Abstract
Objectives
We assessed the evidence relating pre-term delivery (PTD), low birthweight, small for gestational age (SGA), pre-eclampsia and gestational hypertension to five occupational exposures (working hours, shift work, lifting, standing and physical workload). We conducted a systematic search in MEDLINE and EMBASE (1966–2011), updating a previous search with a further six years of observations.
Methods
As before, combinations of keywords and MeSH terms were used. Each relevant paper was assessed for completeness of reporting and potential for important bias or confounding, and its effect estimates abstracted. Where similar definitions of exposure and outcome existed we calculated pooled estimates of relative risk in meta-analysis.
Results
Analysis was based on 86 reports (32 cohort investigations, 57 with usable data on PTD, 54 on birthweight and 11 on pre-eclampsia/gestational hypertension); 33 reports were new to this review. For PTD, findings across a substantial evidence base were generally consistent, effectively ruling out large effects (e.g. RR>1.2). Larger and higher quality studies were less positive, while meta-estimates of risk were smaller than previously and best estimates pointed to modest or null effects (RR 1.04 to 1.18). For SGA, the position was similar but meta-estimates were even closer to the null (eight of nine RRs ≤ 1.07). For pre-eclampsia/gestational hypertension the evidence base remains insufficient.
Conclusions
The balance of evidence is against large effects for the associations investigated. As the evidence base has grown, estimates of risk in relation to these outcomes have become smaller.
Keywords: pregnancy, occupation, review, meta-analysis
In the UK, as in most parts of the world, women make up a substantial proportion of the workforce (50% in 20101). Almost 70% of women work through their reproductive years,2 amounting to some 350,000 pregnant workers in any one year.3 The impetus and legal onus to assess health and safety risks to pregnant workers, and where possible to minimise them, is thus considerable.
As strategies have evolved to manage the risks associated with well-established but uncommon reproductive hazards (e.g. ionising radiation, lead), so attention has turned to everyday occupational exposures, relating to working hours, shift work, standing, lifting, and physical workload.
In theory, such common exposures could affect the outcomes of pregnancy. For example, disrupted circadian rhythms from shift working could trigger neuroendocrine changes that affect fetal growth and timing of parturition, while raised noradrenaline levels from heavy physical exertion could increase uterine contractility and risks of preterm labour. Set against this, however, considerable physiological adaptations to the demands of pregnancy tend to preserve constant fetal oxygen consumption, and a growing body of evidence suggests that moderate physical exercise in pregnancy can be beneficial;4-7 several authoritative clinical bodies now recommend it.8,9
Previously10 we reviewed the evidence (to December 2005) relating five common occupational exposures (prolonged working hours, shift work, lifting, standing, and heavy physical workload) to five clinically important adverse outcomes of pregnancy (pre-term delivery, small for gestational age (SGA), low birthweight (LBW), pre-eclampsia and gestational hypertension). Subsequently, a request by the Royal College of Physicians of London (RCP) to prepare national clinical guidelines on pregnancy and work afforded us the opportunity to update our search over several more years in a surprisingly active area of research inquiry. We report here on the considerably enlarged body of evidence, and present new meta-estimates of effect for exposures and outcomes of interest.
METHODS
Search strategy
Previously we conducted a systematic search in Medline and EMBASE from 1966 to December 2005,10 with a partial update in Medline in respect of shift work to February 2010.11 For this review the same search strategy was run in both databases to provide complete coverage from 1966 to December 31st 2011, adding six more years of data.
As before, medical subject headings (MeSH terms) and key words representing each outcome and exposure of interest were combined. The MeSH terms used were: pregnancy, reproductive health, pre-eclampsia, infant-premature, labour-premature, birth weight, gestational age, small for gestational age, fetal growth retardation, labour complications, pregnancy complications (as outcomes); and lifting, work schedule tolerance, exercise, fatigue, work, workload, employment and occupational exposure (as exposures). Several simple search terms also supplemented the inquiry: occupational activity, standing, manual lifting, heavy lifting and shift work (as exposures). Searches were limited to papers with an abstract in English. Titles and abstracts were examined and all potentially relevant primary reports and reviews were obtained. The references of retrieved papers and a major report in the area by RCP and NHS Plus,12 published since our last review, were also checked for relevant material. These procedures and the steps below were replicated independently by two of us and differences were resolved by consensus. Papers finally included were those which compared an exposed with a less heavily or unexposed reference group for at least one exposure-outcome combination of interest and which provided estimates of effect or the data to calculate these.
