Abstract
Purkinje cells have specialized intrinsic ionic conductances that generate high-frequency action potentials. Disruptions of their Ca or Ca-activated K (KCa) currents correlate with altered firing patterns in vitro and impaired motor behavior in vivo. To examine the properties of somatic KCa currents, we recorded voltage-clamped KCa currents in Purkinje cell bodies isolated from postnatal day 17–21 mouse cerebellum. Currents were evoked by endogenous Ca influx with approximately physiological Ca buffering. Purkinje somata expressed voltage-activated, Cd-sensitive KCa currents with iberiotoxin (IBTX)-sensitive (>100 nS) and IBTX-insensitive (>75 nS) components. IBTX-sensitive currents activated and partially inactivated within milliseconds. Rapid, incomplete macroscopic inactivation was also evident during 50- or 100-Hz trains of 1-ms depolarizations. In contrast, IBTX-insensitive currents activated more slowly and did not inactivate. These currents were insensitive to the small- and intermediate-conductance KCa channel blockers apamin, scyllatoxin, UCL1684, bicuculline methiodide, and TRAM-34, but were largely blocked by 1 mM tetraethylammonium. The underlying channels had single-channel conductances of ∼150 pS, suggesting that the currents are carried by IBTX-resistant (β4-containing) large-conductance KCa (BK) channels. IBTX-insensitive currents were nevertheless increased by small-conductance KCa channel agonists EBIO, chlorzoxazone, and CyPPA. During trains of brief depolarizations, IBTX-insensitive currents flowed during interstep intervals, and the accumulation of interstep outward current was enhanced by EBIO. In current clamp, EBIO slowed spiking, especially during depolarizing current injections. The two components of BK current in Purkinje somata likely contribute differently to spike repolarization and firing rate. Moreover, augmentation of BK current may partially underlie the action of EBIO and chlorzoxazone to alleviate disrupted Purkinje cell firing associated with genetic ataxias.
Keywords: cerebellum, calcium-activated potassium, Kca, action potential, voltage-clamp, SK, EBIO
calcium-activated potassium (KCa) channels regulate action potentials in cerebellar Purkinje neurons. Spike waveforms and firing patterns can be altered by either the BK (large-conductance KCa) antagonist iberiotoxin (IBTX) or the SK (small-conductance KCa) antagonist apamin. These antagonists reduce the magnitude of afterhyperpolarizations (AHPs) and accelerate and/or irregularize firing of simple spikes, promoting burst firing (Cingolani et al. 2002; Edgerton and Reinhart 2003; Swensen and Bean 2003; Womack and Khodakhah 2002, 2003, 2004; Womack et al. 2009). Moreover, genetic mutations that eliminate BK channels (Chen et al. 2010; Sausbier et al. 2004) or reduce currents through SK channels (Alviña and Khodakhah 2010; Walter et al. 2006) lead to cerebellar ataxias. Despite many studies demonstrating roles for these channels in Purkinje cells, direct voltage-clamp recordings of the macroscopic currents themselves are surprisingly sparse (Khaliq et al. 2003; Raman and Bean 1999; Swensen and Bean 2003).
Recordings of BK currents in Purkinje neurons provide mixed data on whether these currents are inactivating or non-inactivating. In inside-out patches from Purkinje cells, Ca application elicits BK currents with no apparent fast inactivation (Widmer et al. 2003; Womack and Khodakhah 2002). Whole cell recordings, however, suggest that IBTX-sensitive currents indeed inactivate (Khaliq et al. 2003), leaving open the question of how these currents behave during repeated spikelike depolarizations. The nature of SK currents in Purkinje cells likewise remains elusive. Apamin alters Purkinje firing patterns in intact Purkinje neurons in cerebellar slices, indicative of SK channel expression (Cingolani et al. 2002; Edgerton and Reinhart 2003; Hosy et al. 2011; Womack and Khodakhah 2003). Additionally, compounds that augment SK currents, such as 1-ethyl-2-benzimidazolinone (EBIO) and chlorzoxazone, tend to regularize Purkinje cell firing, particularly when Ca currents are reduced by ataxia-inducing mutations (Alviña and Khodakhah 2010; Walter et al. 2006). Despite a prominent role for Purkinje somata in regulating firing patterns of simple spikes (Khaliq and Raman 2006), somatic SK currents have rarely been resolved (Swensen and Bean 2003) and are present only in a small fraction of cells tested (Khaliq et al. 2003). Collectively, these data hint that SK expression may be high in dendritic compartments, but low or sporadic in somata. They further raise the possibility that the SK channel agonists may influence spiking through multiple mechanisms.
Here, we have recorded voltage-clamped KCa currents from acutely dissociated Purkinje somata from postnatal day 17 (P17)–P21 mice. We find that the KCa current of Purkinje somata includes a rapidly gating IBTX-sensitive BK conductance that inactivates within a few milliseconds and a more slowly gating IBTX-insensitive, tetraethylammonium (TEA)-sensitive, voltage-dependent BK conductance that does not inactivate, but that is enhanced by EBIO. SK currents do not account for a large fraction of the somatic current. The BK conductances are activated at different phases of the action potential firing cycle, such that the IBTX-sensitive current is likely to contribute to spike repolarization and the IBTX-insensitive current is suited to regulate the interspike interval.
MATERIALS AND METHODS
Preparation of dissociated Purkinje cells.
Experimental procedures were carried out in accordance with institutional guidelines approved by the Northwestern University IACUC. Cerebellar Purkinje cells were acutely dissociated from P17–P21 C57BL6 mice (Charles River, Wilmington, MA) (Raman et al. 1997; Regan 1991), an age range in which Purkinje somatic firing properties resemble those of adult animals (Levin et al. 2006). Mice of both sexes were anesthetized with isoflurane and decapitated. The superficial layers of the cerebellum were removed and minced in an ice-cold dissociation solution containing (in mM) 82 Na2SO4, 30 K2SO4, 5 MgCl2, 10 HEPES, 10 glucose, and 0.001% phenol red (pH 7.4 with NaOH). The tissue was incubated in dissociation solution including 3 mg/ml protease XXIII (pH readjusted to 7.4) for 7 min at 31°C with oxygen blowing over the surface of the fluid, then washed twice and microdissected in 1 mg/ml bovine serum albumin and trypsin inhibitor (pH readjusted to 7.4), and finally transferred to Tyrode solution, containing (in mM) 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose (pH 7.4). The tissue was then triturated with polished Pasteur pipettes. Cells settled in the recording chamber on the microscope, and recordings were made 1–6 h after trituration. Purkinje somata were identified based on their size and morphology.
Electrophysiology.
Borosilicate pipettes (1.5–3 MΩ, A-M Systems) were wrapped in Parafilm to reduce capacitance and filled with an intracellular solution containing (in mM): 120 KCH3SO3, 10 NaCl, 2 MgCl2, 10 HEPES, 0.5 EGTA, 14 Tris creatine PO4, 4 MgATP, and 0.3 Tris-GTP (pH 7.35 with KOH). Setting EGTA at 0.5 mM provides an approximation of the mobile Ca buffer calbindin D28K (Benton and Raman 2009; Fierro and Llano 1996). In a subset of experiments, intracellular Ca was set at 30 μM by including 0.5 mM Fluo-5N as the Ca buffer (Kd = 90 μM), instead of 0.5 EGTA, with 155 μM added Ca. Whole cell recordings were made at 22–26°C, except where higher temperatures are indicated, with an Axopatch 200B amplifier (voltage clamp) or a Multiclamp 700B amplifier (current clamp; Molecular Devices). Data were low-pass filtered at 5 kHz, digitized at 50 kHz, and acquired with a Digidata 1440A and pClamp 10. Whole cell series resistance was 3–5 MΩ, compensated by >60%, usually 80%. Given an average compensation of ∼75% leaves 0.75–1.25 MΩ uncompensated resistance. The error is therefore estimated at 0.75–1.25 mV for 1-nA currents, or 7.5–12.5 mV with 10-nA currents. Currents were evoked every 5 s for current-voltage relationships and every 15 s for other protocols.
Whole cell recordings were made with cells positioned in front of an array of four to six gravity-driven flow pipes made of quartz capillary tubing connected with polyethylene and Tygon tubing to B-D syringe reservoirs. The control extracellular solution for all voltage-clamp experiments contained (in mM) 150 NaCl, 3.5 KCl, 1.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.4), with 300 nM tetrodotoxin (TTX) and 5 mM 4-aminopyridine (4-AP) to block voltage-gated Na and K channels, respectively. With these solutions, measured reversals were close to the predicted K equilibrium potential (EK) of −93 mV. Divalent concentrations were selected to mimic physiological solutions as closely as possible (Hansen 1985). The net Ca and KCa current was obtained as the current blocked by 300 μM CdCl2 (“Cd”); IBTX-sensitive current was that blocked by 200 nM IBTX; and TEA-sensitive current was that blocked by 1 mM TEA. Subtraction of currents measured in constant IBTX with and without Cd gave the IBTX-insensitive, Cd-sensitive (KCa and Ca) currents. In initial experiments, the onset of block by IBTX was monitored by recording currents evoked by 10-ms step depolarizations to −20 mV applied at 5-s intervals. In four cells, maximal blockade was observed in 15–30 s. In all recordings thereafter, cells were exposed to IBTX for at least 30 s before recordings were made.
