Highlights
► Bacterial ribosomes have an intrinsic regulatory capacity. ► Ribosomes can vary in their protein and/or rRNA complement. ► Variations in rRNA and r-protein modifications likewise could lead to heterogeneity. ► Heterogeneous ribosomes can exhibit a functional specificity.
Abstract
Translation of the mRNA-encoded genetic information into proteins is catalyzed by the intricate ribonucleoprotein machine, the ribosome. Historically, the bacterial ribosome is viewed as an unchangeable entity, constantly equipped with the entire complement of RNAs and proteins. Conversely, several lines of evidence indicate the presence of functional selective ribosomal subpopulations that exhibit variations in the RNA or the protein components and modulate the translational program in response to environmental changes. Here, we summarize these findings, which raise the functional status of the ribosome from a protein synthesis machinery only to a regulatory hub that integrates environmental cues in the process of protein synthesis, thereby adding an additional level of complexity to the regulation of gene expression.
Current Opinion in Microbiology 2013, 16:133–139
This review comes from a themed issue on Cell regulation
Edited by Bonnie Bassler and Jörg Vogel
For a complete overview see the Issue and the Editorial
Available online 14th February 2013
1369-5274/$ – see front matter, © 2013 Elsevier Ltd. All rights reserved.
Introduction
The bacterial ribosome is a large ribonucleoprotein particle with an approximate molecular mass of 2.5 MDa and a diameter of 200–250 Å. This intricate macromolecular machine is conducting the functionally complex translation of the mRNA-based genetic information into the amino acid sequence of proteins. This process is conceptually divided into four phases: first, initiation, where the ribosome — equipped with initiator tRNA — is positioned at the translation initiation region of the mRNA; second, elongation, when polypeptide synthesis occurs; and third, termination, where the newly synthesized protein is released after the ribosome encounters a stop codon; these steps are followed by; fourth, recycling of the ribosome into its subunits for the next round of protein synthesis. The underlying structural complexity of the ribosome became apparent by the work of Waller in 1964, who demonstrated the presence of a vast number of different ribosomal proteins (r-proteins) [1]. This finding initiated a long-term debate on the homogeneity of bacterial ribosomes [2,3], which was challenged by several studies performed in the early 1970s indicating the existence of ribosomal subpopulations that vary in their protein complement in response to different growth rates and environmental conditions [4–8]. Despite these intriguing observations, bacterial ribosomes are traditionally still viewed as homogeneous entities that have to be equipped with the same complement of r-proteins and rRNA molecules to precisely accomplish all steps in protein synthesis. This concept was further perpetuated and strengthened by the determination of atomic resolution structures of the ribosome at the beginning of the millennium [9–12]. Consequently, ribosomes were not considered to have an intrinsic regulatory capacity, and the efficiency of translation was suggested to be determined either by features inherent to the mRNA or mediated by protein or RNA regulators.
In contrast to this perception recently emerging evidence reinforced the notion that subpopulations of heterogeneous and functionally specialized ribosomes are engendered when bacteria encounter environmental stress. In this review we summarize these discoveries and discuss several principles of ribosome heterogeneity, which underline the intrinsic capacity of ribosomes to act as key regulators of translation rather than just as protein synthesis machineries. Besides the ribosome diversification induced by external cues, we likewise discuss the concept of intrinsic ribosome heterogeneity conveyed by variations of the modification of ribosomal RNA and protein components (Box 1; Figure 1). By virtue of these alterations, which could potentially also be regulated, the bacterial cell might be equipped with functional diverse translational machineries even under relaxed conditions.
Box 1. More ways toward ribosome heterogeneity: modification of rRNAs and r-proteins.