Data abstraction
Details were abstracted from each relevant paper on the study populations, setting, timing of investigation, study design, exposure contrasts, methods for assessment of exposure(s) and outcome(s), response rates, confounders considered, and estimates of effect. Where a paper provided frequencies but not estimates of relative risk (RR), odds ratios (ORs) with exact 95% confidence intervals (95%CIs) were calculated using STATA software. Similarly, where birthweight was presented as a continuous measure, with group means and standard deviations, the mean difference between exposure groups was calculated with 95%CIs. Where several sub-analyses were presented, analysis focused on exposure contrasts that were most comparable across studies.
Quality assessment
Each paper was rated for completeness of reporting and each exposure-outcome permutation for its potential for significant confounding or “inflationary” bias, as defined previously.10 In brief, completeness of reporting was graded according to nine items that were clearly defined (study design, sampling frame and procedures, inclusion/exclusion criteria, main characteristics of the study population, numbers and response rates, method(s) of assessment of exposure and of outcome, method of analysis, measures of association with 95%CI and numbers in the analysis): studies for which ≥3 items were missing or unclear were classed as poorer in information quality. Potentially important confounders were identified from among risk factors that were reasonably prevalent, unlikely to reflect the effects of occupational exposure or lie on the causal pathway between exposure and health, and which carried a reasonable RR (the choice of confounders varied by outcome as described below). “Inflationary” bias (bias that could cause important overestimation of RRs) was considered most likely when exposures were self-reported retrospectively (especially if of a type difficult to recall), and were being related to outcomes that were self-reported or were clearly adverse. Thus, retrospective studies with self-reported exposures were assigned one point for each of: (1) self-reported outcome; (2) outcome of pre-eclampsia, gestational hypertension, or low birthweight; (3) exposure related to physical workload (standing, lifting, activity score). Exposure-outcome pairings were scored 0 to 3, and scores ≥2 were considered indicative for potential inflationary bias. By these criteria, exposure-outcome combinations were counted as of poorer quality if they had significant potential for confounding or bias or came from studies with incomplete reporting. In summarising findings, we also distinguished risk estimates based on >1000 deliveries from smaller analyses. (With an alpha of <0.05, this cut-point should provide a ≥95% power to detect an OR in case-control studies of 2.0 for exposures such as working >40 hrs/week and shift work ‘most of the time’, and a RR in cohort studies of 2.0 for pre-term delivery and SGA (details available on request).)
Meta-analysis
For studies with similar definitions of exposure and outcome, pooled estimates of RR were calculated by weighting log RRs or log ORs by the inverse of their variances. Meta-analysis was performed using the Sharp and Sterne STATA macro. A fixed effects model was chosen unless there was evidence of heterogeneity (P<0.1), whereupon a random effects (DerSimonian-Laird) model was selected instead. Overall meta-estimates for possible exposure-outcome combinations were computed as well as a sensitivity analysis excluding papers of lower quality. Where possible, estimates were also made for occupational exposures continuing into the second or third trimesters of pregnancy. Where studies provided estimates of effect for several trimesters, the estimate earliest in pregnancy was used for the overall analysis and that latest in pregnancy for the second and third trimester analysis.
RESULTS
Our earlier review identified 53 reports (covering 49 studies).13-65 The updated search, together with a review of the bibliographies of published papers, identified a further 33 reports66-98 relating to 30 studies – in all, 86 reports, 57 with usable data on pre-term delivery, 54 on birthweight (including SGA) and 11 concerning pre-eclampsia or gestational hypertension (some reports covered several exposures and/or outcomes). The additional material comprised 28 reports published after the index date in December 200566-93 and five94-98 from before it identified from citations in papers retrieved by this search.