In experiments with 30 μM intracellular Ca, 30 μM CdCl2 was included in the control extracellular solution. This concentration of Cd2+ is sufficient to block most high-voltage-activated channels in Purkinje cells, regardless of the charge carrier. T-type currents are less sensitive to Cd2+, but block of T-type currents by Cd2+ is more potent with Ba2+ than Ca2+ as the charge carrier (Lacinová et al. 2000; I. M. Raman, unpublished observations for Purkinje cells). Therefore, the 1.5 mM Ca2+ was replaced with 1 mM Ba2+ and 0.5 mM Mg2+, to keep divalent cations constant. Records were obtained in this control solution without and with pharmacological agents as noted, and currents of interest were isolated by subtraction.
Single-channel recordings were made at room temperature in inside-out patches pulled from isolated Purkinje cells with pipettes of resistance 5–10 MΩ and coated with Sylgard. The extracellular (pipette) solution contained (in mM) 150 KCl, 10 HEPES, 2 MgCl2, 5 4-AP, 5 glucose, 0.03 CdCl2, as well as 100 nM TTX and 200 nM IBTX, buffered to pH 7.4 with NaOH (<1 mM). Intracellular solutions were applied through flow pipes as above and consisted of 133 KCH3SO3, 10 HEPES, 10 NaCl, 2 MgCl2, 1 HEDTA, 5.5 sucrose, 3.5 Tris creatine PO4, 1 MgATP, 0.075 Tris GTP, buffered to a pH of 7.35 with KOH for a total K+ concentration of 171 mM, and a predicted EK of −3.3 mV. Ca was added where indicated by calculating the total Ca necessary to achieve the desired free Ca concentration (range, 1–210 μM) based on MaxChelator (http://maxchelator.stanford.edu/). Single-channel current amplitudes were measured as the major unitary peak from all-points histograms made from data at all potentials at which individual openings were evident (1–5 voltages in each condition) and scaled by driving force. Single-channel conductance was estimated as the mean conductance measured at the multiple voltages for each patch.
Action potentials were recorded from whole cells filled with standard intracellular solution (0.5 mM EGTA) and bathed in the voltage-clamp control extracellular solution, without TTX, with other drugs as indicated. In 11 of the 18 cells in which spikes were recorded, input resistances were estimated from the voltage change elicited by 25–50 pA hyperpolarizing current injections that prevented firing and was 1.2 ± 0.1 GΩ, consistent with the high input resistances in isolated Purkinje cells (Raman and Bean 1999). Note that the input resistance during spiking is likely to be lower, as other conductances are activated.
Chemicals were obtained from Sigma-Aldrich except the following: apamin (Sigma-Aldrich, Peptides International, and EMD Chemicals), scyllatoxin and IBTX (Peptides International), EBIO, UCL1684, 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34), bicuculline methiodide, and N-cyclohexyl-N-2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-4-pyrimidinamine (CyPPA) (Tocris Bioscience); TTX (Alomone Laboratories); and Fluo 5N (Invitrogen). Stocks of IBTX, apamin, and scyllatoxin were prepared by dissolving the peptides in water (100–300 μM), then aliquoting to volumes of 15–50 μl in 0.5 ml Eppendorf vials. Stocks of EBIO, CyPPA, UCL 1684, and TRAM-34 were prepared by dissolving the drugs in DMSO (10–20 mM), then aliquoting to volumes of 50–100 μl in 0.5-ml Eppendorf vials. Stocks were stored at −20°C for less than 3 mo before use. All experimental solutions were prepared immediately before the experiment in 15-ml polypropylene centrifuge tubes by diluting stocks (1:100 or greater) into warmed (30°C) solutions. In a subset of experiments, 1 mg/ml cytochrome C was added to the solutions to minimize the possibility of drugs adhering to the tubing (e.g., Khaliq et al. 2003). No changes in drug efficacy were noted, and data plus or minus cytochrome C with the same drug have been pooled.
Analysis.
Data were analyzed with IgorPro (Wavemetrics, Lake Oswego, OR) and are reported as means ± SE. Conductance-voltage data were obtained by dividing current amplitudes by driving force to obtain chord conductances and fitting with a sigmoid equation of the form G = Gmax/{1 + exp[−(V − V1/2)/k]}, where G is conductance, Gmax is the maximal conductance, V1/2 is the voltage at which the current reaches half its maximal amplitude, and k is the slope factor. Statistical significance was assessed with Student's two-tailed paired t-tests, except as noted, with an alpha level of 0.05. Capacitative transients have been blanked for clarity in figures showing responses to trains of stimuli.
RESULTS
Total Ca and KCa current in Purkinje somata.
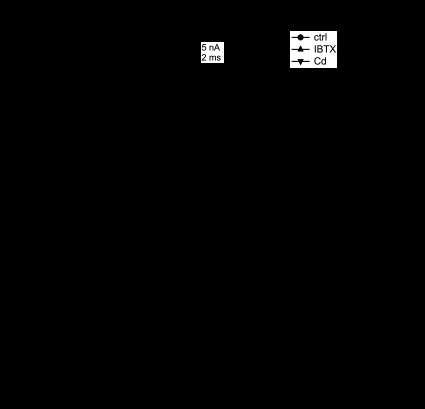
To measure the properties of Ca and KCa current in cerebellar Purkinje somata, we made whole cell voltage clamp recordings from acutely dissociated neurons. Currents were first evoked in control solutions, which included 5 mM 4-AP to block Ca-independent, voltage-gated K currents, by 20-ms step depolarizations in 5-mV increments from a holding potential of −65 mV. Steps were then repeated in IBTX, which blocks BK channels that lack β4-subunits (Behrens et al. 2000; Meera et al. 2000) and finally in Cd (Fig. 1A). In control solutions, currents were first detectable near −40 mV, and the peak current reached 13.1 ± 1.1 nA at 0 mV (n = 11, Fig. 1B). Application of IBTX reduced the current and altered the kinetics, so that the residual current rose more slowly (Fig. 1, A and B). Currents evoked in Cd verified that most of the voltage-gated K current was blocked by the 4-AP in the extracellular solutions (Fig. 1, A and B). Above 0 mV, the net outward current often exceeded 20 nA, saturating the amplifier. The maximal Ca and KCa conductances in the Purkinje cell soma are expected to be even greater, because the P-type current, which is the primary current that couples to KCa currents in Purkinje cells (Womack et al. 2004), is already partially inactivated at −65 mV (Regan 1991); under the conditions we used, it was reduced from −2.8 ± 0.3 nA at −90 mV to −2.0 ± 0.2 nA at −60 mV (n = 6, measured as Cd-sensitive current with Cs+ replacing intracellular K+).
Fig. 1.
Total Ca and Ca-activated K (KCa) current in Purkinje somata during steps and trains. A: representative raw currents evoked by 20-ms steps from −65 mV in 5-mV increments, in control (ctrl) solution (top), iberiotoxin (IBTX; middle), and in 300 μM Cd (bottom). Δ, Step increment. B: current-voltage relations for the maximal step depolarization-evoked currents for all three conditions (n = 11). C, left: Cd-sensitive currents (summed Ca and KCa), obtained as the difference of records evoked in control and Cd, by 50- (top) or 100-Hz (bottom) 1-s trains of 1-ms pulses to +20 mV [holding potential (Vhold) = −60 mV]. Right: the first few responses on an expanded time base. D: peak train-evoked Cd-sensitive currents, normalized to the response to the first pulse (n = 8). For 100 Hz, every second point is plotted. Values in all figures are plotted as mean ± standard error.
To test whether the total Cd-sensitive (KCa + Ca) current in Purkinje neurons is stable or modulated during spiking, we applied trains of brief (1-ms) depolarizing steps (from −60 to +20 mV) to approximate the voltages reached during action potential firing. Recordings were made in control solutions and Cd, and subtractions gave the total Cd-sensitive current. As reported previously, the net Ca and KCa current was outward throughout the step at all membrane potentials (Raman and Bean 1999). During the 50-Hz train, the peak outward current evoked by each step decreased over the first 5 steps to 65 ± 6% of the first response, and then remained stable for the duration of the train, changing further by only −2 ± 1% (n = 8) (Fig. 1, C and D). Upon repolarization, outward tail currents were present, which decayed during the interstep interval (quantified below). With a 100-Hz train, the peak step-evoked currents also decreased over the first 5 steps (to 66 ± 7% of the first response at 100 Hz, P = 0.4, paired vs. 50 Hz), but tended to increase slightly by 7 ± 4%, as the train progressed, (P = 0.064, paired vs. 50 Hz, Fig. 1D).