In all living organisms including bacteria both RNA and protein complement of the ribosome are subjected to a variety of chemical modifications. The majority of post-transcriptional modifications of rRNA are restricted to the process of ribosome biogenesis [40], where they are suggested to serve as quality control check points during the maturation of the ribosome [41]. In general, these modifications cluster at functionally important regions of the ribosomal subunits, the decoding region on the 30S and the peptidyl transferase center on the 50S subunit, where they can influence both ribosome structure and function [42,43,44••]. Unlike most of the 23S rRNA modifications [45], the modifications present in the 16S rRNA are not essential for cell viability or ribosome assembly [46]. Therefore, it is tempting to speculate that variations in the modification pattern of the 16S rRNA introduced during ribosome biogenesis could represent a valuable means for the functional diversification of ribosomes. This assumption is supported by the conditional methylation of an adenine residue of the 23S rRNA located in the nascent peptide exit tunnel conferring resistance against macrolide antibiotics. As the modification retards translation, the residue is only modified when macrolides are present. Besides ribosome stalling, macrolides interfere with 50S assembly thus leading to the accumulation of precursor particles that represent the substrates for the Erm-type methyltransferases [47]. Hence, the conditional alteration of the drug binding site introduced during ribosome assembly ensures the continuous protein synthesis in the presence of macrolides. Moreover, there is evidence for conditional rRNA modification of mature ribosomal subunits in response to stress conditions [48]. The heat-inducible methyltransferase FtsJ of E. coli was identified to introduce a ribose methylation at nucleotide U2552 of the 23S rRNA in assembled 50S subunits [48]. The temperature sensitive phenotype of the ftsJ deletion mutant strain indicates the importance of the modification under heat shock conditions. However, it is still not explored whether this methylation confers a structural stabilization or in addition results in a functional specificity of the ribosome.In addition to rRNA modification there is a growing number of evidence that r-proteins in E. coli are post-translationally modified [49,50•]. Moreover, one study indicates that several r-proteins are differentially acetylated when comparing proteins from E. coli cells grown at exponential or stationary phase [51]. However, as the authors only performed a global proteome analysis, it still remains to be elucidated whether the identified modified proteins are present on the ribosome. Nevertheless, in light of the potential exchange mechanism observed for some r-proteins of mature ribosomes [39], it is conceivable that diverse modifications of r-proteins could likewise contribute to the modulation and fine tuning of the selectivity and activity of bacterial ribosomes in response to varying growth conditions.
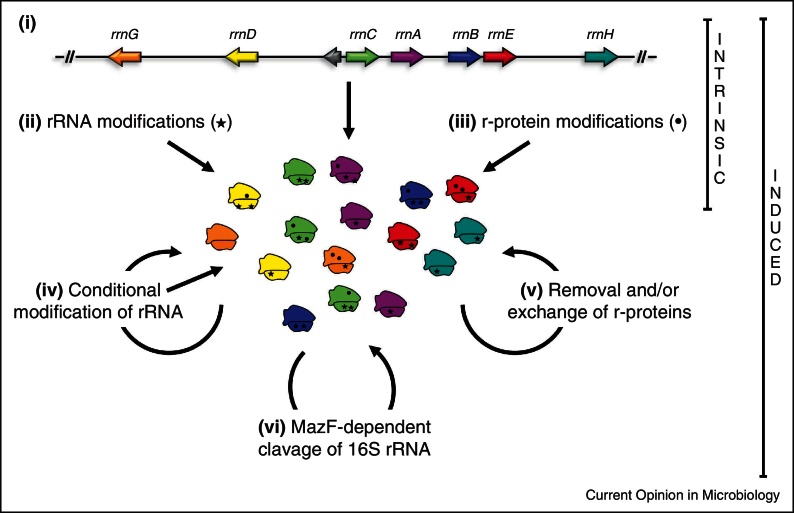
Figure 1.
Known and predicted mechanisms underlying ribosome heterogeneity in E. coli. Intrinsic ribosome heterogeneity under relaxed conditions could be attributed to (i) the presence of seven rrn operons that slightly differ in sequence (sequence micro-heterogeneity; indicated by different colors of the arrows representing the rrn operons; not drawn to scale), and (ii) diverse modifications of the 16S rRNA (indicated by stars) or (iii) the assembly of modified r-proteins (indicated by dots) during ribosome biogenesis. However, as the expression of the rrn operons as well as of the modifying enzymes can be affected by external signals, these mechanisms could likewise result in induced ribosome heterogeneity, thereby specifically adapt translational activity and/or specificity to environmental conditions. In response to stress conditions ribosomes can be altered by (iv) conditional modification of the rRNA either during ribosome biogenesis [47] or on mature ribosomal subunits [48], (v) removal [28] or exchange of r-proteins by a mechanism shown by [39], and by (vi) the truncation of the 16S rRNA via the stress-induced endoribonuclease MazF [34••]. Mechanisms that contribute to the generation of heterogeneity during biogenesis are depicted by straight arrows, whereas circular arrows indicate the alteration of mature ribosomes.
General features of the ribosome
The bacterial ribosome (70S) is composed of two asymmetric subunits, the 30S and the 50S subunit, which assemble at the ribosome binding site on the mRNA during translation initiation. Each subunit contributes to specific functions in protein synthesis. In Escherichia coli the small 30S subunit is composed of the 16S rRNA consisting of 1542 nucleotides (nts), and 21 proteins. Its shape is largely determined by the RNA component, which forms the three domains, the head, the platform, and the body (Figure 2a) [11]. It mediates the step of initiation and contains the binding sites for the three initiation factors as well as the messenger decoding center, where the respective codons of the mRNA are base-paired with the anticodon of the cognate tRNA. The large 50S subunit consists of two different rRNAs, the 23S rRNA (2904 nts) and the 5S rRNA (120 nts), and 34 proteins. It catalyzes peptide bond formation at the peptidyl transferase center, provides the binding sites for the elongation factors and comprises the exit tunnel for the nascent peptide chain.