For reasons of parsimony, we tabulate here only a descriptive summary of risk estimates across the full material (1966 to December 2011), overall and for larger higher quality studies (Table 1), and associated meta-estimates where these could be derived (Table 2). Online supplementary tables S1 to S7 provide a complete listing, covering the design features of all 79 studies, our assessment of their study quality, and associated risk estimates from the 86 reports, enumerated separately by pregnancy outcome [link to be added]. Unless otherwise stated, our description of the findings and discussion cover the entire search period.
Table 1.
Descriptive summary of the associations between reviewed activities and pregnancy outcomes (1966-2011)*
| All studies |
Higher quality larger studies§ |
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcome/exposure | N Studies |
Median (IQR) | Range | N estimates (RR ≥2.0/all estimates) |
N Studies |
Median (IQR) | Range | N estimates (RR ≥2.0/all estimates) |
| Pre-term delivery (RR) | ||||||||
| Working hours | 25 | 1.18 (1.00 to 1.34) | 0.30 to 3.69 | 2/30 | 11 | 1.10 (1.01 to 1.21) | 0.30 to 1.60 | 0/15 |
| Shift work | 21 | 1.10 (0.67 to 1.60) | 0.67 to 5.60 | 3/33 | 9 | 1.03 (0.94 to 1.16) | 0.67 to 1.80 | 0/19 |
| Standing | 28 | 1.16 (1.00 to 1.35) | 0.58 to 4.10 | 3/36 | 10 | 1.09 (0.92 to 1.23) | 0.76 to 1.69 | 0/12 |
| Lifting | 17 | 1.12 (0.90 to 1.30) | 0.55 to 2.91 | 1/22 | 11 | 1.02 (0.90 to 1.30) | 0.55 to 1.49 | 0/15 |
| Physical activity | 33 | 1.20 (1.10 to 1.70) | 0.71 to 4.10 | 4/35 | 8 | 1.10 (1.04 to 1.16) | 0.87 to 1.25 | 0/9 |
|
| ||||||||
| SGA (RR) | ||||||||
| Working hours | 14 | 1.10 (1.00 to 1.27) | 0.80 to 2.10 | 1/18 | 6 | 1.10 (1.00 to 1.10) | 0.99 to 1.19 | 0/9 |
| Shift work | 11 | 1.25 (0.94 to 1.49) | 0.70 to 3.31 | 2/18 | 6 | 1.00 (0.92 to 1.25) | 0.70 to 1.50 | 0/11 |
| Standing | 12 | 1.00 (0.93 to 1.26) | 0.86 to 2.00 | 1/17 | 4 | 1.06 (0.98 to 1.24) | 0.89 to 1.42 | 0/4 |
| Lifting | 7 | 1.03 (0.73 to 1.15) | 0.50 to 1.20 | 0/11 | 4 | 1.08 (1.04 to 1.17) | 0.65 to 1.20 | 0/6 |
| Physical activity | 13 | 1.00 (0.82 to 1.38) | 0.70 to 2.40 | 2/14 | 5 | 0.88 (0.81 to 1.00) | 0.76 to 1.20 | 0/6 |
|
| ||||||||
| Low birth weight (RR) | ||||||||
| Working hours | 8 | 1.34 (1.20 to 1.65) | 0.96 to 1.80 | 0/10 | 0 | - | - | - |
| Shift work | 7 | 1.28 (1.02 to 1.47) | 0.71 to 2.10 | 1/9 | 1 | - | 1.02 | 0/1 |
| Standing | 9 | 1.13 (0.70 to 1.58) | 0.50 to 1.92 | 0/13 | 1 | - | 0.5 | 0/1 |
| Lifting | 7 | 1.10 (0.70 to 1.26) | 0.50 to 2.40 | 1/9 | 3 | 0.75 (0.73 to 1.58) | 0.70 to 2.40 | 1/3 |
| Physical activity | 10 | 1.13 (1.04 to 1.80) | 0.60 to 4.32 | 2/11 | 1 | - | 0.99 to 1.13 | 0/2 |
|
| ||||||||