In previous studies, we found that identical train protocols elicit P-type Ca currents that flow largely as tail currents upon repolarization. Tail current amplitudes remain constant for the first 5–10 pulses, before inactivating by only 5% at 50 Hz, or 10% at 100 Hz, and decay fully in the interstep interval (Benton and Raman 2009). Thus the changes in outward current are unlikely to reflect changes in the underlying inward Ca current. Instead, it seems more likely that the total Cd-sensitive current includes a rapidly activating component that inactivates with repeated brief depolarizations, as well as a component that deactivates slowly enough to summate over time with high-frequency stimulation.
IBTX-sensitive BK current.
To segregate the KCa currents pharmacologically, we isolated currents blocked by IBTX (“IBTX-sensitive current”) by subtracting records obtained in control and IBTX, as well as the residual KCa current (“IBTX-insensitive current”) by subtracting records obtained in IBTX and Cd. The IBTX-sensitive current rose more rapidly and had an inactivating component, whereas the IBTX-insensitive current, which contains both Ca current and IBTX-resistant KCa current, activated more slowly and was sustained throughout the 20-ms step (Fig. 2A, same cell as Fig. 1A; Fig. 2B). Since these two currents were kinetically and pharmacologically distinct, we examined them sequentially.
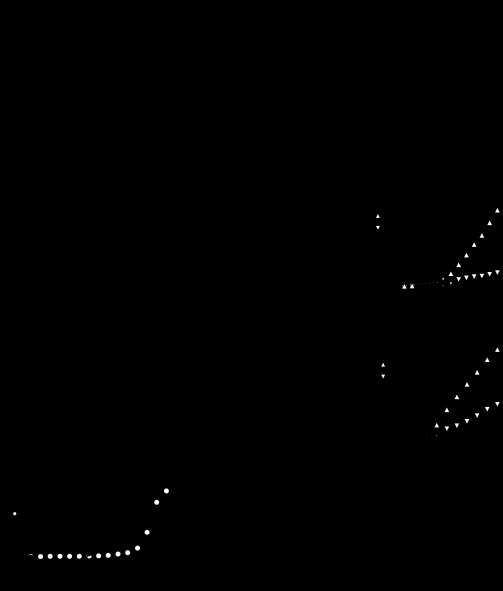
Fig. 2.
Inactivation of IBTX-sensitive large-conductance KCa currents. A: difference currents from Fig. 1, illustrating IBTX-sensitive currents (left) and IBTX-insensitive, Cd-sensitive currents (right) evoked by 20-ms steps from −65 mV in 5-mV increments. Scale applies to both sets of traces. Insets: tail currents for each set of traces on expanded time base; same scale and voltage steps for both insets. B: current-voltage relation for peak IBTX-sensitive and IBTX-insensitive currents (n = 11). Note that, because the peaks occur at different times, the sum of the two curves is greater than the peak raw currents in Fig. 1B. C: representative IBTX-sensitive currents elicited by 50 (top) or 100 Hz (bottom) 1-s trains of 1-ms pulses to +20 mV (Vhold = −60 mV). D: expansion of the first three responses to 50-Hz steps (black). Dotted line is baseline (80 pA). Vertical scale applies to all panels. E: peak currents normalized to the first response in the train (n = 7). For 100 Hz, every second point is plotted.
The IBTX-sensitive current was first detectable near −40 mV, about 5 mV more depolarized than potentials at which Ca current begins to activate under the same recording conditions (Benton and Raman 2009). After the peak currents were converted to conductances, sigmoid fits to the data estimated the maximal chord conductance at 111 ± 13 nS. The conductance was half-activated at −19.5 ± 0.9 mV, with a slope factor of 6.7 ± 0.2 mV. The total time to peak, from the end of the capacitative artifact to the time of maximal current, was 17.9 ± 0.06 ms at −30 mV, decreasing to 2.9 ± 0.02 ms at 0 mV (n = 11). IBTX-sensitive currents also inactivated rapidly. At −20 mV, currents decayed by 80 ± 5% with a decay time constant (τdecay) of 7.3 ± 1.1 ms (n = 9 of 11 cells; 2 cells did not inactivate at this voltage), and at 0 mV, currents decayed by 83 ± 4% with a τdecay of 5.3 ± 0.6 ms (n = 11; including one cell not examined at 0 mV that was measured at −5 mV). Upon repolarization from −25 mV to −65 mV, currents deactivated with a single exponential time constant of 2.5 ± 0.8 ms (n = 11; Fig. 2A, inset).
Since inactivation of BK channels can be induced by auxiliary subunits, such as the β2- or β3-subunit (Uebele et al. 2000; Wallner et al. 1999; Xia et al. 1999; Zeng et al. 2008), the inactivation may be an intrinsic property of IBTX-sensitive BK channels. The inactivation that we observe is on the time scale of a few milliseconds, however, which is considerably faster than seen in chromaffin cells or in expression systems with constant Ca (e.g., Ding et al. 1998; Solaro et al. 1995; Wallner et al. 1999; Xia et al. 1999; Zeng et al. 2008). To investigate whether the decay phase in Purkinje cells may simply reflect the time course of buffering by 0.5 mM EGTA, we tested whether raising the buffer concentration from 0.5 mM EGTA to 5 mM EGTA would accelerate the decay phase, recorded with a step to 0 mV at 32°C. Even this 10-fold variation in EGTA did not change the time course of inactivation (for 0.5 EGTA, 5 EGTA: rise time, 1.1 ± 0.04, 1.1 ± 0.04 ms; τdecay, 2.5 ± 0.3, 3.0 ± 0.3; extent of inactivation, 81 ± 5%, 76 ± 4%; P > 0.25 all comparisons, n = 6, 4), supporting the idea that channel properties rather than buffering determined the inactivation rate.
During trains of depolarizations, IBTX-sensitive BK current generated several nanoamperes of current within the 1-ms step, which decayed fully between steps applied at 50 Hz (Fig. 2, C and D). Over the first 5 steps, the peak BK currents decreased to 81.3 ± 5.0% at 50 Hz, and to 76.6 ± 6.1% at 100 Hz (n = 7), after which further decline was negligible (Fig. 2E). Since the Ca current does not change appreciably during these steps (Benton and Raman 2009), the decrease cannot be attributed to a reduction in Ca influx, but instead suggests that Purkinje IBTX-sensitive current undergoes inactivation over repeated, spikelike depolarizations.
IBTX-insensitive KCa current.
The IBTX-insensitive, Cd-sensitive current consisted of a transient, inward current that was overcome by a slowly rising, non-inactivating outward current. Replacement of Ca with Ba as the permeant divalent cation eliminated the outward flux, leaving only an inward current (n = 3). Both the Cd and Ba sensitivity confirm that the inward component is carried by Ca channels, and the outward component is a KCa current. We examined the voltage dependence of the IBTX-insensitive, Cd-sensitive current by stepping the voltage in 5-mV increments to +60 mV. As shown in Fig. 3, A and B, outward current amplitudes increased with progressively larger depolarizations up to +30 mV, reaching a maximum of 9.5 ± 0.7 nA (n = 8), after which current amplitudes decreased, as previously described for Ca-dependent currents (Marty and Neher 1985; Neely and Lingle 1992).
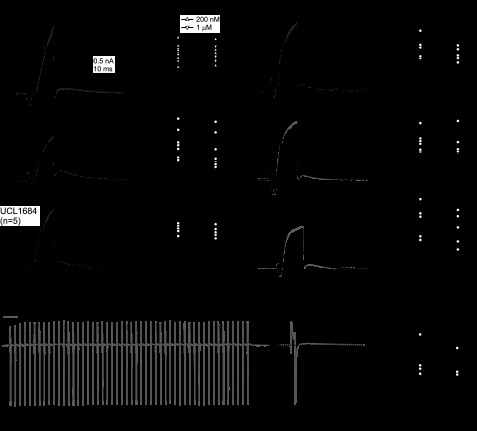
Fig. 3.
IBTX-insensitive currents. A: representative IBTX-insensitive, Cd-sensitive (summed Ca and KCa) currents evoked by 20-ms steps from −65 mV in 5-mV increments. Inset: tail currents on expanded time base. Only records before the turnover of the current-voltage curve are included for clarity. B: current-voltage relation for peak step-evoked currents (n = 11). C: currents evoked with constant Ca (30 μM intracellular Ca; 30 μM extracellular Cd) by voltage steps from −65 mV to +60 mV, in −10-mV increments, in control (IBTX-free) solutions (left) and IBTX (middle). Right: IBTX-sensitive current obtained by subtraction. D: mean current-voltage relations (n = 5), indicating the peak current in control (upward triangles), the instantaneous current in control (downward triangles), and the peak current in IBTX (circles). In IBTX, no data were recorded at −50 and −60 mV. E: Cd-sensitive currents evoked with standard extracellular Ca and intracellular EGTA in IBTX (left), in IBTX with 1 mM tetraethylammonium (TEA; middle). Right: Cd and TEA-sensitive, IBTX-insensitive current obtained by subtraction. F: mean current-voltage relations for the three conditions (n = 9). G: macroscopic current-voltage relation for an inside-out patch exposed to either 0 or 30 μM Ca intracellularly, with IBTX and Cd on the extracellular face. Currents were measured as the average current amplitude between 300 and 400 ms of the voltage step. H: representative IBTX-insensitive single K channels recorded at −90 mV in 0 or 30 μM Ca. Conductance, 164 pS. Same patch as in G. EK, K equilibrium potential.
To test whether this component of KCa current was likely to be an IBTX-insensitive BK current or an SK current, we took advantage of the fact that both BK and SK channels are Ca-dependent, but only BK channels are voltage-dependent. Therefore, we conditioned channels with Ca by replacing EGTA with 0.5 mM Fluo-5N with Ca added to set the intracellular free Ca concentration near 30 μM. We reasoned that, under these conditions, voltage-independent SK currents should be tonically activated, which would be detectable as the instantaneous current evident upon depolarization as the driving force on K changed. In contrast, a BK component would be likely to include a voltage-dependent, noninstantaneous increase in current amplitude upon depolarization (Cui et al. 1997). Cells were bathed in extracellular Cd to block voltage-gated Ca influx, and recordings were made first in IBTX-free (“control”) and then repeated in IBTX-containing (“in IBTX”) solutions (32°C). In control solutions, currents evoked at 5-s intervals by 20-ms voltage steps from −65 to +60 mV ran down over the first 2 min after the whole cell configuration had been established; in cells in which this run-down was measured systematically, currents stabilized at 42 ± 11% of their initial amplitude (n = 4). Full current-voltage curves were recorded in Cd-containing control and IBTX solutions only after currents had stabilized. In both conditions, step depolarizations evoked relatively little instantaneous current; in fact, the instantaneous current was likely overestimated because capacitative transients were not subtracted from the raw records. Therefore, it seems unlikely that non-inactivating, voltage-independent KCa currents were present at high densities (Fig. 3, C and D). Instead, in IBTX-free solutions, currents activated within a few milliseconds, and in IBTX-containing solutions, they continued to rise throughout the 20-ms step. These data suggest that most of the IBTX-insensitive KCa current is likely to be voltage sensitive rather than exclusively Ca gated, consistent with its being carried largely by BK rather than SK channels.
The IBTX-sensitive component, obtained by subtraction, displayed a slow but detectable inactivation phase only at the most positive potentials. Hyperpolarizing the cell from the holding potential of −65 mV to voltages between −125 and −85 mV for 50 ms did not increase the amplitudes of currents evoked upon depolarization (n = 3). The observations that the total KCa current ran down and that the IBTX-sensitive current is smaller and less strongly inactivating when 30 μM Ca is continuously present is consistent with the idea that prolonged exposure to high Ca facilitates inactivation of these channels (Herrington et al. 1995; Neely and Lingle 1992; Solaro et al. 1995). They are also consistent with the observations of Womack and Khodakhah (2002), who found only non-inactivating BK channels upon depolarization in excised inside-out patches chronically exposed to 30 μM Ca.
To test pharmacologically whether the residual, IBTX-insensitive KCa current is indeed carried by BK channels, we examined its sensitivity to 1 mM TEA, which blocks most BK currents with minimal effects on SK currents (Lang and Ritchie 1990; Neely and Lingle 1992). Recordings were made at 32°C with standard intracellular (0.5 mM EGTA) and extracellular solutions, and cells were exposed sequentially to 1) IBTX, 2) IBTX + TEA, 3) IBTX + Cd, and 4) IBTX + Cd + TEA, which permitted the isolation of either IBTX-insensitive (1–3) or IBTX- and TEA-insensitive (2–4) KCa + Ca currents. TEA reduced the IBTX-insensitive outward current by 66 ± 2% (n = 9) from 6.1 ± 0.6 nA to 2.1 ± 0.3 nA at 0 mV (Fig. 3, E and F), supporting the idea that a large fraction of the current is carried by IBTX-insensitive BK channels. In addition, the TEA-sensitive component of IBTX-insensitive current did not inactivate during the 20-ms step, suggesting that it may arise from BK channels that include the β4-subunit that confers IBTX insensitivity and slows activation (Behrens et al. 2000; Meera et al. 2000) but lack β2- and β3-subunits that induce inactivation (Uebele et al. 2000; Wallner et al. 1999; Xia et al. 1999).
If the IBTX-insensitive KCa current is indeed primarily a BK current, then the underlying single channels should have the high conductances typical of BK channels. Therefore, we recorded single-channel activity in inside-out patches pulled from Purkinje cells. Recordings were made with high intracellular and extracellular K+, to set the calculated EK near −3 mV, and with constant Ca applied to the intracellular membrane. The pipette contained IBTX and CdCl2, to block IBTX-sensitive BK channels and prevent Ca influx through voltage-gated Ca channels. Patches were held at −70 mV. After a 100-ms step to −90 or −120 mV to reduce ongoing channel activity, the potential was stepped for 1 s to voltages ranging from −120 mV to +120 mV. In the presence of 30 μM or more Ca, step depolarizations evoked macroscopic currents that reversed at −2.0 ± 0.7 mV (n = 4), close to the predicted EK, and that had amplitudes of a few hundred picoamperes at potentials above +30 mV, indicative of multiple channels in each patch (Fig. 3G). At more negative potentials (less than −75 mV), where the probability of channel opening was low, individual high-conductance (150 ± 15 pS, n = 4) single-channel openings could be resolved throughout the step (Fig. 3H). When the Ca-containing solution was replaced with solutions with no added Ca, the macroscopic current shifted its voltage-dependence strongly positively and the high-conductance events seen at negative potentials disappeared (Fig. 3, G and H). In three patches with sufficiently low noise, single channel openings reappeared with step depolarizations beyond +30 mV. The mean conductance estimated from the channels carrying outward rather than inward current was 185 ± 13 pS, an increase in conductance that is close to that predicted by the 14% higher concentration of internal K+ ions. These results provide direct evidence of high-conductance, IBTX-insensitive, voltage-dependent, non-inactivating KCa channels in Purkinje cell somata.
While the voltage-dependence, TEA-sensitivity, and single-channel conductance provide evidence that the IBTX-sensitive current is carried in part by BK channels, some portion of the total current may nevertheless be carried by SK channels. We explored this possibility by applying a series of SK channel blockers to Purkinje cells (Fig. 4A). Currents were activated with steps to −20 mV, a potential at which Ca current amplitudes in this preparation are maximal (Benton and Raman 2009). The outward current, however, was largely insensitive to SK channel antagonists. It was not reliably blocked by apamin, even at concentrations as high as 1 μM (200 nM, n = 7, P = 0.17; 1 μM, n = 4, P = 0.20, range of block with both concentrations 0–12%), nor was it consistently reduced by three more known blockers of SK channels, scyllatoxin (200 nM, n = 10, P = 0.19), UCL1684 (1 μM, n = 7, P = 0.27) or bicuculline methiodide (100 μM, n = 5, P = 0.12; Khawaled et al. 1999). The absence of effects did not seem to result from either an inactivity of the drugs or a consequence of dissociation, since the same batch of apamin was effective in hippocampal CA1 pyramidal neurons acutely dissociated with the same method. In CA1 cells, 300 nM apamin fully eliminated the outward current of 1.23 ± 0.46 nA, revealing the underlying inward Ca current of −0.15 ± 0.087 nA (n = 3). Finally, testing antagonists from different manufacturers and/or different lots did not affect the results in Purkinje cells.
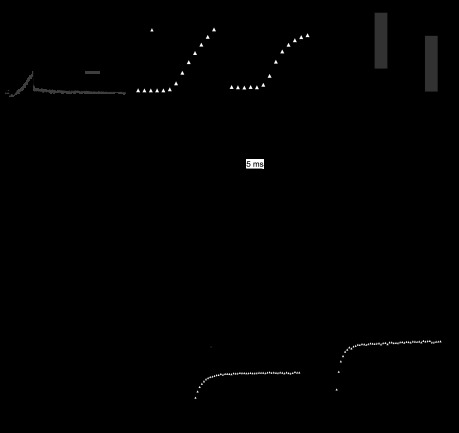
Fig. 4.
Lack of effect of small-conductance KCa antagonists on IBTX-insensitive current. A, left: representative raw (unsubtracted) responses of IBTX-insensitive Ca and KCa currents evoked by 10-ms depolarizing steps from −65 mV to −20 mV, before (black) and after (gray) antagonist application (as labeled). Calibration bars apply to all traces. Right: summary of peak outward current amplitudes for each cell before and after antagonist. Individual cells, open symbols; mean values, closed symbols. Only curare produced a statistically significant decrease in the current. B, left, top: raw currents evoked by 50 trains of depolarizing pulses as in Fig. 1, in control (IBTX-containing) solutions (black) and in 200 nM apamin (gray). The black trace is largely obscured by the gray. Left, bottom: difference current, illustrating the apamin-sensitive component. Middle: expanded response to the last depolarizing pulse from the left traces. Arrow, time of maximal outward current after the train, from which measurements of posttrain current were taken. Note that the difference current is tiny and inward during the step, likely from a small rundown of Ca current. Right: peak posttrain current in 4 cells, before and after apamin. BMI, bicuculline methiodide; TRAM, 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole.
Recent reports have indicated that intermediate-conductance KCa (IK) (SK4) current is present in Purkinje cells (Engbers et al. 2012). Although this channel should be blocked by high concentrations of apamin (Vogalis et al. 1998), which had no effect on the IBTX-insensitive current, we also tested a high concentration of the IK channel blocker TRAM-34 (1 μM). This drug did not reliably decrease the current either, such that the peak remained 95 ± 4% of control (P = 0.27, n = 6). Thus it seems unlikely that the somatic current includes a TRAM-34-sensitive IK component.
Lastly, we tested curare, another antagonist of SK channels (Köhler et al. 1996; Strøbaek et al. 2000). This drug had a small but reliable effect, blocking 711 ± 145 pA of the IBTX-insensitive KCa current (20 ± 5% of the total current, n = 5, P = 0.008), raising the possibility that a fraction of the current may be carried by SK channels. Curare, however, also blocks BK currents (Rossokhin et al. 1996), and the curare-sensitive current activated with a time course similar to the TEA-sensitive component of IBTX-insensitive current, making it seem likely that curare exerted at least some of its effect at BK channels.
Although the KCa current evoked by 10-ms steps lacked the expected pharmacological profile of SK channels, it is possible that classical SK current is indeed present in Purkinje somata, but requires repeated or prolonged depolarizations to be activated. Therefore, we tested the apamin sensitivity of currents evoked by 50-Hz, 1-s trains of spikelike depolarizations, like those in Figs. 1 and 2. In 4 of 4 cells, however, little train-evoked current was blocked by 200 nM apamin (Fig. 4B). The peak outward current after the last step in the train, where SK current should be maximal (Fig. 4B, right, arrow), was 84.9 ± 17.2 pA in control and 73.5 ± 12.0 pA in 200 nM apamin (n = 4, P = 0.13). Thus the IBTX-insensitive current is resistant to several SK channel antagonists, suggesting a low contribution of known SK channels to the total somatic KCa current. We cannot rule out the possibility, however, that some fraction of the somatic current is carried by SK channels with an unusual pharmacology, or that alternative stimulation protocols might be more effective at revealing SK currents.
Modulation of spontaneous and driven action potential firing by EBIO.
KCa channel agonists, such as EBIO, chlorzoxazone, and CyPPA (Cao et al. 2001; Hougaard et al. 2007), can modulate firing patterns of Purkinje cells. In cerebellar slices, in which firing is much less regular than in isolated cells (Häusser and Clark 1997; Raman and Bean 1999), EBIO slows and regularizes firing in Purkinje cells, both in young wild-type rats and in mutant ducky and leaner mutant mice in which P-type Ca influx is reduced (Cingolani et al. 2002; Walter et al. 2006). Since SK channel modulation affects Purkinje cell firing (Womack and Khodakhah 2003) and since these compounds are known to prolong the mean open time of SK channels (Pedarzani et al. 2001), their effect on spiking in mutant animals is ascribed to changes in SK current (Walter et al. 2006). These drugs, however, can also increase the open probability of BK channels (Liu et al. 2003). Since the current evoked during high-frequency trains that flowed in the interstep interval was largely IBTX-insensitive BK current, we tested whether it might be sufficiently sensitive to these agonists to modulate action potential firing.
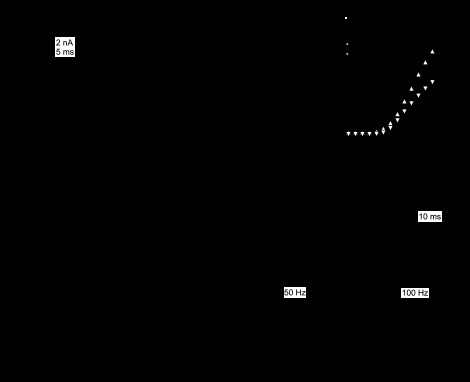
Indeed, application of 20 μM EBIO reliably and significantly enhanced the IBTX-insensitive, Cd-sensitive KCa current at voltages at which activation was submaximal (Fig. 5, A and B). At −25 mV, the current increased by 45%, from 1.3 ± 0.1 nA to 1.9 ± 0.2 nA (n = 6, P = 0.002). In 3 cells, addition of 200 nM apamin after EBIO application did not reduce the current, indicating that the EBIO did not act to unmask or augment SK current (control, 1.7 ± 0.2 nA; EBIO, 2.2 ± 0.2 nA; apamin, 2.2 ± 0.1 nA; control vs. EBIO, P = 0.008; EBIO vs. apamin, P = 0.5). EBIO also induced a modest but reliable hyperpolarization of the half-maximal activation voltage of the IBTX-insensitive KCa conductance measured 1–2 ms after repolarization (from −21 ± 0.6 to −25 ± 0.7 mV, P = 0.002), with no change in slope factor or maximal chord conductance at 0 mV (from k = 4.2 ± 0.2 to 4.6 ± 0.1 mV, EBIO, P = 0.25; from Gmax = 72.4 ± 7.8 to 71.7 ± 7.6 nS, P = 0.091; Fig. 5C). In addition, EBIO slowed the decay time constant of the outward tail current at −65 mV (from τ = 9.6 ± 0.23 to 17.4 ± 0.36 ms, P = 0.003). A similar prolongation of tail current was induced by chlorzoxazone (from 10.9 ± 0.4 ms to 17.4 ± 0.6 ms in 60 μM drug, P = 0.029, n = 3) and CyPPA (from 9.0 ± 0.5 ms to 18.2 ± 1.8 ms in 10 μM drug, P = 0.012, n = 4).
Fig. 5.
Augmentation of IBTX-insensitive, Cd-sensitive current by 1-ethyl-2-benzimidazolinone (EBIO). A, left: representative Cd-sensitive current recorded in IBTX evoked by a 20-ms step from −65 to −25 mV before (gray) or during (black) application of 20 μM EBIO. B: current-voltage relationships for the step (left) and tail (right) currents before (closed symbols) or after (open symbols) application of EBIO (n = 6). C: half-activation (V1/2) and slope factor (k) of the conductance-voltage curve, obtained from tail currents, with and without EBIO (n = 6). D, left: responses to a 50-Hz, 1-s train of 1-ms pulses to +20 mV (Vhold = −60 mV) before (top) or during (bottom) application of EBIO. Right traces are expansions of the current evoked by the final step. E: expansion of boxed region in D. F: interstep currents following the 20th pulse in D. G: peak interstep currents, measured 1 ms before the next step, during 50- or 100-Hz stimulation (n = 6). NS, nonsignificant.
As shown in Fig. 5D and expanded in Fig. 5, E and F, the total IBTX-insensitive current evoked by 50-Hz trains of brief depolarizing steps was inward during the depolarizing step, likely because the Ca current was activated, but the KCa current turned on only slowly. Repolarization elicited a large, rapidly deactivating inward Ca tail current, followed by a small, slower outward component. The outward current increased slightly although consistently over the first four stimuli, but decayed by ∼90% in the 20-ms interstep interval, so the current measured 1 ms before the onset of the next step did not accumulate greatly during the train. In the presence of EBIO, however, the increased amplitude and slowed deactivation of the current led to summation of outward current (Fig. 5, D, E, and F). As a consequence, by the end of the 50-Hz train, outward K tail current exceeded the inward Ca current during each step, and the amplitude of the peak outward current measured in the interstep interval had more than tripled (55 ± 5 pA vs. 190 ± 11 pA, n = 6, P = 0.002, Fig. 5G, left). Again, addition of apamin had almost no effect on the EBIO-enhanced tail current after 50-Hz trains (n = 2; current amplitude in EBIO relative to control, 402% and 614%, and in apamin relative to EBIO, 95% and 98%). With 100-Hz trains, the current that accumulated in control conditions was increased, and the effect of EBIO was greater than for 50-Hz trains (Fig. 5G, right). Thus, while EBIO has a relatively modest effect on peak currents evoked by a single long-step depolarization, it is likely to enlarge the outward currents in interspike intervals during repetitive firing.
To test whether EBIO altered the spontaneous activity of isolated Purkinje neurons, we recorded 5 s of spontaneous action potentials before and after application of 10 μM EBIO (Fig. 6, A and B). Within 1 s of application, EBIO decreased the firing rate and induced a small but consistent increase in the depth of the maximal AHP. Across cells (n = 18), the mean firing frequency dropped from 30 ± 3 Hz to 20 ± 2 Hz (P = 0.00035, Fig. 6C, left). The depth of the AHP increased only slightly, although significantly, from −71.1 ± 0.4 to −72.4 ± 0.2 mV (P = 0.0096, Fig. 6C, middle). The magnitude of the AHP change was not correlated with input resistance (R2 = 0.03). These results suggest either that this small difference in AHP is sufficient to delay the next spike and thereby slow firing, or that EBIO may exert additional effects on other currents that reduce the firing rate. Measuring the maximal rate of rise and decay (dV/dt) of the action potential in the presence or absence of EBIO, however, illustrated that neither the maximal slope of the upstroke nor the downstroke of the action potential was changed by the drug (Fig. 6C, right). This result is consistent with the idea that the effect of EBIO is largely restricted to increasing the interspike outward current, which, in turn, delays the approach to threshold and reduces the firing rate.
Fig. 6.

Modulation of spontaneous firing by EBIO. A: spontaneous action potentials of a Purkinje cell body before and during application of 10 μM EBIO (bar). B: a single action potential showing the small increase in the afterhyperpolarizations (AHP) in EBIO (gray). C: relationship between spontaneous firing rate (left), AHP amplitude (middle), and the maximal rate of rise and decay of a spike (dV/dt; right) in the absence or presence of EBIO (n = 18).
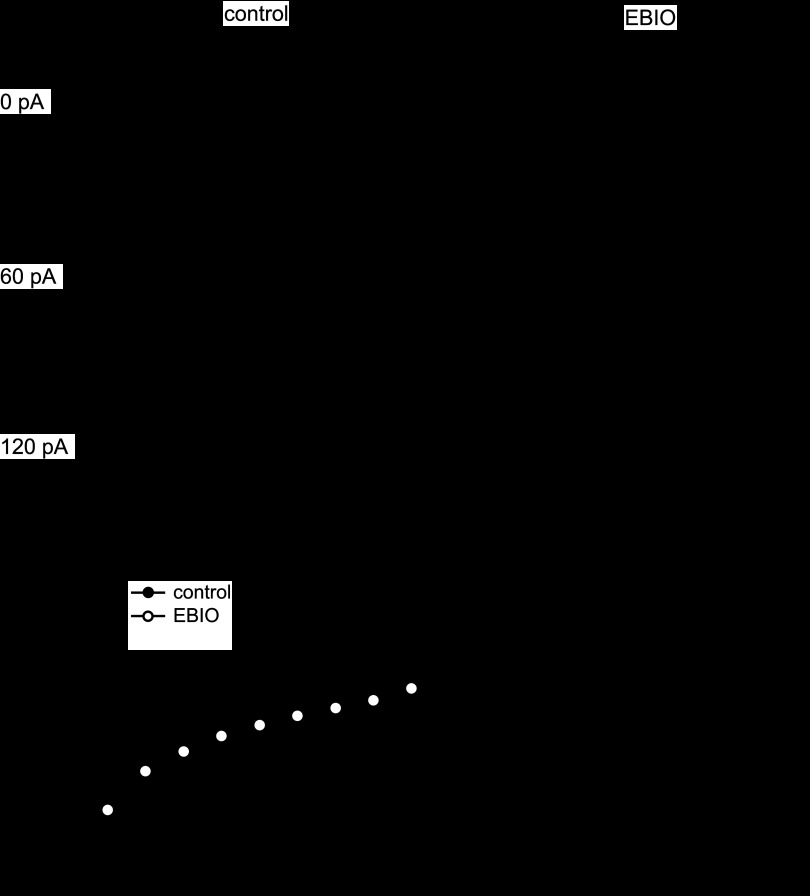
Given that the accumulation of IBTX-insensitive current was more pronounced with higher frequencies of stimulus trains, it seems likely that the greater the drive to fire, the more of a role this current will play. We, therefore, recorded input-output relationships (f-I curves) in the presence and absence of EBIO. Spike rate was modulated above and below the spontaneous rate with 200-ms current injections of −20 to +140 pA (in 20-pA increments; Fig. 7A, left). Under control conditions, progressively larger current injections increased the mean firing above the spontaneous rate at 4.7 ± 0.9 Hz/10 pA (n = 7). At all current injections, EBIO reduced the firing rate relative to control (Fig. 7A, right). The effect was greater at larger current injections, decreasing the slope of the f-I curve to 2.9 ± 0.4 Hz/10 pA in EBIO (P = 0.03, n = 7), such that, at the maximal current injection, the firing rate was only 66 ± 5 Hz, compared with 109 ± 9 Hz in control (P = 0.004, n = 7), and the action potential amplitude decreased through the step. Consistent with the voltage-clamp experiments, these results suggest that the EBIO-sensitive somatic current contributes most strongly to regulating the firing pattern in the face of external depolarization, effectively limiting maximal firing rate of Purkinje cells.
Fig. 7.
Modulation of the input-output curve by EBIO. A: spontaneous and driven action potentials evoked by 200-ms current injections (amplitudes as labeled) before (left) and after (right) application of 10 μM EBIO. Calibration bars apply to all traces. B: input-output curves in the absence (closed symbols) or presence (open symbols) of EBIO (n = 7).
DISCUSSION
The present data demonstrate that Purkinje somata express at least two distinct BK conductances: a rapidly activating and inactivating IBTX-sensitive current, and a slowly activating, non-inactivating IBTX-insensitive current. SK current, as defined by voltage independence and pharmacology, is not reliably present at high densities in Purkinje cell somata in this age range. In response to trains of depolarizations that approximate the voltage deflection and duration of action potentials, IBTX-sensitive BK channels generate several nanoamperes of outward current, but deactivate within a few milliseconds of repolarization. With repeated brief depolarizations, this current inactivates by 20–30% within a few steps. In contrast, the IBTX-insensitive BK current, which activates more slowly, does not activate greatly during brief spikelike depolarizations. With repeated brief steps, however, the tail currents can sum: the more rapid the train, the more the current accumulates in the interstep interval. Thus the properties of Purkinje cell IBTX-sensitive currents tailor them to participate in action potential repolarization and the early AHP, whereas IBTX-insensitive currents likely regulate activity at higher firing rates, curbing the acceleration of firing induced by depolarizing stimuli.
Properties of IBTX-sensitive BK currents.
IBTX-sensitive channels are probably expressed primarily in the somata of Purkinje cells, since dendritic perfusion of IBTX only slightly affects Purkinje cell firing (Womack et al. 2009). Moreover, immunogold labeling identifies BK channel clusters only on the soma and proximal dendrite, although scattered labeling is present throughout the somatodendritic membrane (Kaufmann et al. 2009). The primarily somatic location of these channels likely helps to restrict Ca spikes to the dendrites while preventing them from occurring in the soma.
IBTX-sensitive BK currents inactivated incompletely on a time scale of milliseconds. Inactivating BK currents have been observed in other cells, including chromaffin cells, some pancreatic β-cell lines, and hippocampal neurons (Hicks and Marrion 1998; Li et al. 1999; Neely and Lingle 1992; Solaro et al. 1995). Channels formed by α-subunits of BK channels (slo) generate non-inactivating currents, but inactivation can be induced via pore-block by β2- or β3-subunits (kcnmb2, 3), of which only β2 is found in brain (Brenner et al. 2000; Orio et al. 2002; Wallner et al. 1999; Xia et al. 1999, 2000). Whether Purkinje cells express β2 is unknown. In both expression systems and native tissues, however, inactivation of BK currents has generally been observed to require tens or hundreds of milliseconds, unlike the much briefer time course seen here, raising the question of whether this fast inactivation seen in Purkinje cells is a real gating property of the underlying channels and, if so, whether it is mediated by a similar mechanism. Evidence in favor of the former idea is that inactivation rates increased with larger depolarizations and with temperature, as expected for a true inactivation process. It was not accelerated by a 10-fold increase in EGTA, suggesting that it was not dictated by Ca buffering. It did not result from inactivating voltage-gated Ca currents, as T-type currents were inactivated and the remaining Ca currents do not inactivate on this time scale (Benton and Raman 2009). In response to brief steps, which evoke large, transient Ca tails rather than a sustained Ca influx (Benton and Raman 2009), the IBTX-sensitive current also inactivated rapidly and incompletely, indicating that the decay phase was not a function of the prolonged step. A rapid inactivation phase was absent from the larger total KCa current, indicating that it was not a clamp error. Finally, inactivation was present only in the IBTX-sensitive and not the IBTX-insensitive component, indicating that the phenomenon is specific to a defined fraction of KCa current.
One difference between the present study and much previous work is that the Ca source was influx through native voltage-gated Ca channels, generating a variable concentration at the channels, unlike exposure to a constant concentration of Ca by direct application or preloading through Ca channels (e.g., Solaro et al. 1995; Xia et al. 1999). Since Ca facilitates inactivation (Herrington et al. 1995; Neely and Lingle 1992; Xia et al. 2000), one possibility is that the fast component of inactivation seen in Purkinje neurons is only evident with a step from low to high Ca. Consistent with this idea, with high internal Ca, the total KCa current in Purkinje cells ran down, and IBTX-sensitive component of current lacked the millisecond-scale inactivation phase. Native Ca influx in neurons does not necessarily induce a rapid decay phase, however. In studies of dorsal root ganglion cells, IBTX-sensitive current evoked with depolarizations and normal Ca influx did not decay within milliseconds (Hendrich et al. 2012). Rapid block of BK currents is nevertheless not without precedent. In single-channel recordings from hippocampal neurons, depolarization of an open channel led to channel blockade within 3–4 ms, even in constant Ca (Hicks and Marrion 1998). It is possible, therefore, that other attributes of the recordings here reveal a concerted transition of open Purkinje BK channels to blocked states. Additional possibilities are that other factors in the Purkinje cell BK channel complex accelerate the time course of macroscopic inactivation or that a lower affinity of a blocking subunit permits a faster equilibration into inactivated states.
BK channels of Purkinje cells have previously been reported not to inactivate (Womack and Khodakhah 2002). In that study, channel openings were examined either by applying Ca within 50 ms to multichannel, inside-out patches at fixed potentials, or by subtracting responses to voltage steps applied in the presence and absence of Ca (cf., Widmer et al. 2003). With both methods, openings were still evident after sustained depolarization, indicative of non-inactivating channels. The present data are consistent with the idea that non-inactivating BK channels are present in Purkinje cells, but further suggest that the protocols of previous work might have precluded detection of rapidly inactivating BK channels.
Inactivation of the IBTX-sensitive component of Purkinje BK current was only partial, however. In contrast, in single-channel recordings from chromaffin cells (Solaro et al. 1995), BK channels either inactivate completely or not at all, and, in expression systems, the inclusion of inactivating β-subunits leads channels to inactivate completely (Wallner et al. 1999; Xia et al. 1999). Macroscopic current recordings from chromaffin cells further demonstrate that a single inactivation domain is required for channels to inactivate (Ding et al. 1998). The incomplete decay of IBTX-sensitive currents may result from the brevity of the voltage step (20 ms), if a slower component of inactivation requires a longer period of depolarization. Alternatively, the residual current may result from non-inactivating, IBTX-sensitive BK channels, which would presumably lack β2-, β3-, and β4-subunits.
Properties of IBTX-insensitive currents.
The IBTX-insensitive currents of Purkinje somata differ from IBTX-sensitive currents in that they activate more slowly upon depolarization and fail to inactivate. About two-thirds of the IBTX-insensitive current is blocked by 1 mM TEA, and 20% is blocked by curare, but the current is not significantly reduced by apamin, scyllatoxin, UCL1684, bicuculline methiodide, or TRAM-34. With constant, high intracellular Ca, large standing outward currents are absent, but depolarizing steps elicit voltage-gated currents, and, in the presence of IBTX, single-channel openings with conductances on the order of 150 pS are evident and increase with depolarization and Ca. Together, these data suggest that, while the SK current density is relatively low, Purkinje somata also express non-inactivating BK channels. These channels are likely to include the β4-subunit, which slows the activation rate and generates IBTX resistance (Behrens et al. 2000; Meera et al. 2000). The presence of inactivating, IBTX-sensitive BK channels, which likely include β2- (or β2-like) subunits, but no β4, as well as non-inactivating, IBTX-insensitive BK channels, which likely include β4-subunits, but no β2, is interesting in the context of work demonstrating that BK channels can assemble with four or fewer β-subunits (Wang et al. 2002). In expression systems, the number of β-subunits per α-subunit varies with ratio of transfection, whereas the present results suggest that Purkinje neurons have regulatory mechanisms that segregate subunits, even within the same cellular compartment.
Purkinje cells indeed express SK currents, although their location and developmental expression are still under investigation. In cerebellar slices, perfusion of distal Purkinje dendritic trees with apamin modulates firing (Womack and Khodakhah 2003). The effects of apamin are intensified by perfusing the entire cell, but whether the additional channels are in the proximal dendrite or soma remains indeterminate. Purkinje somata can express SK channels, as Swensen and Bean (2003) recorded scyllatoxin- and apamin-sensitive currents in cells isolated from P13–P17 mice. Recording at P14–P20, however, Khaliq et al. (2003) found SK current in only 12% of cells, under nominally the same recording conditions. The rarity of current blocked by SK antagonists in the present study, which examined P17–P21 mice, suggests that somatic expression of SK channels may decrease over the second postnatal week. Indeed, in situ hybridizations demonstrate a decrease of SK2 transcripts in rat Purkinje cells over the first 24 postnatal days, as well as loss of SK2 antibody labeling and decreased effects of apamin on firing between P12 and P60 (Cingolani et al. 2002). Physiological studies have demonstrated adult expression of apamin-sensitive current from somatodendritic perfusion of apamin, while immunolabeling reveals SK channels primarily in the dendritic arbor (Hosy et al. 2011; Womack and Khodakhah 2003). Together, the data are consistent with a developmental decrease of somatic SK channel expression in Purkinje cells.
KCa channels and regulation of Purkinje cell firing.
The somatic localization and high density of IBTX-sensitive and -insensitive BK currents positions them to regulate spiking, since Purkinje cell action potentials are driven largely by somatic currents and initiated in the initial segment and/or soma (Foust et al. 2010; Khaliq and Raman 2006; Palmer et al. 2010). Additionally, genetic deletion of BK channels produces ataxia and disrupted cerebellar learning (Sausbier et al. 2004). Interestingly, this loss of BK channels slows spontaneous firing in Purkinje cells, presumably because of a loss of hyperpolarizing drive and consequent depolarization block (Sausbier et al. 2004). Even when the knockout is restricted to Purkinje cells, the modified firing alters the cerebellar circuit (Chen et al. 2010).
The IBTX-sensitive current activates on the time scale of a spike, consistent with its known role in action potential repolarization (Edgerton and Reinhart 2003). The IBTX-insensitive current activates more slowly and apparently makes its primary contribution in the interspike interval. Its sensitivity to EBIO, chlorzoxazone, and CyPPA is potentially significant, since these drugs are useful in alleviating the symptoms of certain classes of ataxia. Specifically, in the ducky, leaner, and tottering mutations of P/Q-type Ca channels, which are models of episodic ataxia type 2, Ca influx is reduced, Purkinje firing patterns are irregular, and affected animals have movement disorders (Alviña and Khodakhah 2010; Walter et al. 2006). Application of EBIO or the clinically approved chlorzoxazone can regularize firing and reduce dyskinesia (Alviña and Khodakhah 2010; Walter et al. 2006). Since these drugs affect SK channels, these results have led to the conclusion that the increasing SK currents are the primary mechanism by which these compounds exert their therapeutic effect. The present data, however, suggest that a complementary action of these ataxia-relieving drugs may be to enhance BK currents, which, in turn, influence Purkinje cell output.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS39395 (I. M. Raman) and F31 NS073340 (M. D. Benton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.B., A.H.L., J.S.B., and I.M.R. conception and design of research; M.D.B., A.H.L., and J.S.B. performed experiments; M.D.B., A.H.L., J.S.B., and I.M.R. analyzed data; M.D.B., A.H.L., J.S.B., and I.M.R. interpreted results of experiments; M.D.B., A.H.L., J.S.B., and I.M.R. prepared figures; M.D.B. and I.M.R. drafted manuscript; M.D.B., A.H.L., J.S.B., and I.M.R. approved final version of manuscript; A.H.L., J.S.B., and I.M.R. edited and revised manuscript.
REFERENCES
- Alviña K, Khodakhah K. KCa channels as therapeutic targets in episodic ataxia type-2. J Neurosci 30: 7249–7257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens R, Nolting A, Reimann F, Schwarz M, Waldschütz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett 474: 99–106, 2000 [DOI] [PubMed] [Google Scholar]
- Benton MD, Raman IM. Stabilization of Ca current in Purkinje neurons during high-frequency firing by a balance of Ca-dependent facilitation and inactivation. Channels (Austin) 3: 393–401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterisation of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000 [DOI] [PubMed] [Google Scholar]
- Cao Y, Dreixler JC, Roizen JD, Roberts MT, Houamed KM. Modulation of recombinant small-conductance Ca(2+)-activated K(+) channels by the muscle relaxant chlorzoxazone and structurally related compounds. J Pharmacol Exp Ther 296: 683–689, 2001 [PubMed] [Google Scholar]
- Chen X, Kovalchuk Y, Adelsberger H, Henning HA, Sausbier M, Wietzorrek G, Ruth P, Yarom Y, Konnerth A. Disruption of the olivo-cerebellar circuit by Purkinje neuron-specific ablation of BK channels. Proc Natl Acad Sci USA 107: 12323–12328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Gymnopoulos M, Boccaccio A, Stocker M, Pedarzani P. Developmental regulation of small-conductance Ca2+-activated K+ channel expression and function in rat Purkinje neurons. J Neurosci 22: 4456–4467, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Cox DH, Aldrich RW. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels. J Gen Physiol 109: 647–673, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JP, Li ZW, Lingle CJ. Inactivating BK channels in rat chromaffin cells may arise from heteromultimeric assembly of distinct inactivation-competent and noninactivating subunits. Biophys J 74: 268–289, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol 548: 53–69, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbers JDT, Anderson D, Asmara H, Reha R, Mehaffey WH, Hameed S, McKay BE, Kruskic M, Zamponi GW, Turner RW. Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells. Proc Natl Acad Sci U S A 109: 2601–2606, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro L, Llano I. High endogenous calcium buffering in Purkinje cells from rat cerebellar slices. J Physiol 496: 617–625, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust A, Popovic M, Zecevic D, McCormick DA. Action potentials initiate in the axon initial segment and propagate through axon collaterals reliably in cerebellar Purkinje neurons. J Neurosci 30: 6891–6902, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron 19: 665–678, 1997 [DOI] [PubMed] [Google Scholar]
- Hendrich J, Alvarez P, Chen X, Levine JD. GDNF induces mechanical hyperalgesia in muscle by reducing I(BK) in isolectin B4-positive nociceptors. Neuroscience 219: 204–213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J, Solaro CR, Neely A, Lingle CJ. The suppression of Ca(2+)- and voltage-dependent outward K+ current during mAChR activation in rat adrenal chromaffin cells. J Physiol 485: 297–318, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GA, Marrion NV. Ca2+-dependent inactivation of large conductance Ca2+-activated K+ (BK) channels in rat hippocampal neurones produced by pore block from an associated particle. J Physiol 508: 721–734, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E, Piochon C, Teuling E, Rinaldo L, Hansel C. SK2 channel expression and function in cerebellar Purkinje cells. J Physiol 589: 3433–3440, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard C, Eriksen BL, Jørgensen S, Johansen TH, Dyhring T, Madsen LS, Strøbaek D, Christophersen P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol 151: 655–665, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WA, Kasugai Y, Ferraguti F, Storm JF. Two distinct pools of large-conductance calcium-activated potassium channels in the somatic plasma membrane of central principal neurons. Neuroscience 169: 974–986, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Gouwens NW, Raman IM. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J Neurosci 23: 4899–4912, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Raman IM. Relative contributions of axonal and somatic Na channels to action potential initiation in cerebellar Purkinje neurons. J Neurosci 26: 1935–1944, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflügers Arch 438: 314–321, 1999 [DOI] [PubMed] [Google Scholar]
- Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273: 1709–1714, 1996 [DOI] [PubMed] [Google Scholar]
- Lacinová L, Klugbauer N, Hofmann F. Regulation of the calcium channel alpha(1G) subunit by divalent cations and organic blockers. Neuropharmacology 39: 1254–1266, 2000 [DOI] [PubMed] [Google Scholar]
- Lang DG, Ritchie AK. Tetraethylammonium blockade of apamin-sensitive and insensitive Ca2+-activated K+ channels in a pituitary cell line. J Physiol 425: 117–132, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SI, Khaliq ZM, Aman TK, Grieco TM, Kearney JA, Raman IM, Meisler MH. Impaired motor function in mice with cell-specific knockout of sodium channel Scn8a (NaV1.6) in cerebellar Purkinje neurons and granule cells. J Neurophysiol 96: 785–793, 2006 [DOI] [PubMed] [Google Scholar]
- Li ZW, Ding JP, Kalyanaraman V, Lingle CJ. RINm5f cells express inactivating BK channels whereas HIT cells express noninactivating BK channels. J Neurophysiol 81: 611–624, 1999 [DOI] [PubMed] [Google Scholar]
- Liu YC, Lo YK, Wu SN. Stimulatory effects of chlorzoxazone, a centrally acting muscle relaxant, on large conductance calcium-activated potassium channels in pituitary GH3 cells. Brain Res 959: 86–97, 2003 [DOI] [PubMed] [Google Scholar]
- Marty A, Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol 367: 117–141, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A 97: 5562–5627, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely A, Lingle CJ. Two components of calcium-activated potassium current in rat adrenal chromaffin cells. J Physiol 453: 97–131, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci 17: 156–161, 2002 [DOI] [PubMed] [Google Scholar]
- Palmer LM, Clark BA, Gründemann J, Roth A, Stuart GJ, Häusser M. Initiation of simple and complex spikes in cerebellar Purkinje cells. J Physiol 588: 1709–1717, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem 276: 9762–9769, 2001 [DOI] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron 19: 881–891, 1997 [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci 19: 1663–1674, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan LJ. Voltage-dependent calcium currents in Purkinje cells from rat cerebellar vermis. J Neurosci 11: 2259–2269, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossokhin A, Teodorescu G, Grissmer S, Zhorov BS. Interaction of d-tubocurarine with potassium channels: molecular modeling and ligand binding. Mol Pharmacol 69: 1356–1365, 1996 [DOI] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA 101: 9474–9478, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro CR, Prakriya M, Ding JP, Lingle CJ. Inactivating and noninactivating Ca2+- and voltage-dependent K+ current in rat adrenal chromaffin cells. J Neurosci 15: 6110–6123, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøbaek D, Jørgensen TD, Christophersen P, Ahring PK, Olesen SP. Pharmacological characterization of small-conductance Ca(2+)-activated K(+) channels stably expressed in HEK 293 cells. Br J Pharmacol 129: 991–999, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J Neurosci 23: 9650–9663, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, Austin CP, Bennett PB, Swanson R. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J Biol Chem 275: 23211–23218, 2000 [DOI] [PubMed] [Google Scholar]
- Vogalis F, Zhang Y, Goyal RK. An intermediate conductance K+ channel in the cell membrane of mouse intestinal smooth muscle. Biochim Biophys Acta 1371: 309–316, 1998 [DOI] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc Natl Acad Sci USA 96: 4137–4142, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YW, Ding JP, Xia XM, Lingle CJ. Consequences of the stoichiometry of Slo1 α and auxiliary β subunits on functional properties of large-conductance Ca2+-activated K+ channels. J Neurosci 22: 1550–1591, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter JT, Alviña K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci 9: 389–397, 2006 [DOI] [PubMed] [Google Scholar]
- Widmer HA, Rowe IC, Shipston MJ. Conditional protein phosphorylation regulates BK channel activity in rat cerebellar Purkinje neurons. J Physiol 552: 379–391, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci 22: 10603–10612, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar Purkinje neurons. J Neurosci 23: 2600–2607, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci 24: 3511–3521, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci 24: 8818–1822, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Hoang C, Khodakhah K. Large conductance calcium-activated potassium channels affect both spontaneous firing and intracellular calcium concentration in cerebellar Purkinje neurons. Neuroscience 162: 989–1000, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci 19: 5255–5264, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Zeng XH, Duan KL, Lingle CJ. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel beta subunit. J Neurosci 20: 4890–4903, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Xia XM, Lingle CJ. Species-specific differences among KCNMB3 BK beta3 auxiliary subunits: some beta3 N-terminal variants may be primate-specific subunits. J Gen Physiol 132: 115–129, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]