Abstract
Using a mouse brain slice preparation, we studied the modulatory effects of a feedback projection from higher visual cortical areas, mostly or exclusively area LM (or V2), on two inputs to layer 4 cells in the first visual area (V1). The two inputs to these cells were geniculocortical and an unspecified intracortical input, possibly involving layer 6 cells. We found that activation of metabotropic glutamate receptors (mGluRs) from stimulation of the feedback projection reduced the evoked excitatory postsynaptic currents of both of these inputs to layer 4 but that this modulation acts in an input-specific way. Reducing the strength of the geniculocortical input in adults involved both presynaptic and postsynaptic group I mGluRs (although in younger animals presynaptic group II mGluRs were also involved), whereas modulation of the intracortical input acted entirely via postsynaptic group II mGluRs. These results demonstrate that one of the effects of this feedback pathway is to control the gain of geniculocortical transmission.
Keywords: modulation, thalamocortical, metabotropic glutamate
in thalamic and cortical circuitry, glutamatergic pathways can be divided in two main groups, Class 1 and Class 2, on the basis of their synaptic properties (Sherman and Guillery 2006, 2011). We have suggested that Class 1 afferents represent the main information to be processed, whereas Class 2 afferents serve a mainly modulatory function. Much of this Class 2 function is related to the fact that they can activate metabotropic glutamate receptors (mGluRs). To try to understand how this might serve to modulate circuit activity, we have begun to test the effects of mGluR activation on modulation of various inputs in cortex. We have recently reported that mGluR activation from local Class 2 inputs within a region of cortex reduces the amplitude of evoked thalamocortical excitatory postsynaptic currents (EPSCs) from Class 1 inputs to the same target neuron (De Pasquale and Sherman 2012; Lee and Sherman 2012). In the present study, we wanted to extend this understanding by studying the effects of a Class 2 input from the lateromedial visual area (LM) (Berezovskii et al. 2011; Coogan and Burkhalter 1993), which we refer to as V2 for simplicity (see materials and methods for terminology), to the primary visual area, or V1. We tested the modulatory properties of mGluR activation by this feedback pathway on two inputs to layer 4 cells in V1, one Class 1, which we conclude to be geniculocortical, and the other Class 2, which we conclude to be intracortical and possibly largely from layer 6. We found that EPSCs evoked from both inputs were reduced by mGluR activation from the feedback pathway, but different mGluR types and locations, presynaptic and postsynaptic, were involved in these effects. We propose that modulatory feedback projections from V2 control the gain of thalamocortical inputs to V1 through the activation of metabotropic glutamatergic receptors.
MATERIALS AND METHODS
Preparation and Maintenance of Brain Slices
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Chicago. Techniques used have been described previously (De Pasquale and Sherman 2011, 2012) and are briefly repeated here. We performed all experiments on tissue slices prepared from BALB/c mice of either sex, most fully adult (Harlan; 60–65 days postnatal) except for some described in results that were juvenile (Harlan; 10–13 days postnatal). Animals were deeply anesthetized by inhalation of isoflurane (AErrane; Baxter Pharmaceuticals) and then decapitated. The brain was quickly removed and submerged in cooled (0°C) oxygenated (5% CO2-95% O2) modified artificial cerebrospinal fluid (ACSFmodified; in mM: 206 sucrose, 25 NaHCO3, 2.5 KCl, 10 MgSO4, 1.25 NaH2PO4, 0.5 CaCl2, and 11 d-glucose). Brains were blocked and vibratome sectioned (Campden Instruments, Lafayette, IN). Slices (∼500 μm thick) were cut at a 20–25° anterior tilt relative to the coronal plane and rapidly transferred to a holding chamber with artificial cerebrospinal fluid (ACSF; in mM: 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, and 25 d-glucose). The slices were kept oxygenated for 1 h at room temperature (20–25°) prior to recording. The slices were then placed in a submersion-type recording chamber on a modified microscope stage and maintained at 32°C with constant perfusion of ACSF. In experiments designed to block synaptic activity, we used an ACSF containing low Ca2+ and high Mg2+ concentrations (ACSFLow Calcium; in mM: 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 6 MgCl2, 0.2 CaCl2, and 25 d-glucose).
Identification of Visual Cortical Areas
We identified visual cortical areas and cortical layers following the same criteria we used previously (De Pasquale and Sherman 2011). Briefly, different areas (V1 and LM or V2) and layers were identified by differential interference contrast microscopy. V1 had a higher cell body density than did V2. Different packing densities also served to distinguish cortical layers. Layer 1 was typically thin and aneuronal. Layer 4 and layer 5b showed dense packing of cell bodies. Layers 2/3 can be recognized as located between layers 1 and 4, so layer 5a was identified as lying between layers 4 and 5b. Layer 6 had a lower cell density than layer 5b and was located between that layer and the white matter. As we have noted previously, what we refer to as “V2” is equivalent to the area “LM” defined by others (Berezovskii et al. 2011; Coogan and Burkhalter 1993; Wang and Burkhalter 2007), but we want to make it clear that we use the term provisionally, because the homology between LM and V2 is not completely established (see Wang and Burkhalter 2007).
Electrophysiological Recordings
Whole cell recordings were obtained from neurons of layer 4 of primary visual cortex (V1). Recording pipettes were fabricated from borosilicate glass (Garner Glass) with input resistances of ∼6–10 MΩ and were filled with intracellular solution (in mM: 135 K-gluconate, 7 NaCl, 10 HEPES, 2 Na2ATP, 0.3 Na3GTP, 2 MgCl2, 1 DNDS, and 0.1–0.5% biocytin; at a pH of 7.3 obtained with KOH and osmolality of 290 mosM obtained with distilled water).
In all our recordings, we used 4,4′-dinitrostilbene-2,2′-disulfonic acid (DNDS; Molecular Probes) to isolate excitatory inputs by blocking GABAergic inputs to the recorded cell without affecting the inhibitory circuitry more generally (Covic and Sherman 2011; De Pasquale and Sherman 2011, 2012; Dudek and Friedlander 1996). DNDS is placed into the recording pipette and diffuses into the recorded neuron. In some experiments designed to interfere with the postsynaptic activity of mGluRs, we added GDPβS (1 mM) to the intracellular solution in the recording pipette. GDPβS is a nonhydrolyzable GTP analog that blocks G protein-coupled activity.
All experiments were performed using a visualized slice setup under a differential interference contrast-equipped Axioscop 2FS microscope (Carl Zeiss Instruments). Recordings were made by using a Multiclamp 700B amplifier and pClamp software (Axon Instruments). Only cells having a stable access resistance of <20 MΩ were kept for recording. We injected hyperpolarizing currents to identify IH and depolarizing currents to identify regular, tonic, or bursting spike patterns. Postsynaptic responses were recorded in both current and voltage clamp.
Laser Uncaging of Glutamate
We used our previously described methods for photostimulation to identify inputs connected to each recorded neuron (Covic and Sherman 2011; De Pasquale and Sherman 2011, 2012; Lam and Sherman 2005). Data acquisition and photostimulation were controlled by a program written in MATLAB (The MathWorks, Natick, MA). Nitroindolinyl-caged glutamate (Sigma-RBI; Canepari et al. 2001) was added to recirculating ACSF with a concentration of 0.37 mM during recording. Focal photolysis of caged glutamate was accomplished by a pulsed UV laser (355-nm wavelength, frequency-tripled Nd:YVO4, 100-kHz pulse repetition rate; DPSS Laser, San Jose, CA) to provide three 1-ms, 100-pulse light stimuli. Neutral density filters controlled the intensity of the stimuli. The laser beam (5-mW intensity) was directed into the side port of the Axioscop 2FS microscope using a pair of mirror galvanometers (Cambridge Technology, Cambridge, MA) and then focused onto the brain slice using a low-magnification objective lens (×10/0.3 NA/water UMPlanFl or ×4/0.13 NA/air UPlanFl; Olympus). Angles of the galvanometers were computer controlled and determined the position stimulated by the laser. The optics was designed to generate a nearly cylindrical beam in the slice to keep the mapping two dimensional. The Q-switch of the laser and a shutter (LS3-ZM2; Vincent Associate) controlled the timing of the laser pulse for stimulation.
During experiments, a variable neutral density wheel (Edmund, Barrington, NJ) controlled the power of photostimulation at different levels by attenuating the intensity of the laser. A thin microscope coverslip in the laser path reflected a small portion of the laser onto a photodiode. The photodiode output was amplified, acquired by the computer, and used to monitor the laser intensity during the experiment. The photodiode output was calibrated to the laser power at the back focal plane of the objective. The laser power was measured using a power meter (Thorlabs). The beam expansion was limited to a twofold gain through the scan lens–tube lens pair. The beam underfilled the objective and could be focused on a spot in the specimen plane that was ∼10 μm in the x-y dimension and larger (≫100 μm) in the z-axis. Voltage traces were recorded and quantified during the 100-ms period following UV stimulus onset, using custom software.
In our experiments, we followed two different protocols of photostimulation. The first protocol was used to map regions of the second cortical visual area (V2) to localize the excitatory inputs to cells of layer 4 in V1. Our criteria for monosynaptically evoked responses from photostimulation included a response latency <3 ms, latency jitter <1 ms, and very rare failures. A preset stimulation grid was used to map all layers and regions of V2. Stimulation was organized according to a sequence that maximized the distance and time between consecutive trials to avoid receptor desensitization, local caged-glutamate depletion, and excitotoxicity. The second protocol of photostimulation was used in experiment aimed to isolate direct stimulation by using the low-Ca2+, high-Mg2+ ACSF. Here we evoked postsynaptic responses by directly applying photostimulation on to the recorded cell. We used a preset grid that limited stimulation to an area within 50 μm from the recorded neuron.
Electrical Stimulation
In most of our experiments, we electrically stimulated inputs to neurons recorded in layer 4 of V1. We did this by using bipolar concentric electrodes (FHC; 125-μm pole distance). Stimulating electrodes were placed in the white matter below V1 and/or in V2. In the first case, the electrode was placed in the white matter below layer 6, ventrally in line with the cell recorded in layer 4. In the second case, the electrode was placed in V2 in a location previously guided by photostimulation. Electrical stimulation consisted of 0.1- to 0.2-ms pulses and ranged from 5 to 130 Hz. We only included in our data responses we determined to be monosynaptic by meeting the following criteria: latencies <3.5 ms, latency jitter <1 ms, and no or very rare failures for stimulation levels above threshold. The intensity of stimulation was gradually increased from a subthreshold level by steps of 5 μA until a response was evoked. The intensity was then further increased by 5 μA for data recording. We used stimulation intensities between 100 and 300 μA.
Pharmacology
Various agonists and antagonists to mGluRs were bath applied. The choices of chemicals and concentrations were based on our previous experience with these agents (Covic and Sherman 2011; De Pasquale and Sherman 2011, 2012; Lee and Sherman 2008. 2009a; Reichova and Sherman 2004). Agonist and antagonist stock solutions were prepared in distilled water or DMSO and diluted in ACSF to their final concentration just before use. During the drug application, the system of tubes providing ACSF to the recording chamber was connected to a 5-ml container of ACSF. Agents were bath applied by slowly injecting a 0.2-ml bolus into the tube providing ACSF to the recording chamber. The ACSF was continuously added to the chamber at 1.7 ml/min by a motorized peristaltic pump in a recirculating mode. We calculated the final bath concentration on the basis of the total volume of the bolus, the recording chamber, the tubes, and the small container of ACSF.
Specific antagonists (TOCRIS, Ellisville, MO) were used to block the activity of mGluRs: the type 1 mGluR antagonist LY-367385 (50 μM), the type 5 mGluR antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP; 30 μM), and the group II mGluR antagonist LY-341495 (100 μM). In some experiments, we activated mGluR pharmacologically, using the following agonists (TOCRIS): the general mGluR agonist 1-aminocyclopentane-1,3-dicarboxylic acid (ACPD; 100 μM), the group I mGluR agonist 5-dihydroxyphenylglycine (DHPG; 100 μM), and the group II mGluR agonist 4-aminopyrrolidine-2,4-dicarboxylate (APCD; 100 μM). In some experiments, antagonists to ionotropic glutamate receptors (iGluRs; TOCRIS) were also used: the AMPA receptor antagonist 7-dinitroquinoxaline-2,3-dione (DNQX; 50 μM) and the NMDA receptor antagonist MK-801 (40 μM). All stimulation protocols were performed 5 min after the end of the application of each antagonist. In some experiments, we tested more than one antagonist on the same cell. In these cases, each new antagonist was administrated only after the previous one had been washed out for 10 min.
Each cell was tested with one, two, or three mGluR antagonists, depending on the period the cell could be held in a healthy condition. This period was on average 30 min.
Identification of Glutamatergic Inputs and Their Terminology
The synaptic properties we tested in the present study are those we had previously adopted to identify glutamatergic pathways as members of two classes: Class 1 or Class 2 (Covic and Sherman 2011; De Pasquale and Sherman 2011, 2012; Lee and Sherman 2008, 2009a; Reichova and Sherman 2004; Sherman and Guillery 1998; Viaene et al. 2011b, 2011c). This classification was originally made in thalamus (Sherman and Guillery 1998, 2006) and proved to be useful to study the functional role of glutamatergic connections. Among other differences, Class 1 inputs show paired-pulse depression, whereas Class 2 inputs show paired-pulse facilitation; Class 1 inputs evoke a larger initial EPSC; Class 2 inputs are activated in a more graded manner; and Class 1 inputs activate only iGluRs, whereas Class 2 inputs also activate mGluRs. In practice, we have found that not all criteria need be evaluated to identify an input as Class 1 or 2. Thus an input showing paired-pulse depression and an all-or-none activation profile is sufficient to be identified as Class 1; likewise, an input showing paired-pulse facilitation and a graded activation profile is sufficient to be identified as Class 2.
Although not generally relevant to this study, in other pathways, we have shown subtypes to the Class 1 group (Covic and Sherman 2011; De Pasquale and Sherman 2011; Lee and Sherman 2008; Petrof and Sherman 2009; Reichova and Sherman 2004; Viaene et al. 2011a, 2011b, 2011c). Class 1A inputs have a strictly all-or-none activation pattern, whereas Class 1B inputs have a pattern intermediate between all-or-none and the graded pattern seen in Class 2 inputs, and Class 1C inputs have a curious paired-pulse pattern whereby the first two EPSP/Cs show facilitation and the remainder in a train show depression. Because this subclassification is not generally relevant to this study, we shall simply use the term “Class 1” for these inputs. For completeness, however, the Class 1 input we describe in V1 from the lateral geniculate nucleus to layer 4 is Class 1A, and that from V2 to V1 is Class 1B.
RESULTS
All data were collected by recording from cells patched in layer 4 of V1. Whole cell recordings of a total of 99 cells used for analysis in this study were performed in voltage and current clamp. All data obtained from voltage-clamp recordings were collected from neurons clamped at −65 mV. Neurons had resting membrane potentials of −66.56 ± 0.47 mV and input resistances of 212.18 ± 0.73 MΩ. All neurons had a regular spiking pattern. Ten of the recorded neurons were successfully filled with biocytin, and all were found to be pyramidal.
Synaptic Properties of Inputs from White Matter to Layer 4 of V1
We performed a preliminary study of the synaptic properties of inputs from the white matter to neurons of layer 4 in V1. We activated those inputs, thereby evoking EPSCs, by placing a stimulating electrode in the white matter below layer 6 and aligned vertically to the patched cell in layer 4. We only included in our data responses having properties consistent with monosynaptic activation as defined in materials and methods. On the basis of these criteria, we sampled from a total of 1,388 cells to obtain the 99 (7.1%) for which monosynaptic inputs could be evoked; only these 99 cells are considered further. Subsets of the 99 cells for various experiments in this study are indicated below.
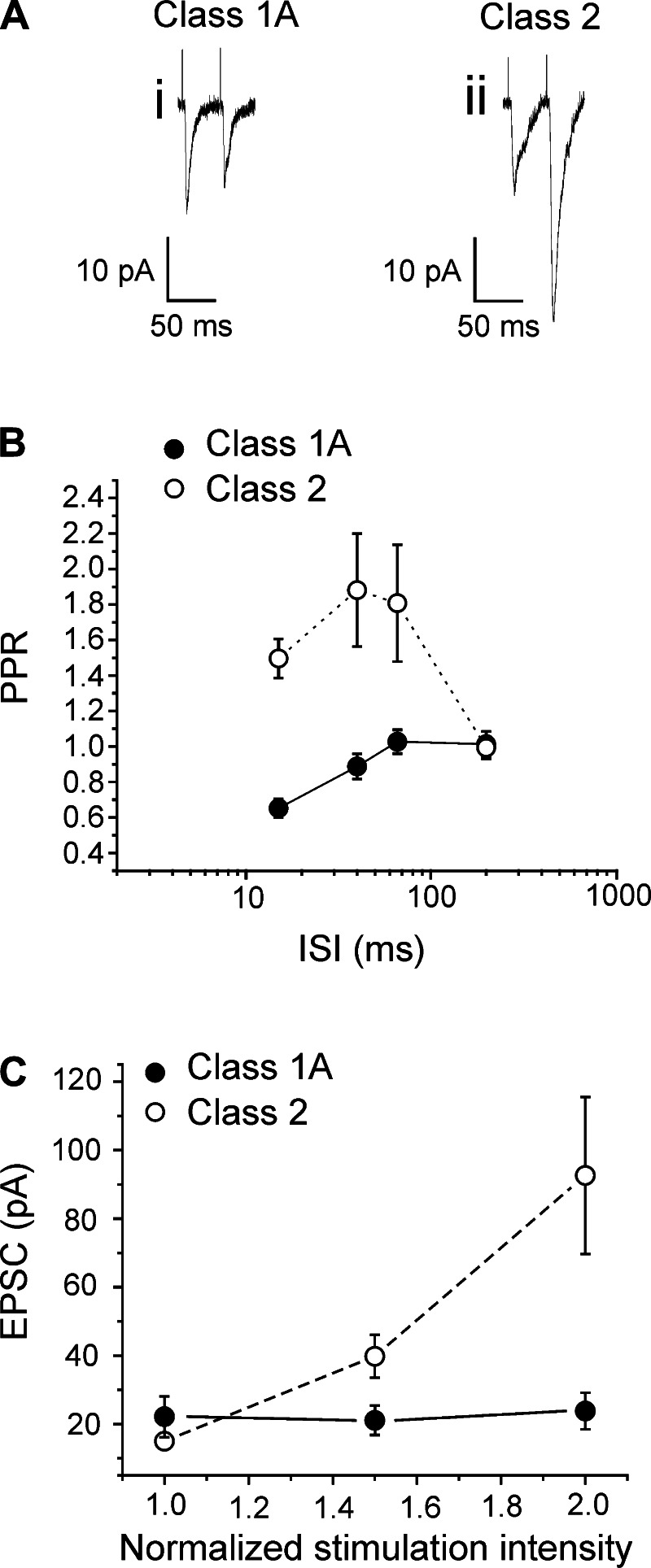
In this way, synaptic properties of inputs were measured initially in 13 neurons located in layer 4. When stimulated at various appropriate frequencies, nine cells showed paired-pulse depression and the other four, paired-pulse facilitation (Fig. 1, A and B). The difference between the two groups, in terms of paired-pulse ratio, was greater at higher frequencies of stimulation (2-way ANOVA, P < 0.01; Fig. 1B). The effect of paired-pulse depression did not depend on the amplitude of the response [P > 0.1 for interstimulus interval (ISI) = 40 ms and for ISI = 15 ms on a Pearson significance test]. The cells exhibiting depression also showed all-or-none responses, whereas those showing facilitation had a graded activation pattern (Fig. 1C), and these activation patterns were statistically different (2-way ANOVA, P < 0.01). These findings indicate that nine of these cells received Class 1 inputs from the white matter, whereas the other four received Class 2 inputs (see materials and methods for the criteria used to identify these classes). In fact, because of the all-or-none activation patterns seen with this white matter stimulation, we identify these as a subtype of Class 1 input referred to as Class 1A (Covic and Sherman 2011; De Pasquale and Sherman 2011; Viaene et al. 2011b, 2011c). Class 1A and Class 2 responses were recorded from cells having similar membrane properties. Cells receiving Class 1 inputs were not statistically different from cells receiving Class 2 inputs either in terms of input resistance and capacitance or in various other responses to injected currents (t-test, P > 0.1 for all tests). As explained in more detail in discussion, we conclude that the Class 1A responses reflect activation of geniculocortical axons, which are known to produce Class 1A responses in layer 4 neurons, whereas those exhibiting Class 2 responses are more difficult to identify as to origin but plausibly include layer 6 corticogeniculate axons, which branch to innervate layer 4 (Lee and Sherman 2008, 2009b; Stratford et al. 1996) (see discussion).
Fig. 1.
Synaptic properties of inputs from the white matter to layer 4 of primary visual area (V1). A: examples of Class 1 response (i), showing paired-pulse depression, and Class 2 response (ii) showing paired-pulse facilitation. B: graph showing how the paired-pulse ratio [PPR; amplitude of the second excitatory postsynaptic current (EPSC) divided by that of the first] in both Class 1 and Class 2 responses (mean ± SE) increased with the decrease of the interval between the 2 stimuli. The difference between the 2 classes is greater at shorter interstimulus intervals (ISI; 2-way ANOVA, P < 0.01). C: graph showing the activation patterns of Class 1 and Class 2 inputs. The average first EPSC amplitude (mean ± SE) is plotted against the stimulation intensity normalized to threshold value, meaning that the abscissa is divided into units that are multiples of the threshold current. The activation patterns of the 2 groups are significantly different (2-way ANOVA, P < 0.01).
Effects of mGluR Activation on Class 1 Input from White Matter to Layer 4
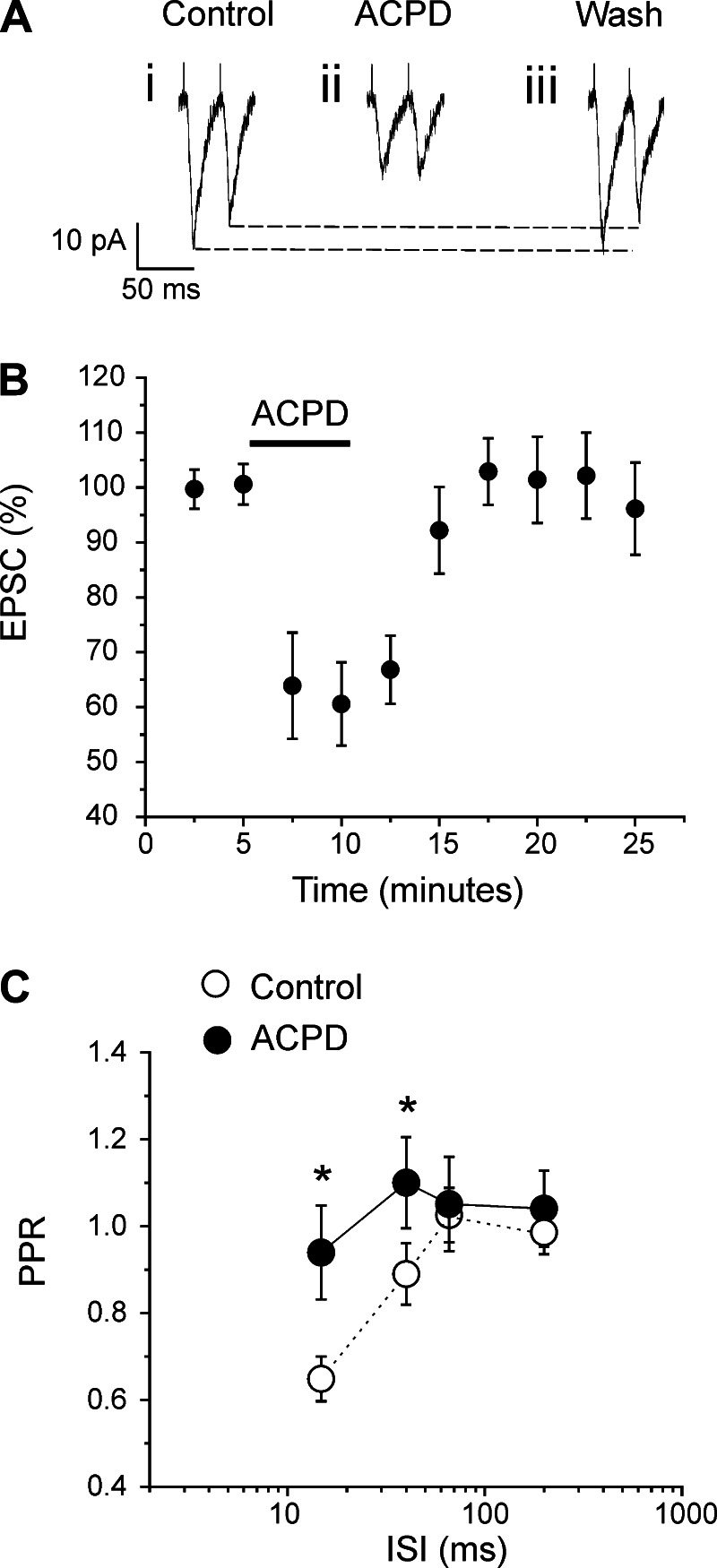
Because Class 2 inputs are known to innervate layer 4 cells, and because these inputs activate mGluRs (Covic and Sherman 2011; Lee and Sherman 2009b; Petrof et al. 2012), we investigated the role of such activation on the Class 1 input from the white matter to layer 4 of V1. We did this by testing the effects of the general mGluR agonist ACPD on nine cells, and Fig. 2 shows the results. The agonist was administrated for 5 min, and EPSCs were recorded before, during, and after the drug application (Fig. 2B). Two minutes after bath application, ACPD caused a roughly 40% decrease of EPSC amplitudes (Fig. 2, A and B; P < 0.001, repeated-measures ANOVA).
Fig. 2.
Effects of the general muscarinic glutamate receptor (mGluR) agonist 1-aminocyclopentane-1,3-dicarboxylic acid (ACPD) on the synaptic properties of Class 1 inputs to layer 4 cells in V1 activated from the white matter. A: example from 1 cell showing effects of ACPD on synaptic responses. B: graph showing population data in the form of normalized amplitudes of the first EPSC before, during, and after ACPD application. The EPSC amplitude (mean ± SE) was significantly decreased by ACPD (P < 0.001, repeated-measures ANOVA). C: graph showing that ACPD application greatly increased PPR (mean ± SE, see Fig. 1; *P < 0.05, repeated-measures ANOVA, for ISI = 15 and 40 ms). With ACPD, such depression was abolished at all intervals (P > 0.1, on a repeated measure ANOVA).
Activation of mGluRs is known in some systems to affect short-term plasticity by affecting paired-pulse ratios (Bandrowski et al. 2002; Gerber et al. 2000; O'Leary et al. 1997). We thus used the same nine cells mentioned above to study the action of ACPD on this parameter on the Class 1 input by measuring the amplitudes of the first two evoked EPSCs at different stimulation frequencies. For each frequency, 10 trials were recorded and averaged. Between each trial was a 3-s waiting period. For the control condition, paired-pulse protocols were applied 3 min before the application of the drug. For the ACPD condition, the same protocols were applied 3 min after the administration of ACPD.
ACPD caused a significant, roughly 40% decrease of both evoked EPSCs at long ISIs (200 and 66 ms; P < 0.001, repeated-measures ANOVA for the first EPSC; P < 0.01, repeated-measures ANOVA for the second EPSC). At a 40-ms interval, the second EPSC was less reduced (∼20%) than the first (∼40%) (P < 0.001, repeated-measures ANOVA for the first EPSC; P < 0.05, repeated-measures ANOVA for the second EPSC). At a 15-ms interval, only the first EPSC was significantly decreased (P < 0.001, repeated-measures ANOVA for the first EPSC; P > 0.1, repeated-measures ANOVA for the second EPSC). Thus, at short ISIs (15 and 40 ms), the first EPSC is more affected then the second. This leads, at these stimulation frequencies, to an increase in the paired-pulse ratio, which is the amplitude of the second EPSC divided by that of the first, and this means a reduced paired-pulse depression (P < 0.05, repeated-measures ANOVA; Fig. 2C).
Characterization of Inputs from V2 to Layer 4 of V1
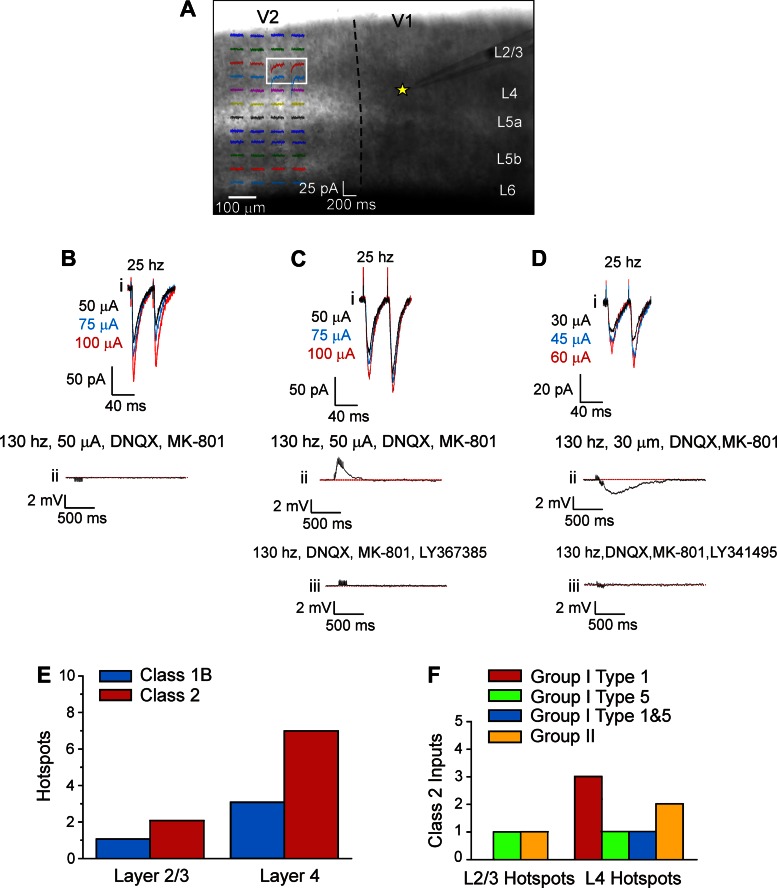
We recently showed that neurons of layer 4 of V1 receive Class 2 inputs that activate mGluRs from V2 (De Pasquale and Sherman 2011). Because that study was performed in juvenile mice (postnatal days 11–19), we aimed to verify this general finding in fully adult animals using the same techniques as we did previously. We thus activated inputs from V2 onto 11 neurons in layer 4 of V1, using photostimulation (i.e., laser uncaging of glutamate) to map the regions in V2 in which the afferent cell bodies were present. In these regions, referred to as the “hotspots,” photostimulation evoked a significant response in the form of EPSCs (Fig. 3A), and this confirmed the presence of an afferent pathway from the hotspot to the recorded cell, because photostimulation only activates cells at proximal dendrites or cell bodies (Callaway 1998). We then placed a concentric bipolar stimulating electrode on the hotspot identified in V2 to evoke EPSCs for further evaluation. Only EPSCs deemed to be monosynaptic, on the basis of the criteria defined in materials and methods, are considered further.
Fig. 3.
Synaptic properties of inputs from secondary visual area (V2) to layer 4 of V1. A: example of localization of a hotspot in V2 in a typical experiment. Photostimulation in layer 4 of V2 evoked responses in a recorded cell in layer 4 of V1 (yellow star). The white box covers the location from which photostimulation evoked clear EPSCs. B: example of a Class 1 response recorded in one layer 4 cell of V1. Stimulation at 25 Hz elicited paired-pulse depression and a graded response (i). High-frequency stimulation with ionotropic glutamate receptor (iGluR) antagonists failed to evoke activation of mGluRs (ii). C: example of a Class 2 response recorded in another layer 4 of V1. Stimulation at 25 Hz caused paired-pulse facilitation and a graded response (i). High-frequency stimulation with iGluR antagonists elicited a depolarizing mGluR response (ii), which was blocked by the mGluR1 antagonist LY-367385 (iii). D: example of a Class 2 response recorded from a cell in yet another layer 4 cell of V1. Conventions are as in C. Here the mGluR response was hyperpolarizing (ii) and blocked by the group II mGluR antagonist LY-341495 (iii). E: laminar distribution of the hotspots in V2 evoking responses in layer 4 of V1. F: Class 2 inputs having different types of activated mGluRs. DNQX, 7-dinitroquinoxaline-2,3-dione.
Each of the 11 cells recorded in V1 had its hotspot in layer 4 and/or layers 2/3 of V2. In particular, 8 cells had their hotspot in layer 4, 1 cell had the hotspot in layers 2/3, and 2 cells had two hotspots, one in layer 4 and the other in layers 2/3. Consequently, a total of 13 afferent inputs from V2 to layer 4 of V1 were recorded. Each afferent input could be identified as a member of either Class 1 (4 inputs) or Class 2 (9 inputs). The two cells having two hotspots received only Class 2 inputs. Figure 3B illustrates a Class 1 response of a cell recorded in layer 4 of V1 to stimulation of layer 4 of V2. Electrical stimulation at 25 Hz elicited paired-pulse depression (Fig. 3Bi). To detect the possible presence of mGluRs, we applied high-frequency stimulation at 130 Hz (Covic and Sherman 2011; De Pasquale and Sherman 2011; McCormick and Von Krosigk 1992) in the presence of antagonists to iGluRs. We found no evidence for mGluR activation (Fig. 3Bii). All the four Class 1 inputs from V2 to layer 4 of V1 share the same profile shown in Fig. 3Bi. Here Class 1 inputs have a graded activation pattern similar to that of Class 2. As we already documented in our previous works (De Pasquale and Sherman 2011, 2012), in cortical circuitry, Class 1 inputs exhibit graded responses rather than an all-or-none activation pattern.
Figure 3C shows a Class 2 input pattern for another cell recorded in V1 and activated from layer 4 of V2. Electrical stimulation at 25 Hz evoked a response having paired-pulse facilitation (Fig. 3Ci). High-frequency stimulation in the presence of iGluR blockers caused a long, slow depolarization corresponding to a type 1 mGluR (mGluR1) response (Fig. 3, Cii and Ciii). Of the nine Class 2 inputs from V2 so recorded in V1, six showed a similar depolarizing mGluR response, whereas the remaining three inputs had a hyperpolarizing mGluR response, reflecting group II mGluR activation (Fig. 3D; Covic and Sherman 2011; De Pasquale and Sherman 2011). Each of these nine inputs showed a graded response profile, meaning that increasing stimulus intensity evokes increasingly large EPSCs (Fig. 3, Bi, Ci, and Di).
Class 2 inputs were more frequent than Class 1 (Fig. 3E), and this is consistent with what we previously found in younger animals (De Pasquale and Sherman 2011), with no significant difference seen (P > 0.1, Fisher's exact test). All Class 2 responses showed activation of mGluRs, and three types were seen: group I, type 1 (mGluR1), group I, type 5 (mGluR5), and group II, types 2 and/or 3. Activation of both mGluR1s and mGluR5s produced prolonged membrane depolarization (Fig. 3, Cii and Ciii), whereas group II mGluR activation produced prolonged membrane hyperpolarization (Fig. 3, Dii and Diii). As we have documented previously (De Pasquale and Sherman 2011), four types of mGluR response were found. Three of them involved sole activation of mGluR1s, mGluR5s, or group II mGluRs, and the fourth involved both mGluR1s and mGluRs5 (Fig. 3F).
Effects of High-Frequency Stimulation of V2 on Class 1 Input from White Matter to Layer 4
Because high-frequency stimulation of V2 activates mGluRs on cells in layer 4 of V1, and because ACPD suppresses the Class 1 input from the white matter to these layer 4 cells, we tested whether mGluRs have the same modulatory action when activated by high-frequency stimulation of the Class 2 input from V2. We thus chose the Class 2 input from layer 4 of V2 to test for this effect (see Fig. 3E). Before each experiment for this question, we used photostimulation as described above to identify the hotspot in layer 4 of V2 for a Class 2 input.
With such an experimental design, we recorded from a total of 19 neurons in layer 4 of V1 that receive a Class 1 input from the white matter and a Class 2 input from layer 4 of V2. We followed the protocol shown in Fig. 4A. Two concentric bipolar stimulating electrodes were used, one placed in the layer 4 hotspot of V2 and the other placed in the white matter below V1. In the control condition, only V1 was stimulated (2 pulses, 66 Hz; Fig. 4Ai). In the modulation condition, V2 was stimulated first (20 pulses, 130 Hz), followed by stimulation of white matter under V1 (2 pulses, 66 Hz) 20 ms after the last stimulation pulse of V2 (Fig. 4Aii). The entire protocol was repeated for 10 trials alternating between control and modulation with 5-s intervals. The amplitude of the first EPSC before modulation was 38.7 ± 6.4 pA. In the modulation condition, the first EPSC was significantly reduced by about 40% (Fig. 4, B and C; P < 0.001, paired t-test), whereas the second EPSC was significantly reduced by roughly 20% (Fig. 4B; P < 0.01, paired t-test).
Fig. 4.
Modulation of the Class 1 input to layer 4 of V1 via high-frequency stimulation of V2. A: example of traces showing the modulation protocol. Stimulating electrodes were placed in V1 (white matter) and in V2 (layer 4). In the control condition, V1 white matter was stimulated with 2 pulses at 66 Hz (i). In the modulation condition, V2 was stimulated first at high frequency (20 pulses at 130 Hz). Stimulation of V1 white matter (2 pulses at 66 Hz) followed 20 ms after the last stimulation pulse delivered in V2, which means that the responses to V1 white matter stimulation were evoked during the activation of mGluRs from V2 stimulation (ii). B: same example shown in A with focus on the effects of modulation on the amplitudes of the EPSCs. Stimulation of V1 white matter alone (control) at 66 Hz induced paired-pulse depression. Pairing this with V2 stimulation as in A (modulation) decreased both EPSCs (i). This effect of modulation was prevented by bath application of the mGluR1 antagonist LY-367385 (ii). C: graph showing the effects of modulation (means ± SE) with and without various mGluR antagonists on the amplitude of the first EPSC. *P < 0.05, paired t-test. MPEP, 2-methyl-6-(phenylethynyl)pyridine.
We used specific antagonists to identify the involvement of particular mGluR types in the modulation of inputs to layer 4 cells. From the 19 cells mentioned above, 8 cells were tested with the specific mGluR1 antagonist LY-367385, 7 cells were tested with the specific mGluR5 antagonist MPEP, and 7 cells were tested with the group II mGluR antagonist LY-341495. Three cells were tested with two different antagonists.
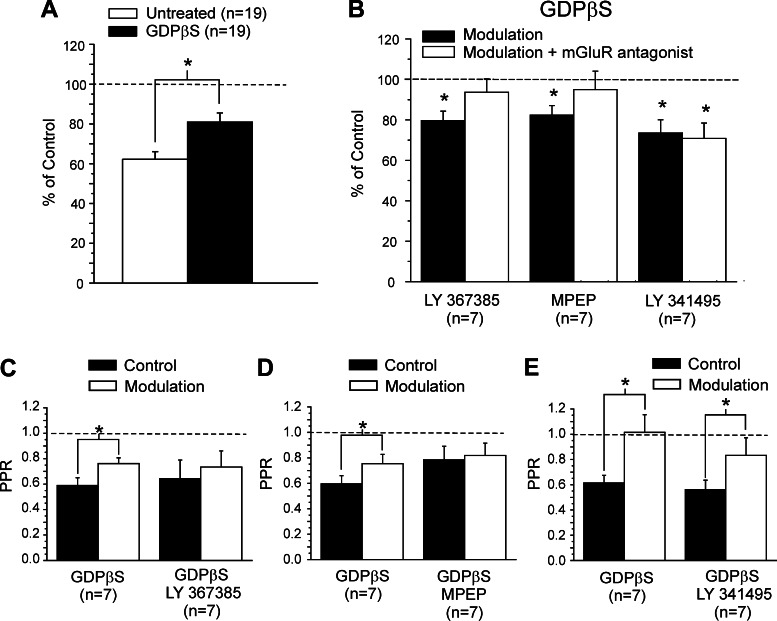
Figure 4C summarizes our results. We found that LY-367385 blocked the modulation (Fig. 4C; P > 0.1, paired t-test compared with control condition). Similarly, the specific mGluR5 antagonist MPEP also blocked the modulatory effect (Fig. 4C; P > 0.1, paired t-test compared with control condition). We found that the group II mGluR antagonist LY-341495 affected the modulation, but less than did application of the group I mGluR antagonists, so that the effects with LY-341495 showed significant reduction of the EPSC, but less than that seen with LY-367385 or MPEP modulation to affect the modulation significantly (Fig. 4C; P < 0.05, paired t-test for each comparison).
To investigate the involvement of presynaptic and postsynaptic mGluR locations to this modulation, we performed a set of experiments with GDPβS administered intracellularly in the recording pipette. GDPβS blocks postsynaptic G protein-coupled activity, thereby interfering with the postsynaptic activity of mGluRs. We recorded from 19 neurons in layer 4 of V1, testing the effects of modulation on the Class 1 input from white matter to layer 4, following the same protocol described above to activate mGluRs via high-frequency stimulation of V2, but with the presence of GDPβS in the recording electrode. We found that the presence of GDPβS did not change the amplitude of the EPSC before modulation, compared with the untreated condition (P > 0.1, t-test). Modulation with GDPβS significantly reduced the first evoked EPSC by about 20% (Fig. 5A; P < 0.05, paired t-test), but there was no reduction of the second EPSC (P > 0.1, paired t-test). This reduction is significantly smaller compared with the effect of modulation without GDPβS (roughly 40%; P < 0.01, t-test). Since the second EPSC was unaffected, the paired-pulse depression was significantly reduced (Fig. 5C; P < 0.05, paired t-test).
Fig. 5.
Effects of GDPβS on modulation of the Class 1 input to layer 4 of V1 via high-frequency stimulation of V2. A: graph showing the effects of modulation on the amplitude of the first EPSC (mean ± SE) for untreated neurons and for GDPβS-treated cells. The amplitudes are normalized to the values measured in the control condition. B: graph showing the effects of modulation on the amplitude (means ± SE) of the first EPSC for GDPβS-treated cells with and without various mGluR antagonists. The amplitudes are normalized to the values measured in the control condition. C: graph showing the effects of modulation on the PPR (means ± SE) for cells treated with GDPβS, with and without the mGluR1 antagonist LY-367385. D: graph showing the effects of modulation on the PPR (mean ± SE) for cells treated with GDPβS, with and without the mGluR5 antagonist MPEP. E: graph showing the effects of modulation on the PPR (means ± SE) for cells treated with GDPβS, with and without the mGluR1 antagonist LY-341495. *P < 0.05, paired t-test.
We tested these effects of modulation resulting from activation of specific mGluRs by also using specific mGluR antagonists in addition to the GDPβS. To achieve this purpose, we used 14 of the 19 cells mentioned above. We tested 7 cells for every antagonist. Seven cells were tested with two different antagonists. The amplitude of EPSC for GDPβS-treated neurons was 50.1 ± 9.4 pA and was not significantly different compared with that for untreated neurons (P > 0.1, t-test). The effects of modulation were prevented by the application of the mGluR1 antagonist LY-367385 (Fig. 5, B and C) or the mGluR5 antagonist MPEP (Fig. 5, B and D; P > 0.1, paired t-test for both compared with control condition). However, in the presence of the group II mGluR antagonist LY-341495, the EPSC was significantly reduced by about 20% (Fig. 5B; P < 0.05, paired t-test), and the paired-pulse depression was also significantly reduced (Fig. 5E; P < 0.05, paired t-test).
From these data, we can form the following conclusions. The observation that GDPβS in the electrode partly but not completely prevents the modulation suggests that both presynaptic and postsynaptic mGluRs are involved. That the addition of group I mGluR antagonists (i.e., to mGluR1s and mGluR5s) further reduces the effects of modulation suggests that a presynaptic component to the effect is mainly due to activation of these mGluRs. That the addition of group II mGluR antagonists does not further impair the modulation effects suggests that presynaptic group II mGluRs are not much involved.
Modulation of the Class 2 Input to Layer 4 of V1 via High-Frequency Stimulation of V2
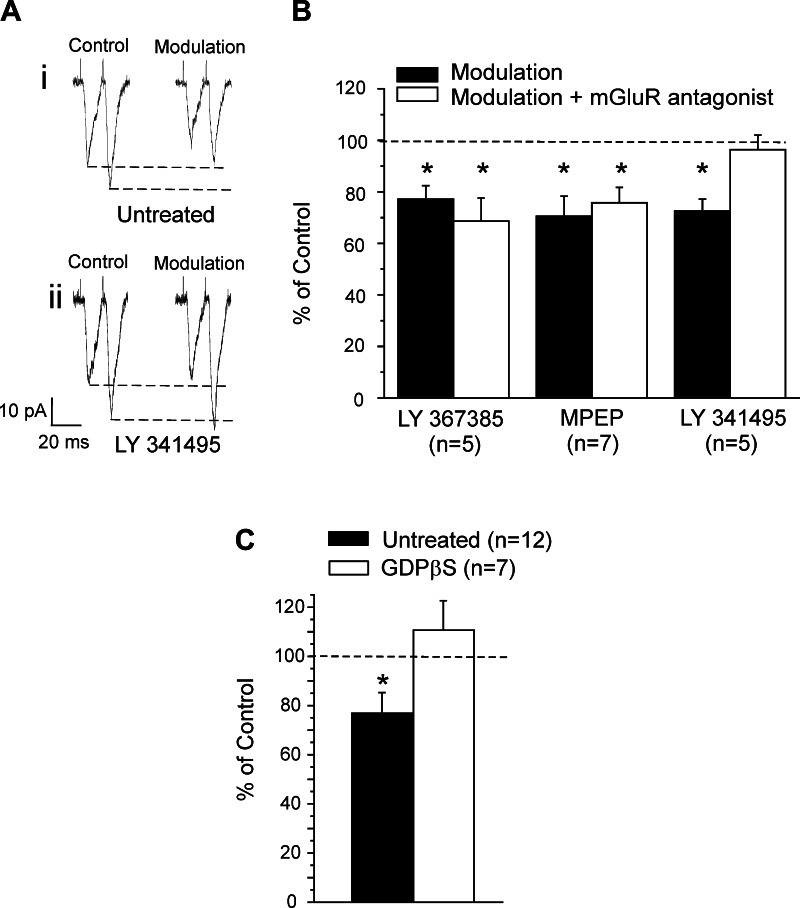
As noted above, both Class 1 and Class 2 inputs can be activated from the white matter to cells in layer 4 of V1 (Fig. 1). We sought to determine if the Class 2 inputs are also modulated by activation of mGluRs and, if so, how this modulation compares to that just described for the Class 1 inputs. We therefore recorded from 12 cells in layer 4 of V1, testing the effects of modulation on the Class 2 input from the white matter, and followed the same protocol described above for Class 1 inputs: we compared the EPSCs evoked from this white matter stimulation by itself (control) and during activation of mGluRs via high-frequency stimulation of layer 4 of V2. We found that modulation significantly decreases both EPSCs of the Class 2 input by about 25% (Fig. 6, A and B; P < 0.05, paired t-test). We used 11 cells of the 12 mentioned above to test the effects of mGluRs blockers on modulation. Six cells were tested with two different antagonists. In five cells, we found that application of the group II mGluR antagonist LY-341495 reduced the effect of this modulation (Fig. 6, A and B; P > 0.1, paired t-test). We used five cells and seven cells to test modulation during the application of the mGluR1 and mGluR5 antagonists, respectively. Application of either these group I mGluR antagonists failed to prevent the effect of modulation (Fig. 6B; P > 0.1, paired t-test).
Fig. 6.
Modulation of the Class 2 input to layer 4 of V1 via high-frequency stimulation of V2. A: example of traces showing the effects of modulation on the amplitudes of the EPSCs. Stimulation at 66 Hz caused paired-pulse facilitation, and both EPSCs were similarly decreased by modulation (i). Effects of modulation were blocked by group II mGluR antagonist LY-341495 (ii). B: graph showing the effect of modulation on the amplitude of the first EPSC; conventions as in Fig. 5B. C: graph showing the effect of modulation on the amplitude of the first EPSC for untreated neurons and for GDPβS-treated cells. Amplitudes (means ± SE) are normalized to the values measured in the control condition. *P < 0.05, paired t-test.
The relative contribution of presynaptic and postsynaptic mechanisms was also investigated for this modulation of the Class 2 input. We recorded from seven neurons with GDPβS in the recording electrode and found that this completely blocked the effect of modulation (Fig. 6C; P > 0.1, paired t-test). Both the lack of effect of the modulation on the paired-pulse facilitation and the complete prevention of the modulatory effect by GDPβS suggest that the modulation of this Class 2 input is strictly based on postsynaptic group II mGluRs.
Modulation of the Class 1 Input to Layer 4 of V1 via High-Frequency Stimulation of V2 in Young Animals
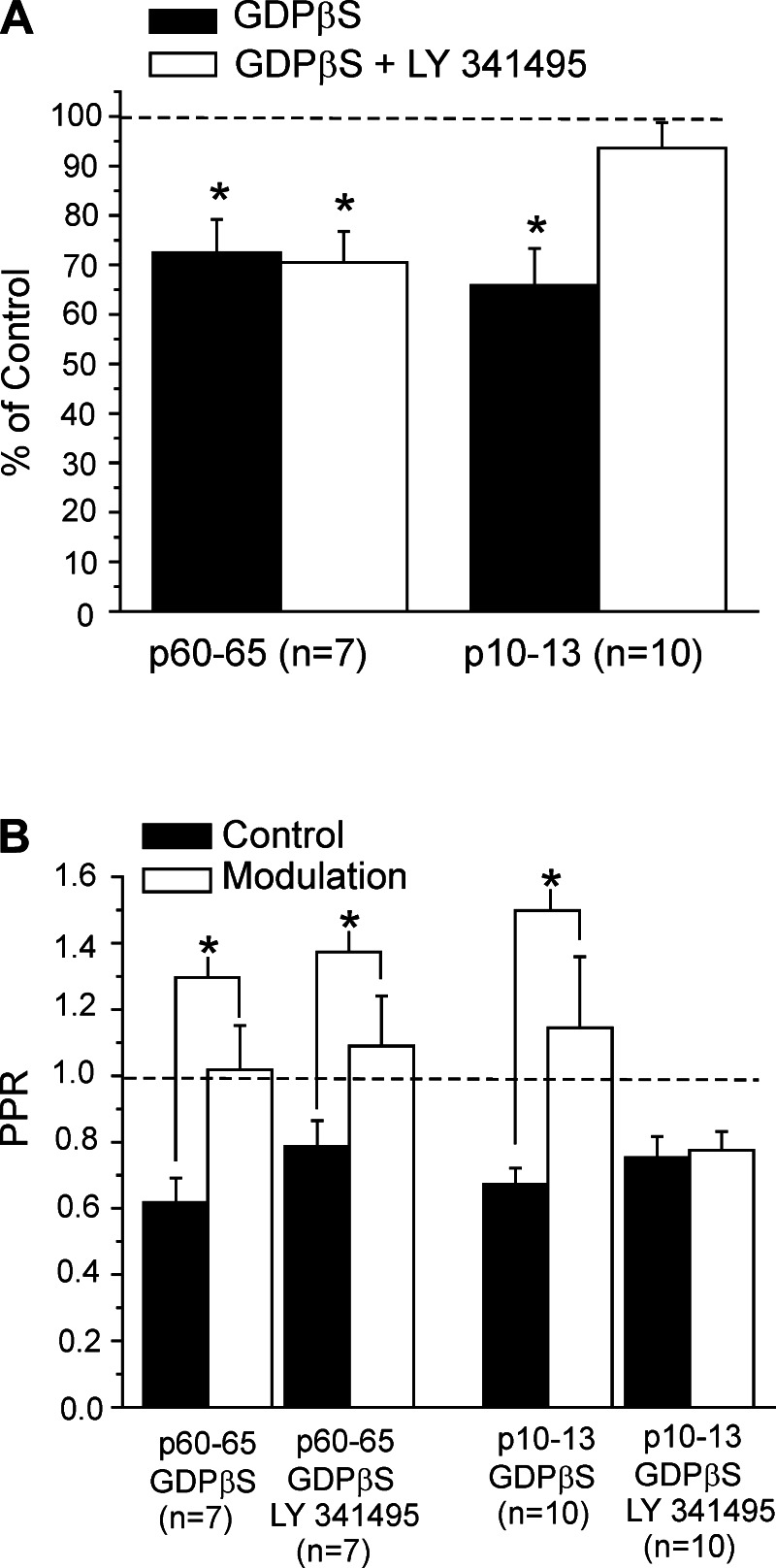
Our data indicate that the Class 1 input is largely modulated by the activity of presynaptic and postsynaptic group I mGluRs. However, presynaptic group II mGluRs on thalamocortical terminals have been described in layer 4 role during development (Mateo and Porter 2007). Since our experiments were done in fully adult animals, this suggests the possibility that our failure to find evidence of a contribution of presynaptic group II mGluRs could be related to age. To test this, we performed a set of experiments on the Class 1 input from white matter in 10 cells from younger animals (postnatal days 10–13), using the same protocol as described above to test for the involvement of presynaptic group II mGluRs in modulation of geniculocortical inputs. As above, we only describe data here for layer 4 cells for which we evoked monosynaptic inputs from white matter stimulation; this was roughly 20% of the recorded cells for younger animals. We added GDPβS in the recording electrode to isolate presynaptic mGluRs, and we measured the effects of modulation with and without the group II mGluR antagonist LY-341495.
In these young animals, the amplitude of EPSC was 42.8 ± 11.8 pA and was not significantly different compared with that in adult animals (P > 0.1, t-test). On the other hand, modulation significantly decreased the first EPSC by about 35% (Fig. 7A; P < 0.05, paired t-test), whereas the second EPSC was unaffected (P > 0.1, paired t-test). As a consequence, the paired-pulse depression was significantly reduced (Fig. 7B; P < 0.05, paired t-test). However, whereas these effects were not much changed in adults with the addition of the group II mGluR antagonist, they were in the young animals (Fig. 7; P > 0.1, paired t-test compared with control condition). These findings suggest that the involvement of presynaptic group II mGluRs activity in modulating thalamocortical synapses is age dependent.
Fig. 7.
Involvement of presynaptic group II mGluRs in the modulation of the Class 1 input to layer 4 in juvenile vs. adult animals. In these experiments, GDPβS was in the recording electrode to isolate presynaptic mGluR activation. A: graph showing the EPSC amplitude (means ± SE) normalized to the values measured in the control condition. At both ages, modulation significantly decreased the EPSC (*P < 0.05, paired t-test), indicating a presynaptic affect. In juvenile animals (postnatal days 10–13, p10–13), but not in adults (p60–65), this effect was reduced in the presence of the group II mGluR antagonist LY-341495 (*P > 0.1, paired t-test). B: graph showing effects on the PPR (means ± SE). Modulation increased the PPR at both ages, again indicating presynaptic mGluR involvement (*P < 0.05, paired t-test), but this was prevented by the group II mGluR antagonist in juvenile, but not adult, animals.
Effects of Pharmacological Activation of mGluRs on Direct Stimulation of Cells in Layer 4
In the present study, we described a modulatory effect of V2 on neurons of V1 that partly involves the activity of postsynaptic Group I and Group II mGluRs. To further support this conclusion, we tested the pharmacological activation of postsynaptic mGluRs in layer 4 cells. We used direct photostimulation to measure the effect of agonists on responses evoked on these cells (Fig. 8). These experiments were performed in a low-Ca2+ and high-Mg2+ bathing medium that blocked synaptic transmission, thereby ensuring that the response to uncaged glutamate reflects direct activation of the recorded cell.
Fig. 8.
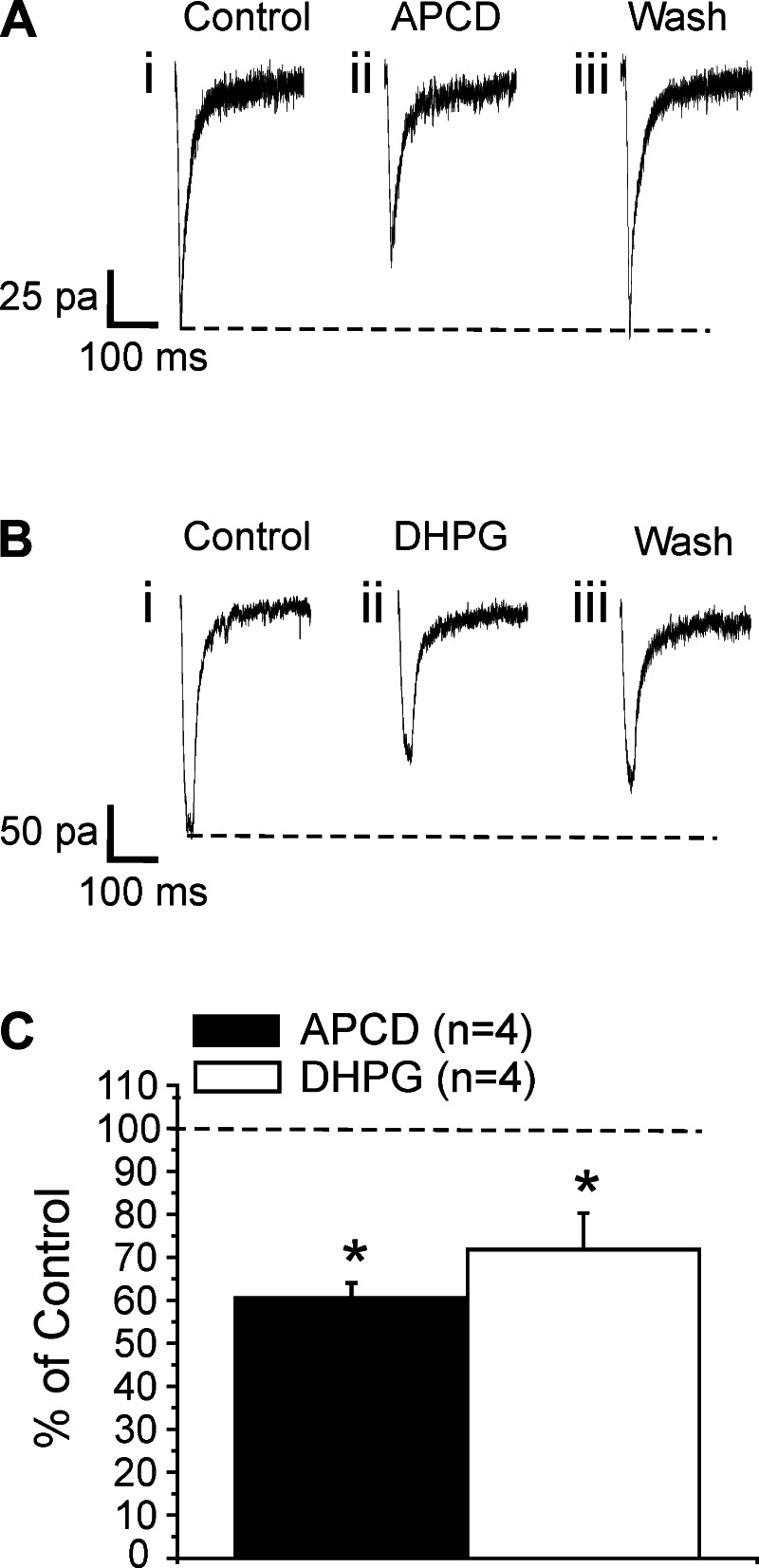
Effects of 4-aminopyrrolidine-2,4-dicarboxylate (APCD) and 5-dihydroxyphenylglycine (DHPG) on direct photostimulation of neurons in layer 4 of V1. Shown in A and B are traces recorded in a layer 4 cell of V1 evoked by direct photostimulation in experiments in which synaptic transmission was blocked by a low-Ca2+ and high-Mg2+ bathing medium. A: bath application of the group II mGluR agonist APCD notably reduced the amplitude of the evoked responses (i vs. ii), and this effect disappeared after the drug was washed out (iii). B: bath application of the group I mGluR agonist DHPG also decreased the amplitude of the response (i vs. ii), and this effect somewhat persisted after washout (iii). C: graph showing the effects of APCD and DHPG on responses evoked with direct photostimulation. Amplitudes (means ± SE) are normalized to the mean of the baseline values. APCD and DHPG significantly decreased the amplitude of the EPSC (*P < 0.05, repeated-measures ANOVA for both agonists).
We recorded from four cells in this fashion before and after adding the specific group II mGluR agonist APCD for 5 min. The agonist application decreased the direct responses by about 40% (Fig. 8, A and C; P < 0.05, repeated-measures ANOVA). The effect disappeared after washout of the antagonist (Fig. 8Aiii). We followed the same protocol to record from another four cells and test the effects of the specific group I mGluR agonist DHPG. DHPG reduced evoked response by roughly 30% (Fig. 8, B and C; P < 0.05, repeated-measures ANOVA). This effect persisted in part after washout of the drug (Fig. 8Biii). The long-term effect of DHPG has been documented and is likely to be caused by mechanisms of long-term synaptic plasticity (Fitzjohn et al. 1999; Huber et al. 2000; Sergeeva et al. 2007; Watabe 2002). Together, these data support the involvement of postsynaptic mGluRs in modulating the synaptic responses evoked in layer 4 cells.
DISCUSSION
The present study focused on the modulatory effect of certain Class 2 feedback projections from secondary visual cortex (V2) on cells of layer 4 of the primary visual cortex (V1), noting such effects on two other inputs to these cells, one thought to be from the lateral geniculate nucleus of thalamus and the other from an unspecified intracortical source that plausibly involves mostly branches of layer 6 axons. These latter two inputs were activated by applying electrical stimulation to the white matter below the recorded layer 4 cell in V1. We found that such white matter stimulation elicited two different types of responses in neurons of layer 4: most had paired-pulse depression and all-or-none responses, consistent with Class 1 properties, whereas a minority had paired-pulse facilitation and graded responses, consistent with Class 2 properties (reviewed in Sherman and Guillery 1998, 2006, 2011). We have provisionally concluded that the Class 1 input is thalamic, from the lateral geniculate nucleus, and that Class 2 is mostly from antidromic activation of layer 6 corticogeniculate axons that branch to also innervate layer 4 (Lee and Sherman 2008, 2009b). As we have reported previously, we found that activation of layer 4 of V2 provides a Class 2 input to layer 4 cells of V1 and that this input can activate mGluRs (De Pasquale and Sherman 2011), which act to reduce EPSC amplitudes evoked by presumptive geniculocortical or layer 6 inputs to these cells. Such modulation acts in an input-specific way. In modulation of thalamocortical input, both presynaptic and postsynaptic group I mGluRs are involved, whereas modulation of the intracortical input acts via postsynaptic group II mGluRs.
Technical Issues
Use of BALB/c mice.
We used BALB/c mice, which are albino, to be consistent with our previous work that forms the basis of these experiments (De Pasquale and Sherman 2011, 2012). However, recent work points to behavioral deficiencies in these mice (Yeritsyan et al. 2012), which may be attributed to lack of ocular pigmentation that would degrade the image and a deficiency of ipsilateral retinal projections (a common feature of albinos), with the known deficiencies being largely limited to retinofugal projections (Guillery and Kaas 1971; Sanderson et al. 1974). Indeed, as Yeritsyan et al. (2012) stated, “visual cortical maps induced by stimulation of the contralateral eye were normal in both activation strength and retinotopic map quality.” We therefore tentatively conclude that the BALB/c mouse involves little or no significant alteration in the cortical circuitry we are testing any more than other strains might. Nonetheless, extending these observations to other strains, species, and areas remains an issue to be resolved.
Nature of recorded cells in V1.
Although only a minority of our recorded cells were successfully filled with biocytin, all were found to be pyramidal. Nonetheless, we cannot rule out the possibility that some recordings derived from interneurons. However, the main goal of this work was the study of the modulatory action of mGluRs on thalamocortical input. In layer 4, the great majority of interneurons receiving thalamocortical inputs are fast spiking (Amitai and Connors 1995; Connors and Gutnick 1990; Gibson et al. 1999; Nowak et al. 2003; Schiff and Reyes 2012), and all neurons we used to collect our data had a regular firing pattern. For these reasons, we think that it is unlikely that many, if any, interneurons were recorded in our experiments.
Interpreting effects of electrical activation.
Many of our recordings were obtained from responses evoked by electrical stimulation. This raises questions about precisely what neuronal populations were actually activated to contribute to postsynaptic responses seen and how relevant the results may be to behaving animals.
ACTIVATION OF INPUTS TO LAYER 4 OF V1 FROM THE WHITE MATTER.
To activate thalamocortical inputs, we placed concentric bipolar stimulating electrodes in the white matter. The plausible glutamatergic inputs activated from white matter are geniculocortical axons, axon branches to layer 4 from layer 6 corticogeniculate axons, or unspecified corticocortical axons from other areas running in the white matter. We identified two inputs types from this white matter stimulation, and they are separately considered here.
We believe the most likely source of the Class 1 inputs activated are geniculocortical for the following reasons. Layer 6 axon inputs to layer 4 cells are Class 2 (Lee and Sherman 2009a, 2009b), so these axons as the source of Class 1 input can be ruled out. Furthermore, corticocortical inputs to layer 4 show a Class 1B pattern (meaning a graded response pattern), and what we observed from white matter was a Class 1A pattern (an all-or-none activation pattern; see Fig. 1C). Figure 1C also shows for the Class 2 input that a graded activation pattern can be evoked if present from the white matter. Since all thalamocortical inputs to layer 4 so far described in the mouse show the Class 1A pattern (Lee and Sherman 2008; Viaene et al. 2011a), it logically follows that the most likely source of Class 1A input that we found from activating white matter to layer 4 cells is the lateral geniculate nucleus.
The source of the Class 2 inputs are harder to pin down in our studies, but we emphasize that the main purpose of this study was to identify modulatory effects of the V2 feedback projection to layer 4 of V1, particularly on geniculocortical inputs, and effects on the Class 2 inputs serve mainly as a basis for comparison. These Class 2 inputs could be from either layer 6 cells or other cortical areas (Covic and Sherman 2011; De Pasquale and Sherman 2011), or both. We provisionally suggest that a layer 6 source is more likely because they do provide a strictly Class 2 input to layer 4 cells (Lee and Sherman 2009b), and evidence suggests that corticocortical inputs to middle layers (such as layer 4) tend to run through the gray matter of cortex rather than in the white matter (reviewed in Thomson and Bannister 2003).
Stimulation of the white matter below the primary visual cortex has been previously shown to elicit three classes of inputs (Stratford et al. 1996). Stratford et al. identified a geniculocortical input and two different intracortical inputs. We found only one presumed intracortical input. This discrepancy with our study could be due to species differences (their study was in cats) or a different postsynaptic population (they focused their study on stellate cells, whereas those we identified were all pyramidal).
ACTIVATION OF THE V2 TO V1 PATHWAY.
To activate feedback connections from V2, stimulating electrodes were placed on hotspots localized by photostimulation. For every corticocortical input, we assessed the presence of connectivity between the patched neuron in V1 and the afferent cell bodies and proximal dendrites in V2.
We cannot exclude the possibility that we stimulated axons of passage providing sources of input other than the ones we provisionally identified. However, we believe that this possibility does not greatly affect our general conclusions for the following reasons. Regarding fibers of passage in activating the inputs from V2 to V1, we have discussed this in detail previously: because this connectivity is topographic (Schuett et al. 2002; Wang and Burkhalter 2007), axons passing through from distant sites are unlikely to innervate the patched cell, a point that we demonstrated via control experiments (De Pasquale and Sherman 2011).
ACTIVATION OF mGluRs VIA ELECTRICAL STIMULATION.
To activate mGluRs in V1 by stimulating V2 afferents, we used tetanus, or high-frequency stimulation. Such an approach has proved to be routine and can be traced back at least two decades (Beierlein et al. 2000; Grassi et al. 2002; Long et al. 2004; McCormick and Von Krosigk 1992; Rush et al. 2002; Sung et al. 2001). This is done to maximize the effects and thereby ensure that they do occur.
It should be noted that cortical cells that fire at roughly 130 Hz or more (the tetanus frequency we used) exist, including bursting cells (Brumberg et al. 2000; DeBusk et al. 1997; Jacob et al. 2012; Llano and Sherman 2009; Tateno et al. 2004). Furthermore, a recent study showed that activation of mGluRs via Class 2 inputs (as in this study) can be achieved with brief trains activated at lower frequencies (e.g., ≥10–15 Hz) and that although higher frequency stimulation increases the amplitude of the mGluR response, it is not necessary to initially evoke it (Viaene et al. 2013). Nevertheless, more study is needed to determine the range of activation patterns able to evoke similar effects as a necessary step in evaluating the full significance of these results. What we see as the main contribution here is the demonstration that specific pathways can induce mGluR-related modulation as an advance over earlier studies limited mostly to agonist application (for review, see Sherman 2013).
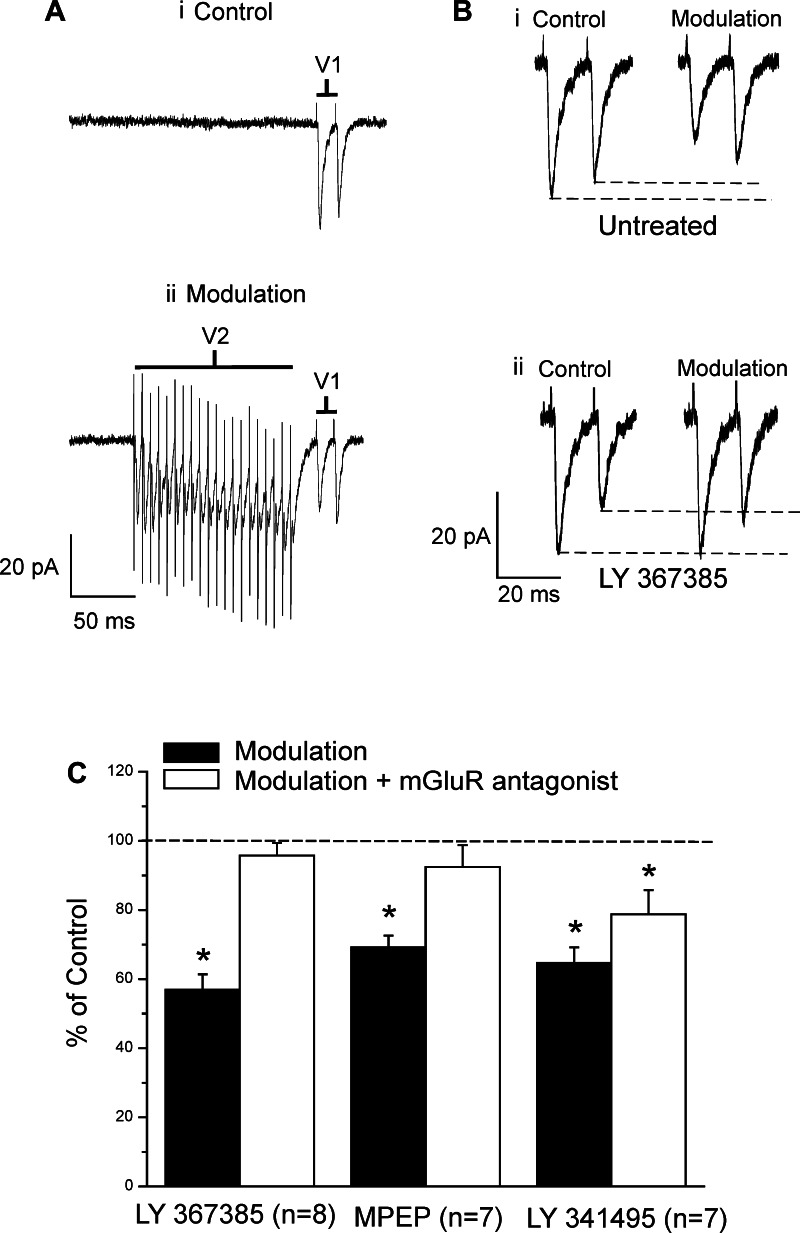
Effects of mGluR Activation
Evidence has accrued for some time that activation of both group I and group II mGluRs by agonist application in cortex can affect responsiveness and synaptic transmission, typically in a suppressive manner (Beaver et al. 1999; Cahusac 1994; Cahusac and Wan 2007; Reid and Daw 1997). However, such studies begged the question as to how these mGluRs might be activated by circuit activity: what specific glutamatergic afferents activate them to produce such effects? Our laboratory has begun to explore this. We recently reported two examples of local Class 2 inputs affecting synaptic transmission of other, Class 1 inputs in cortex, showing that mGluRs activated from layer 6 inputs to layer 4 cells reduce the amplitude of EPSP/Cs activated in those cells from thalamus (Lee and Sherman 2012) and that Class 2 inputs from layers 2/3 onto cells in that layer reduce the amplitude of Class 1 inputs from layer 4 (De Pasquale and Sherman 2012). We can now add to that the current example of modulation via activation by Class 2 inputs of mGluRs involving a feedback circuit from V2 to V1.
These data help to define some of the ways by which Class 2 inputs can modulate circuit activity, particularly via activation of mGluRs. Clearly, the data are from far too few examples to generalize concerning these processes, but it is interesting that, so far, these involve both presynaptic and postsynaptic mGluRs of both group I and group II varieties. Furthermore, all of the effects so far documented on synaptic transmission involve reducing the amplitude of evoked postsynaptic responses. The involvement of group I mGluRs in reducing synaptic transmission has been observed previously in the hippocampus (Fitzjohn et al. 1999; Huber et al. 2000; Watabe et al. 2002) and in corticostriatal transmission (Sergeeva et al. 2007). Our findings support this idea and add that group I mGluRs have an important role in reducing synaptic transmission in thalamocortical circuitry.
Our data show that the effects of mGluRs on synaptic transmission of the presumed geniculocortical input are largely limited to the first evoked response when the frequency of stimulation is relatively high. This caused a shift of the paired-pulse ratio from depression to facilitation. This effect of mGluRs on short-term plasticity already has been documented in sensory cortical circuitry (Bandrowski et al. 2001; De Pasquale and Sherman 2012; Lee and Sherman 2012). These findings suggest that the modulatory action of mGluRs in cortical areas might be more effective in suppressing low-frequency signals, which are typical of nonarousal cortical states.
Conclusions
We have shown that a Class 2 feedback projection from V2 reduces EPSCs evoked from both Class 1 inputs (thought to be thalamocortical) and Class 2 inputs (thought to be intracortical and likely from layer 6) to layer 4 cells of V1. The presumed thalamocortical input is principally modulated through group I mGluRs, involving both presynaptic and postsynaptic mechanisms. The Class 2 intracortical input is modulated only via postsynaptically activated group II mGluRs. Thus, although both inputs are affected, the mechanisms and mGluR types involved differ. Overall, these findings suggest that feedback projections from V2 can modulate visual information processing in V1 through activation of mGluRs.
There have been many suggestions regarding the possible function of feedback pathways in cortex involving various ideas of modulatory functions, such as attentional processes, predictive coding, etc., but very few studies of how such feedback can affect synaptic properties of local circuits. Our evidence should be seen as an early example of how such feedback might work at the synaptic level, but it seems likely that many other forms of synaptic modulation remain to be discovered. Furthermore, these observations need to be tested for generality by studying other mouse lines, mammalian species, and cortical areas.
GRANTS
This work was supported by National Institutes of Health Grants R01 DC008794 and R01 EY022338.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.D.P. and S.M.S. conception and design of research; R.D.P. performed experiments; R.D.P. and S.M.S. analyzed data; R.D.P. and S.M.S. interpreted results of experiments; R.D.P. and S.M.S. prepared figures; R.D.P. and S.M.S. drafted manuscript; R.D.P. and S.M.S. edited and revised manuscript; R.D.P. and S.M.S. approved final version of manuscript.
REFERENCES
- Amitai Y, Connors BW. Intrinsic physiology and morphology of single neurons in neocortex. In: Cerebral Cortex, edited by Jones EG, Diamond IT. New York: Plenum, 1995, p. 299–331 [Google Scholar]
- Bandrowski AE, Aramakis VB, Moore SL, Ashe JH. Metabotropic glutamate receptors modify ionotropic glutamate responses in neocortical pyramidal cells and interneurons. Exp Brain Res 136: 25–40, 2001 [DOI] [PubMed] [Google Scholar]
- Bandrowski AE, Moore SL, Ashe JH. Activation of metabotropic glutamate receptors by repetitive stimulation in auditory cortex. Synapse 44: 146–157, 2002 [DOI] [PubMed] [Google Scholar]
- Beaver CJ, Ji Q, Daw NW. Effect of the group II metabotropic glutamate agonist, 2R,4R-APDC, varies with age, layer, and visual experience in the visual cortex. J Neurophysiol 82: 86–93, 1999 [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci 3: 904–910, 2000 [DOI] [PubMed] [Google Scholar]
- Berezovskii VK, Nassi JJ, Born RT. Segregation of feedforward and feedback projections in mouse visual cortex. J Comp Neurol 519: 3672–3683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg JC, Nowak LG, McCormick DA. Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. J Neurosci 20: 4829–4843, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahusac PM. Cortical layer-specific effects of the metabotropic glutamate receptor agonist 1S,3R-ACPD in rat primary somatosensory cortex in vivo. Eur J Neurosci 6: 1505–1511, 1994 [DOI] [PubMed] [Google Scholar]
- Cahusac PM, Wan H. Group II metabotropic glutamate receptors reduce excitatory but not inhibitory neurotransmission in rat barrel cortex in vivo. Neuroscience 146: 202–212, 2007 [DOI] [PubMed] [Google Scholar]
- Callaway EM. Local circuits in primary visual cortex of the macaque monkey. Annu Rev Neurosci 21: 47–74, 1998 [DOI] [PubMed] [Google Scholar]
- Canepari M, Nelson L, Papageorgiou G, Corrie JE, Ogden D. Photochemical and pharmacological evaluation of 7-nitroindolinyl- and 4-methoxy-7-nitroindolinyl-amino acids as novel, fast caged neurotransmitters. J Neurosci Methods 112: 29–42, 2001 [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13: 99–104, 1990 [DOI] [PubMed] [Google Scholar]
- Coogan TA, Burkhalter A. Hierarchical organization of areas in rat visual cortex. J Neurosci 13: 3749–3772, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covic EN, Sherman SM. Synaptic properties of connections between the primary and secondary auditory cortices in mice. Cereb Cortex 21: 2425–2441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBusk BC, DeBruyn EJ, Snider RK, Kabara JF, Bonds AB. Stimulus-dependent modulation of spike burst length in cat striate cortical cells. J Neurophysiol 78: 199–213, 1997 [DOI] [PubMed] [Google Scholar]
- De Pasquale R, Sherman SM. Synaptic properties of corticocortical connections between the primary and secondary visual cortical areas in the mouse. J Neurosci 31: 16494–16506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pasquale R, Sherman SM. Modulatory effects of metabotropic glutamate receptors on local cortical circuits. J Neurosci 32: 7364–7372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Friedlander MJ. Intracellular blockade of inhibitory synaptic responses in visual cortical layer IV neurons. J Neurophysiol 75: 2167–2173, 1996 [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Kingston AE, Lodge D, Collingridge GL. DHPG-induced LTD in area CA1 of juvenile rat hippocampus; characterisation and sensitivity to novel mGlu receptor antagonists. Neuropharmacology 38: 1577–1583, 1999 [DOI] [PubMed] [Google Scholar]
- Gerber G, Zhong J, Youn D, Randic M. Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn. Neuroscience 100: 393–406, 2000 [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999 [DOI] [PubMed] [Google Scholar]
- Grassi S, Frondaroli A, Pettorossi VE. Different metabotropic glutamate receptors play opposite roles in synaptic plasticity of the rat medial vestibular nuclei. J Physiol 543: 795–806, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW, Kaas JH. A study of normal and congenitally abnormal retinogeniculate projections in cats. J Comp Neurol 143: 73–100, 1971 [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288: 1254–1257, 2000 [DOI] [PubMed] [Google Scholar]
- Jacob V, Petreanu L, Wright N, Svoboda K, Fox K. Regular spiking and intrinsic bursting pyramidal cells show orthogonal forms of experience-dependent plasticity in layer V of barrel cortex. Neuron 73: 391–404, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Mapping by laser photostimulation of connections between the thalamic reticular and ventral posterior lateral nuclei in the rat. J Neurophysiol 94: 2472–2483, 2005 [DOI] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical inputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol 100: 317–326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Glutamatergic inhibition in sensory neocortex. Cereb Cortex 19: 2281–2289, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Modulator property of the intrinsic cortical projection from layer 6 to layer 4. Front Syst Neurosci 3: 1–5, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Intrinsic modulators of auditory thalamocortical transmission. Hear Res 287: 43–50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Differences in intrinsic properties and local network connectivity of identified layer 5 and layer 6 adult mouse auditory corticothalamic neurons support a dual corticothalamic projection hypothesis. Cereb Cortex 19: 2810–2826, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Landisman CE, Connors BW. Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J Neurosci 24: 341–349, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Z, Porter JT. Group II metabotropic glutamate receptors inhibit glutamate release at thalamocortical synapses in the developing somatosensory cortex. Neuroscience 146: 1062–1072, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci USA 89: 2774–2778, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak LG, Azouz R, Sanchez-Vives MV, Gray CM, McCormick DA. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J Neurophysiol 89: 1541–1566, 2003 [DOI] [PubMed] [Google Scholar]
- O'Leary DM, Cassidy EM, O'Connor JJ. Group II and III metabotropic glutamate receptors modulate paired pulse depression in the rat dentate gyrus in vitro. Eur J Pharmacol 340: 35–44, 1997 [DOI] [PubMed] [Google Scholar]
- Petrof I, Sherman SM. Synaptic properties of the mammillary and cortical afferents to the anterodorsal thalamic nucleus in the mouse. J Neurosci 29: 7815–7819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof I, Viaene AN, Sherman SM. Two populations of corticothalamic and interareal corticocortical cells in the subgranular layers of the mouse primary sensory cortices. J Comp Neurol 520: 1678–1686, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol 92: 2185–2197, 2004 [DOI] [PubMed] [Google Scholar]
- Reid SN, Daw NW. Activation of metabotropic glutamate receptors has different effects in different layers of cat visual cortex. Vis Neurosci 14: 83–88, 1997 [DOI] [PubMed] [Google Scholar]
- Rush AM, Wu J, Rowan MJ, Anwyl R. Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro. J Neurosci 22: 6121–6128, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson KJ, Guillery RW, Shackelford RM. Congenitally abnormal visual pathways in mink (Mustela vison) with reduced retinal pigment. J Comp Neurol 154: 225–248, 1974 [DOI] [PubMed] [Google Scholar]
- Schiff ML, Reyes AD. Characterization of thalamocortical responses of regular-spiking and fast-spiking neurons of the mouse auditory cortex in vitro and in silico. J Neurophysiol 107: 1476–1488, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuett S, Bonhoeffer T, Hübener M. Mapping retinotopic structure in mouse visual cortex with optical imaging. J Neurosci 22: 6549–6559, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva OA, Doreulee N, Chepkova AN, Kazmierczak T, Haas HL. Long-term depression of cortico-striatal synaptic transmission by DHPG depends on endocannabinoid release and nitric oxide synthesis. Eur J Neurosci 26: 1889–1894, 2007 [DOI] [PubMed] [Google Scholar]
- Sherman SM. The function of metabotropic glutamate receptors in thalamus and cortex. Neuroscientist. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci USA 95: 7121–7126, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus and its Role in Cortical Function. Cambridge, MA: MIT Press, 2006 [Google Scholar]
- Sherman SM, Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol 106: 1068–1077, 2011 [DOI] [PubMed] [Google Scholar]
- Stratford KJ, Tarczy-Hornoch K, Martin KAC, Bannister NJ, Jack JJB. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature 382: 258–261, 1996 [DOI] [PubMed] [Google Scholar]
- Sung KW, Choi S, Lovinger DM. Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J Neurophysiol 86: 2405–2412, 2001 [DOI] [PubMed] [Google Scholar]
- Tateno T, Harsch A, Robinson HP. Threshold firing frequency-current relationships of neurons in rat somatosensory cortex: type 1 and type 2 dynamics. J Neurophysiol 92: 2283–2294, 2004 [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cereb Cortex 13: 5–14, 2003 [DOI] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Properties of the thalamic projection from the posterior medial nucleus to primary and secondary somatosensory cortices in the mouse. Proc Natl Acad Sci USA 108: 18156–18161, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to layers 2/3 in primary somatosensory and auditory cortices. J Neurophysiol 105: 279–292, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to the subgranular layers of primary somatosensory and auditory cortices in the mouse. J Neurosci 31: 12738–12747, 2011c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Activation requirements for metabotropic glutamate receptors. Neurosci Lett. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QX, Burkhalter A. Area map of mouse visual cortex. J Comp Neurol 502: 339–357, 2007 [DOI] [PubMed] [Google Scholar]
- Watabe AM, Carlisle HJ, O'Dell TJ. Postsynaptic induction and presynaptic expression of group 1 mGluR-dependent LTD in the hippocampal CA1 region. J Neurophysiol 87: 1395–1403, 2002 [DOI] [PubMed] [Google Scholar]
- Yeritsyan N, Lehmann K, Puk O, Graw J, Lowel S. Visual capabilities and cortical maps in BALB/c mice. Eur J Neurosci 36: 2801–2811, 2012 [DOI] [PubMed] [Google Scholar]