| Birth weight (gms diff) | ||||||||
| Working hours | 7 | −60 (−74 to −70) | −84 to −32 | (N=9) | 3 | −45 (−53 to −44) | −60 to −43 | (N=3) |
| Shift work | 6 | 10 (−273 to 39) | −438 to 195 | (N=13) | 1 | 37 (21 to 57) | 2 to 91 | (N=4) |
| Standing | 8 | −25 (−31 to 0.5) | −49 to 20 | (N=11) | 3 | −36 (−42 to −29) | −49 to −18 | (N=4) |
| Lifting | 3 | −21 (−24 to 11) | −44 to 19 | (N=8) | 0 | - | - | - |
| Physical activity | 8 | −59 (−148 to −29) | −216 to 183 | (N=14) | 2 | - | −21 to 51 | (N=2) |
|
| ||||||||
|
Pregnancy-induced
hypertension |
||||||||
| Working hours | 5 | 1.10 (0.85 to 1.10) | 0.76 to 1.18 | 0/5 | 1 | - | 0.76 | 0/1 |
| Shift work | 2 | - | 0.90 to 1.10 | 0/2 | 0 | - | - | - |
| Standing | 4 | 1.05 (0.93 to 1.14) | 0.70 to 1.26 | 0/4 | 1 | - | 1.26 | 0/1 |
| Lifting | 2 | - | 1.10 to 1.10 | 0/2 | 0 | - | - | - |
| Physical activity | 4 | 1.15 (1.00 to 1.77) | 0.70 to 3.47 | 1/4 | 0 | - | - | - |
|
| ||||||||
| Pre-eclampsia | ||||||||
| Working hours | 2 | - | 0.96 to 1.20 | 0/2 | 1 | - | 0.96 | 0/1 |
| Shift work | 2 | - | 1.00 to 1.30 | 0/2 | 1 | - | 1.30 | 0/1 |
| Standing | 4 | 0.77 (0.72 to 1.34) | 0.70 to 2.90 | 1/4 | 1 | - | 0.72 | 0/1 |
| Lifting | 3 | 1.1 | 0.68 to 1.70 | 0/3 | 0 | - | - | - |
| Physical activity | 3 | 0.75 | 0.70 to 2.10 | 1/3 | 0 | - | - | - |
See online supplementary tables S1-S7 [ADD LINK] for a complete listing of the reports and associated risk estimates summarised in this table
After excluding estimates with higher potential for bias or confounding, involving <1000 deliveries, or from incompletely reported studies
Table 2.
Relationship between working hours, standing, shift work and two pregnancy outcomes (pre-term delivery and small for gestational age): meta-estimates of relative risk (1966-2011)*
| Working hours (> v <40h/w) |
Standing (>4 v <4 h/d) |
Shift work (Yes v No) |
||||
|---|---|---|---|---|---|---|
| N | RR (95%CI) | N | RR (95%CI) | N | RR (95%CI) | |
| Preterm delivery | ||||||
| Overall meta-estimate | 17 | 1.23 (1.13 to 1.34) | 12 | 1.22 (1.12 to 1.33) | 19 | 1.14 (1.01 to 1.30) |
| Sensitivity analysis§ | 11 | 1.18 (1.05 to 1.33) | 7 | 1.13 (0.99 to 1.29) | 12 | 1.04 (0.94 to 1.15) |
| Later pregnancy | 6 | 1.17 (0.94 to 1.45) | 7 | 1.15 (0.96 to 1.37) | 8 | 1.17 (0.86 to 1.60) |
| SGA | ||||||
| Overall meta-estimate | 8 | 1.04 (0.94 to 1.16) | 7 | 1.07 (0.94 to 1.22) | 10 | 1.01 (0.92 to 1.10) |
| Sensitivity analysis§ | 6 | 0.99 (0.88 to 1.11) | 5 | 1.16 (0.97 to 1.38) | 7 | 0.98 (0.90 to 1.08) |
| Later pregnancy | 4 | 0.99 (0.83 to 1.19) | 5 | 0.95 (0.76 to 1.20) | 5 | 1.05 (0.94 to 1.18) |
Excluding studies with a higher potential for bias or confounding, or which reported incompletely.