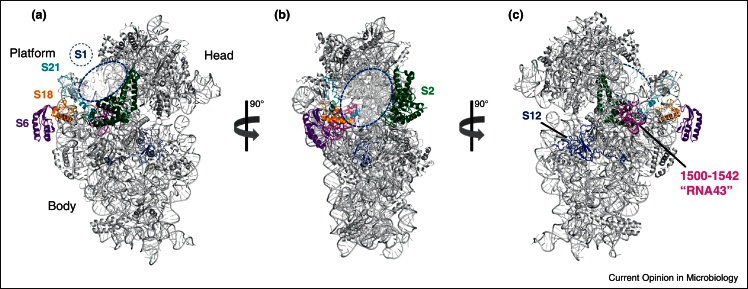
Figure 2.
The ‘hot spots’ of induced ribosome heterogeneity. Structure of the 30S subunit from the solvent side (a), rotated about 90° around the vertical axis (b), and from the subunit interface (c). The stoichiometry of r-proteins S1 (tentative binding site on the ribosome is indicated by a blue circle), S2 (blue), S6 (purple), S12 (green), S18 (pink), and S21 (yellow) is affected in response to environmental cues or by the presence of the aminoglycoside antibiotic kasugamycin [5,7,28]. Nucleotides 1500–1542 of the 16S rRNA (termed ‘RNA43’ when cleaved off by MazF) are shown in magenta. Other r-proteins and the 16S rRNA are shown in gray. The structure was modeled using Polyview 3D molecular system software [52] and PDB file 2AVY [53].
Variation of the ribosomal protein complement
In light of this structural and functional complexity a variety of different mechanisms leading to heterogeneity and consequently to the generation of functionally specialized ribosomes can be envisaged. As already suggested by studies mentioned above, ribosomes could differ in the stoichiometry of r-proteins [4–8]. Despite the catalytic activity of the rRNA in protein synthesis, the variation of or deficiency in some r-proteins could contribute to a fine tuning of ribosome function and in particular to its selectivity for distinct transcripts, a mechanism that is, well established in eukaryotes: a study performed already in 1981 in the slime mold Dictiostelium discoideum revealed that ribosomes from vegetative amoeboid cells differ in their protein content from ribosomes derived from spores [13]. This idea was further put forward by Mauro and Edelman in their ‘ribosome filter hypothesis’ in which the ribosome acts like a filter that selects for specific mRNAs and hence modulates translation [14]. Later on, the existence of ribosome protein paralogs in Saccharomyces cerevisiae, which contribute to the generation of functionally distinct ribosome populations, supported the new level of complexity in the regulation of gene expression and the term ‘ribosome code’ was coined [15]. Conversely, in prokaryotes the ribosome was generally not considered as a source for translational regulation, despite the first indications of heterogeneity due to alteration in the stoichiometry of r-proteins made almost 40 years ago [3,5,16,17]. In 1972, Deusser and Wittmann described a growth-rate-dependent variation in the protein composition of E. coli ribosomes. Their data revealed that the amount of proteins S6, S21, and L12 differs significantly, when ribosomes were prepared from cells grown either in rich or minimal media [5,7]. These observations are consistent with results published by Weber in 1972 [18] and Milne et al. in 1975 [8]. Moreover, protein S1, which is essential for translation initiation on canonical mRNAs [19], was found to be present in substoichiometric amounts on 70S ribosomes resulting in functional ribosome heterogeneity [16,20]. Surprisingly, since these early times in ribosome research only little attention has been dedicated to the regulatory role of the ribosome in bacterial translation.
More recently, several lines of evidence for the functional specificity of ribosomes harboring a reduced protein complement emerged from studies performed to enlighten the requirements for translation of leaderless mRNAs (lmRNAs), which harbor a 5′-terminal AUG start codon and are thus devoid of canonical ribosome recognition signals [21–26]. Collectively, these studies suggest a model wherein a subpopulation of ribosomes lacking protein S1 is responsible for translation of leaderless transcripts in vivo [24]. This notion was further underlined by a study performed to decipher the phenomenon of enhanced translational efficiency of lmRNAs in an E. coli rpsBts mutant strain conditionally deficient for r-protein S2 [21]. The results reveal that S2 is required for binding of protein S1 to the ribosome. Consequently, the lack of S1 can readily explain the inhibition of bulk (canonical) mRNA translation in the E. coli rpsBts mutant strain under non-permissive conditions [21]. Taken together with the observation that S1 is present in substoichiometric amounts on 70S ribosomes [20], these results implicated for the first time that ribosome heterogeneity might contribute to the functional selectivity of the translational machinery under distinct conditions. Recently, this notion gained further support by additional data substantiating the presence of a subpopulation of S1-depleted ribosomes under normal physiological conditions in E. coli cells [27]. Thus, it is tempting to speculate that conditions that might increase the S1-deficient ribosomal subpopulation could specifically stimulate the translation of lmRNAs.
In line with these observations, a follow-up study performed to explore the selective effect of the aminoglycoside antibiotic kasugamycin on translation initiation of lmRNA revealed the appearance of ribosomes in E. coli that lack several r-proteins [28]. These ribosomes are depleted for at least six proteins from the 30S subunit, among which are the functionally important proteins S1 and S2. However, the characterization of these protein-depleted 61S particles revealed that they are competent in translation of lmRNAs even in the presence of kasugamycin in vivo and in vitro [28]. Notwithstanding the artificial conditions, which trigger the release of proteins S1, S2, S6, S18, S12, and S21 from the mature 70S ribosome (Figure 1), this study presented the first evidence for the functionality of ribosomes devoid of multiple proteins, and puts forward the idea that the protein complement of the ribosome can vary in response to changed physiological conditions, which in turn leads to a modulation of the activity and specificity of the protein synthesis machinery. However, it is important to note that in contrast to the formation of 61S particles, where the r-proteins are removed from mature 70S ribosomes in the presence of kasugamycin, it still remains elusive whether the observed generation of protein-depleted ribosomes [3–8,16–18,20] can be attributed to the incorporation of premature subunits or whether these r-proteins are removed or lost from mature ribosomes.
Variation of the ribosomal RNA complement
Considering the catalytic activity of the rRNA in ribosome function, it is conceivable that variability within the RNA component could likewise represent a potential mechanism to establish functional ribosome heterogeneity. The presence of multiple individual rrn operons in bacteria and the observed differential regulation of the seven rRNA operons present in E. coli, which slightly differ in sequence, add significant weight to this argument [29–31] (Figure 1). Taken together these data support a model wherein sequence micro-heterogeneities of the rrn operons might contribute to the modulation of activity and specificity of the ribosome in response to environmental changes [29]. This hypothesis can be exemplified by the observation that during the morphological development of the Gram-positive bacterium Streptomyces coelicolor the heterogeneous rRNA molecules encoded by distinct rRNA operons are differentially expressed and assembled into the ribosomes [32,33]. Considering that the differentiation of Streptomycetes generally coincides with the production of antibiotics, some of which target the protein synthesis machinery, these results underscore the possibility that variations in the rRNA may play a significant role in the control of gene expression in response to developmental cues [32]. However, it is still an open question whether an alteration in translational specificity can be attributed to ribosomes containing different rRNA species.
The surprising discovery that the 16S rRNA of assembled ribosomes becomes 3′-terminally truncated when E. coli cells encounter stress completely changed the perception of ribosome-mediated regulation of protein synthesis [34••]. Stress-induced activation of the endoribonuclease MazF, the toxin component of the toxin-antitoxin system mazEF [35], results in cleavage of the 16S rRNA at the ACA-site located at positions 1500–1502 (Figure 3) [34••]. Since thereby the anti-Shine Dalgarno sequence, which is crucial for translation initiation on canonical mRNAs, is removed, the altered ribosomes selectively translate lmRNAs that are likewise generated by MazF [34••]. It is intriguing to note that this specialization of the translational machinery does neither rely on de novo ribosome biogenesis nor on the synthesis of regulatory RNA or protein factors, since the MazF-mediated cleavage occurs on mature 30S subunits or 70S ribosomes. Hence, this ribosome modification represents a paradigm for a highly economical and fast-reacting regulatory mechanism, which is conceptually related to the translational regulation by riboswitches or ribozymes [36]. Moreover, as the variation is achieved by virtue of RNA cleavage, which is independent of cell growth, this post-transcriptional stress response pathway might be of physiological relevance for cells encountering hostile conditions, e.g. during host infection. Thus, it is tempting to speculate that the shift of ribosome specificity during bacteriostasis provides a means to prepare the translational program of stressed cells for recuperation under ameliorated conditions, which would, for example, facilitate growth recovery of bacterial pathogens when the antibiotic treatment is stopped.
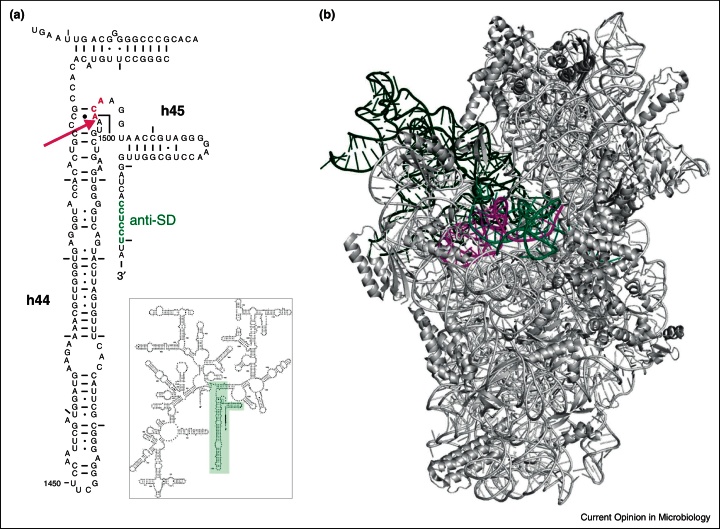
Figure 3.
MazF-dependent generation of stress-ribosomes. (a) The secondary structure of the Escherichia coli 16S rRNA is depicted. The helices 44 and 45 are enlarged, the ACA-site is indicated in magenta and the position of MazF cleavage between nucleotides A1499 and A1500 is indicated by an arrow. (b) The structure of the 30S subunit as seen from the solvent side. The tRNAs are shown in black, and the mRNA bound to the ribosomal subunit is shown in green. RNA43 of the 16S rRNA, which is removed upon MazF cleavage (shown in magenta), interacts via formation of the Shine and Dalgarno duplex with the mRNA. The structure was modeled using Polyview 3D molecular system software [52] and PDB file 2HGP [54].
Conclusion and future directions
The results summarized in this review emphasize that the bacterial ribosome can effectively and economically integrate environmental signals in the regulatory network of protein synthesis. Since the ‘hot spots’ of ribosome variability are mainly clustered at the 30S subunit, in close proximity to the decoding center (Figure 2), these studies collectively indicate that ribosome heterogeneity can be considered as a potential means to fine tune translation in response to environmental cues at the level of initiation, the major rate limiting step in protein synthesis [37,38]. Surprisingly, this regulatory role of the bacterial ribosome was not appreciated until recently, what might be attributed at least partially to the main focus on the structural and functional characterization of the translational apparatus, which was intentionally based on the purification of homogeneous ribosome populations from exponentially growing cells. In light of this consideration, the identification of more variations can be anticipated when studying the composition of ribosomes purified from bacteria that encountered adverse conditions. Moreover, the vast amount of data that becomes available by global RNA-seq analyses performed under diverse conditions could likewise indicate the differential regulation of ribosomal components and thus potentially facilitate the search for heterogeneous ribosome populations.
In light of the enormous amount of energy required for ribosome biogenesis, one puzzling question raised by these findings, namely the fate of the specialized ribosomes, still remains elusive. A single study addressing the mechanism of ribosome repair provided evidence that the replacement of damaged r-proteins restores translational activity of chemically inactivated ribosomes [39]. Since the majority of the proteins involved in ribosome heterogeneity — with the exception of proteins S6 and S18 — are determined to be exchangeable, this study strongly corroborates the regulatory function of the ribosome and provides a possible mechanism to reverse the specialization of the translational machinery. Therefore, the unexpected observation that the specificity of fully assembled ribosomes, which are already translationally active, can be modulated has opened up new avenues for the bacterial stress response, as the ribosome represents a novel hub in the complex regulatory network of cell physiology.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The authors would like to thank all colleagues and past and present members of the Moll group who contributed to these studies. The work was supported by grants P20112-B03 and P22249-B20 from the Austrian Science Fund to I.M.
References
- 1.Waller J.P. Fractionation of the ribosomal protein from Escherichia coli. J Mol Biol. 1964;10:319–336. doi: 10.1016/s0022-2836(64)80050-4. [DOI] [PubMed] [Google Scholar]
- 2.Moore P.B., Traut R.R., Noller H., Pearson P., Delius H. Ribosomal proteins of Escherichia coli. II. Proteins from the 30 s subunit. J Mol Biol. 1968;31:441–461. doi: 10.1016/0022-2836(68)90420-8. [DOI] [PubMed] [Google Scholar]
- 3.Kurland C.G., Voynow P., Hardy S.J., Randall L., Lutter L. Physical and functional heterogeneity of E. coli ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:17–24. doi: 10.1101/sqb.1969.034.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Bickle T.A., Howard G.A., Traut R.R. Ribosome heterogenecity. The nonuniform distribution of specific ribosomal proteins among different functional classes of ribosomes. J Biol Chem. 1973;248:4862–4864. [PubMed] [Google Scholar]
- 5.Deusser E. Heterogeneity of ribosomal populations in Escherichia coli cells grown in different media. Mol Gen Genet. 1972;119:249–258. doi: 10.1007/BF00333862. [DOI] [PubMed] [Google Scholar]
- 6.Deusser E., Weber H.J., Subramanian A.R. Variations on stoichiometry of ribosomal proteins in Escherichia coli. J Mol Biol. 1974;84:249–256. doi: 10.1016/0022-2836(74)90583-x. [DOI] [PubMed] [Google Scholar]
- 7.Deusser E., Wittmann H.G. Ribosomal proteins: variation of the protein composition in Escherichia coli ribosomes as function of growth rate. Nature. 1972;238:269–270. doi: 10.1038/238269a0. [DOI] [PubMed] [Google Scholar]
- 8.Milne A.N., Mak W.W., Wong J.T. Variation of ribosomal proteins with bacterial growth rate. J Bacteriol. 1975;122:89–92. doi: 10.1128/jb.122.1.89-92.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ban N., Nissen P., Hansen J., Moore P.B., Steitz T.A. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science. 2000;289:905–920. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- 10.Schluenzen F., Tocilj A., Zarivach R., Harms J., Gluehmann M., Janell D., Bashan A., Bartels H., Agmon I., Franceschi F. Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution. Cell. 2000;102:615–623. doi: 10.1016/s0092-8674(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 11.Wimberly B.T., Brodersen D.E., Clemons W.M., Jr., Morgan-Warren R.J., Carter A.P., Vonrhein C., Hartsch T., Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000;407:327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- 12.Yusupov M.M., Yusupova G.Z., Baucom A., Lieberman K., Earnest T.N., Cate J.H., Noller H.F. Crystal structure of the ribosome at 5.5 A resolution. Science. 2001;292:883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 13.Ramagopal S., Ennis H.L. Regulation of synthesis of cell-specific ribosomal proteins during differentiation of Dictyostelium discoideum. Proc Natl Acad Sci USA. 1981;78:3083–3087. doi: 10.1073/pnas.78.5.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauro V.P., Edelman G.M. The ribosome filter hypothesis. Proc Natl Acad Sci USA. 2002;99:12031–12036. doi: 10.1073/pnas.192442499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komili S., Farny N.G., Roth F.P., Silver P.A. Functional specificity among ribosomal proteins regulates gene expression. Cell. 2007;131:557–571. doi: 10.1016/j.cell.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Duin J., van Knippenberg P.H. Functional heterogeneity of the 30 S ribosomal subunit of Escherichia coli. 3. Requirement of protein S1 for translation. J Mol Biol. 1974;84:185–195. doi: 10.1016/0022-2836(74)90221-6. [DOI] [PubMed] [Google Scholar]
- 17.Voynow P., Kurland C.G. Stoichiometry of the 30S ribosomal proteins of Escherichia coli. Biochemistry. 1971;10:517–524. doi: 10.1021/bi00779a026. [DOI] [PubMed] [Google Scholar]
- 18.Weber H.J. Stoichiometric measurements of 30S and 50S ribosomal proteins from Escherichia coli. Mol Gen Genet. 1972;119:233–248. doi: 10.1007/BF00333861. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen M.A., Fricke J., Pedersen S. Ribosomal protein S1 is required for translation of most, if not all, natural mRNAs in Escherichia coli in vivo. J Mol Biol. 1998;280:561–569. doi: 10.1006/jmbi.1998.1909. [DOI] [PubMed] [Google Scholar]
- 20.van Knippenberg P.H., Hooykaas P.J., van Duin J. The stoichiometry of E. coli 30S ribosomal protein S1 on in vivo and in vitro polyribosomes. FEBS Lett. 1974;41:323–326. doi: 10.1016/0014-5793(74)81239-1. [DOI] [PubMed] [Google Scholar]
- 21.Moll I., Grill S., Grundling A., Blasi U. Effects of ribosomal proteins S1, S2 and the DeaD/CsdA DEAD-box helicase on translation of leaderless and canonical mRNAs in Escherichia coli. Mol Microbiol. 2002;44:1387–1396. doi: 10.1046/j.1365-2958.2002.02971.x. [DOI] [PubMed] [Google Scholar]
- 22.Moll I., Grill S., Gualerzi C.O., Blasi U. Leaderless mRNAs in bacteria: surprises in ribosomal recruitment and translational control. Mol Microbiol. 2002;43:239–246. doi: 10.1046/j.1365-2958.2002.02739.x. [DOI] [PubMed] [Google Scholar]
- 23.Moll I., Hirokawa G., Kiel M.C., Kaji A., Blasi U. Translation initiation with 70S ribosomes: an alternative pathway for leaderless mRNAs. Nucleic Acids Res. 2004;32:3354–3363. doi: 10.1093/nar/gkh663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moll I., Resch A., Blasi U. Discrimination of 5′-terminal start codons by translation initiation factor 3 is mediated by ribosomal protein S1. FEBS Lett. 1998;436:213–217. doi: 10.1016/s0014-5793(98)01131-4. [DOI] [PubMed] [Google Scholar]
- 25.Moll I., Huber M., Grill S., Sairafi P., Mueller F., Brimacombe R., Londei P., Blasi U. Evidence against an interaction between the mRNA downstream box and 16S rRNA in translation initiation. J Bacteriol. 2001;183:3499–3505. doi: 10.1128/JB.183.11.3499-3505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrgazov K., Manoharadas S., Kaberdina A.C., Vesper O., Moll I. Direct interaction of the N-terminal domain of ribosomal protein S1 with protein S2 in Escherichia coli. PLoS ONE. 2012;7:e32702. doi: 10.1371/journal.pone.0032702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delvillani F., Papiani G., Deho G., Briani F. S1 ribosomal protein and the interplay between translation and mRNA decay. Nucleic Acids Res. 2011;39:7702–7715. doi: 10.1093/nar/gkr417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaberdina A.C., Szaflarski W., Nierhaus K.H., Moll I. An unexpected type of ribosomes induced by kasugamycin: a look into ancestral times of protein synthesis? Mol Cell. 2009;33:227–236. doi: 10.1016/j.molcel.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillebrand A., Wurm R., Menzel A., Wagner R. The seven E. coli ribosomal RNA operon upstream regulatory regions differ in structure and transcription factor binding efficiencies. Biol Chem. 2005;386:523–534. doi: 10.1515/BC.2005.062. [DOI] [PubMed] [Google Scholar]
- 30.Condon C., Philips J., Fu Z.Y., Squires C., Squires C.L. Comparison of the expression of the seven ribosomal RNA operons in Escherichia coli. EMBO J. 1992;11:4175–4185. doi: 10.1002/j.1460-2075.1992.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Condon C., Liveris D., Squires C., Schwartz I., Squires C.L. rRNA operon multiplicity in Escherichia coli and the physiological implications of rrn inactivation. J Bacteriol. 1995;177:4152–4156. doi: 10.1128/jb.177.14.4152-4156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H.L., Shin E.K., Kim H.M., Ryou S.M., Kim S., Cha C.J., Bae J., Lee K. Heterogeneous rRNAs are differentially expressed during the morphological development of Streptomyces coelicolor. FEMS Microbiol Lett. 2007;275:146–152. doi: 10.1111/j.1574-6968.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim H.L., Song W.S., Kim K., Lee K. Characterization of heterogeneous LSU rRNA profiles in Streptomyces coelicolor under different growth stages and conditions. Curr Microbiol. 2008;57:537–541. doi: 10.1007/s00284-008-9238-1. [DOI] [PubMed] [Google Scholar]
- 34••.Vesper O., Amitai S., Belitsky M., Byrgazov K., Kaberdina A.C., Engelberg-Kulka H., Moll I. Selective translation of leaderless mRNAs by specialized ribosomes generated by MazF in Escherichia coli. Cell. 2011;147:147–157. doi: 10.1016/j.cell.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work describes a novel paradigm for a post-transcriptional stress response mechanism in E. coli, which is based on the 3′-terminal truncation of the 16S rRNA by the toxin MazF. Since the ribosomes become selective for specific mRNAs, the translational program is modulated in response to environmental cues.
- 35.Engelberg-Kulka H., Hazan R., Amitai S. mazEF: a chromosomal toxin–antitoxin module that triggers programmed cell death in bacteria. J Cell Sci. 2005;118:4327–4332. doi: 10.1242/jcs.02619. [DOI] [PubMed] [Google Scholar]
- 36.Nudler E., Mironov A.S. The riboswitch control of bacterial metabolism. Trends Biochem Sci. 2004;29:11–17. doi: 10.1016/j.tibs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Gualerzi C.O., Pon C.L. Initiation of mRNA translation in prokaryotes. Biochemistry. 1990;29:5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- 38.Noller H.F. Structure of the bacterial ribosome and some implications for translational regulation. In: Hershey J.W.B., Sonenberg N., Mathews M., editors. Translational Control in Biology and Medicine. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2007. pp. 41–58. [Google Scholar]
- 39.Pulk A., Liiv A., Peil L., Maivali U., Nierhaus K., Remme J. Ribosome reactivation by replacement of damaged proteins. Mol Microbiol. 2010;75:801–814. doi: 10.1111/j.1365-2958.2009.07002.x. [DOI] [PubMed] [Google Scholar]
- 40.Kaczanowska M., Ryden-Aulin M. Ribosome biogenesis and the translation process in Escherichia coli. Microbiol Mol Biol Rev. 2007;71:477–494. doi: 10.1128/MMBR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodson S.A. RNA folding and ribosome assembly. Curr Opin Chem Biol. 2008;12:667–673. doi: 10.1016/j.cbpa.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brimacombe R., Mitchell P., Osswald M., Stade K., Bochkariov D. Clustering of modified nucleotides at the functional center of bacterial ribosomal RNA. FASEB J. 1993;7:161–167. doi: 10.1096/fasebj.7.1.8422963. [DOI] [PubMed] [Google Scholar]
- 43.Decatur W.A., Fournier M.J. rRNA modifications and ribosome function. Trends Biochem Sci. 2002;27:344–351. doi: 10.1016/s0968-0004(02)02109-6. [DOI] [PubMed] [Google Scholar]
- 44••.Shajani Z., Sykes M.T., Williamson J.R. Assembly of bacterial ribosomes. Annu Rev Biochem. 2011;80:501–526. doi: 10.1146/annurev-biochem-062608-160432. [DOI] [PubMed] [Google Scholar]; This review provides an overview of 50 years of ribosome assembly with a special focus on the ribosomal cofactors which are involved in ribosome biogenesis.
- 45.Green R., Noller H.F. Reconstitution of functional 50S ribosomes from in vitro transcripts of Bacillus stearothermophilus 23S rRNA. Biochemistry. 1999;38:1772–1779. doi: 10.1021/bi982246a. [DOI] [PubMed] [Google Scholar]
- 46.Krzyzosiak W., Denman R., Nurse K., Hellmann W., Boublik M., Gehrke C.W., Agris P.F., Ofengand J. In vitro synthesis of 16S ribosomal RNA containing single base changes and assembly into a functional 30S ribosome. Biochemistry. 1987;26:2353–2364. doi: 10.1021/bi00382a042. [DOI] [PubMed] [Google Scholar]
- 47.Pokkunuri I., Champney W.S. Characteristics of a 50S ribosomal subunit precursor particle as a substrate for ermE methyltransferase activity and erythromycin binding in Staphylococcus aureus. RNA Biol. 2007;4:147–153. doi: 10.4161/rna.4.3.5346. [DOI] [PubMed] [Google Scholar]
- 48.Bügl H., Fauman E.B., Staker B.L., Zheng F., Kushner S.R., Saper M.A., Bardwell J.C., Jakob U. RNA methylation under heat shock control. Mol Cell. 2000;6:349–360. doi: 10.1016/s1097-2765(00)00035-6. [DOI] [PubMed] [Google Scholar]
- 49.Soufi B., Soares N.C., Ravikumar V., Macek B. Proteomics reveals evidence of cross-talk between protein modifications in bacteria: focus on acetylation and phosphorylation. Curr Opin Microbiol. 2012;15:357–363. doi: 10.1016/j.mib.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 50•.Nesterchuk M.V., Sergiev P.V., Dontsova O.A. Posttranslational modifications of ribosomal proteins in Escherichia coli. Acta Naturae. 2011;3:22–33. [PMC free article] [PubMed] [Google Scholar]; This review summarizes the all known posttranslational modifications of ribosomal proteins in Escherichia coli and discusses certain enzymes responsible for the modifications and mechanisms of enzymatic reactions.
- 51.Yu B.J., Kim J.A., Moon J.H., Ryu S.E., Pan J.G. The diversity of lysine-acetylated proteins in Escherichia coli. J Microbiol Biotechnol. 2008;18:1529–1536. [PubMed] [Google Scholar]
- 52.Porollo A., Meller J. Versatile annotation and publication quality visualization of protein complexes using POLYVIEW-3D. BMC Bioinformatics. 2007;8:316. doi: 10.1186/1471-2105-8-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuwirth B.S., Borovinskaya M.A., Hau C.W., Zhang W., Vila-Sanjurjo A., Holton J.M., Cate J.H. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- 54.Yusupova G., Jenner L., Rees B., Moras D., Yusupov M. Structural basis for messenger RNA movement on the ribosome. Nature. 2006;444:391–394. doi: 10.1038/nature05281. [DOI] [PubMed] [Google Scholar]