Abstract
Background: There are potential conflicts between authorities and companies to fund new premium priced drugs especially where there are safety and/or budget concerns. Dabigatran, a new oral anticoagulant for the prevention of stroke in patients with non-valvular atrial fibrillation (AF), exemplifies this issue. Whilst new effective treatments are needed, there are issues in the elderly with dabigatran due to variable drug concentrations, no known antidote and dependence on renal elimination. Published studies have shown dabigatran to be cost-effective but there are budget concerns given the prevalence of AF. There are also issues with potentially re-designing anticoagulant services. This has resulted in activities across countries to better manage its use.
Objective: To (i) review authority activities in over 30 countries and regions, (ii) use the findings to develop new models to better manage the entry of new drugs, and (iii) review the implications for all major stakeholder groups.
Methodology: Descriptive review and appraisal of activities regarding dabigatran and the development of guidance for groups through an iterative process.
Results: There has been a plethora of activities among authorities to manage the prescribing of dabigatran including extensive pre-launch activities, risk sharing arrangements, prescribing restrictions, and monitoring of prescribing post-launch. Reimbursement has been denied in some countries due to concerns with its budget impact and/or excessive bleeding. Development of a new model and future guidance is proposed to better manage the entry of new drugs, centering on three pillars of pre-, peri-, and post-launch activities.
Conclusion: Models for introducing new drugs are essential to optimize their prescribing especially where there are concerns. Without such models, new drugs may be withdrawn prematurely and/or struggle for funding.
Keywords: critical drug evaluation, dabigatran, demand-side measures, drug and therapeutics committees, managed introduction new medicines, pharmacovigilance, registries, risk sharing
BACKGROUND
New medicines are of real value to patients when they improve their health either because they are more effective, have less side-effects, or are easier to administer than current standards. European health authorities also wish new drugs to be cost-effective (Garattini et al., 2008; Godman et al., 2008, 2009c, 2012d; Wettermark et al., 2008, 2010a; Coma et al., 2009; Sermet et al., 2010; Garuoliene et al., 2011b; Voncina and Strizrep, 2011; Vončina et al., 2011; Cheema et al., 2012; Markovic-Pekovic et al., 2012). Continued pressure on resources is already resulting in some countries unable to fund new premium priced drugs (Garuoliene et al., 2011a,b; Godman et al., 2011c, 2012b; Taylor, 2011), with the number of countries likely to increase with new drugs now being launched at US$300,000 (€228,000) per patient per year or more (Kaiser, 2012). Premium prices are of concern among authorities struggling to maintain, and potentially incompatible with, the European ideals of comprehensive and equitable healthcare (Garattini et al., 2008; Adamski et al., 2010; Godman et al., 2012b).
This may result in conflicts between authorities and pharmaceutical companies with the latter keen to re-coup the considerable monies spent on research and development as soon as possible through rapid reimbursement (DiMasi and Grabowski, 2007; Abraham, 2008; Jaroslawski and Toumi, 2011; Persson et al., 2012) as well as maintain profitability with established products (Shuchman, 2006; Abraham, 2008; Godman et al., 2010, 2011b,b; Vončina et al., 2011; Baumgärtel et al., 2012; Jackevicius et al., 2012). However, this can be at odds with the aims of health authorities and health insurance companies struggling to meet European ideals within available resources (Shuchman, 2006; Abraham, 2008; Garattini et al., 2008; Godman et al., 2010, 2011b, 2012b; Sermet et al., 2010; Garuoliene et al., 2011b; Voncina and Strizrep, 2011; Vončina et al., 2011; Baumgärtel et al., 2012; Ozierański et al., 2012). Marketing activities are seen as important by companies to achieve their aims in an increasingly competitive environment (Civaner, 2012); but these can involve considerable spending. Published studies suggest marketing costs can be as high as one-third of a company’s income (Civaner, 2012), with companies spending US$53bn (€40.2) in the US alone in 2004 marketing to physicians (Lexchin and Kohler, 2011; Godman et al., 2012b; Godman and Gustafsson, 2013). In addition, there have been concerns with aggressive lobbying and other indirect strategies by some companies (Mello et al., 2012; Ozierański et al., 2012), as well as with some of the marketing (Department of Justice, 2010; Griffin and Segal, 2010; Lexchin and Kohler, 2011; Fisk et al., 2012; Davies and Abraham, 2013) and other activities (Jackevicius et al., 2012; Baumgärtel et al., 2012; Ozierański et al., 2012; Davies and Abraham, 2013) to achieve their aims. This is despite the imposition of multi-million dollar fines (Davies and Abraham, 2013).
These conflicts can be greater when there are safety concerns with new drugs, and they are subsequently prescribed in a wider population than studied in randomized clinical trials. Typically Phase III clinical trials are conducted under ideal and highly controlled conditions to seek high internal validity to maximize the chance of demonstrating clinical benefit (Fritz and Cleland, 2003). However, this may lead to substantial differences from their subsequent use in clinical practice. Typically Phase III clinical trials do not include treatment preferences and/or multimodal treatment programs (Wells, 1999; Guthrie, 2000; Fritz and Cleland, 2003). Phase III clinical trials may also include a placebo group as a comparator in order to isolate the effects of a particular intervention (Fritz and Cleland, 2003). These situations can lead to concerns with the generalizability of the findings when new drugs are being considered as an alternative to current treatments, especially once prescribed in patients with greater co-morbidities than those enrolled into Phase III clinical trials.
For example, both cerivastatin and mibefradil had favorable benefit–risk profiles at market authorization, but their use in clinical practice, coupled with physicians ignoring recommended guidance, caused their withdrawal from the market (Friedman et al., 1999; Eichler et al., 2011). Previously in the 1980s zimelidine, the first selective serotonin re-uptake inhibitor, was withdrawn from the market due to hypersensitivity reactions and febrile reactions in connection to liver function disturbances, which later evolved into Guillain–Barré syndrome (GBS; Carlson, 1999, 2000). This withdrawal might have been avoided if zimelidine had been introduced in a stepwise fashion, as there was an average increase of GBS-risk of 25-fold among patients receiving zimelidine compared with the natural incidence of the disorder (Fagius et al., 1985).
Rofecoxib was also withdrawn following growing evidence of increased cardiovascular events such as heart attacks and stroke with long-term treatment (Merck, 2004; MHRA UK, 2004). Rofecoxib was seen as the most selective COX-II inhibitor among the first generation of this class with minimal COX-I activity (Davies and Jamali, 2004). Whilst this reduces gastrointestinal (GI) side-effects, this also reduced the cardioprotective effect of COX-I inhibitors that is similar to low-dose aspirin (Davies and Jamali, 2004; Bresalier et al., 2005). This protective effect of COX-I inhibitors led to a reduction in the risk of thrombotic cardiovascular events in patients treated with naproxen compared to rofecoxib (Reicin et al., 2001; Weir et al., 2003; Davies and Jamali, 2004), documented in the VIGOR study (Bombardier et al., 2000). The study specifically excluded patients who were taking concomitant aspirin or other antiplatelet drugs such as those with a recent history of myocardial infarction or stroke (Bombardier et al., 2000; Merck, 2002). The findings led to a caution being added to the product label in May 2002 in patients with a medical history of ischemic heart disease (FDA, 2002; Merck, 2002). The concerns with increased cardiovascular events associated with long-term rofecoxib therapy also led to the instigation of the APPROVe study (Bresalier et al., 2005). The findings of increased cardiovascular risk with rofecoxib (Bresalier et al., 2005) subsequently led to its withdrawal (Davies and Jamali, 2004; Merck, 2004; MHRA UK, 2004). There are ongoing debates whether the withdrawal of rofecoxib may have been avoided if there had not been appreciable marketing activities, including considerable direct to consumer advertising in the US, promoting the safety of COX-II inhibitors (Calfee and Pinell, 2005).
Natalizumab was withdrawn soon after its launch despite improved effectiveness in patients with relapsing multiple sclerosis. This was due to the development of progressive multifocal leukoencephalopathy (PML) in some patients (Kappos et al., 2011; Keegan, 2011). However, it was re-launched some 2 years later in Europe, but under strict prescribing regulations and with the instigation of research programs to clarify the benefit:risk ratios (Kappos et al., 2011; Keegan, 2011). More recently, rimonabant has been withdrawn from the market. Patients prescribed rimonabant experienced a higher incidence of anxiety, depression, and insomnia (Moreira et al., 2009; O’Shaughnessy, 2009; Ioannides-Demos et al., 2011). This led to advice that patients prescribed rimonabant should be investigated first for psychiatric illness and that rimonabant should not be prescribed in patients with mental illness (Moreira et al., 2009; O’Shaughnessy, 2009; Ioannides-Demos et al., 2011). However, this advice was sometimes ignored leading to its withdrawal due to increased risk of depression and suicidal ideation (European Medicines Agency [EMA], 2009; Godman et al., 2009c; Dietrich and Horvath, 2012; Wong et al., 2012). It may be that greater knowledge of the role of the hypothalamus in enabling the central nervous system to adapt to the changing environment could facilitate the discovery of new agents that are more effective and have a more acceptable benefit–risk profile (Wong et al., 2012). However, this remains to be seen.
New oral anticoagulants (NOACs) illustrate some of these tensions as they show promise in the prevention of stroke in patients with atrial fibrillation (AF), offering an alternative to warfarin without the need for INR (International Normalized Ratio) monitoring (Baetz and Spinler, 2008; Connolly et al., 2009; Malmström, 2009; Pink et al., 2011; Scottish Medicines Consortium, 2011; Banerjee et al., 2012; Godman et al., 2012d; Kansal et al., 2012; Mannuci et al., 2012; National Institute for Health and Clinical Excellence, 2012; Davidson et al., 2013; Joppi et al., 2013; Marshall et al., 2013; Rodriguez et al., 2013). This is in addition to venous thromboembolism prophylaxis for patients undergoing hip and knee surgery, and in the treatment of acute deep vein thrombosis and pulmonary embolism (Marshall et al., 2013). However, there are safety concerns especially in the elderly (Malmström, 2009; Pink et al., 2011; Godman et al., 2012d; Mannuci et al., 2012; Stollberger and Finsterer, 2013) in addition to potential compliance (Marshall et al., 2013; Rodriguez et al., 2013) and storage issues (Stollberger and Finsterer, 2013).
Atrial fibrillation is the most common clinically significant cardiac arrhythmia with an estimated prevalence of 1–2% of the population (Marshall et al., 2013). One in four adults over the age of 40 is likely to develop AF in their lifetime, higher in those aged over 80 (Lloyd-Jones et al., 2004; Stewart et al., 2004; Camm et al., 2010; Pink et al., 2011; Mannuci et al., 2012; Davidson et al., 2013). Current estimates suggest there are 4.5 million people in Europe with AF and 3.03 million in the US (Marshall et al., 2013), with the prevalence of AF likely to double in the next 50 years with ageing populations (Go et al., 2001; Lloyd-Jones et al., 2004; Luengo-Fernandez et al., 2006; Kirchhof et al., 2007; Connolly et al., 2009; Camm et al., 2010; Pink et al., 2011; Marshall et al., 2013). New drugs are needed since patients with AF have a fivefold increased risk of cardioembolic stroke compared with those in sinus rhythm (Stewart et al., 2004; Camm et al., 2010; Pink et al., 2011), with a cardioembolic stroke resulting in approximately 20% of patients dying in the acute phase and 60% developing severe disability (Mannuci et al., 2012). Incurred costs also tend to be higher in stroke patients with AF, with those patients who survive left more disabled by their stroke and more likely to have a recurrence than those with other causes of stroke (Luengo-Fernandez et al., 2006; Camm et al., 2010; Kansal et al., 2012). Initial incurred secondary care costs averaged GB£9667/patient (2005 costs) in patients with AF compared with an average of GB£5824 in other stroke patients (Luengo-Fernandez et al., 2006). As a consequence, the risk of death from AF related strokes is doubled compared with other forms of stroke, and the overall cost of care increased 1.5-fold (Kirchhof et al., 2007; Camm et al., 2010; Scottish Medicines Consortium, 2011; National Institute for Health and Clinical Excellence, 2012). Anticoagulant therapy with vitamin K antagonists (VKAs) can reduce by at least 60% the risk of stroke (Camm et al., 2010; Mannuci et al., 2012). However, there are concerns with warfarin due to the potential of bleeding, the need to tailor doses to the individual with too high a dose potentially causing serious complications and too low a dose losing protection, and the difficulties with maintaining some patients within INRs (Pink et al., 2011; Mannuci et al., 2012; Marshall et al., 2013).
Dabigatran received EU marketing authorization in August 2011 (Boehringer Ingelheim, 2011a; Marshall et al., 2013) for the prevention of stroke and systemic embolism/clot formation in adult patients with non-valvular AF with one or more of the following risk factors:
• Previous stroke, transient ischemic attack, or systemic embolism/clot formation
• Left ventricular ejection fraction <40%
• Symptomatic heart failure > New York Heart Association (NYHA) Class 2
• Age > 75 years
• Age > 65 years in combination with additional vascular risk, i.e., patients with diabetes mellitus, coronary artery disease, or arterial hypertension
Published studies showed a 9% reduction in the prevention of stroke or systemic embolism with dabigatran 110 mg twice daily and 34% for the 150 mg twice daily (Horsley, 2010; Mannuci et al., 2012; Davidson et al., 2013; Marshall et al., 2013). Overall mortality was also reduced by 12% for the highest dose of dabigatran, which reached statistical significance (Horsley, 2010; Mannuci et al., 2012). There was also an appreciable and consistent reduction in the risk of hemorrhagic stroke ranging from 69 to 74% depending on the dose of dabigatran (Horsley, 2010; Pink et al., 2011; Mannuci et al., 2012), with the 150 mg twice daily dose of dabigatran also providing a statistical significant reduction in ischemic stroke (24% risk reduction; Horsley, 2010; Pink et al., 2011; Mannuci et al., 2012). Dabigatran could also potentially require no monitoring compared with warfarin (Pink et al., 2011; Godman et al., 2012d; Mannuci et al., 2012; Marshall et al., 2013). As a result, dabigatran has the potential to be an important new treatment, especially where regular monitoring with warfarin is problematic or where there are adverse events or other patient issues with warfarin.
These improvements, coupled with potential savings with dabigatran with the opportunity to reduce patient monitoring, resulted in incremental cost-effectiveness ratios (ICERs) of GB£4831 (€5560)/quality adjusted life year (QALY) in patients under 80 versus warfarin and GB£7090 (€8150) above 80 (Kansal et al., 2012). A similar study in Sweden estimated the cost/QALY gained for dabigatran versus warfarin as €7742, increasing to €12,449 in patients who were well controlled with warfarin (Davidson et al., 2013). Other authors have published higher ICERs, i.e., GB£23,082 (€26,700)/QALY for high dose dabigatran versus warfarin (Pink et al., 2011; Scottish Medicines Consortium, 2011; Marshall et al., 2013). The manufacturer’s submission to the Scottish Medicines Consortium (SMC) suggested a cost/QALY of GB£6986 (€8030) versus warfarin. This estimate was based on the sequencing of dabigatran, starting with 150 mg twice daily for patients under the age of 80 who were subsequently switched to 110 mg twice daily when they reached 80 years (Scottish Medicines Consortium, 2011). The ICER increased to GB£13,347 (€15,350) when the model was adjusted to lower the potential savings from reduced INR monitoring to a more appropriate figure (Scottish Medicines Consortium, 2011; Marshall et al., 2013). The Evidence Review Group (ERG) of the National Institute for Health and Clinical Excellence (NICE) also had concerns with the model provided by the manufacturer and the cost of anticoagulation therapy (National Institute for Health and Clinical Excellence, 2012; Marshall et al., 2013). Under different assumptions, the ERG believed the base case ICER for dabigatran 150 mg twice daily increased from GB£6264 (€7200) to GB£24,173–29,131 (€27,790–33,490)/QALY (National Institute for Health and Clinical Excellence, 2012). This was due to two main weaknesses in the submitted model (National Institute for Health and Clinical Excellence, 2012). These included the lack of any potential switching of treatment from dabigatran back to warfarin as well as an overstatement of the costs of monitoring patients prescribed warfarin in practice (National Institute for Health and Clinical Excellence, 2012). There were also concerns that patient heterogeneity would be greater in practice than allowed for in the submitted models (National Institute for Health and Clinical Excellence, 2012; Marshall et al., 2013). However, both organizations recommended dabigatran as an alternative to warfarin in patients who meet the criteria outlined in the marketing authorization (Scottish Medicines Consortium, 2011; National Institute for Health and Clinical Excellence, 2012; Marshall et al., 2013), with NICE also recommending that dabigatran should only be prescribed after an informed discussion between clinicians and patients (National Institute for Health and Clinical Excellence, 2012). The National Centre for Pharmacoeconomics (NCPE) in Ireland recently concluded that the ICER for dabigatran versus warfarin was €6311/QALY in patients under 80 years and €20,654/QALY in patients 80 years or older. Extracranial hemorrhage was an important cost driver (versus warfarin in those 80 years and over) and disability costs were important across all comparisons (National Centre for Pharmacoeconomics, 2011).
However, there have been concerns with the rapid introduction of dabigatran, which led to an appreciable number of serious adverse events with the first 12 weeks of availability in the US (Institute for Safe Medication Practices, 2011). These were principally serious bleeding events or blood clots in the elderly (Institute for Safe Medication Practices, 2011). These concerns and others led the FDA to explore correlating reductions in stroke events with increasing plasma correlations alongside bleeding event rates (Thompson, 2010), with the guidance available when dabigatran was launched in Europe. In addition, re-examining and comparing the bleeding rates with warfarin and dabigatran (Southworth et al., 2013). These concerns have arisen due to dabigatran’s low mean oral bioavailability, considerable variation in plasma drug concentrations, and the complete dependence on renal elimination of the active metabolite (Stangier et al., 2008; Stangier and Clemens, 2009; Thompson, 2010; Liesenfeld et al., 2011; Douxfils et al., 2012; Huisman et al., 2012; Mannuci et al., 2012; Ten Cate, 2012). Consequently, any accumulation of dabigatran in patients with reduced renal function will increase their risk of excessive bleeding (Malmström, 2009; Legrand et al., 2011; Pink et al., 2011; Garber et al., 2012; Godman et al., 2012d; Harper et al., 2012; Huisman et al., 2012; Mannuci et al., 2012; Ten Cate, 2012), complicated by no known antidote (Rolfe et al., 2010; Institute for Safe Medication Practices, 2011; Pink et al., 2011; Godman et al., 2012d; Huisman et al., 2012; Mannuci et al., 2012; Marshall et al., 2013). This is important in this situation as patients in clinical practice are likely to be more elderly, have greater co-morbidities, and have reduced hepatic and renal functions, compared to the patients in the clinical trials (Joppi et al., 2013). There are also concerns with its budget impact given the growing prevalence of AF (Pink et al., 2011; Godman et al., 2012d; Midlands Therapeutic Review and Advisory Committee [MTRAC], 2012; Marshall et al., 2013). A number of health authorities across Europe have recognized these issues and initiated extensive pre- and peri-launch programs to educate physicians and the public regarding the optimal use of dabigatran, especially in elderly patients with poor renal function.
The principal objective of this paper is to review health authority and health insurance company activities across Europe pre-launch to post-launch of dabigatran for the prevention of stroke as an exemplar for developing future models to better manage the entry of new premium priced drugs. Subsequently, to use these strategies to suggest future activities that all key stakeholder groups could undertake to reduce the likelihood of new drugs being removed from the market place where there are concerns with their use in a wider patient population. Finally, to suggest activities that better manage expenditure on new drugs where there are concerns with their budget impact. This is important as concerns with the budget impact of new drugs are growing. This especially given the number of new drugs in development including new biological drugs (EvaluatePharma, 2012; Godman, 2013), which are now costing up to US$10,000–25,000 (€7580–18,960) per patient per month (Selyukh, 2011; Yukhananov, 2011; Godman et al., 2012b; Kaiser, 2012; UK Medicines Information, 2012; UKMi Medicines Information, 2013). This potentially inhibits the ability of governments to continue to provide equitable and comprehensive healthcare within current budgets.
METHODOLOGY
A descriptive review of national, regional or local health authority, health insurance company or physician association activities across Europe regarding dabigatran up to and including the beginning of 2013 was conducted by one of the co-authors (Brian B. Godman). This was undertaken by collating and appraising relevant published papers and internal documents known to the co-authors as well as any pertinent documents available on the internet. Direct feedback was provided by the co-authors where there was limited or no data available in a particular country. The information provided by the co-authors was subsequently re-checked (Brian B. Godman) to enhance its accuracy. In total, information was collected from over 30 European countries and regions. We have used this methodological approach in previous publications involving health authority and health insurance company personnel when there has been a paucity of published data (Cheema et al., 2012; Godman et al., 2011b,c, 2010, 2012a,b; Adamski et al., 2010; Baumgärtel et al., 2012). The countries were chosen to provide differences in geography, epidemiology, financing of healthcare, available resources for healthcare as well as different approaches to the pricing and reimbursement of new drugs (Godman et al., 2008, 2010, 2011b, 2012d; Wettermark et al., 2008, 2010a; Coma et al., 2009; Sermet et al., 2010; Garuoliene et al., 2011a,b; Voncina and Strizrep, 2011; Cheema et al., 2012; Markovic-Pekovic et al., 2012; Godman and Gustafsson, 2013). This included both national and regional authorities in some countries, recognizing ongoing budget devolution, e.g., England, Scotland, Spain, and Sweden, as well as differences with the organization and funding of anticoagulant services.
Demand-side initiatives and reforms were collated under four different activities named the four Es – Education, Engineering, Economics, and Enforcement (Wettermark et al., 2009a) – to provide comparisons with measures used to improve the quality and efficiency of the prescribing of existing drugs across Europe (Godman et al., 2009b,c, 2010, 2011a,b, 2012b,c,f; Wettermark et al., 2009a,b, 2010b; World Health Organisation, 2009; McGinn et al., 2010; Gustafsson et al., 2011; Vončina et al., 2011; Baumgärtel et al., 2012; Bennie et al., 2012; Kalaba et al., 2012; Markovic-Pekovic et al., 2012; Medicine Balance [MEDICIJNBALANS], 2012; van Woerkom et al., 2012); they include:
• Educational activities – these range from simple distribution of printed material to intensive strategies including academic detailing and monitoring of prescribing habits usually by professional medical networks. Examples include local, regional, and national formularies, guidance and guidelines including those from Drug and Therapeutic Committees
• Engineering activities – organizational or managerial issues to influence change, e.g., quality and efficiency prescribing targets
• Economic interventions – financial incentives. These include financial incentives for physicians if they achieve agreed prescribing targets in a class, devolution of drug budgets to local GP groups combined with regular monitoring of prescribing behavior, as well as fines for prescribing costs above agreed limits. Initiatives also include patient co-payments, especially if patients wish a more expensive product than the current reference priced product for the molecule (Anatomical Chemical Therapeutic – ATC – Level 5) or the class/group (ATC Level 3 or 4)
• Enforcement – regulations by law such as compulsory International Non-proprietary Name (INN) prescribing, compulsory generic substitution, or prescribing restrictions such as those instigated for patented statins in Austria, Finland, and Norway and the angiotensin receptor blockers (ARBs) in Austria, Croatia, Lithuania, the Republic of Srpska, and Sweden
No attempt has been made to critique the initiatives, including comparing and contrasting the potential influence of the multiple initiatives across the countries and regions to provide future guidance. This is because this would require a thorough analysis of drug utilization patterns alongside associated health policies (Coma et al., 2009; Godman et al., 2009b, 2010, 2011a,b,2012c; Wettermark et al., 2009b, 2010b; McGinn et al., 2010; Garuoliene et al., 2011b; Vončina et al., 2011; Bennie et al., 2012; Kalaba et al., 2012; Markovic-Pekovic et al., 2012; van Woerkom et al., 2012). This will be undertaken in future research projects. However, documented initiatives were used to derive suggested models and potential guidance for all key topics and stakeholder groups to improve the managed entry of new drugs in the future. Initial models and draft guidance were subsequently amended and refined through an iterative process. This involved several rounds with the co-authors until all co-authors were satisfied and agreed with the proposed new model and guidance provided.
RESULTS
HEALTH AUTHORITY AND HEALTH INSURANCE COMPANY ACTIVITIES
Table 1 summarizes some of the health authority/health insurance company activities pre-, peri-, and post-launch up till the end of 2012. Unless stated, the indications are those contained in the EMA marketing authorization (Boehringer Ingelheim, 2011a; Marshall et al., 2013). Table A1 in the Appendix provides a comprehensive summary of examples from regions and countries across Europe.
Table 1.
Summary of key activities across Europe to improve the quality and efficiency of prescribing of dabigatran (Godman et al., 2008, 2009a,c, 2011a, 2012d; Holmström et al., 2009; Janusinfo, 2009, 2012a,b; KVH - Aktuell, 2010; Martikainen et al., 2010; Wettermark et al., 2010a,c; Boehringer Ingelheim, 2011b; Gustafsson et al., 2011; Neue Arzneimittel, 2011; Stockholms läns landsting, 2011; Vončina et al., 2011; Keele University School of Pharmacy, 2012; Medicin & Läkemedel, 2012; Midlands Therapeutic Review and Advisory Committee [MTRAC], 2012; Östergötland, 2012; Persson et al., 2012; Davidson et al., 2013).
| Timing | Examples of activities among European countries and regions |
|---|---|
| Pre-launch (principally education) | Swedish counties: |
| (A) Östergötland County Council | |
|
|
| (B) Stockholm County Council | |
|
|
| Peri-launch (principally education) | (A) West Midlands (Region – England) |
|
|
| (B) Germany | |
|
|
| (C) Slovenia | |
|
|
| Post-launch (principally education and enforcement) | (A) Austria (enforcement) |
|
|
| (B) Finland (enforcement) | |
|
|
| (c) Slovenia | |
|
Similarly in Australia, the Department of Health and Ageing in the Ministry of Health recently undertook a review of NOACs in the management of stroke risk in patients with AF (Australian Government Department of Health and Ageing, 2012). They recommended to the Pharmaceutical Benefits Advisory Committee (PBAC) the following based on their belief that the net overall benefit of NOACs in clinical practice, and the subsequent impact on cost-effectiveness, is uncertain at this stage:
• Initiating a managed entry scheme taking into account the identified uncertainties while acknowledging the clinical need for effective alternatives to warfarin. This includes the entry price that addresses the uncertainties
• New oral anticoagulants are only reimbursed in patients unable to tolerate warfarin therapy and/or who are unable to obtain satisfactory INR control despite specific measures. This would require a definition of “satisfactory INR control” together with potential price–volume arrangements that address the risk to the Australian Government of use beyond such restrictions
This recommendation has resulted in PBAC undertaking further analysis as it reviews its previous decisions (NPS MEDICINEWISE, 2012).
PROPOSED MODEL AND ASSOCIATED ACTIVITIES
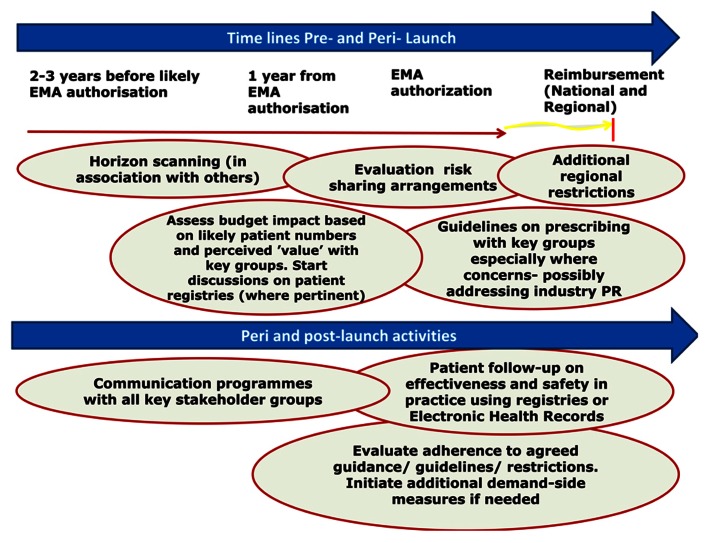
Figure 1 outlines the suggested new model to better manage the entry of new drugs in the future. This is based on the extensive knowledge and experience of the co-authors shared across healthcare institutions. This builds on the three pillars of pre, peri-, and post-launch activities (Wettermark et al., 2010a; Godman et al., 2012d).
FIGURE 1.
Proposed model for optimizing the managed entry of new drugs across Europe incorporating national and regional stakeholder groups where pertinent building on the example of dabigatran.
This starts with horizon scanning activities pre-launch and continues to post-launch monitoring, benchmarking, and registries. Potential activities for each stakeholder group are discussed later in Table 4.
Table 4.
Key considerations among stakeholder groups to optimize the managed entry of new drugs (Garattini et al., 2008; Godman et al., 2008, 2009c, 2012a,b,d; Wettermark et al., 2008, 2010a,c; Coma et al., 2009; Adamski et al., 2010; Sermet et al., 2010; Gustafsson et al., 2011; Kolasa et al., 2011; Vončina et al., 2011; Cheema et al., 2012; Siviero et al., 2012; Godman and Gustafsson, 2013).
| Stakeholder | Key considerations among stakeholder groups to optimize the managed entry of new drugs |
|---|---|
| Health authorities/health insurance companies/physician associations | Pre-launch |
|
|
| Peri-launch | |
|
|
| Post-launch | |
|
|
| Physicians | Peri-launch |
|
|
| Post-launch | |
|
|
| Patient organizations | Pre-launch |
|
|
| Pre- and peri-launch | |
|
|
| Post-launch | |
|
|
| Commercial organizations | Pre-launch |
|
|
| Peri- and post-launch | |
|
There has been a growth in risk sharing arrangements across Europe as health authorities and health insurance companies struggle to fund new premium priced products within available funds (Adamski et al., 2010; Godman, 2011; Jaroslawski and Toumi, 2011; Klemp and Frønsdal, 2011; Cheema et al., 2012; Hirschler, 2012; Jommi, 2012; Siviero et al., 2012; Vogler et al., 2012). Risk sharing has previously been defined as agreements concluded by payers and pharmaceutical companies to diminish the impact on the payer’s budget of new and existing medicines brought about by either the uncertainty of the value of the medicine and/or the need to work within finite budgets (Adamski et al., 2010; Godman, 2011). Consequently, the agreement lies in setting the scope and realizing the mutual obligations amongst both payers and pharmaceutical companies depending on the occurrence of an agreed condition – the “risk,” which varies by situation (Adamski et al., 2010; Godman, 2011).
In view of the concerns with some of these schemes, coupled with the experiences with dabigatran across Europe (Table 1 and Table A1 in the Appendix), national and regional health authorities and health insurance companies should consider a number of key issues when appraising risk sharing schemes in the future (Table 2). These considerations do not apply to price:volume agreements, straight discounts or rebates, which are easier to administer (Jaroslawski and Toumi, 2011).
Table 2.
Key issues for health authorities and health insurance companies to consider when appraising risk sharing arrangements proposed by pharmaceutical companies for new drugs.
| Key issues regarding risk sharing arrangements |
|---|
|
Health authorities and health insurance companies also need to consider a number of key issues before implementing patient registries. Key considerations and issues are shown in Table 3. Patient registries can subsequently be used to assess the effectiveness, safety and cost-effectiveness of new treatments in routine clinical care. The nature and extent of data collected will depend on the objectives of any study.
Table 3.
Key issues for authorities to consider when planning patient registries post-launch.
| Events/timing | Key considerations regarding patient registries |
|---|---|
| Funding and other considerations | Funding |
|
|
| Legal considerations | |
|
|
| Ownership | |
|
|
| Endorsement | |
|
|
| Timing |
|
|
Overall, there are a number of activities that each key stakeholder group should consider pre-, peri-, and post-launch to better manage the entry of new drugs. This is especially important where there are potential safety and/or resource issues (Table 4). These build on the three pillars and a brief outline of activities discussed in Figure 1.
EMA AND FDA ACTIVITIES
The low mean bioavailability of dabigatran (Douxfils et al., 2012; Mannuci et al., 2012; Ten Cate, 2012), as well as studies demonstrating considerable variation in plasma drug concentrations in practice, led the FDA in 2010 to explore the relationship between dabigatran concentrations in plasma and the risks of suffering a stroke or major bleeding (Thompson, 2010; Ten Cate, 2012). These publications also demonstrated it is important to avoid too low or too high levels of dabigatran (Mismetti and Laporte, 2010; Thompson, 2010). Consequently similar to warfarin, certain patients on dabigatran and other NOACs should be monitored to reduce potential side-effects (Mismetti and Laporte, 2010; Douxfils et al., 2012; Mannuci et al., 2012; Ten Cate, 2012).
The EMA in their Risk Minimization Plan for dabigatran issued in 2011 also defined a cut-off for the risk of bleeding with the 150 mg bid regimen of 200 ng/mL dabigatran in plasma at Ctrough (Heidbüchel et al., 2011).
DISCUSSION
Dabigatran and the other NOACs are the result of a long search for an alternative to warfarin to prevent strokes in patients with AF. However, the weighing of the advantages and disadvantages associated with dabigatran, especially in the elderly with poor renal function, needs to be judged carefully and handled appropriately alongside the additional acquisition costs of dabigatran. These challenges led to an extensive range of activities among national and regional health authorities, health insurance companies, and physician associations across Europe pre-, peri-, and post-launch to enhance its appropriate use (Table 1 and Table A1 in the Appendix).
The main medical concerns were the risk of excessive bleeding in elderly patients with AF with no known antidote, variable plasma drug concentrations in practice exacerbated by low bioavailability, and the dependence on renal elimination of the active metabolite (Baetz and Spinler, 2008; Malmström, 2009; Legrand et al., 2011; Liesenfeld et al., 2011; Banerjee et al., 2012; Douxfils et al., 2012; Godman et al., 2012d; Harper et al., 2012; Huisman et al., 2012; Mannuci et al., 2012; Ten Cate, 2012; Marshall et al., 2013). Cases of major bleeding and deaths were seen with dabigatran soon after its launch (Malmström, 2009; Institute for Safe Medication Practices, 2011; EMA, 2011; Legrand et al., 2011; Wood, 2011; Garber et al., 2012; Godman et al., 2012d; Harper et al., 2012; Mannuci et al., 2012; Lothian Prescribing Bulletin, 2012; Marshall et al., 2013). The EMA reported on November 6, 2011 that there had already been 256 spontaneous reports of serious bleeding resulting in deaths in the EudraVigilance database (EMA, 2011).
Table 1 and Table A1 in the Appendix document the extensive range of activities initiated across Europe. These include educational activities pre-launch in Stockholm County Council, Sweden, as well as post-launch activities among regions and localities in Germany, Spain, Sweden, and the UK. There were also prescribing restrictions in some countries alongside the development of shared care protocols between ambulatory and hospital care to improve interface management and enhance the subsequent quality of care (Godman et al., 2012e). It is suggested that these activities reduced subsequent bleeding among patients in practice, especially among those with poor renal function and, as a result, potentially helped preserve the availability of dabigatran across Europe. This is unlike that situation seen with a number of drugs described earlier including zimelidine, COX-II inhibitor drugs, cerivastatin, and rimonabant (Fagius et al., 1985; Carlson, 1999, 2000; Friedman et al., 1999; FDA, 2002; Merck, 2002, 2004; MHRA UK, 2004; Calfee and Pinell, 2005; European Medicines Agency [EMA], 2009; Moreira et al., 2009; O’Shaughnessy, 2009; Eichler et al., 2011; Ioannides-Demos et al., 2011; Kappos et al., 2011; Keegan, 2011; Dietrich and Horvath, 2012). However, it is difficult to substantiate this without definite research. Having said this, reimbursement of dabigatran has recently been rejected in Poland due to concerns with excessive bleeding and deaths (Table A1 in the Appendix).
There have also been issues with the additional costs of dabigatran versus warfarin at GB£919.80 (€1060) per patient (UK) given the growing prevalence of AF with currently over 4.5–6 million patients across Europe and rising (Lloyd-Jones et al., 2004; Stewart et al., 2004; Camm et al., 2010; Pink et al., 2011; Mannuci et al., 2012; Marshall et al., 2013). However, there is less of a budget differential in Sweden (Davidson et al., 2013). These combined issues led to (i) prescribing restrictions in some countries alongside prior authorization schemes, e.g., Austria, Belgium, Finland, NHS Bury (initially), Slovakia, and Slovenia, (ii) delays with reimbursement in others including Croatia (still undergoing review), the Netherlands, Norway (only just reimbursed), and Portugal (150 mg); as well as (iii) price:volume and other agreements (risk sharing) to lower the price of dabigatran, e.g., Ireland, the Netherlands, and Slovenia as well as potentially in Croatia (Table 1 and Table A1 in the Appendix). These concerns have also resulted in dabigatran not being reimbursed/not preferred as an alternative in some countries and regions including Estonia, Lithuania, Lothian Health Board (Scotland), NHS Cornwall Community Health, the Republic of Serbia, and Turkey (Table A1 in the Appendix). Prescribing restrictions and risk sharing arrangements are no doubt preferred by manufacturers versus not having their drugs reimbursed.
The weighing up of the benefits and concerns with dabigatran make it increasingly important for European countries and regions to develop and refine models to further improve the managed entry of new premium priced drugs, even if they do not have a tradition of Health Technology Assessment (HTA). The alternative could be reduced resources to fund new drugs in the future, especially with a growing elderly population, which is already happening (Garuoliene et al., 2011a,b; Godman et al., 2011c, 2012b; Taylor, 2011). As mentioned previously, budgetary pressures are growing as a result of the number of new biological drugs in development (EvaluatePharma, 2012; Godman, 2013) including new cancer drugs (Nagle et al., 2008; National Cancer Institute, 2010; Sullivan et al., 2011; Mullard, 2012), which are now costing up to US$10,000–25,000 (€7580–18,960) per patient per month (Selyukh, 2011; Yukhananov, 2011; Kaiser, 2012; UK Medicines Information, 2012; UKMi Medicines Information, 2013). Such models may also reduce the possibility of new drugs such as dabigatran being withdrawn from the market due to a greater level of side-effects in a wider co-morbid population than that included in the clinical trials (Joppi et al., 2013). None of these alternative scenarios are in the best interests of any key stakeholder group.
Moreover, it is critical that health authorities and health insurance companies take full advantage of the increasing availability of standard drugs as generics to help fund increased volumes and new premium priced drugs in the future (Frank, 2007; Jack, 2008; Godman et al., 2012a,b,f). For example, expenditure on proton pump inhibitors (PPIs) and statins would have been GB£449 million (€520 million) higher in Scotland in 2010 without appreciable demand-side measures enhancing the prescribing of low cost generics for its 5.2 million population (Bennie et al., 2012; Godman et al., 2012b). This activity is driven by global sales of products likely to lose their patents between 2008 and 2013 estimated at US$50–100bn (€38–76bn), and considerably higher for subsequent years (Frank, 2007; Jack, 2008; Godman et al., 2012b,f), out of global sales of pharmaceuticals estimated at US$820bn (€625bn) in 2009 (EATG, 2009).
The next stage of our research will be to assess the influence of the plethora of health authority and health insurance company activities (Table 1 and Table A1 in the Appendix) on subsequent utilization of dabigatran and other NOACs, alongside ongoing reforms, to further refine the suggested model (Figure 1). This should also help with future recommendations regarding potential demand-side measures that could be introduced to further improve the managed entry of new drugs, based around the four Es (Wettermark et al., 2009a). This includes the implications for all key stakeholder groups (Table 4). We are already seeing health authorities and health insurance companies monitor the effectiveness and safety of patients prescribed dabigatran and other NOACs, and this will grow.
In the meantime, we hope we have demonstrated why it is imperative that health authorities and health insurance companies continue to develop and refine new models to better manage the entry of new drugs in the future. In addition, we hope we have provided direction to all key stakeholder groups based on our considerable experience to further stimulate this debate in this critically important area. This especially as the constant introduction of new premium priced drugs is seen as the greatest challenge to the continued provision of equitable and comprehensive healthcare in Europe (Garattini et al., 2008; Godman et al., 2012d).
CONCLUSION
There have been multiple activities pre- to post-launch among authorities across Europe to improve the prescribing of dabigatran, especially in elderly patients where there are concerns with their renal function. In addition, address potential concerns with the budget impact of dabigatran through for instance price:volume agreements and prescribing restrictions.
We believe and recommend, based on the experiences with dabigatran and other new premium priced drugs, that it is essential for authorities to develop new models to better manage the entry of new drugs in the future (Figure 1). This is becoming critical given the number of new premium priced drugs in development.
Critical activities for health authorities and health insurance agencies pre-launch in the future involve horizon scanning and budget planning activities. This includes identifying products likely to lose their patent within the next 1–2 years. In addition, educational materials and clinical guidance need to be developed pre-launch with the help of physicians and patient groups. Key peri-launch activities include developing prescribing indicators for new treatments as well as the critical appraisal of any proposed risk sharing arrangements, assessed against the criteria documented in Table 2. Essential post-launch activities include monitoring of prescribing against agreed guidance. Increasingly also entering patients into registries to monitor the effectiveness and safety of new drugs in wider patient populations having considered key issues (Table 3).
Without such models, authorities may well struggle to maintain the European ideals of equitable and comprehensive healthcare as well as ensuring funding for new “valued” treatments in target populations. Consequently, the development of new models to better manage the entry of new drugs should be in the interest of all key stakeholder groups.
Conflict of Interest Statement
There are no conflicts of interest from any author. However, the majority of authors are employed by health authorities, health insurance companies, universities, or Physician Associations or are advisers to them. The content of the paper and the conclusions are those of each author and may not necessarily reflect those of the organization that employs them.
Acknowledgments
This work was in part supported by grants from the Karolinska Institutet, Sweden. The authors would like to thank Elina Asola and Jaan Martikainen from Finland for their help with the data from Finland and critiquing previous drafts. No writing assistance was provided for this paper.
Appendix
Table A1.
Examples of health authority and health insurance company activities regarding dabigatran for the prevention of stroke in adults with non-valvular AF among European countries to the beginning of 2013 (building on EU marketing authorisation – (Boehringer Ingelheim, 2011a; Marshall et al., 2013).
| Country | Date dabigatran reimbursed for AF | Summary of activities |
|---|---|---|
| Austria (Godman et al., 2008, 2009a; Wettermark et al., 2009a; Vončina et al., 2011; Therapie Tipps, 2012) | February 2012 | Post-launch |
| Enforcement – Ex ex-ante approval by the head physician of the patient’s social health insurance fund before reimbursement of dabigatran; otherwise 100% co-payment (mirroring other situations). This is now fully automated, with the first prescription typically taking approximately 30 min to approve | ||
| The renal function has to be assessed and recorded prior to initiation of therapy with dabigatran through determining Creatinine-Clearance (CrCl) levels to exclude patients with severe renal dysfunction (= CrCl < 30ml/min). In addition during treatment, renal function has to be monitored where a decline is envisaged, e.g., patients with hypovolaemia, dehydration and the use of specific additional medication, and renal function has to be assessed at least once a year in patients aged 75 or older, and/or in patients with compromised renal function. Otherwise 100% co-payment | ||
| Health Insurers (WGKK – Vienna) have also stated that patients who are well adjusted on Vitamin K antagonists should not be switched to dabigatran as there is no additional clinical benefit, enhanced by currently no known antidote. | ||
| Belgium (Federal Agency for Medicines and Health Products (FAMHP), 2011) | August 2012 | (i) Reimbursement |
|
||
| (ii) Education (pre-launch) | ||
|
||
| Peri-launch (Enforcement) | ||
|
||
| Post-launch (Education, Engineering) | ||
|
||
| Croatia | Under evaluation | Reimbursed for the prevention of venous thromboembolism in patients undergoing hip or knee surgery, and only in hospitals, with prescriptions traced in hospitals if abuse is suspected. |
| Under consideration for the prevention of stroke and systemic embolism in adult non-valvular AF patients including price:volume agreements and/ or co-payments. There are also ongoing discussions regarding safety issues | ||
| England (Horsley, 2010; Elton et al., 2011; Keele University School of Pharmacy, 2012; Midlands Therapeutic Review and Advisory Committee [MTRAC], 2012; National Institute for Health and Clinical Excellence, 2012; Marshall et al., 2013; NHS Improvement. Guidance on Risk Assessment and Stroke Prevention for Atrial Fibrillation (GRASP – AF), 2013) Coventry and Warwickshire Area Prescribing Committee (2012); Interface – A monthly medicines and prescribing bulletin for healthcare professionals in East Lancashire focusing on new therapies (2011); East Lancashire NHS Medicines Management Health Economy New Medicines and Treatments Group - Dabigatran for prevention of stroke in non-valvular atrial fibrillation (2012); NHS Cumbria, NHS Lancashire (2012a),b; | August 2011 | Post-launch |
| (A) National – NICE: | ||
| Dabigatran is recommended in line with the licenced indication, with the decision whether to start treatment made after an informed discussion between clinicians and patients about the risks and benefits of dabigatran versus warfarin. | ||
| For patients already on warfarin, the potential risks and benefits of switching to dabigatran should be considered in light of current INR control. | ||
| (B) Regions (Midlands – MTRAC) – Education | ||
| Guidance stating that warfarin remains the first-line option for anticoagulation in patients with AF at high risk of a stroke, and PCTs should ensure optimal existing warfarin therapy services – including access to INR clinics, use of computerised decision-support software, and access to drugs for patients who are allergic to warfarin (the latter is rare in practice) | ||
| In view of the considerable financial implications, dabigatran treatment should only be prescribed for those patients: | ||
|
||
| Alongside this, patient follow-up via agreed shared care protocols with ongoing monitoring of prescribing costs and feedback from Pharmaceutical Advisers. | ||
| (C) Localities | ||
| (i) Coventry and Warwickshire Area Prescribing Committee (Education, engineering): | ||
|
||
| (ii) East Lancashire (Education): | ||
| Initially not approved (October 2011); but subsequently approved for usage in January 2012. As part of this: | ||
|
||
| (iii) NHS Bury | ||
|
||
| (iv) NHS Cornwall Health Community (Education) | ||
|
||
| (v) NHS Lancashire/ NHS Cumbria (Education, economics): | ||
|
||
| Estonia | Not reimbursed | Dabigatran rejected for this indication as not seen as sufficiently cost-effective versus warfarin in view of its high acquisition costs |
| Finland (Martikainen et al., 2010; Godman et al., 2011a) | April 2012 | Post-launch (enforcement) |
| Reimbursement restrictions (Enforcement) – limiting the reimbursement of dabigatran to patients with risk factors where satisfactory control has not been reached with warfarin; alternatively, warfarin cannot be prescribed due to side-effects or contra-indications. | ||
| Enforcement at the pharmacy with on average 16 days needed for requests to be centrally reviewed and authorised. 100% co-pay without authorisation. | ||
| France (Sermet et al., 2010; Godman et al., 2012a; Haute Authorite de Sante – Commission de la Transparence, 2012) | ASMR Rating February 2012 | Peri-launch |
| Dabigatran classified as ASMR V (no additional therapeutic value) compared with current therapies for the prevention of strokes in adults at risk who have “non-valvular atrial fibrillation” and are considered to be at risk of stroke. | ||
| Post-launch | ||
| (a) April 2012 – Education: | ||
| (i) Publication of information about dabigatran from the authorities including a warning from the medicine agency ANSM (ex afssaps): | ||
|
||
| (ii) Publication of advice for (1) change of prescribing from or to other anticoagulants, (2) patients undergoing surgery | ||
| (b) May 2102 | ||
| Translation of the latest advice from the EMA | ||
| Once reimbursed, patients will be followed up to assess the effectiveness and safety of dabigatran in practice (pharmacovigilance) | ||
| Germany (IQWiG, 2010; Kreatinin-Clearance Rechner (Creatinine Clearance calculator), 2013) | August 2011 | Peri- and post-launch |
| Activities (education and engineering) included the following: | ||
|
||
| Ireland (National Centre for Pharmacoeconomics, 2011; Burke, 2012; Health Service Executive (HSE) Ireland. Primary Care Reimbursement Service (PCRS) Online Services, 2012) | July 2012 | Peri- and post-launch |
| August 2011: The National Centre for Pharmacoeconomics (NCPE) stated that “dabigatran etexilate could be considered a cost effective treatment for the prevention of stroke and systemic embolism for adult patients with atrial fibrillation and one or more of the specified risk factors. However there are uncertainties associated with some of the clinical input data and the model assumptions in addition to the considerable opportunity cost, in the region of €13 million over 10 years.” In view of this a reduction in price is recommended to ensure value for money for the health service in Ireland (HSE). | ||
| November 2011: A HSE Statement advises that the drug will not be reimbursed if prescribed for any new patients for SPAF (Stroke prevention in patients with AF). | ||
| July 2012: A HSE Statement states that: | ||
| Warfarin is the recommended first line agent for stroke prevention in atrial fibrillation. Dabigatran should be reserved for: | ||
|
||
| As part of the implementation, the physician responsible has to make a specific application for each patient to HSE. Otherwise, pharmacists will not be reimbursed for dispensing dabigatran to patients for SPAF without prior reimbursement approval (enforcement) | ||
| October 2012: Following the NCPE pharmacoeconomic assessment (August 2011), the manufacturer reduced the price of dabigatran. At this revised price, the NCPE now considers dabigatran to be cost effective in this situation | ||
| Italy (Adamski et al., 2010; Cheema et al., 2012; Jommi, 2012; Siviero et al., 2012) | Undergoing evaluation | Pre-launch |
|
||
| Post-launch | ||
|
||
| Lithuania | Not reimbursed | Reimbursement was rejected at the end of 2012 as dabigatran was not seen as sufficiently cost-effective versus warfarin |
| Netherlands (Medicine Balance [MEDICIJNBALANS], 2012) | December 2012 | Peri- to post-launch(education, economics) |
|
||
| Norway | January 2013 | Peri-launch |
|
||
| Poland (Agencja Oceny Technologii Medycznych Rada Przejrzystości, 2012) | Not reimbursed | The Transparency Council of the Polish HTA Agency assessed dabigatran for its potential inclusion in the national reimbursement list |
| The Transparency Council subsequently rejected reimbursement due principally to safety concerns. The Council was concerned about the number of serious bleeds and deaths that had already occurred in the United States and New Zealand soon after its launch in these countries. | ||
| Portugal | August 2011 for 110 mg (when PL granted) |
|
| The Republic of Serbia | Not reimbursed | Dabigatran is currently not reimbursed in Serbia principally due to concerns with its price/ budget impact versus warfarin for the prevention of stroke in patients with AF and the perceived limited benefits in practice. |
| Republic of Srpska | Not currently reimbursed |
|
| Scotland (NHS Tayside, 2011; Fife Area Drug and Therapeutics Committee (2012); Health Improvement Scotland (2012); NHS Highland - The Pink One (2012); Scottish Medicines Consortium, 2011; Oral anticoagulants, 2012; Marshall et al., 2013) | August 2011 | Peri-launch |
| National (SMC) – Dabigatran is accepted for use in accordance with the approved indication as it was seen to be at least as effective as standard oral anticoagulation at preventing stroke or systemic embolism and was not associated with an increased risk of major bleeding. | ||
| Post-launch (among the Health Boards) (educational) | ||
| (a) Fife (December 2011) | ||
| Dabigatran should only be prescribed in line with advice from Healthcare Improvement Scotland, i.e., on balance of risks and benefits of dabigatran, warfarin remains the anticoagulant of clinical choice for moderate or high risk atrial fibrillation patients (CHA2DS2-VASc = 2) with good INR control, and clinicians should only consider prescribing dabigatran in patients with: | ||
|
||
| (b) Highlands (December 2011) | ||
|
||
| (c) Tayside (December 2011) | ||
|
||
| (d) Lothian (May 2012 Bulletin) | ||
|
||
| Slovakia | April 2012 | Peri-launch |
|
||
| Post-launch | ||
|
||
| Slovenia | August 2012 | Peri-launch |
| Reimbursement in line with the licensed indication in conjunction with a complex price: volume agreement | ||
| Post-launch | ||
| Demand-side activities included: | ||
|
||
| Spain (Coma et al., 2009; Ostazen, 2011) | November 2011 | (A) Post-launch activities (Basque Country) |
| (a) Assessment | ||
|
||
| (b) Engineering/ enforcement | ||
|
||
| (c) Post-launch activities also included (education, engineering) | ||
|
||
| (B) Post-launch activities (Catalonia) | ||
| (a) Education | ||
|
||
| (b) Engineering | ||
|
||
| (c) Economics | ||
|
||
| Post-launch activities (education, engineering) also included: | ||
|
||
| Sweden (Godman et al., 2009c, 2012d; Holmström et al., 2009; Janusinfo, 2009; Stockholms läns landsting, 2011; Janusinfo, 2012a; Medicin & Läkemedel, 2012; östergötland, 2012; Davidson et al., 2013), SBU (Swedish Council on Health Technology Assessment, 2011) | November 2011 | (I) National – peri-launch
|
| (II) Regional/ County activities | ||
| (i) östergötland County Council | ||
| Pre-launch activities (educational, economics) | ||
|
||
| Peri-launch/ post-launch | ||
|
||
| (ii) Stockholm County Council | ||
| Pre-launch | ||
|
||
| Peri-launch/ post-launch | ||
|
||
| Turkey | Not reimbursed |
|
AUTHOR CONTRIBUTIONS
All authors critiqued successive drafts to develop robust recommendations. Additional specific areas include: Brian B. Godman – developed the first draft and co-ordinated activities and inputs regarding additional drafts; Rickard E. Malmström, Eduard Diogene, Christoph Baumgärtel, and Lars L. Gustafsson – provided input regarding dabigatran and NOACs, as well as comments regarding patient registries, clinical trial design and physician activities; Marion Bennie, Iain Bishop, and Jan Jones – provided data regarding Scotland. Jan Jones also critiqued the health economic section particularly regarding SMC; Anna Brzezinska, Kamila Malinowska, and Magdalena Wladysiuk – provided data regarding Poland; Anna Bucsics and Jutta Piessnegger – provided data regarding Austria; Stephen Campbell – provided specific input regarding the development of quality indicators along with Menno van Woerkom; Alessandra Ferrario and Alexander Finlayson – critiqued successive drafts using their expertise in health services research across a range of countries; Jurij Fürst – provided data on Slovenia; Kristina Garuoliene – provided data on Lithuania; Miguel Gomes – provided data on Portugal; Iñaki Gutiérrez-Ibarluzea, Eduard Diogene, and Corrine Zara – provided data on the Spanish regions; Iñaki Gutiérrez-Ibarluzea, Alan Haycox, Laura McCullagh, Ken Paterson, and Magdalena Wladysiuk – provided guidance regarding the health economic data; Alan Haycox, Julie Lonsdale, Andrew Martin, and Michael Wilcock – provided data on England. Krystyna Hviding and Christina Kvalheim – provided data on Norway; Harald Herholz, Irene Langner, and Gisbert Selke – provided data on Germany; Mikael Hoffmann, Lars L. Gustafsson, Rickard E. Malmström, and Sven-Åke Lööv – provided data on county council activities in Sweden; Saira Jan provided data regarding post launch activities in the US; Roberta Joppi – provided data on Italy as well as data on the characteristics of patients with AF compared to the clinical trial populations; Marija Kalaba – provided data on the Republic of Serbia; Ott Laius – provided data on Estonia; Laura McCullagh – provided data on Ireland; Vanda Markovic-Pekovic – provided data on the Republic of Srpska; Catherine Sermet – provided data on France; Steven Simoens – provided data on Belgium; Cankat Tulunay – provided data on Turkey; Dominik Tomek – provided data on Slovakia; Luka Vončina and Vera Vlahovic-Palcevski – provided data on Croatia; Janet Wale – critiqued comments regarding potential patient involvement in new models; Menno van Woerkom – also provided data on the Netherlands.
REFERENCES
- Abraham J. (2008). Sociology of pharmaceuticals development and regulation: a realist empirical research programme. Sociol. Health Illn. 30 869–885 [DOI] [PubMed] [Google Scholar]
- Adamski J., Godman B., Ofierska-Sujkowska G., Osinska B., Herholz H., Wendykowska K., et al. (2010). Review of risk sharing schemes for pharmaceuticals: considerations, critical evaluation and recommendations for European payers. BMC Health Serv. Res. 10:153 10.1186/1472-6963-10-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agencja Oceny Technologii Medycznych Rada Przejrzystości. (2012). Dabigatran. Available at: http://www.aotm.gov.pl/assets/files/rada/rekomendacje_stanowiska/2012_SRP/R-08-2012-Pradaxa/Stanowisko_RP_AOTM_8_2012_Pradaxa_(dabigatran)_prewencja.pdf (accessed January 2013) [Google Scholar]
- Australian Government Department of Health and Ageing. (2012). Review of Anticoagulation Therapies in Atrial Fibrillation. Available at: http://www.pbs.gov.au/reviews/atrial-fibrillation-files/report-anticoagulation.pdf (accessed January 2013) [Google Scholar]
- Baetz B. E., Spinler S. A. (2008). Dabigatran etexilate: an oral direct thrombin inhibitor for prophylaxis and treatment of thromboembolic diseases. Pharmacotherapy 28 1354–1373 [DOI] [PubMed] [Google Scholar]
- Banerjee A., Lane D. A., Torp-Pedersen C., Lip G. Y. (2012). Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb. Haemost. 107 584–589 [DOI] [PubMed] [Google Scholar]
- Baumgärtel C., Godman B., Malmstrom R. E., Andersen M., Abuelkhair M., Abdu S., et al. (2012). What lessons can be learned from the launch of generic clopidogrel? GaBi J. 1 58–68 [Google Scholar]
- Bennie M., Godman B., Bishop I., Campbell S. (2012). Multiple initiatives continue to enhance the prescribing efficiency for the proton pump inhibitors and statins in Scotland. Expert Rev. Pharmacoecon. Outcomes Res. 12 125–130 [DOI] [PubMed] [Google Scholar]
- Boehringer Ingelheim. (2011a). Breakthrough Therapy PRADAXA® (Dabigatran Etexilate) First Drug in 50 Years to Gain Approval for Stroke Prevention in Atrial Fibrillation in EU. Available at: http://www.boehringer-ingelheim.com/news/news_releases/press_releases/2011/04_aug_2011_dabigatranetexilate.html (accessed December 2012) [Google Scholar]
- Boehringer Ingelheim. (2011b). Mitteilung an die Angehörigen der Heilberufe zur Bedeutung einer überprüfung der Nierenfunktion von Patienten, die mit Pradaxa® (Dabigatranetexilat) behandelt warden. Available at: http://www.akdae.de/Arzneimittelsicherheit/RHB/Archiv/2011/20111027.pdf (accessed December 2012) [Google Scholar]
- Bombardier L., Laine L., Reicin A., Shapiro D., Burgos-Vargas R., Davis B., et al. (2000). Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N. Engl. J. Med. 343 1520–1528 [DOI] [PubMed] [Google Scholar]
- Bresalier R., Sandler R., Quan H., Bolognese J. A., Oxenius B., Horgan K., et al. (2005). Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 352 1092–1102 [DOI] [PubMed] [Google Scholar]
- Burke P. (2012). Communications (by letter) from Mr. P. Burke (Primary Care Reimbursement Services, Health Services Executive, Ireland) to All Chairpersons/Secretaries of Drug and Therapeutic (Pharmacy and Therapeutic) Committees, Consultant (Cardiologists, Geriatricians, Haematologists, Neurologists, Stroke Physicians), Hospital Chief Pharmacists, Ireland, July 2012. [Google Scholar]
- Calfee J. E., Pinell X. (2005). Prepared for a Conference on Consumers, Information, and the Evolving Healthcare Market Place. Available at: http://www.aei.org/papers/health/the-significance-of-the-vioxx-withdrawal/ (accessed December 2012) [Google Scholar]
- Camm A. J., Kirchhof P., Lip G. Y., Schotten U., Savelieva I., Ernst S., et al. (2010). ESC Guidelines for the management of atrial fibrillation the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 31 2369–2429 [DOI] [PubMed] [Google Scholar]
- Carlson A. (1999). “The discovery of SSRIs: a milestone in neuropsychopharmacology and rational drug design,” in Selective Serotonin Reuptake Inhibitors: Past, Present and Future, ed. Standford S. C. (Austin: R.G. Landes Company; ) 1–7 Available at: http://elib.fk.uwks.ac.id/asset/archieve/e-book/FISIOLOGI%20-%20FAAL%20-%20PHISIOLOGY%20-%20PATHOFISIOLOGY/Selective%20Serotonin%20Reuptake%20Inhibitors.pdf (accessed January 2013) [Google Scholar]
- Carlson A. (2000). A half century of neurotransmitter research; impact on neurology and psychiatry. Nobel lecture December 2000. Available at: http://www.nobelprize.org/nobel_prizes/medicine/laureates/2000/carlsson-lecture.pdf (accessed January 20123) [DOI] [PubMed] [Google Scholar]
- Cheema P., Gauvra S., Migus M., Godman B., Yeung L., Trudeau M. E., et al. (2012). International variability in the reimbursement of cancer drugs by publically funded drug programs. Curr. Oncol. 19 e165–e176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civaner M. (2012). Sale strategies of pharmaceutical companies in a ‘pharmerging’ country: the problems will not improve if the gaps remain. Health Policy 106 225–232 [DOI] [PubMed] [Google Scholar]
- Coma A., Zara C., Godman B., Augusti A., Diogene E., Wettermark B., et al. (2009). Policies to enhance the efficiency of prescribing in the Spanish Catalan Region: impact and future direction. Expert Rev. Pharmacoecon. Outcomes Res. 9 569–581 [DOI] [PubMed] [Google Scholar]
- Connolly S. J., Ezekowitzs M. D., Yusuf S., Eikelboom J., Oldgren J., Parekh A., et al. (2009). Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361 1139–1151 [DOI] [PubMed] [Google Scholar]
- Coventry and Warwickshire Area Prescribing Committee. (2012). Dabigatran (Pradaxa®) in Atrial Fibrillation. Available at: http://www.coventry.nhs.uk/CmsDocuments/f32f3f79-d837-4aed-a3cc-d854cd 58063e.pdf (accessed December, 2012) [Google Scholar]
- Davidson T., Husberg M., Janzon M., Oldgren J., Levin A.-K. (2013). Cost-effectiveness of dabigatran compared with warfarin for patients with atrial fibrillation in Sweden. Eur. Heart J. 34 177–183 [DOI] [PubMed] [Google Scholar]
- Davies C., Abraham J. (2013). Is there a cure for corporate crime in the drug industry? Effective enforcement of regulations requires more resources and determination to impose robust sanctions. BMJ 346:f755 10.1136/bmj.f755 [DOI] [PubMed] [Google Scholar]
- Davies N., Jamali F. (2004). Cox-II selective inhibitors cardiac toxicity: getting to the heart of the matter. J. Pharm. Pharm. Sci. 7 332–336 [PubMed] [Google Scholar]
- Department of Justice. (2010). Settlement Agreement between United States and AstraZeneca. Available at: http://www.justice.gov/usao/pae/Pharma-Device/astrazeneca_settlementagreement.pdf (accessed January 2013) [Google Scholar]
- Dietrich M., Horvath T. (2012). Limitations in anti-obesity drug development: the critical role of hunger-promoting neurons. Nat. Rev. Drug Discov. 11 675–691 [DOI] [PubMed] [Google Scholar]
- DiMasi J. A., Grabowski H. G. (2007). The cost of biopharmaceutical R&D: is biotech different? Manag. Decis. Econ. 28 469–479 [Google Scholar]
- Douxfils J., Mullier F., Robert S., Chatelain C., Chatelain B, Dogné J.-M. (2012). Impact of dabigatran on a large panel of routine or specific coagulation assays – laboratory recommendations for monitoring of dabigatran etexilate. Thromb. Haemost. 107 985–997 [DOI] [PubMed] [Google Scholar]
- EATG. (2009). 2009 World Pharma Sales Forecast to Top $820 Billion. Available at: http://www.eatg.org/eatg/Global-HIV-News/Pharma-Industry/2009-world-pharma-sales-forecast-to-top-820-billion (accessed December 2012) [Google Scholar]
- East Lancashire NHS Medicines Management Health Economy New Medicines and Treatments Group - Dabigatran for prevention of stroke in non-valvular atrial fibrillation (2012). Available at: http://www.elmmb.nhs.uk/formularies/joint-medicines-formulary/2/2-8/ (accessed December, 2012) [Google Scholar]
- Eichler H.-G., Abadie E., Breckenridge A., Flamion B., Gustafsson L. L., Leufkens H., et al. (2011). Bridging the efficacy–effectiveness gap: a regulator’s perspective on addressing variability of drug response. Nat. Rev. 10 495–506 [DOI] [PubMed] [Google Scholar]
- Elton P., Ratcliffe J., Fitchet A., White A., Sutton A., Budden P. (2011). Re: cost of dabigatran for atrial fibrillation. Rapid response. BMJ. Available at: http://www.bmj.com/content/343/bmj.d6980?tab=responses (accessed December 2012) [Google Scholar]
- European Medicines Agency (EMA). (2008). CHMP Assessment Report for Pradaxa (Dabigatran). EMEA/174363/2008. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000829/WC500041062.pdf (accessed December 2012) [Google Scholar]
- European Medicines Agency (EMA). (2009). Withdrawal of the Marketing Authorisation in the European Union. EMEA/39457/2009. Available at: http://www.emea.europa.eu/docs/en_GB/document_library/Public_statement/2009/11/WC500012189.pdf (Accessed December 2012) [Google Scholar]
- European Medicines Agency (EMA). (2011). Update on Safety of Pradaxa. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/11/news_detail_001390.jsp&mid=WC0b01ac058004d5c1 (accessed January 2013) [Google Scholar]
- European Medicines Agency (EMA). (2012). Pradaxa – Dabigatran. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000829/human_med_000981.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d125 (accessed December 2012) [Google Scholar]
- EvaluatePharma. (2012). Surveying Tomorrow’s BioPharma Landscape. The NASDAQ Biotech Index Up Close. Available at: http://info.evaluatepharma.com/rs/evaluatepharmaltd/images/EvaluatePharma_NBI_Up_Close_2012.pdf (accessed January 2013) [Google Scholar]
- Fagius J., Osterman P. O., Siden A., Wiholm B. E. (1985). Guillain–Barré syndrome following zimelidine treatment. J. Neurol. Neurosurg. Psychiatry 48 65–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. (2002). Vioxx (Rofecoxib). Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154520.htm (accessed January 2013) [Google Scholar]
- Federal Agency for Medicines and Health Products (FAMHP). (2011). Dabigatran Etexilate (PRADAXA): Updated Information. Available at: http://www.fagg-afmps.be/en/news/news_dabigatran_pradaxa.jsp?referer=tcm:292-153431-64 (accessed February 2013) [Google Scholar]
- Fife Area Drug and Therapeutics Committee. (2012). Area Drug & Therapeutics Bulletin. Prescribing of newer oral anticoagulants. December 2011–March 2012. Available at: http://www.fifeadtc.scot.nhs.uk/bulletins/2012/ADTC%20Bulletin%20Dec-Mar.pdf [accessed December, 2012] [Google Scholar]
- Fisk M., Feeley J., Voreacos D. (2012). J&J Said to Agree to $2.2 Billion Drug Marketing Accord. Available at: http://www.bloomberg.com/news/2012-06-11/j-j-said-to-pay-2-2-billion-to-end-risperdal-sales-probe.html (accessed January 2013) [Google Scholar]
- Frank R. (2007). The ongoing regulation of generic drugs. N. Engl. J. Med. 357 1993–1996 [DOI] [PubMed] [Google Scholar]
- Friedman M. A., Woodcock J., Lumpkin M. M., Shuren J. E., Hass A. E., Thompson L. J. (1999). The safety of newly approved medicines: do recent market removals mean there is a problem? JAMA 281 1728–1734 [DOI] [PubMed] [Google Scholar]
- Fritz J., Cleland J. (2003). Effectiveness versus efficacy: more than a debate over language. J. Orthop. Sports Phys. Ther. 33 163–165 [DOI] [PubMed] [Google Scholar]
- Garattini L., Casadei G. (2011). Risk sharing arrangements: what lessons from Italy? Int. J. Technol. Assess 27 169–172 [DOI] [PubMed] [Google Scholar]
- Garattini S., Bertele V., Godman B., Haycox A., Wettermark B., Gustafsson L. L. (2008). Enhancing the rational use of new medicines across European healthcare systems – a position paper. Eur. J. Clin. Pharmacol. 64 1137–1138 [DOI] [PubMed] [Google Scholar]
- Garber S. T., Sivakumar W., Schmidt R. H. (2012). Neurosurgical complications of direct thrombin inhibitors – catastrophic haemorrhage after mild traumatic brain injury in a patient receiving dabigatran. J. Neurosurg. 116 1093–1096 [DOI] [PubMed] [Google Scholar]
- Garuoliene K., Alonderis T, Marcinkevičius M. (2011a). Pharmaceutical policy and the effects of the economic crisis: Lithuania. Eurohealth 17 1–4 [Google Scholar]
- Garuoliene K., Godman B., Gulbinovič J., Wettermark B., Haycox A. (2011b). European countries with small populations can obtain low prices for drugs: Lithuania as a case history. Expert Rev. Pharmacoecon. Outcomes Res. 11 341–347 [DOI] [PubMed] [Google Scholar]
- Go A. S., Hylek E. M., Phillips K. A., Chang Y., Henault L. E., Selby J. V., et al. (2001). Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA 285 2370–2375 [DOI] [PubMed] [Google Scholar]
- Godman B. (2011). Risk Sharing Arrangements – Guidance for the Future. Available at: http://www.ceestahc.org/pliki/uoc/20110519/uoc20110519_godman.pdf (accessed December 2012) [Google Scholar]
- Godman B. (2013). Health authority perspective on biosimilars. GaBi J. 10.5639/gabij.2013.0201.010[Epubaheadofprint]. [DOI] [Google Scholar]
- Godman B., Abuelkhair M., Vitry A., Abdu S., Bennie M., Bishop I., et al. (2012a). Payers endorse generics to enhance prescribing efficiency: impact and future implications, a case history approach. GaBi J. 1 69–83 [Google Scholar]
- Godman B., Bennie M., Baumgärtel C., Sović Brkičić L., Burkhardt T., Fürst J., et al. (2012b). Essential to increase the use of generics in Europe to maintain comprehensive health care? Farmecon. Health Econ. Ther. Pathw. 13(Suppl. 3) 5–20 [Google Scholar]
- Godman B., Malmstrom R. E., Bennie M., Sakshaug S., Burkhardt T., Campbell S., et al. (2012c). Prescribing restrictions – a necessary strategy among some European countries to enhance future prescribing efficiency? Rev. Health Care 3 5–16 [Google Scholar]
- Godman B., Paterson K., Malmstrom R., Selke G., Fagot J. P., Mrak J. (2012d). Improving the managed entry of new medicines: sharing experiences across Europe. Expert Rev. Pharmacoecon. Outcomes Res. 12 439–441 [DOI] [PubMed] [Google Scholar]
- Godman B., Wettermark B., Bennie M., Diogéne E., Van Ganse E., Gustafsson L. L. (2012e). Critical role for clinical pharmacologists and hospital pharmacists with enhancing prescribing efficiency for new and existing drugs. (E)Hospital 14 1–2 (pharma special) [Google Scholar]
- Godman B., Wettermark B., Bishop I., Burkhardt T., Fürst J., Garuoliene K., et al. (2012f). European payer initiatives to reduce prescribing costs through use of generics. GaBi J. 1 22–27 [Google Scholar]
- Godman B., Bucsics A., Burkhardt T., Haycox A., Seyfried H., Wieninger P. (2008). Insight into recent reforms and initiatives in Austria; implications for key stakeholders. Expert Rev. Pharmacoecon. Outcomes Res. 8 357–371 [DOI] [PubMed] [Google Scholar]
- Godman B., Burkhardt T., Bucsics A., Wettermark B., Wieninger P. (2009a). Impact of recent reforms in Austria on utilisation and expenditure of PPIs and lipid-lowering drugs; implications for the future. Expert Rev. Pharmacoecon. Outcomes Res. 9 475–484 [DOI] [PubMed] [Google Scholar]
- Godman B., Schwabe U., Selke G., Wettermark B. (2009b). Update of recent reforms in Germany to enhance the quality and efficiency of prescribing of proton pump inhibitors and lipid lowering drugs. Pharmacoeconomics 27 435–438 [DOI] [PubMed] [Google Scholar]
- Godman B., Wettermark B., Hoffman M., Andersson K., Haycox A., Gustafsson L. L. (2009c). Multifaceted national and regional drug reforms and initiatives in ambulatory care in Sweden; global relevance. Expert Rev. Pharmacoecon. Outcomes Res. 9 65–83 [DOI] [PubMed] [Google Scholar]
- Godman B., Gustafsson L. L. (2013). A new reimbursement systems for innovative pharmaceuticals combining value-based and free market pricing. Appl. Health Econ. Health Policy. 11 79–82 [DOI] [PubMed] [Google Scholar]
- Godman B., Sakshaug S., Berg C., Wettermark B., Haycox A. (2011a). Combination of prescribing restrictions and policies to engineer low prices to reduce reimbursement costs. Expert Rev. Pharmacoecon. Outcomes Res. 11 121–129 [DOI] [PubMed] [Google Scholar]
- Godman B., Shrank W., Andersen M., Berg C., Bishop I., Burkhardt T., et al. (2011b). Policies to enhance prescribing efficiency in Europe: findings and future implications. Front. Pharmacol. 1:141 10.3389/fphar.2010.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godman B., Wettermark B., Bennie M., Burkhardt T., Garuoliene K. (2011c). Enhancing prescribing efficiency through increased utilisation of generics at low prices. (E)Hospital 13 28–31 [Google Scholar]
- Godman B., Shrank W., Andersen M., Berg C., Bishop I., Burkhardt T., et al. (2010). Comparing policies to enhance prescribing efficiency in Europe through increasing generic utilisation: changes seen and global implications. Expert Rev. Pharmacoecon. Outcomes Res. 10 707–722 [DOI] [PubMed] [Google Scholar]
- Griffin D., Segal A. (2010). Feds Found Pfizer too Big to Nail. Available at: http://edition.cnn.com/2010/HEALTH/04/02/pfizer.bextra/index.html [Google Scholar]
- Gustafsson L. L., Wettermark B., Godman B, Andersén-Karlsson E., Bergman U., Hasselström J., et al. (2011). The “Wise List”– a comprehensive concept to select, communicate and achieve adherence to recommendations of essential drugs in ambulatory care in Stockholm. Basic Clin. Pharmacol. Toxicol. 108 224–233 [DOI] [PubMed] [Google Scholar]
- Guthrie E. (2000). Psychotherapy for patients with complex disorders and chronic symptoms. The need for a new research paradigm. Br. J. Psychiatry 177 131–137 [DOI] [PubMed] [Google Scholar]
- Haute Authorite de Sante – Commission de la Transparence. (2012). Pradaxa. Available at: http://www.has-sante.fr/portail/upload/docs/application/pdf/2012-03/pradaxa_15022012_avis_ct10749.pdf (accessed Dece-mber 2012) [Google Scholar]
- Harper P., Young L., Merriman E. (2012). Bleeding risk with dabigatran in the frail elderly. N. Engl. J. Med. 366 864–866 [DOI] [PubMed] [Google Scholar]
- Health Improvement Scotland. (2012). Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation. Available at: www.healthcareimprovementscotland.org/programmes/cardiovascular_disease/dabigatran_consensus_statement.aspx (accessed December, 2012) [Google Scholar]
- Heidbüchel H. T. V., Verhamme P., Hermans C., Peeters A, Scavée C. (2011). Practical Guide Dabigatran – Guidance for Use in Particular Situations. Available at: http://www.thrombosisguidelinesgroup.be (accessed December 2012) [Google Scholar]
- Hirschler B. (2012). Fifty Percent off the First Three months of Cancer Medicine. Buy a Course of Eye Treatment and Get Extra Injections Free. A Money-back Guarantee if Your Erectile Dysfunction Pills Don’t Work. Available at: http://www.reuters.com/article/2012/10/24/us-pharmaceuticals-europe-pricing-idUSBRE89N0GQ20121024 (accessed January 2013) [Google Scholar]
- Holmström M., Johnsson H., Lärfars G., Malmström R., Hjemdahl P. (2009). New drug in atrial fibrillation – how does it function in regular health care? Lakartidningen 106 3019–3020 [PubMed] [Google Scholar]
- Horsley W. (2010). Dabigatran for the Prevention of Stroke in Patient with Non-valvular Atrial Fibrillation: A Cost Analysis for the NHS North East. NHS North East Treatment Advisory Group. Available at: http://www.netag.nhs.uk/files/appraisal-reports/Dabigatran%20NETAG%20cost%20analysis%20-Jan2010-%20-%20web%20version%202.pdf (accessed December 2012) [Google Scholar]
- Huisman M., Lip G., Diener H.-G., Brueckmann M., van Ryn J., Clemens A. (2012). Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb. Haemost. 107 838–847 [DOI] [PubMed] [Google Scholar]
- IQWiG. (2010). Merkblatt: Gerinnungshemmende Medikamente sicher anwenden. Available at: http://www.gesundheitsinformation.de/index.624.de.html (accessed January 2013) [Google Scholar]
- Institute for Safe Medication Practices. (2011). Quarter Watch 2010 Quarter 4. Available at: http://www.ismp.org/QuarterWatch/pdfs/2010Q4.pdf (accessed December 2012) [Google Scholar]
- Interface – A monthly medicines and prescribing bulletin for healthcare professionals in East Lancashire focusing on new therapies. (2011). Available at: www.elmmb.nhs.uk/EasySiteWeb/GatewayLink.aspx?alId=48664 (accessed December 2012) [Google Scholar]
- Ioannides-Demos L., Piccenna L., McNeil J. (2011). Pharmacotherapies for obesity: past, current, and future therapies. J. Obes. 2011 179674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A. (2008). Balancing Big Pharma’s books. BMJ 336 418–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackevicius C., Chou M., Ross J., Shah N., Krumholz H. (2012). Generic atorvastatin and health care costs. N. Eng. Jn. Med. 366 201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusinfo. (2009). Nya riktlinjer & behandlingsstrategier. Rekommendationer avseende antikoagulantiabehandling med warfarin eller dabigatran vid förmaksflimmer. Available at: http://www.janusinfo.se/Documents/Broschyrer/F%C3%B6rskrivarinfo_f%C3%B6rmaksflimmer_A4.pdf (accessed January 2013) [Google Scholar]
- Janusinfo. (2012a). Frågor och svar om dabigatran. Available at: http://www.janusinfo.se/Documents/Expertgruppsdokument/dabigatran_fragor_svar_120208.pdf (accessed January 2013) [Google Scholar]
- Janusinfo. (2012b). Välj i första hand warfarin som tromboemboliprofylax vid förmaksflimmer. Följ riktlinjer för warfarinbehandling. Available at: http://www.janusinfo.se/Global/Kloka%20Rad/warfarin_120320.pdf (accessed January 2013) [Google Scholar]
- Jaroslawski S., Toumi M. (2011). Market access agreements for pharmaceuticals in Europe: diversity of approaches and underlying concepts. BMC Health Serv. Res. 11:259 10.1186/1472-6963-11-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jommi C. (2012). Managed Market Entry for Drugs in Italy. EMAUD Market Access Newsletter. Available at: http://www.slideshare.net/Mondher_Toumi/market-access-newsletter-emaud-june2012 (accessed December 2012) [Google Scholar]
- Joppi R., Cinconze E., Mezzalira M., Pase D., Poggiani C., Rossi E., et al. (2013). Hospitalized patients with atrial fibrillation compared to those included in recent trials on novel oral anticoagulants: A population-based study. Eur. J. Intern. Med. 10.1016/j.ejim.2013.02.018[Epubaheadofprint]. [DOI] [PubMed] [Google Scholar]
- Kaiser J. (2012). New cystic fibrosis drug offers hope, at a price. Science 335 645 [DOI] [PubMed] [Google Scholar]
- Kalaba M., Godman B., Vuksanovic A., Bennie M., Malmstrom R. E. (2012). Possible ways to enhance renin–angiotensin prescribing efficiency: Republic of Serbia as a case history? J. Comp. Eff. Res. 1 539–549 [DOI] [PubMed] [Google Scholar]
- Kansal A. R., Sorensen S. V., Gani R., Robinson P., Pan F., Plumb J. M., et al. (2012). Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart 98 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L., Bates D., Edan G. (2011). Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 10 745–758 [DOI] [PubMed] [Google Scholar]
- Keegan B. (2011). Natalizumab for multiple sclerosis: a complicated treatment. Lancet Neurol. 10 677–678 [DOI] [PubMed] [Google Scholar]
- Keele University School of Pharmacy. (2012). Medicines Management – Effective Shared Care Agreement Toolkit – Dabigatran. Available at: http://www.esca-keele.co.uk/dabigatran/ (accessed January 2013) [Google Scholar]
- Kirchhof P., Auricchio A., Bax J., Crijns H., Camm J., Diener H. C., et al. (2007). Outcome parameters for trials in atrial fibrillation: executive summary. Recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork (AFNET) and the European Heart Rhythm Association (EHRA). Eur. Heart J. 28 2803–2817 [DOI] [PubMed] [Google Scholar]
- Kirsch I., Deacon B. J., Huedo-Medina T. B., Scoboria A., Moore T. J., Johnson B. T., et al. (2008). Initial severity and antidepressant benefits: a meta analysis of data submitted to the Food and Drug Administration. PLoS Med. 5:e45 10.1371/journal.pmed.0050045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemp M, Frønsdal K. (2011). What principles should govern the use of managed entry agreements? Int. J. Technol. Assess. Health Care 27 77–83 [DOI] [PubMed] [Google Scholar]
- Kmietowicz Z. (2013). GSK backs campaign for disclosure of trial data. BMJ 346 f819 [DOI] [PubMed] [Google Scholar]
- Kolasa K., Schubert S., Manca A., Hermanowski T. (2011). A review of Health Technology Assessment (HTA) recommendations for drug therapies issued between 2007 and 2009 and their impact on policymaking processes in Poland. Health Policy 102 145–151 [DOI] [PubMed] [Google Scholar]
- Kreatinin-Clearance Rechner (Creatinine Clearance calculator). (2013). Cocktroft-Gault Formula. Available at: http://www.dialyse-hamburg.de/kreacal.htm (accessed January 2013) [Google Scholar]
- KVH - Aktuell. (2010). Informationsdienst der Kassenärztlichen Vereinigung Hessen Pharmakotherapie. Available at: http://www.kvberlin.de/40presse/50publikation/20pharmakotherapie/2010/pharmakotherapie_1001.pdf (accessed December 2012) [Google Scholar]
- Legrand M., Mateo J., Aibaud A., Ginisty S., Eftekhari P., Huy P. T., et al. (2011). The use of dabigatran in elderly patients. Arch. Intern. Med. 8 432–439 [DOI] [PubMed] [Google Scholar]
- Lexchin J., Kohler J. (2011). The danger of imperfect regulation: OxyContin use in the United States and Canada. Int. J. Risk Saf. Med. 23 233–240 [DOI] [PubMed] [Google Scholar]
- Liesenfeld K. H., Lehr T., Dansirikul C., Reilly P. A., Connolly S. J., Ezekowitz M. D., et al. (2011). Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J. Thromb. Haemost. 9 2168–2175 [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D. M., Wang T. J., Leip E. P., Larson M. G., Levy D., Vasan R. S., et al. (2004). Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 110 1042–1046 [DOI] [PubMed] [Google Scholar]
- Lothian Prescribing Bulletin. (2012) Oral anticoagulants – what’s new? Available at: http://www.ljf.scot.nhs.uk/PrescribingBulletins/Prescribing%20Bulletins/Lothian%20Prescribing%20Bulletin%20Issue%2055%20May%202012%20FINAL.pdf (accessed December 2012) [Google Scholar]
- Luengo-Fernandez R., Gray A. M., Rothwell P. M. (2006). Population-based study of determinants of initial secondary care costs of acute stroke in the United Kingdom. Stroke 37 2579–2587 [DOI] [PubMed] [Google Scholar]
- Malmström R. E. (2009). “New anticoagulants,” in Essential Guide to Blood Coagulation, eds Antovic J. P., Blombäck M. (Oxford: Wiley-Blackwell Publishing; ) 91–102 [Google Scholar]
- Mannuci M., Nobil A., Garattini S. (2012). New drugs for thromboprophylaxis in atrial fibrillation. Eur. J. Intern. Med. 23 1–5 [DOI] [PubMed] [Google Scholar]
- Markovic-Pekovic V., Škrbić R., Godman B., Gustafsson L. L. (2012). Ongoing initiatives in the Republic of Srpska to enhance prescribing efficiency; influence and future direction. Expert Rev. Pharmacoecon. Outcomes Res. 5 661–671 [DOI] [PubMed] [Google Scholar]
- Marshall S., Fearon P., Dawson J., Quinn T. (2013). Stop the clots, but at what cost? Pharmacoeconomics of dabigatran etexilate for the prevention of stroke in subjects with atrial fibrillation: a systematic literature review. Expert Rev. Pharmacoecon. Outcomes Res. 13 29–42 [DOI] [PubMed] [Google Scholar]
- Martikainen J., Saastamoinen L., Korhonen M., Enlund H., Helin-Salmivaara A. (2010). Impact of restricted reimbursement on the use of statins in Finland. Med. Care 48 761–766 [DOI] [PubMed] [Google Scholar]
- Martin D. (2012). Drug Firms are ‘Risking Lives by Hiding Bad Trials and Side Effects of Their Medicines’. Available at: http://www.dailymail.co.uk/news/article-2222220/Drug-firms-risking-lives-hiding-bad-trials-effects-medicines.html?ito=feeds-newsxml) (accessed November 12, 2012) [Google Scholar]
- McGinn D., Godman B., Lonsdale J., Way R., Wettermark B., Haycox A. (2010). Initiatives to enhance the quality and efficiency of statin and PPI prescribing in the UK: impact and implications. Expert Rev. Pharmacoecon. Outcomes Res. 10 73–85 [DOI] [PubMed] [Google Scholar]
- Medicine Balance (MEDICIJNBALANS). (2012). Oral Anticoagulants. Available at: http://www.medicijnbalans.nl/medicijngroepen/orale-anticoagulantia (accessed December 2012) [Google Scholar]
- Medicin & Läkemedel. (2012). E n t i d N I N G f r å n S t o c k h o l m s l ä n s l ä k e m e d e l s k o mm i t t é h ä l s o - o c h s j u k v å r d s f ö r va l t n i n g e n. Frågor & svar om dabigatran Expertråden ger svar på frågor om nytt antikoagulantium. Available at: http://www.janusinfo.se/publikationer/evidens/nr01/files/assets/seo/page1.html (accessed December 2012) [Google Scholar]
- Melander H., Ahlqvist-Rastad J., Meijer G., Beermann B. (2003). Evidence b(i)ased medicine – selective reporting from studies sponsored by pharmaceutical industry: review of studies in new drug applications. BMJ 326 1171–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello M. M., Abiola S., Colgrove J. (2012). Pharmaceutical companies’ role in state vaccination policymaking: the case of human papillomavirus vaccination. Am. J. Public Health. 102 893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck. (2002). Important Prescribing Information. Available at: http://www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM171089.pdf (accessed January 2013) [Google Scholar]
- Merck. (2004). Merck Announces Voluntary Worldwide Withdrawal of VIOXX®. Available at: http://www.pbm.va.gov/vioxx/Dear%20Healthcare%20Professional.pdf (accessed January 2013) [Google Scholar]
- MHRA UK. (2004). Immediate With-drawal of Rofecoxib (Vioxx/Vioxxacutate). Available at: http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON1004263 (accessed January 2013) [Google Scholar]
- Midlands Therapeutic Review and Advisory Committee (MTRAC). (2012). COMMISSIONING GUIDANCE Dabigatran Etexilate for Stroke Prevention in Patients with Atrial Fibrillation. Available at: http://www.keele.ac.uk/media/keeleuniversity/fachealth/fachealthsop/mtrac/documents/summary/Dabigatran%20VS%2012.pdf (accessed December 2012) [Google Scholar]
- Mismetti P., Laporte S. (2010). New oral antithrombotics: a need for laboratory monitoring. J. Thromb. Haemost. 8 621–626 [DOI] [PubMed] [Google Scholar]
- Moreira F., Alexandre J., Crippa S. (2009). The psychiatric side-effects of rimonabant. Rev. Bras. Psiquiatr. 31 145–153 [DOI] [PubMed] [Google Scholar]
- Mullard A. (2012). 2011 FDA drug approvals. Nat. Rev. Drug Discov. 11 91–94 [DOI] [PubMed] [Google Scholar]
- Nagle P. C., Nicita C. A., Gerdes L. A., Schmeichel C. J. (2008). Characteristics of and trends in the late-stage biopharmaceutical pipeline. Am. J. Manag. Care 14 226–229 [PubMed] [Google Scholar]
- National Cancer Institute. (2010). Surveillance Epidemiology and End Results (SEER). Washington, DC: National Cancer Institute [online] Available at: http://seer.cancer.gov/ (accessed December 2012) [Google Scholar]
- National Centre for Pharmacoeconomics. (2011). Dabigatran Etexilate (Pradaxa®) for the Prevention of Stroke and Systemic Embolism in Adult Patients with Atrial Fibrillation. Available at: http://www.ncpe.ie/drugs/dabigatran-etexilate-pradaxa-for-the-prevention-of-stroke-and-systematic-embolisom-in-atrial-fibrillation/ (accessed December 2012) [Google Scholar]
- National Institute for Health and Clinical Excellence. (2012). Dabigatran Etexilate for the Prevention of Stroke and Systemic Embolism in Atrial Fibrillation. NICE Technology Appraisal Guidance 249. Available at: http://www.nice.org.uk/nicemedia/live/13677/58470/58470.pdf (accessed December 2012) [Google Scholar]
- Neue Arzneimittel. (2011). Information der Arzneimittelkommission der deutschen Ärzteschaft (AkdÄ) Pradaxa® (Dabigatran) – neu zugelassene Indikation. Available at: http://www.akdae.de/Arzneimitteltherapie/NA/Archiv/2011030-Pradaxa.pdf (accessed December 2012) [Google Scholar]
- NHS Cumbria NHS Lancashire. (2012a). Consensus statement: Oral anti-coagulant drugs for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation. Available at: http://www.cumbria.nhs.uk/ProfessionalZone/MedicinesManagement/Guidelines/NOAC-Consensus-Statement.pdf (Accessed December 2012) [Google Scholar]
- NHS Cumbria NHS Lancashire. (2012b). Guidance for prescribing of dabigatran in patients with non-valvular AF. Available at: http://www.cumbria.nhs.uk/ProfessionalZone/MedicinesManagement/Guidelines/Prescribing-Guidance-for-NOACs.pdf (accessed December, 2012) [Google Scholar]
- NHS Highland - The Pink One. (2012). Dabigatran recommended for specific patient groups. December 2011 to January 2012. Available at: http://www.nhshighland.scot.nhs.uk/Publications/Documents/Newsletters/Pharmacy/The%20Pink%20One%20No%2095%20December%202011%20-%20January%202012.pdf (accessed December, 2012) [Google Scholar]
- NHS Improvement. Guidance on Risk Assessment and Stroke Prevention for Atrial Fibrillation (GRASP – AF). (2013). Available at: http://www.improvement.nhs.uk/graspaf/ (accessed February 2013) [Google Scholar]
- NHS Tayside. (2011). Dabigatran, Tayside Prescriber Supplement No. 112. December 2011. Available at: http://www.nhstaysideadtc.scot.nhs.uk/approved/bulletin/adtcsupp/2011/No%20112.pdf (accessed December 2012) [Google Scholar]
- NPS MEDICINEWISE. (2012). Delay in PBS Listing of Dabigatran. Available at: http://www.nps.org.au/publications/health-professional/nps-direct/2012/delay-in-PBS-listing?utm_source=nps-direct&utm_medium=email&utm_campaign=issue-3-2013 (accessed January 2013) [Google Scholar]
- Oral anticoagulants. (2012). What’s new? LOTHIAN PRESCRIBING BULLETIN. Issue 65, May 2012. Available at: http://www.ljf.scot.nhs.uk/PrescribingBulletins/Prescribing%20Bulletins/Lothian%20Prescribing%20Bulletin%20Issue%2055%20May%202012%20FINAL.pdf [accessed December 2012] [Google Scholar]
- Ostazen 2011. Consensus statement on Dabigatran - Sociedad Vasco Navarra de Cardiología, Sociedad Vasca de Medicina de Familia y Comunitaria (OSATZEN), Sociedad de Neurología del País Vasco, Asociación del Norte de Hematología-Hemoterapia, Sociedad de Medicina Interna País Vasco, Sociedad Vasca de Farmacia Hospitalaria, y la vocalía de Euskadi de la Sociedad Española de Farmacéuticos de Atención Primaria 2011. (2011). Available at: http://www.osakidetza.euskadi.net/r85-pkcevi02/es/contenidos/informacion/cevime_atencion_primaria/es_cevime/r01hRedirectCont/contenidos/informacion/cevime_nuevo_medicamento/es_nme/adjuntos/Consenso_dabigatran_c_bis. pdf (accessed December, 2012) [Google Scholar]
- O’Shaughnessy. (2009). Adieu Rimonabant. Available at: http://www.beyondthc.com/wp-content/uploads/2012/05/2009AdieuRimonabant.pdf (accessed December 2012) [Google Scholar]
- Östergötland. (2012). Strukturerat införande av nya antikoagulantia i Östergötland – Dabigatran – 111011. Available at: http://www.lio.se/pages/142057/Baseline-formulär%20och%20checklista%20Dabigatran%205%20111011.pdf (accessed December 2012) [Google Scholar]
- Ozierański P., McKee M., King L. (2012). Pharmaceutical lobbying under postcommunism: universal or country-specific methods of securing state drug reimbursement in Poland? Health Econ. Policy Law 7 175–195 [DOI] [PubMed] [Google Scholar]
- Papeix C., Vukusic S., Passante N., Ionescu I., Frangoulis B., Stankoff S., et al. (2012). Natalizumab Discontinuation in Clinical Practice: A Systematic Observational Study from the National TYSEDMUS Cohort of Multiple Sclerosis Patients Treated with Natalizumab in France. Available at: http://registration.akm.ch/einsicht.php?XNABSTRACT_ID=137397&XNSPRACHE_ID=2&XNKONGRESS_ID=150&XNMASKEN_ID=900 (accessed December 2012) [Google Scholar]
- Persson U., Svensson J., Pettersson B. (2012). A new reimbursement system for innovative pharmaceuticals combining value-based and free market pricing. Appl. Health Econ. Health Policy 10 217–225 [DOI] [PubMed] [Google Scholar]
- Pink J., Lane S., Pirmohamed M., Dyfrig A., Hughes D. (2011). Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ 343 d6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reicin A., Barr E., Shapiro D. (2001). Cardiovascular safety profile of rofecoxib: a meta-analysis. Arthritis Rheum. 44 S372;S230;S266 [Google Scholar]
- Rodriguez R., Carrier M., Wells P. (2013). Non-adherence to new oral anticoagulants: a reason for concern during long-term anticoagulation? Int. Soc. Thromb. Haem. 11 390–394 [DOI] [PubMed] [Google Scholar]
- Rolfe S., Papadopoulos S., Cabral K. P. (2010). Controversies of anticoagulation reversal in life-threatening bleeds. J. Pharm. Pract. 23 217–225 [DOI] [PubMed] [Google Scholar]
- Scottish Medicines Consortium. (2011). Dabigatran Etexilate 110 mg and 150 mg Hard Capsules (Pradaxa®) SMC No. (672/11). Available at: http://www.scottishmedicines.org.uk/files/advice/dabigatran_Pradaxa_FINAL_August_2011_Amended_05.09.11_for_website.pdf (accessed December 2012) [Google Scholar]
- Selyukh A. (2011). Seattle Genetics Cancer Drug Price May Top $100,000. Available at: http://www.reuters.com/article/2011/08/22/us-seattlegenetics-idUSTRE77L5EB20110822 (accessed February 2013) [Google Scholar]
- Sermet C., Andrieu V., Godman B., Van Ganse E., Haycox A., Reynier J. P. (2010). Ongoing pharmaceutical reforms in France; implications for key stakeholder groups. Appl. Health Econ. Health Policy 8 7–24 [DOI] [PubMed] [Google Scholar]
- Shuchman M. (2006). Delaying generic competition – corporate payoffs and the future of Plavix. N. Engl. J. Med. 355 1297–1300 [DOI] [PubMed] [Google Scholar]
- Siviero P., Sammarco A., Tafuri G., Pani L. (2012). An HTA approach for pricing and reimbursement decisions: the Italian experience with managed entry agreements. Gac. Sanit. 26 280 [Google Scholar]
- Southworth M., Reichman M., Unger E. (2013). Dabigatran and postmarketing reports of bleeding. N. Engl. J. Med. 10.1056/NEJMp1302834[Epubaheadofprint]. [DOI] [PubMed] [Google Scholar]
- Stangier J., Clemens A. (2009). Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin. Appl. Thromb. Hemost. 15(Suppl. 1) 9S–16S [DOI] [PubMed] [Google Scholar]
- Stangier J., Stahle H., Rathgen K., Fuhr R. (2008). Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin. Pharmacokinet. 47 47–59 [DOI] [PubMed] [Google Scholar]
- Stewart S., Hart C. L., Hole D. J., McMurray J. J. (2001). Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 86 516–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S., Murphy N., Walker A., McGuire A., McMurray J. J. (2004). Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 90 286–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockholms läns landsting. (2011). Prognos över användning och kostnader för läkemedel i SLL 2011–2012. Available at: http://www.janusinfo.se/Documents/Lakemedelsstatistik/Prognos%202011_2012_110420%20final.pdf (accessed January 2013) [Google Scholar]
- Stollberger C., Finsterer J. (2013). Concerns about storage and application of dabigatran and rivaroxaban. Eur. J. Clin. Pharmacol. 69 739–740 [DOI] [PubMed] [Google Scholar]
- Sullivan R., Peppercorn P., Sikora K., Zalcberg J., Meropol N. J., Amir E., et al. (2011). Delivering affordable cancer care in high-income countries. Lancet Oncol. 12 933–980 [DOI] [PubMed] [Google Scholar]
- Swedish Council on Health Technology Assessment. (2011) Dabigatran för att förebygga stroke vid förmaksflimmer. Stockholm: Statens beredning för medicinsk utvärdering (SBU). SBU Alert-rapport nr 2011-04. Available at: http://www.sbu.se; in English: http://www.sbu.se/en/Published/Alert/Dabigatran-to-Prevent-Stroke-in-Patients-With-Atrial-Fibrillation/ (accessed December 2012) [Google Scholar]
- Taylor L. (2011). Australian Govt Blocks Subsidies for New Drugs. Available at: http://www.pharmatimes.com/Article/11-03-15/Australian_govt_blocks_subsidies_for_new_drugs.aspx (accessed December 2012) [Google Scholar]
- Ten Cate H. (2012). Monitoring new oral anticoagulants, managing thrombosis, or both? Thromb. Haemost. 107 803–805 [DOI] [PubMed] [Google Scholar]
- Therapie Tipps. (2012). Wiener Gebietskrankenkasse p. 23 Issue 2. Available at: http://www.wgkk.at/mediaDB/874712_therapietipps_sb_2_2012.pdf (accessed December 2012) [Google Scholar]
- Thompson A. (2010). NDA 22-512 Dabigatran – Efficacy Review. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM226704.pdf?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=dabigatran&utm_content=10 (accessed December 2012) [Google Scholar]
- UK Medicines Information. (2012). New Drugs Online Report for Vemurafenib. Available at: http://www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=5009 (accessed December 2012) [Google Scholar]
- UKMi Medicines Information. (2013). New Drugs Online Report for Crizotinib. Available at: http://www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=5097 (accessed February 2013) [Google Scholar]
- van Woerkom M., Piepenbrink J. F., Godman B., Metz J., Campbell S., Bennie M., et al. (2012). Ongoing measures to enhance the efficiency of prescribing of proton pump inhibitors and statins in The Netherlands: influence and future implications. J. Comp. Eff. Res. 1 527–538 [DOI] [PubMed] [Google Scholar]
- Vogler S., Zimmermann N., Habl C., Piessnegger J., Bucsics A. (2012). Discounts and rebates granted to public payers for medicines in European countries. South Med. Rev. 5 38–46 [PMC free article] [PubMed] [Google Scholar]
- Voncina L., Strizrep T. (2011). Croatia: 2009/2010 pharmaceutical pricing and reimbursement reform. Eurohealth 16 20–22 [Google Scholar]
- Vončina L., Strizrep T., Godman B., Bennie M., Bishop I., Campbell S., et al. (2011). Influence of demand side measures to enhance renin–angiotensin prescribing efficiency in Europe; implications for the future. Expert Rev. Pharmacoecon. Outcomes Res. 11 469–479 [DOI] [PubMed] [Google Scholar]
- Weir M. R., Sperling R. S., Reicin A., Gertz B. J. (2003). Selective COX-2 inhibition and cardiovascular effects: a review of the rofecoxib development program. Am. Heart J. 146 591–604 [DOI] [PubMed] [Google Scholar]
- Wells K. B. (1999). Treatment research at the crossroads: the scientific interface of clinical trials and effectiveness research. Am. J. Psychiatry 156 5–10 [DOI] [PubMed] [Google Scholar]
- Wettermark B., Godman B., Andersson K., Gustafsson L. L., Haycox A., Bertele V. (2008). Recent national and regional drug reforms in Sweden – implications for pharmaceutical companies in Europe. Pharmacoeconomics 26 537–550 [DOI] [PubMed] [Google Scholar]
- Wettermark B., Godman B., Eriksson C., van Ganse E., Garattini S., Joppi R., et al. (2010a). Einführung neuer Arzneimittel in europäische Gesundheitssysteme. GGW 10 24–34 (Introduction of new medicines into European healthcare systems) [Google Scholar]
- Wettermark B., Godman B., Neovius M., Hedberg N., Mellgren T. O., Kahan T. (2010b). Initial effects of a reimbursement restriction to improve the cost-effectiveness of antihypertensive treatment. Health Policy 94 221–229 [DOI] [PubMed] [Google Scholar]
- Wettermark B., Persson M., Wilking N., Kalin M., Korkmaz S., Hjemdahl P., et al. (2010c). Forecasting drug utilization and expenditure in a metropolitan health region. BMC Health Ser. Res. 10:128 10.1186/1472-6963-10-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettermark B., Godman B., Jacobsson B., Haaijer-Ruskamp F. (2009a). Soft regulations in pharmaceutical policymaking – an overview of current approaches and their consequences. Appl. Health Econ. Health Policy 7 137–147 [DOI] [PubMed] [Google Scholar]
- Wettermark B., Pehrsson A., Juhasz-Haverinen M., Veg A., Edlert M., Törnwall-Bergendahl G., et al. (2009b). Financial incentives linked to self-assessment of prescribing patterns – a new approach for quality improvement of drug prescribing in primary care. Qual. Prim. Care 17 179–189 [PubMed] [Google Scholar]
- Wong D., Sullivan K., Heap G. (2012). The pharmaceutical market for obesity therapies. Nat. Rev. Drug Discov. 11 669–670 [DOI] [PubMed] [Google Scholar]
- Wood S. (2011). Deaths Prompt Dabigatran Safety Advisory in Japan. Available at: http://www.theheart.org/article/1264365.do (accessed December 2012) [Google Scholar]
- World Health Organisation (WHO). (2009). Guidelines for ATC Classification and DDD Assignment. Oslo: WHO Collaborating Centre for Drug Statistics Methodology. Available at: www.whocc.no (accessed December 2012) [Google Scholar]
- Yukhananov A. (2011). Reuters. Available at: http://www.reuters.com/article/2011/08/26/fda-pfizer-idUSN1E77P1UG20110826 (accessed December 2012) [Google Scholar]