See online supplementary tables S1 and S2 [ADD LINK] for details of the reports and risk estimates incorporated into these meta-analyses
Identified studies covered 27 countries, a third of reports coming from the USA and a third from Europe. In general, reports had satisfactory completeness of reporting by our criteria. However, for 20/79 (25%) studies the score was ≤6.
Sample sizes varied from small (<50) to extremely large (>350,000), but 57% of the 353 effect estimates across both reviews (Tables S1 to S7) were based on findings from >1000 births. Response rates at baseline (cross-sectional studies) or follow-up (cohort studies) often exceeded 80%-90%, but were <65% or unclear in 21 reports.25-6,32,34-5,37,47,51-4,59,65,79,85,87,89,90,92-3,98
In 29 cohort investigations, occupational history was determined during pregnancy and in three others64,71,77 by record linkage; for the remaining studies information on work exposures was obtained after delivery, mostly through self-report, but in a minority26-7,39,44,47,59,77,80,82,90 using job title as a surrogate index. Issues of measurement error in exposure assessment were seldom considered, only a few studies employed personal diaries to assist self-reporting, and about 40% of studies did not report the timing of exposures during pregnancy. Most studies of working hours, standing and shift work employed similar exposure definitions. However, definitions for lifting and physical workload differed materially between studies.
With few exceptions health outcomes were established objectively (from hospital records, registers, or birth certificates).
Various strategies were used to control for confounding (matching, restriction, stratification, regression modelling), but confounding was ignored altogether in some investigations. Roughly 40% of exposure-outcome pairings carried higher potential for inflationary bias or confounding according to our criteria.
Pre-term delivery
Case definition
Most reports adopted the WHO definition for pre-term delivery: “the birth of a living fetus before 37 completed weeks of gestation”.
Potential confounding factors
Many maternal characteristics have been associated with an increased risk of pre-term delivery (e.g. previous pre-term delivery, multiple gestation, diabetes, pre-eclampsia, bacterial vaginosis, extremes of maternal age), but few such factors are both common and carry a high RR and some (e.g. obstetric events in previous pregnancies) could have arisen from previous work exposures. Smoking and lower social class carry moderate RRs (1.5-2.0) and are prevalent exposures whose frequency could vary systematically by occupational activity. Risk estimates that failed to take account of both of these variables (or proxies of them – e.g. lower educational attainment or income) were classed as having higher potential for confounding.
Scope for meta-analysis
Formal meta-analysis was feasible for associations of pre-term delivery with working hours (>40 hours/week vs. less), shift work (Yes vs No) and standing (>4 hours/day vs. less). For lifting and physical workload, definitions of exposure were too heterogeneous to justify being combined.
Working hours
The relation of working hours to pre-term delivery was considered in 25 studies,16,18-9,21,25,28,32,35,37,44-5,51-4,60,71-2,76,79,81,84,86-7,97 including nine cohort investigations. These provided 30 estimates of RR, the median RR being 1.18 (and 1.10 in 11 large studies of higher quality). In only two of 30 estimates, was the RR ≥2.0.84,87 One of these studies was unusual in its focus on exposure to anaesthetic gases and infective risks,87 and both were small relative to the field (<750 births), with correspondingly wide 95%CIs. By contrast, the eight largest studies (>2000 births) 19,37,51,53,71,76,79,81 all had RRs <1.34. A pooled RR of 1.23 (95%CI 1.13-1.34) (Figure 1) was derived from 17 studies that compared work for at least 40 hours per week with shorter hours.18,19,21,25,35,44,53-4,71-2,76,79,81,84,86-7,97 For the subset of 11 studies judged of higher methodological rigor,18,19,21,44,54,72,76,79,81,84,87 the meta-RR was somewhat lower (1.18 (95%CI 1.05-1.33)), while meta-estimate for exposure continuing into later pregnancy was close to this second value.
Figure 1.
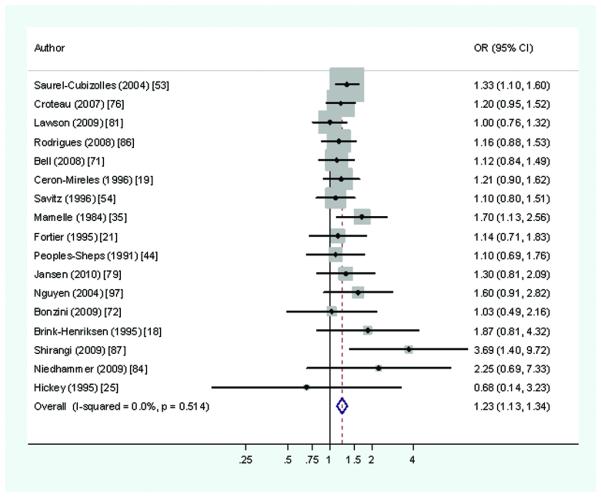
Risk of preterm delivery associated with working >40 hours per week during pregnancy (Forest plot ordered by study size)
Shift work
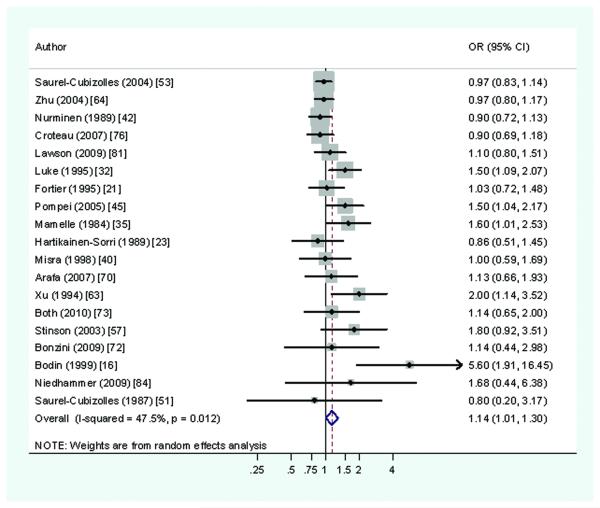
Twenty-one studies16,21,23,32,35,37,40,42,45,51,53,57,63-4,68,70,72-3,76,81,84 were found that considered the association of pre-term delivery with shift work (usually defined either as shift or night work), including nine cohort investigations. Together these provided 33 estimates of effect. In two-thirds the point estimate of RR was near or below unity, although in nine studies16,32,35,45,57,63,68,73,84 the RR was ≥1.5 and in three of these16,63,68 risks were elevated ≥2.0. Among these, one study focussed primarily on exposure to anaesthetic gases in midwives16 and was an outlying observation. A second involved exposure not only to shift working but also to self-reported undefined “physical and chemical hazards”.68 Both this and a third study of textile workers63 were small (<1000 births) relatively. Among the seven largest studies of shift working and pre-term delivery, 21,37,53,64,73,76,81 each involving >4000 births, 13 of 14 RRs were <1.18. The median estimate of RR across all studies was 1.10, but only 1.03 in the nine larger better quality studies; the meta-estimate (based on 19 studies 16,21,23,32,35,40,42,45,51,53,57,63-4,70,72-3,76,81,84) was 1.14 (Figure 2), and that for the 12 studies that met our criteria for higher quality21,40,42,45,57,63-4,72-3,76,81,84 was 1.04 (95%CI 0.94-1.15).
Figure 2.
Risk of preterm delivery associated with working shifts during pregnancy (Forest plot ordered by study size)
Standing
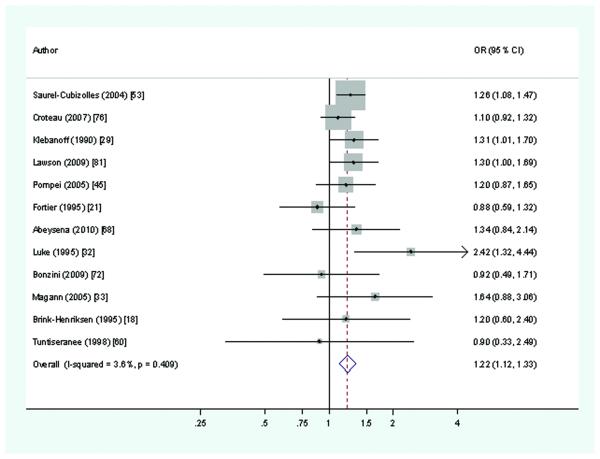
Twenty-eight studies15,18-9,21,23,25,29,31-3,35,37,40-1,45,51-3,59,60,68,70,72,76,81,85-6,98 which considered standing and pre-term delivery, 12 of cohort design, provided 36 estimates of effect. ‘High’ exposure was defined as standing for ≥4 hours/day in 12 studies.17,21,29,32-3,45,53,60,68,72,76,81 Risk estimates exceeded 1.5 in eight studies,31-33,35,41,52,59,98 of which three reported RRs ≥2.0.32,59,98 Of these three, two32,98 were of lower quality, in part because exposures were self-reported after delivery and two59,98 were small (<750 births). In the 10 largest studies (>2000 births), 18,19,21,29,31,37,51,53,76,81,10 of the 11 effect estimates were <1.31. The overall median estimate of RR was 1.16 and 1.09 in larger and better quality studies. The meta-estimate (based on 12 studies) was 1.22 (Figure 3), and that for the seven studies18,21,29,33,60,72,76 of higher quality was 1.13 (95%CI 0.99-1.29).
Figure 3.
Risk of preterm delivery associated with standing at work for >4 hours per day during pregnancy (Forest plot ordered by study size)
Lifting
The relation between occupational lifting and pre-term delivery was examined in 17 studies,13,15,18,21,33,37,40,45,51-3,60,72,76,81,89,98 including eight prospective investigations. Studies differed substantially in their definition of exposure. Twenty-two effect estimates were reported, the median overall being 1.12. In only one of 22 estimates was the RR >2.0;98 this study was rated as more susceptible to confounding and was also relatively small (<500 births). In the 11 higher quality studies with >1000 births,13,18,21,37,40,45,53,60,72,76,89 the median value was 1.02 (IQR 0.90 to 1.30).
Physical workload
Thirty-three studies,19,21,23,25-6,29,31-2,34-5,37,41,43-4,47-8,50-2,57,60,69,71,77,80,82-6,90,94,97 including 12 of cohort design, investigated the link between physical workload and pre-term delivery and provided 35 risk estimates. Exposure was defined variously. For example, six studies25,32,35,37,41,57 used an occupational fatigue score proposed by Mamelle et al, comprising a combination of standing >1 hour/day, work on a machine, carrying loads >10 Kg, mental stress, and chemical or physical exposures at work; while other studies used a physical workload score, calculated as an estimated daily energy expenditure or by grouping self-estimates of physical exertion.
The median effect estimate was 1.20. In four of 35 estimates the RR was >2.0. These came from three studies of relatively small size (<800 births),50,51,83 two of which were classified as having higher potential for confounding.50,51 In the six largest studies (>3000 births),29,31,37,71,77,82 the highest risk estimate was 1.16, the median value being 1.10 (IQR 1.07-1.11). Self-reporting of a subjective exposure (e.g. ‘heavy’ workload) is more than usually susceptible to reporting bias, so ideally occupational history would be taken before pregnancy outcome. The 12 prospective studies 25,29,31,34,41,48,57,60,71,77,84,97 gave a median RR of 1.16; but this provides only a limited guide as seven of the 12 relevant estimates came from small studies (<650 births). The median RR for higher quality studies with >1000 births was 1.10.
Birthweight
Case definition
The 53 identified reports on low birthweight used three different approaches to define outcome: birthweight as a continuous measure, birthweight below a threshold (usually 2,500 grams), or small for gestational age (SGA) by a cut-point on an expected distribution (usually the 10th centile). Several papers presented results for several outcomes and where birthweight was adjusted for gestational age, risk estimates tended if anything to be lower, suggesting that associations with unadjusted birthweight partly reflected effects on gestation. This account therefore focuses on the 24 studies that provided information on occupational risks of SGA,16,19,21-2,28,31,33-4,42,45,54,56,60,64,66,70-2,75,77,79,84,88,91 though additional results (from 38 reports) for other measures of birthweight are presented in the supplementary tables.
Potential confounders
Major risk factors for intrauterine growth retardation in developed countries include smoking, small maternal stature, sub-optimal nutrition and low maternal weight gain; but among these, poor maternal weight gain could lie on the causal pathway between occupational exposures and SGA, while lower socioeconomic status is a proxy for poorer nutrition. Risk estimates were therefore classified as having higher potential for confounding if they failed to take account both of smoking and ≥1 of: socioeconomic status, maternal height, or pre-pregnancy weight.
Scope for meta-analysis
A meta-estimate of risk of SGA was calculated in relation to working hours (>40 hours/week vs. less), standing (>4 hours/day vs. less) and shift work (Yes vs No); but exposure definitions for lifting and physical workload were too heterogeneous to be combined.
Working hours
Fourteen studies16,19,21,28,45,54,56,60,71-2,75,79,84,88 (seven of cohort design), all but three of higher quality, considered weekly working hours and SGA, providing 18 estimates of effect. The median RR was 1.10. In only one of 18 estimates was the RR ≥ 2.0 – in a relatively small study (<1000 births) with higher potential for confounding.60 ‘High’ exposure mostly entailed working for ≥40 hours/week and in eight studies16,19,21,51,71-2,75,79,84 with the exposure that could be combined in meta-analysis the estimated RR was 1.04 (95%CI 0.94-1.16) overall, and 0.99 (95%CI 0.88-1.11) in six studies of higher quality. The estimated effect from this exposure continuing beyond the first trimester was below 1.0.
The median estimated RR for LBW (1.34), based on 10 estimates from eight reports,16,24,37,44,51,60,84,92 was somewhat higher than for SGA, but seven of the 10 estimates derived from smaller studies (<1000 births) and the three larger studies16,44,51 were deemed more susceptible than average to confounding. None of 10 estimated RRs was as much as doubled.
Supplementary Table S6 summarises the outcome in relation to birthweight measured continuously. All seven studies16,24,28,62,79,88,95 found a lower birthweight in women working longer (median 60 gms, range 32-84 gms). Most studies were small (four had <250 births), but in the two largest studies,79,88 both rated of better quality and both prospective, birth weights were on average about 45 gm lower in women with longer working hours.
Shift work
Eleven studies16,21-2,42,45,64,66,70,72,75,84 (eight of higher quality) reported on shift work and SGA. The median RR overall was 1.25 and in only one study (two of 18 estimates) was above 2.0. This study66 was small, had a higher potential for inflationary bias, and defined exposure not only in terms of shift work but also the presence of self-reported “physical or chemical hazards at work”. However, the median RR for larger higher quality studies was 1.0. The pooled estimate of risk was 1.01 (95%CI 0.92–1.10), and 0.98 (95%CI 0.90–1.08) when analysis was restricted to seven studies21-2,45,64,72,75,84 of higher quality.
The median RR for LBW (1.28) was somewhat higher than for SGA, but only one of nine estimates derived from a higher quality study with >1000 births (based on a national birth cohort in Denmark64). In this the RR was 1.01, in keeping with meta-analytic estimates. