron at the tip of the electrode) and a draw from the noise distribution (which represents the information available from a theoretical but identical neuron whose receptive field is in the mirror-symmetric location opposite the fixation point) (Britten et al. 1992). The ideal observer's choice is based on whichever draw is larger and is correct when the larger draw came from the signal distribution. We quantified the performance of the ideal observer by fitting Eq. 4 to the neurometric data, where the fitted parameters α and β describe the neurometric threshold (NT) and slope, respectively. To compare NTs and PTs directly, we calculated the neurometric-to-psychometric threshold ratio (TR) separately for each color direction. Although we typically tested contrasts within 0.25 to two times the QUEST estimate of detection threshold, TRs varied over a wider range, because PTs and NTs were occasionally at the upper and lower ends of this range.
To assess differences in neuronal sensitivity across color directions statistically, we performed a permutation test based on an F-statistic
| (5) |
where Ȳi is the mean neuronal sensitivity (NT or log(TR)) for the ith color direction, Ȳ is the mean across all color directions and neurons, k is the number of color directions, ni is the number of observations for the ith color direction, Yij is the sensitivity of the jth neuron in the ith color direction, and N is the total number of observations. Next, we calculated F-statistics for 5,000 data sets that were permuted by randomly reassigning neuronal sensitivity values (NT or log(TR)) to color directions subject to two constraints: 1) the two values for any given neuron were reassigned to a cardinal direction and an intermediate direction (consistent with our experimental design), and 2) the number of values within each color direction was the same as in the original data set. The permuted data sets thus maintained the statistical dependence within cells that was observed in the real data set but randomly shuffled the association between neural sensitivity values and color. The P value was calculated as the percentage of F-statistics from permuted data sets that exceeded the observed F-statistic.
Choice probability.
For each neuron, we converted firing rates to z-scores within each stimulus condition (color direction × contrast × stimulus location) and then pooled z-scores according to the choice that the monkey expressed at the end of the trial. We calculated a ROC curve from these two distributions of z-scores and defined choice probability (CP) as the area beneath the curve (Britten et al. 1996): CP = 0.5 when the monkey's choice is unrelated to variations in the neuronal response; CP > 0.5 indicates that the monkey reported more often the stimulus to be inside of the RF on trials in which the firing rate was unusually high. Firing rates were z-scored to remove effects of the color direction and contrast of the stimulus in the RF.
Inclusion criteria.
We recorded from 96 V1 neurons in two monkeys performing the 2AFC chromatic detection task (51 from monkey K; 45 from monkey S). Receptive fields were located between 3° and 8° from the fovea (mean ± SD: 5.14 ± 0.88°). A minimum of 16 trials/color-contrast condition was collected from each neuron. For the purposes of fitting CRFs, we included neurons, for which one or more ROC areas exceeded 0.8 (n = 67). We used the same inclusion criteria for the analysis of TRs. For the analysis of CP, we included only those color/contrast conditions in which the monkey made greater than or equal to five choices to each target.
RESULTS
Preliminary psychophysical results.
To test the appropriate contrast ranges during our neurophysiology experiments, it was critical that we first measure PTs in the cardinal and intermediate color directions and across the range of spatial frequencies preferred by parafoveal V1 neurons. Stimuli were centered at [−5°, −3.5°] or [5°, 3.5°] with respect to the fixation point. Consistent with psychophysical results from humans, contrast-sensitivity functions for isoluminant stimuli were spatially low-pass (Fig. 3, A–C), and sensitivity for achromatic stimuli was band-pass (Mullen 1985). Note that the Gaussian envelope (SD = 0.4°) renders stimuli with spatial frequencies less than ∼1 cycle/degree artifactually similar. Nevertheless, we were able to observe an attenuation of achromatic contrast sensitivity below this value. Spatial frequencies >4 cycles/degree were not tested to avoid complexities introduced by chromatic aberrations. The qualitative and quantitative similarities between the human and monkey observers demonstrate that the monkeys were under behavioral control.
Under the cardinal mechanisms model, stimulus modulations in intermediate color directions are detected on the basis of pooled signals from the cardinal mechanisms. As shown in Fig. 1, this should cause detection thresholds in the intermediate directions to be lower than detection thresholds mediated by either of the cardinal mechanisms individually. We tested this prediction by plotting detection thresholds for intermediate colors in threshold units along the cardinal color directions (Fig. 3, D–F). Thresholds in the 135º/315º intermediate direction were qualitatively consistent with the cardinal mechanisms model at all spatial frequencies tested. Thresholds in the 45º/225º intermediate direction at >1 cycle/degree were inconsistent with the model. These psychophysical results are agnostic to the color tuning of individual neurons—an issue that we turn to next.
Testing the cardinal mechanisms model in V1.
We asked whether individual V1 neurons were tuned to the cardinal directions at PT. For two example V1 neurons (Fig. 4, A and B), responses to the L−M cardinal color direction were greater than responses to the intermediate color direction at every contrast tested. This tuning is consistent with a preference for the cardinal direction, but this interpretation depends critically on how stimulus contrast is defined. Implicit in our definition of contrast (Eq. 1) is the equality of contributions from the L-, M-, and S-cone types. Changing the relative weights on the three cone types alters the contrast values, which in turn, can scale CRFs along the contrast axis.
To determine whether V1 neurons conform to the cardinal mechanisms model, we tested a prediction that it makes: spike counts in intermediate color directions should be determined by the projection of the stimulus onto the neuron's preferred cardinal axis. For example, a neuron tuned to the L−M color direction will respond equally to stimulus modulations in the L−M+S and L−M color directions if their L−M components are equal. To test this prediction, we quantified stimulus contrast as the projection onto the preferred cardinal axis and fit CRFs using a model that represents the difference between the CRFs as a scaling of the contrast axis (Eqs. 2 and 3). If V1 neurons respond like the psychophysical cardinal mechanisms, the two CRFs should overlay each other, and the scale factor that relates cardinal and intermediate CRFs (β3 in Eq. 3) should equal one. The CRFs for some neurons were consistent with the cardinal model (Fig. 4C), but the majority of neurons had CRFs that were inconsistent with the cardinal model (Fig. 4D). β3 was significantly >1 for 82% of the neurons individually (Wald tests, P < 0.05) and across the population [geometric mean = 1.50; t-test on log(β3), P < 0.001; Fig. 4E].
As a control analysis, we randomly partitioned responses to cardinal colors into two groups and then fit these randomized data sets with Eqs. 1 and 2. The average β3 parameter from this control analysis was not significantly different from one [t-test on log(β3), P = 0.24], which demonstrates that the tendency that we observed in the data for β3 > 1 is not a trivial consequence of the model-fitting procedure. We conclude that individual V1 neurons respond more strongly to modulations in intermediate color directions than predicted by the cardinal model.
Comparing CRFs equated for stimulus detectability.
The qualitative similarity between neuronal and behavioral chromatic sensitivity led us to ask whether CRFs in cardinal and intermediate color directions might match quantitatively if contrast were represented in units of detection threshold. We scaled contrasts so that a value of “1” in any color direction was detectable 82% of the time and then re-fit Eqs. 2 and 3 to the data. For the few neurons that were well described by the cardinal model, this rescaling necessarily forced a discrepancy between cardinal and intermediate CRFs (Fig. 4F). For most neurons, however, this manipulation yielded a closer correspondence in the CRFs (e.g., Fig. 4G). The average β3 (Eq. 3) for the entire population was not significantly different from one [geometric mean = 0.97, P = 0.59, t-test on log(β3); Fig. 4H]. We conclude that the CRFs of V1 neurons are more closely related to PTs than to the S and L−M components of cone contrast modulations.
Neurometric sensitivity.
Chromatic detection depends on the amplitude and variability of neural responses to low-contrast chromatic stimulation. To compare neuronal sensitivity across colors, we performed an ideal observer analysis that incorporates measurements of neuronal signal and noise (see materials and methods). Specifically, neuronal sensitivity was quantified as the NT derived by fitting a cumulative Weibull function to the performance of a theoretical ideal observer with access to neuronal responses (Fig. 5, A and B; see materials and methods). Every neuron was tested in two color directions during the detection task, and NTs were calculated for each color direction separately.
An analysis of NTs showed that V1 neurons were preferentially sensitive to some color directions relative to others (permutation test on NTs, P < 0.001; Fig. 5C). NTs in the L−M color direction were lower than in any other color direction and were 4.1 times smaller than in the S-cone-isolating color direction [unpaired t-test, P < 0.001; mean for L−M = 0.029; mean for S-cone isolating = 0.12]. This analysis demonstrates that individual neurons are capable of producing reliable signals to near-threshold chromatic stimuli and that the contrast (as defined by Eq. 1) necessary to evoke a reliable response depends on color direction.
Neurometric-to-psychometric TRs.
To quantify the relationship between neuronal sensitivity and behavioral performance, we calculated neurometric-to-psychometric TRs for each neuron in each color direction tested. A TR of one means that the neuron and monkey are equally sensitive. A TR greater than one means that the monkey is more sensitive than the neuron. On average, monkeys were 1.5 times more sensitive than V1 neurons (geometric mean TR = 1.5; 95% confidence interval [1.38, 1.63]; Fig. 6A). Thirty percent of the neurons were insensitive at the monkey's PT (see materials and methods). Their TRs were undefined and did not contribute to our calculation of the geometric mean TR.
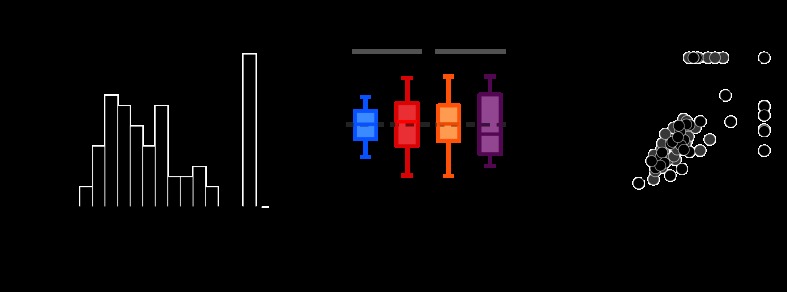
Fig. 6.
Comparison of TRs within and between cells. A: neurometric-to-psychometric TRs across the population of neurons (n = 96). Neurons were tested in 2 color directions but only contribute a single TR to the population histogram (the minimum of the 2). TRs were considered undefined (Und) when the NT could not be measured (no points on the neurometric function > 0.8). The triangle represents the geometric mean. B: distributions of TRs for each color direction across neurons that were responsive at PT. The number of neurons measured in each color direction is indicated in parentheses. The total number of cells included in the analysis was 67 (each neuron contributes a TR to 1 cardinal and/or 1 intermediate color direction). C: TRs measured in each neuron's preferred cardinal and intermediate color direction (black: monkey K, gray: monkey S).
To determine if the relationship between neurometric and psychometric sensitivity depended on color direction, we compared TRs across the four color directions tested. This analysis was necessarily limited to those neurons for which NTs were measureable (see materials and methods). For this subpopulation, the distributions of TRs were highly overlapping (Fig. 6B), and the geometric mean TR did not differ significantly across color direction [permutation test on log(TR), P = 0.42; see materials and methods]. Note that under the cardinal mechanisms model, we would expect TRs to be higher in the intermediate directions than in the cardinal directions (Fig. 1), which we did not observe.
Consistency between neurometric and psychometric sensitivity was also observed within cells. TRs measured in each neuron's preferred cardinal and intermediate color directions were not significantly different (paired t-test, P = 0.60; Fig. 6C). Moreover, the within-cells analysis demonstrated that TRs in the cardinal and intermediate color directions were well correlated (Spearman's r = 0.84, P < 0.01). We conclude that V1 neurons differ widely in their sensitivity relative to the monkey but that the relationship between neuronal and psychophysical sensitivity depends little, if at all, on color direction.
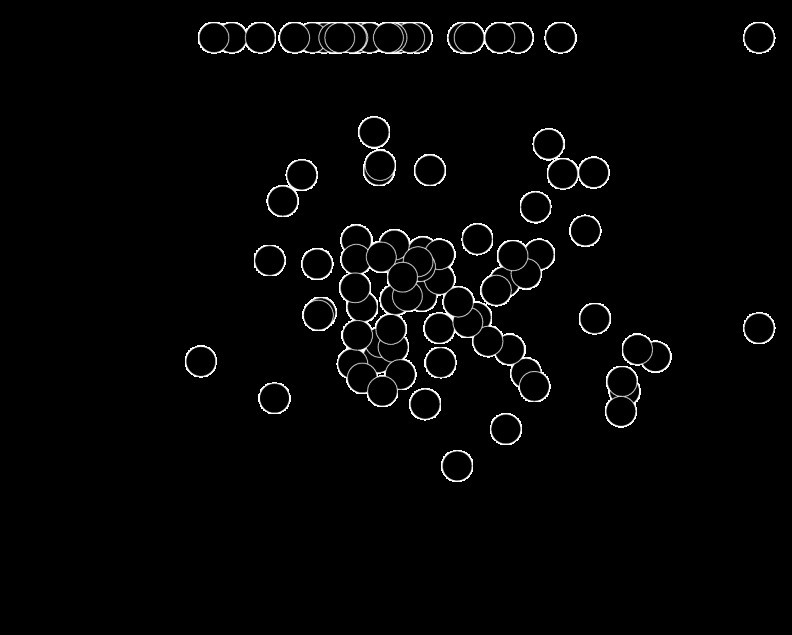
A potential explanation for the consistency of TRs across color directions is that we tested too narrow a range of color directions: each neuron was tested in only two neighboring color directions of the four that we considered. To control for this possibility, we tested 11 neurons in two orthogonal color directions: L−M and S-cone-isolating. Only four of these 11 neurons were sensitive to one of the cardinal color directions and insensitive to the other. The remaining seven neurons were sensitive to both color directions and were often more sensitive than the monkey (Fig. 7A). For these neurons, TRs in response to L−M were slightly smaller than in response to S-cone-isolating stimuli (geometric mean TR for L−M = 0.8 and for S-cone-isolating = 1.05, paired t-test, P = 0.043; Fig. 7B). Thus S-cone-isolating and L−M stimuli produce similarly reliable signals in V1 at the monkeys' detection threshold, both across neurons and within the subpopulation that is sensitive in both color directions.
Fig. 7.
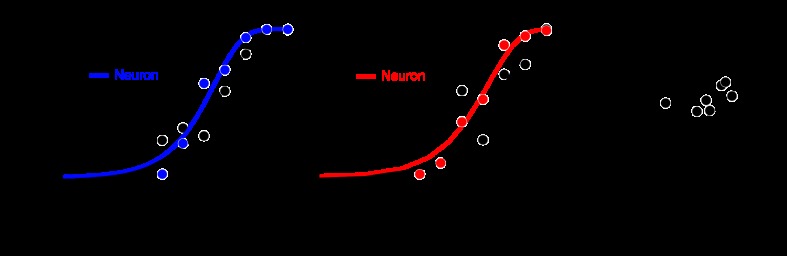
Sensitivity of individual neurons to modulations in the L−M and S-cone-isolating color directions. A: example of psychometric (black) and neurometric (blue or red) performance. This neuron was slightly more sensitive than the monkey in response to modulations in either of the cardinal color directions. B: TRs of the subset of neurons that were tested in the L−M and S-cone-isolating color directions and were responsive at PT to both colors.
Choice probability.
V1 neurons that contribute to chromatic detection must be responsive at the monkeys' chromatic detection threshold; however, the converse is not true: not all chromatically sensitive neurons must contribute to psychophysical contrast detection. To identify V1 neurons that are most likely to contribute to the monkeys' psychophysical judgments, we performed an analysis of CP. This analysis hinges on the logic that neurons that are causally linked to behavior may produce responses that are correlated with the monkeys' behavior on a trial-by-trial basis (Britten et al. 1996; Palmer et al. 2007; but see Nienborg and Cumming 2009).
By this analysis, neuronal responses were correlated with the monkeys' choices only weakly. The average CP was 0.52 ± 0.008 SE, which is slightly but significantly >0.5 (t-test, P < 0.05). Very few individual V1 neurons had significant CPs (n = 4 of 67, Wilcoxon, P < 0.05), and those that did were not obviously unusual in any other way. Specifically, they were not unusually sensitive compared with neurons with insignificant CP [t-test on log(TR), P = 0.44]. Nevertheless, to designate a population of V1 neurons that were the best candidates for contributing to task performance, we extracted the subset of 32 neurons that had a CP > 0.51 (the median value). This subpopulation was neither particularly sensitive [t-test on log(TR), P = 0.71] nor differentially sensitive across color direction [ANOVA on log(TR) F(3,27) = 0.84, P = 0.48]. CP thus appears to provide little leverage into the question of which V1 neurons mediate performance on our task.
Suprathreshold response properties of chromatically sensitive V1 neurons.
The degree to which a V1 neuron participates in color vision has traditionally been inferred from its responses to suprathreshold chromatic stimuli (Johnson et al. 2001; Lennie et al. 1990; Solomon and Lennie 2005), but the extent to which these suprathreshold measurements predict near-threshold sensitivity is unknown.
Following Johnson et al. (2001), we computed a color-sensitivity index (CSI) based on responses to the high-contrast chromatic gratings used in our initial characterization procedure
| (6) |
where FR(Pref Isolum) is the average firing rate in response to the preferred isoluminant stimulus, and FR(L+M) is the average rate in response to a 13% contrast L+M stimulus. Neurons with CSIs ≤ 0.5 were classified as luminance preferring (n = 19). Neurons with CSIs between 0.5 and 2 were classified as color-luminance cells (n = 46), and neurons with CSIs ≥ 2 were classified as color-preferring cells (n = 31). The prevalence of color-preferring neurons in our data set is higher than reported previously (32% vs. 11%) (Conway 2001; Johnson et al. 2001, 2008), and the prevalence of luminance-preferring neurons is lower (20% vs. 60%). This outcome likely reflects our use of four chromatic stimuli (three of which robustly modulate the S-cones) instead of a single red–green isoluminant stimulus. When we redefined color sensitivity as the ratio of responses to the L−M and L+M color directions, the proportion of color-preferring neurons (13%) was closer to those of previous studies (Conway 2001; Johnson et al. 2001, 2008).
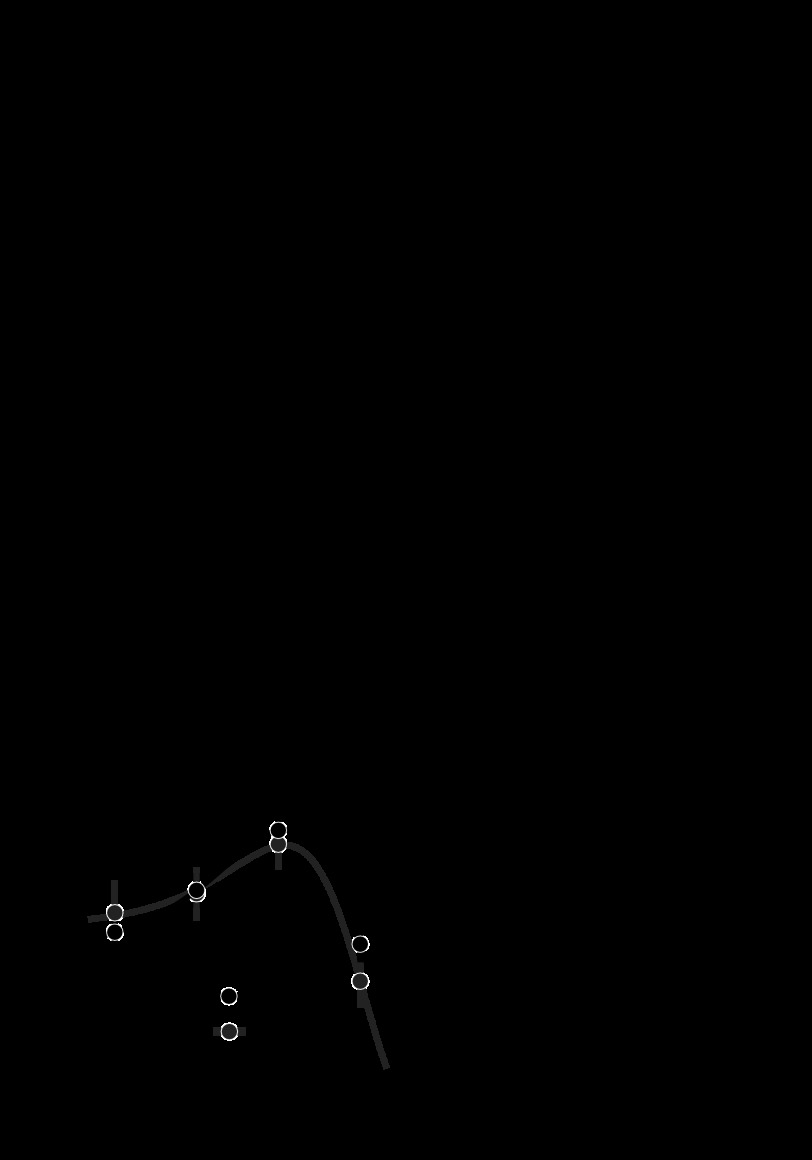
A comparison of CSI and TR (Fig. 8) showed a weak but significant relationship (Spearman's r = −0.28, P = 0.005); neurons that were particularly sensitive during our detection task (i.e., those with the lowest TRs) tended to be color preferring, whereas neurons that were insensitive tended to be color luminance or luminance preferring. As expected, luminance-preferring neurons had higher TRs than color-luminance or color-preferring cells (Wilcoxon tests: P < 0.05), but many (n = 8 of 19) had TR ≤ 3, indicating non-negligible sensitivity to isoluminant modulations. TRs of color-luminance and color-only neurons were statistically indistinguishable (Wilcoxon test, P = 0.17). This result supports a role for color-luminance neurons in chromatic detection and argues against the idea that they can be considered “miscalibrated photometers,” whose sensitivity to chromatic stimuli is small and has no behavioral significance (Billock 1995; Gegenfurtner et al. 1994).
Fig. 8.
The relationship between color-sensitivity index (CSI) and TR. CSIs were calculated on the basis of mean firing rates in response to suprathreshold sinusoidal gratings. CSIs were considered infinite (Inf) for neurons that were unresponsive to the L+M stimulus. TRs were calculated from responses in the 2AFC detection task. The smaller of the 2 TRs from each neuron is shown here, but results were qualitatively similar when we used the larger one (see Fig. 6C).
Color-luminance V1 neurons, unlike color-preferring neurons, tend to have band-pass spatial-frequency tuning (Johnson et al. 2001). Our observation that many color-luminance neurons are sensitive at PT thus implies band-pass spatial-frequency tuning among the most chromatically sensitive V1 neurons. Consistent with this prediction, neurons that were sensitive at the monkeys' detection threshold had a range of spatial-frequency preferences and tuning bandwidths (Fig. 9, A–F). Bandwidths calculated by fitting a difference of Gaussians model to the raw data (Johnson et al. 2001) were poorly correlated with TRs (Spearman's r = 0.14, P = 0.25; data not shown), and the population spatial-frequency tuning function of neurons sensitive at detection threshold (TR ≤ 3) was similar to the tuning function of insensitive neurons (Fig. 9J). For most neurons, spatial-frequency tuning was measured with achromatic gratings, but a similar result was obtained from a subset of neurons (n = 14), whose spatial-frequency tuning was measured with gratings of the preferred isoluminant color direction (Fig. 9, G–I). The existence of chromatically sensitive, spatially band-pass V1 neurons is consistent with psychophysical observations (Bradley et al. 1988; Losada and Mullen 1994; Mullen and Losada 1999) and argues against the view that strongly color-opponent, low-pass, unoriented neurons are the sole mediators of color processing in V1.
Fig. 9.
Spatial-frequency tuning of neurons that were sensitive (i.e., TR ≤ 3) at the monkeys' chromatic detection threshold. A–F: data from 6 individual neurons illustrating low-pass (A and D) and band-pass (B, C, E, and F) tuning in response to achromatic sinusoidal gratings. G–I: data from 3 example neurons illustrating band-pass tuning in response to isoluminant sinusoidal gratings. Curves are the best-fitting difference of Gaussians. The TR and CSI are provided for each neuron. J: population average spatial-frequency tuning curve for neurons that were sensitive (black) and insensitive (gray) at the chromatic detection threshold. Individual tuning functions were normalized by their maximum value and then averaged across neurons. Error bars represent ± 1 SE.
DISCUSSION
Detection psychophysics has been instrumental in developing models of contrast sensitivity on the basis of neural detection mechanisms (Blakemore and Campbell 1969; Boynton et al. 1999; Cole et al. 1993; Geisler and Albrecht 1997; Mullen 1985; Sankeralli and Mullen 1996; Tolhurst et al. 1983). Simultaneous measurement of neuronal responses and psychophysical performance offers a powerful method of testing these models. We used this approach to show that individual V1 neurons are responsive at chromatic detection threshold, but their tuning is inconsistent with the cardinal mechanisms model. CRFs did not match when contrast was defined as the projection of a stimulus onto the preferred cardinal axis but were better matched when contrast was defined in units of detection threshold. An ideal observer analysis showed that NTs were, on average, 1.5 times greater than the monkeys' PTs, and this result was consistent across the four color directions that we tested. In contrast to the independence between the cardinal mechanisms demonstrated psychophysically (Krauskopf and Farell 1990; Krauskopf et al. 1982; Sankeralli and Mullen 1997) and supported by the physiology and anatomy of the visual system prior to V1 (Chatterjee and Callaway 2003; De Valois et al. 1966; Derrington et al. 1984), some V1 neurons were sensitive at detection threshold in both L−M and S-cone-isolating color directions. Lastly, we found that many V1 neurons that responded to high-contrast luminance stimuli were also well driven by isoluminant stimuli at the monkeys' PT. This suggests a role for neurons that are jointly tuned for color and luminance in chromatic detection.
Relationship to psychophysically defined detection mechanisms.
Psychophysical experiments have demonstrated that chromatic detection is mediated by multiple visual mechanisms, but the tuning and number of these mechanisms are controversial [for review, see Eskew (2009) and Stockman and Brainard (2009)]. Evidence in favor of the cardinal mechanisms model comes from a variety experimental techniques, including color matching (LeGrand 1949), detection (Cole et al. 1993; Sankeralli and Mullen 1996), chromatic habituation (Krauskopf et al. 1982), noise masking (Eskew et al. 2001; Giulianini and Eskew 1998; Sankeralli and Mullen 1997), and motion-coherence judgments (Krauskopf and Farell 1990). Other studies that used essentially the same techniques have acknowledged a dominant role for the cardinal mechanisms in chromatic detection but suggest the existence of an unknown number of “higher-order” mechanisms that also contribute to color vision (Krauskopf and Gegenfurtner 1992; Krauskopf et al. 1986, 1996; Stoughton et al. 2012; Webster and Mollon 1994). A third group of experiments found evidence of higher-order mechanisms and argued against a dominant role for the cardinal mechanisms (D'Zmura and Knoblauch 1998; Gegenfurtner and Kiper 1992; Hansen and Gegenfurtner 2006; Krauskopf et al. 1996).
The higher-order chromatic detection mechanisms that are revealed psychophysically may result, in part, from cone signal processing in V1. Many previous studies have documented noncardinal color tuning in V1 (Horwitz et al. 2007; Johnson et al. 2004; Lennie et al. 1990). Our study confirms and extends these previous studies in three ways. First, we studied neurons in the contrast regime most appropriate for revealing detection mechanisms. Second, we analyzed the signal and noise components of neuronal responses in a way that is directly comparable with the psychophysical performance of the observer. Third, we measured neuronal responses directly, thereby obviating the need for stimulus manipulations to isolate detection mechanisms (e.g., habituation and noise masking), which can have complex effects on the responses of individual V1 neurons (Tailby et al. 2008) and may affect task strategy (D'Zmura and Knoblauch 1998; Gegenfurtner and Kiper 1992). We conclude that even under these conditions, V1 neurons do not conform to the cardinal mechanisms model.
One potential concern with this conclusion is that detection might be mediated exclusively by the subset of neurons tuned to the cardinal color directions (e.g., those with β3 near one; see Eqs. 2 and 3). Although this possibility is consistent with our data, it is not strongly supported by them: neurons tuned to the cardinal axes were uncommon and only moderately sensitive to chromatic contrast. TRs of neurons with β3, within ±1 SD of one, were not significantly different than TRs of neurons with β3 outside of this range (t-test, P = 0.78). Similarly, β3s were not significantly correlated to TRs (Spearman's r = 0.02, P = 0.85). We thus find no evidence that V1 neurons tuned to the cardinal axes have a special role in chromatic detection.
An additional concern is that our main conclusion—that V1 neurons do not behave as the cardinal mechanisms at detection threshold—is dependent on the spatial properties of the stimulus that we used. Indeed, color tuning is not, in general, separable from spatial tuning (Johnson et al. 2001; Lennie et al. 1990). For example, LGN neurons are tuned to the cardinal color directions when tested with full-field stimuli but not when tested with stimuli that are spatially band limited, such as sinusoidal gratings (De Valois et al. 2000; Lennie et al. 1990). Although it is possible that we might have obtained a different result by using a different spatial stimulus, we found that restricting our analysis to neurons with a preferred spatial frequency < 1 cycle/degree did not change our main conclusion (data not shown).
Our use of spatially band-limited stimuli was motivated by two factors: first, neurophysiological investigations have shown that some V1 neurons are chromatically and spatially opponent. For example, Johnson et al. (2008) showed clear examples of double-opponent V1 neurons that appear to behave in accordance with the cardinal mechanisms model [e.g., Fig. 1 in Johnson et al. (2008)]. Spatially band-limited stimuli drive these cells more strongly than full-field stimuli and are thus presumably a better choice for revealing the contribution of these cells to visual processing. Second, we wanted to compare fairly the neurometric sensitivity of color-preferring and color-luminance V1 neurons. These two groups of neurons tend to be tuned for different ranges of spatial frequency (Johnson et al. 2001), and their respective contribution to color vision is a matter of debate. Fixing the spatial frequency of the stimulus would likely have biased the result in favor of one group or the other (lower spatial frequencies favoring the color-preferring group). By tailoring the spatial frequency to the neuron under study, we found that neurons in both categories were approximately equally sensitive. This result suggests a role for band-pass V1 neurons in the detection of isoluminant stimuli and is consistent with psychophysical evidence postulating spatial frequency-selective mechanisms for chromatic detection (Bradley et al. 1988; Losada and Mullen 1994; Mullen and Losada 1999; Webster et al. 1990).
We presented all stimuli binocularly, but had we occluded one eye, we expect that PTs would have increased by a factor of ∼1.5 (Legge 1984; Simmons and Kingdom 1998). How this manipulation would affect neuronal sensitivity is unclear, and we did not measure ocular dominance. For monocular neurons, this manipulation would not have affected NTs. Because PTs would increase, the neurometric-to-psychometric TR for monocular neurons would be closer to one. However, binocular, color-sensitive neurons are common in V1 and likely have higher NTs under monocular stimulation (Landisman and Ts'o 2002; Peirce et al. 2008).
We looked for trial-to-trial covariations between neuronal responses and psychophysical behavior (CP) but did not find compelling evidence for this relationship among the V1 neurons that we studied. Although one study of V1 (Palmer et al. 2007) has reported significant CP for the population of V1 neurons taken as a whole, CP has not been established solidly as a characteristic of individual V1 neurons. Another notable study failed to find significant CP in V1 (Nienborg and Cumming 2006). Another reason that we may have failed to find significant CP in this study is that z-scoring distributions of low spike counts can corrupt CP measurements (Kang and Maunsell 2012). Improved methods of measuring weak relationships between neuronal responses and behavior might reveal such a pattern in our data, but for our purposes, such a method would have to be sufficiently sensitive at the level of individual neurons to be useful for identifying those that are tightly linked to behavior.
Small signal linearity.
A motivation to study color vision at detection threshold is that the complexities of adaptation and gain control are minimized, potentially leading to a closer-to-linear color-vision system (Solomon and Lennie 2005; Solomon et al. 2004; Tailby et al. 2008). This is undoubtedly true at many levels of the visual system, but we were surprised by clear nonlinearities in V1 responses even in the low-contrast regime.
One nonlinearity that all V1 neurons exhibit is a spike threshold that naturally exerts a strong effect at low contrasts. This nonlinearity was expected and was thus included in the model that we used to fit CRFs (Eqs. 1 and 2). The model also assumes that CRFs in pairs of color directions can be equated by scaling the contrast axis. This would be the case for a linear neuron with a static output nonlinearity (e.g., spiking threshold) but was demonstrably untrue for 39% of the neurons that we recorded. For these neurons, an analysis of deviance rejected the simple contrast-scaling model in favor of a more complex model, in which the spiking threshold and contrast gain parameters were allowed to differ between color directions (data not shown).
An additional nonlinearity was the width of color tuning observed for some V1 neurons. Seven of the 11 neurons that we tested in the L−M and S-cone-isolating directions were sensitive to both color directions at detection threshold. A linear model fit to the TRs for these neurons predicts an average TR in the intermediate color direction of 0.65, which is lower than any of the TRs that we measured when we directly tested each neuron's preferred intermediate color direction.
We have shown previously that the responses of many V1 neurons to high chromatic contrast are poorly described by a linear model (Horwitz and Hass 2012), and the current data suggest that this observation extends to near-threshold contrasts. Some V1 neurons in the current study were sensitive at PT to both L−M and S-cone-isolating color directions. These neurons may have had isoresponse surfaces that resemble ellipsoids or hyperboloids of one sheet. A few neurons (n = 17) were sensitive at PT to one of the colors that we tested but not the neighboring color direction (45° away in the color space of Fig. 2). These neurons may have had hyperbolic isoresponse surfaces. Unfortunately, we were unable to draw stronger connections between these two data sets, despite the fact that we tested 20 neurons in both experimental paradigms. Isoresponse surfaces measured at near-threshold contrasts were too noisy to be interpreted, and isoresponse surfaces measured at higher contrasts [those described in Horwitz and Hass (2012)] were insufficient to infer neural responses at detection threshold.
Effects of stimulus eccentricity.
Most chromatic detection experiments in humans have been performed at the fovea, but our experiments were performed at ∼5° eccentricity. The spatial distribution of cones and their convergence onto downstream neurons depend critically on eccentricity, leaving open the possibility that our results could change if we had performed our study at a different eccentricity. A decisive test of this idea would require repeating our experiment, recording from a different region of V1. However, psychophysical evidence from humans suggests that the cone inputs to the detection mechanisms that operate 18° in the periphery are similar to those at the fovea (Newton and Eskew 2003), although the relative sensitivity of these mechanisms depends on eccentricity (Mullen and Kingdom 1996, 2002).
We controlled the activity of the L-, M-, and S-cones using the method of silent substitution. Our use of human cone fundamentals in the silent substitution calculations is justified by the similarity in photopigment absorption spectra between monkeys and humans (Baylor et al. 1987). Nevertheless, it is likely that the 2° cone fundamentals that we used in the majority of our experiments resulted in incomplete cone isolation due to the relatively large eccentricity of the neurons that we recorded (mean RF eccentricity = 5.14° SD = 0.88°). The 10° fundamentals presumably provided better cone isolation.
The major difference between the 2° and 10° cone fundamentals is that the 2° fundamentals implicitly assume a higher density of macular pigment, which is dense in the fovea but is largely absent >5° from the fovea (Snodderly et al. 1984). Because it absorbs short-wavelength light preferentially, macular pigment affects the shape of the S-cone fundamental. As a result, high-contrast, nominally S-cone-isolating stimuli based on the 2° fundamentals can have appreciable luminance artifacts when presented outside of the fovea (Cottaris 2003; Sun et al. 2006).
The impact of this error on the results of our experiments is minimal, because stimulus contrasts were low. The highest PT for S-cone-isolating stimuli corresponded to the point [0, 0, 0.17] in L-, M-, and S-cone contrast space when represented using the 2° fundamentals but corresponds to the point [0.0029, 0.0072, 0.172] when calculated using the 10° fundamentals. This introduces an L+M artifact of 0.71% cone contrast and an L−M artifact of 0.30% cone contrast. These contrasts were below detection threshold for both monkeys across all spatial frequencies that we tested (see Fig. 3). Moreover, the error in S-cone contrast (0.20% cone contrast) is small, relative to the SD of detection thresholds for S-cone-isolating lights, based on 2° cone fundamentals (average SD across spatial frequencies and monkeys = 0.93% contrast). As an extra precaution, we used the 10° fundamentals (Stockman et al. 1993) in a subset of experiments. The data from these experiments (n = 27) were neither qualitatively nor quantitatively different than the data collected using the 2° fundamentals. Future studies of color processing in the macaque cerebral cortex will benefit from accurate measurements of corneal cone fundamentals specifically for these animals.
GRANTS
Support for this work was provided by a National Institute of General Medical Science training grant (C. A. Hass), the ARCS Foundation (C. A. Hass), the McKnight Foundation (G. D. Horwitz), and National Center for Research Resources Grant RR-000166 and National Eye Institute Grant EY-018849 (G. D. Horwitz).
DISCLOSURES
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Author contributions: C.A.H. and G.D.H. conception and design of research; C.A.H. performed experiments; C.A.H. and G.D.H. analyzed data; C.A.H. and G.D.H. interpreted results of experiments; C.A.H. prepared figures; C.A.H. drafted manuscript; C.A.H. and G.D.H. edited and revised manuscript; C.A.H. and G.D.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Elise Grover and Leah Tait for excellent technical assistance and Geoff Boynton, Yasmine El-Shamayleh, and Patrick Weller for valuable comments on the manuscript.
REFERENCES
- Anzai A, Ohzawa I, Freeman RD. Neural mechanisms for processing binocular information I. Simple cells. J Neurophysiol 82: 891–908, 1999 [DOI] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol 390: 145–160, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billock VA. Cortical simple cells can extract achromatic information from the multiplexed chromatic and achromatic signals in the parvocellular pathway. Vision Res 35: 2359–2369, 1995 [DOI] [PubMed] [Google Scholar]
- Blakemore C, Campbell FW. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol 203: 237–260, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Demb JB, Glover GH, Heeger DJ. Neuronal basis of contrast discrimination. Vision Res 39: 257–269, 1999 [DOI] [PubMed] [Google Scholar]
- Bradley A, Switkes E, De Valois K. Orientation and spatial frequency selectivity of adaptation to color and luminance gratings. Vision Res 28: 841–856, 1988 [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997 [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996 [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12: 4745–4765, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature 371: 511–513, 1994 [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Callaway EM. Parallel colour-opponent pathways to primary visual cortex. Nature 426: 668–671, 2003 [DOI] [PubMed] [Google Scholar]
- Cole GR, Hine T, McIlhagga W. Detection mechanisms in L-, M-, and S-cone contrast space. J Opt Soc Am A 10: 38–51, 1993 [DOI] [PubMed] [Google Scholar]
- Conway BR. Spatial structure of cone inputs to color cells in alert macaque primary visual cortex (V-1). J Neurosci 21: 2768–2783, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BR, Livingstone MS. Spatial and temporal properties of cone signals in alert macaque primary visual cortex. J Neurosci 26: 10826–10846, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottaris NP. Artifacts in spatiochromatic stimuli due to variations in preretinal absorption and axial chromatic aberration: implications for color physiology. J Opt Soc Am A Opt Image Sci Vis 20: 1694–1713, 2003 [DOI] [PubMed] [Google Scholar]
- De Valois RL, Abramov IA, Jacobs GH. Analysis of response patterns of LGN cells. J Opt Soc Am 56: 966–977, 1966 [DOI] [PubMed] [Google Scholar]
- De Valois RL, Cottaris NP, Elfar SD, Mahon LE, Wilson JA. Some transformations of color information from lateral geniculate nucleus to striate cortex. Proc Natl Acad Sci USA 97: 4997–5002, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in the lateral geniculate nucleus of macaque. J Physiol 357: 241–265, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Zmura M, Knoblauch K. Spectral bandwidths for the detection of color. Vision Res 38: 3117–3128, 1998 [DOI] [PubMed] [Google Scholar]
- Eskew RT. Higher order color mechanisms: a critical review. Vision Res 49: 2686–2704, 2009 [DOI] [PubMed] [Google Scholar]
- Eskew RT, Newton JR, Giulianini F. Chromatic detection and discrimination analyzed by a Bayesian classifier. Vision Res 41: 893–909, 2001 [DOI] [PubMed] [Google Scholar]
- Estévez O, Spekreijse H. The “silent substitution” method in visual research. Vision Res 22: 681–691, 1982 [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR, Kiper DC. Contrast detection in luminance and chromatic noise. J Opt Soc Am A 9: 1880–1888, 1992 [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR, Kiper DC, Beusmans JMH, Carandini M, Zaidi Q, Movshon JA. Chromatic properties of neurons in macaque MT. Vis Neurosci 11: 455, 1994 [DOI] [PubMed] [Google Scholar]
- Geisler WS, Albrecht DG. Visual cortex neurons in monkeys and cats: detection, discrimination, and identification. Vis Neurosci 14: 897–919, 1997 [DOI] [PubMed] [Google Scholar]
- Giulianini F, Eskew RTJ. Chromatic masking in the (delta L/L, delta M/M) plane of cone-contrast space reveals only two detection mechanisms. Vision Res 38: 3913–3926, 1998 [DOI] [PubMed] [Google Scholar]
- Graham N. Visual detection of aperiodic spatial stimuli by probability summation among narrow band channels. Vision Res 17: 637–652, 1977 [DOI] [PubMed] [Google Scholar]
- Hanazawa A, Komatsu H, Murakami I. Neural selectivity for hue and saturation of colour in the primary visual cortex of the monkey. Eur J Neurosci 12: 1753–1763, 2000 [DOI] [PubMed] [Google Scholar]
- Hansen T, Gegenfurtner KR. Higher level chromatic mechanisms for image segmentation. J Vis 6: 239–259, 2006 [DOI] [PubMed] [Google Scholar]
- Hawken MJ, Parker AJ. Detection and discrimination mechanisms in the striate cortex of the Old-World monkey. In: Vision: Coding and Efficiency, edited by Blakemore C. Cambridge, UK: Cambridge University Press, 1990, p. 103–116 [Google Scholar]
- Heeger DJ. Half-squaring in responses of cat striate cells. Vis Neurosci 9: 427–443, 1992 [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Chichilnisky EJ, Albright TD. Blue-yellow signals are enhanced by spatiotemporal luminance contrast in macaque V1. J Neurophysiol 93: 2263–2278, 2005 [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Chichilnisky EJ, Albright TD. Cone inputs to simple and complex cells in V1 of awake macaque. J Neurophysiol 97: 3070–3081, 2007 [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Hass CA. Nonlinear analysis of macaque V1 color tuning reveals cardinal directions for cortical color processing. Nat Neurosci 15: 913–919, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. Cone inputs in macaque primary visual cortex. J Neurophysiol 91: 2501–2514, 2004 [DOI] [PubMed] [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. The orientation selectivity of color-responsive neurons in macaque V1. J Neurosci 28: 8096–8106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. The spatial transformation of color in the primary visual cortex of the macaque monkey. Nat Neurosci 4: 409–416, 2001 [DOI] [PubMed] [Google Scholar]
- Kang IK, Maunsell JM. Potential confounds in estimating trial-to-trial correlations between neuronal response and behavior using choice probabilities. J Neurophysiol 108: 3403–3415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauskopf J, Farell B. Influence of colour on the perception of coherent motion. Nature 348: 328–331, 1990 [DOI] [PubMed] [Google Scholar]
- Krauskopf J, Gegenfurtner K. Color discrimination and adaptation. Vision Res 32: 2165–2175, 1992 [DOI] [PubMed] [Google Scholar]
- Krauskopf J, Williams DR, Heeley DW. Cardinal directions of color space. Vision Res 22: 1123–1131, 1982 [DOI] [PubMed] [Google Scholar]
- Krauskopf J, Williams DR, Mandler MB, Brown AM. Higher order color mechanisms. Vision Res 26: 23–32, 1986 [DOI] [PubMed] [Google Scholar]
- Krauskopf J, Wu HJ, Farell B. Coherence, cardinal directions and higher-order mechanisms. Vision Res 36: 1235–1245, 1996 [DOI] [PubMed] [Google Scholar]
- Landisman CE, Ts'o DY. Color processing in macaque striate cortex: electrophysiological properties. J Neurophysiol 87: 3138–3151, 2002 [DOI] [PubMed] [Google Scholar]
- Legge GE. Binocular contrast summation–I. Detection and discrimination. Vision Res 24: 373–383, 1984 [DOI] [PubMed] [Google Scholar]
- LeGrand Y. Les seuils différentiels de couleurs dans la théorie de Young. Rev Opt 28: 261–278, 1949 [PubMed] [Google Scholar]
- Lennie P, Krauskopf J, Sclar G. Chromatic mechanisms in striate cortex of macaque. J Neurosci 10: 649–669, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada MA, Mullen KT. The spatial tuning of chromatic mechanisms identified by simultaneous masking. Vision Res 34: 331–341, 1994 [DOI] [PubMed] [Google Scholar]
- Mullen KT. The contrast sensitivity of human colour vision to red-green and blue-yellow chromatic gratings. J Physiol 359: 381–400, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen KT, Kingdom FA. Differential distributions of red-green and blue-yellow cone opponency across the visual field. Vis Neurosci 19: 109–118, 2002 [DOI] [PubMed] [Google Scholar]
- Mullen KT, Kingdom FA. Losses in peripheral colour sensitivity predicted from “hit and miss” post-receptoral cone connections. Vision Res 36: 1995–2000, 1996 [DOI] [PubMed] [Google Scholar]
- Mullen KT, Losada MA. The spatial tuning of color and luminance peripheral vision measured with notch filtered noise masking. Vision Res 39: 721–731, 1999 [DOI] [PubMed] [Google Scholar]
- Nagy AL, Eskew RT, Boynton RM. Analysis of color-matching ellipses in a cone-excitation space. J Opt Soc Am A 4: 756–768, 1987 [DOI] [PubMed] [Google Scholar]
- Newton JR, Eskew RT. Chromatic detection and discrimination in the periphery: a postreceptoral loss of color sensitivity. Vis Neurosci 20: 511–521, 2003 [DOI] [PubMed] [Google Scholar]
- Nienborg H, Cumming BG. Decision-related activity in sensory neurons reflects more than a neuron's causal effect. Nature 459: 89–92, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienborg H, Cumming BG. Macaque V2 neurons, but not V1 neurons, show choice-related activity. J Neurosci 26: 9567–9578, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C, Cheng SY, Seidemann E. Linking neuronal and behavioral performance in a reaction-time visual detection task. J Neurosci 27: 8122–8137, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW, Solomon SG, Forte JD, Lennie P. Cortical representation of color is binocular. J Vis 8: 6.1–6.10, 2008 [DOI] [PubMed] [Google Scholar]
- Sachs MB, Nachmias J, Robson JG. Spatial-frequency channels in human vision. J Opt Soc Am 61: 1176–1186, 1971 [DOI] [PubMed] [Google Scholar]
- Sankeralli MJ, Mullen KT. Estimation of the L-, M-, and S-cone weights of the postreceptoral detection mechanisms. J Opt Soc Am A 13: 906–915, 1996 [Google Scholar]
- Sankeralli MJ, Mullen KT. Postreceptoral chromatic detection mechanisms revealed by noise masking in three-dimensional cone contrast space. J Opt Soc Am A Opt Image Sci Vis 14: 2633–2646, 1997 [DOI] [PubMed] [Google Scholar]
- Simmons DR, Kingdom FA. On the binocular summation of chromatic contrast. Vision Res 38: 1063–1071, 1998 [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci 25: 674–685, 1984 [PubMed] [Google Scholar]
- Solomon SG, Lennie P. Chromatic gain controls in visual cortical neurons. J Neurosci 25: 4779–4792, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SG, Peirce JW, Lennie P. The impact of suppressive surrounds on chromatic properties of cortical neurons. J Neurosci 24: 148–160, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman A, Brainard DH. Color vision mechanims. In: The Optical Society Of America Handbook Of Optics, edited by Bass M, DeCusatis C, Enoch J, Lakshminarayanan V, Li G, Macdonald C, Mahajan V, Stryland EV. New York: McGraw Hill, 2009 [Google Scholar]
- Stockman A, MacLeod DI, Johnson NE. Spectral sensitivities of the human cones. J Opt Soc Am A Opt Image Sci Vis 10: 2491–2521, 1993 [DOI] [PubMed] [Google Scholar]
- Stoughton CM, Lafer-Sousa R, Gagin G, Conway BR. Psychophysical chromatic mechanisms in macaque monkey. J Neurosci 32: 15216–15226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Smithson HE, Zaidi Q, Lee BB. Specificity of cone inputs to macaque retinal ganglion cells. J Neurophysiol 95: 837–849, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailby C, Solomon SG, Lennie P. Functional asymmetries in visual pathways carrying S-cone signals in macaque. J Neurosci 28: 4078–4087, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst DJ, Movshon JA, Dean AF. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res 23: 775–785, 1983 [DOI] [PubMed] [Google Scholar]
- Wandell BA. Color measurement and discrimination. J Opt Soc Am A 2: 62–71, 1985 [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys 33: 113–120, 1983 [DOI] [PubMed] [Google Scholar]
- Webster MA, De Valois KK, Switkes E. Orientation and spatial-frequency discrimination for luminance and chromatic gratings. J Opt Soc Am A 7: 1034–1049, 1990 [DOI] [PubMed] [Google Scholar]
- Webster MA, Mollon JD. The influence of contrast adaptation on color appearance. Vision Res 34: 1993–2020, 1994 [DOI] [PubMed] [Google Scholar]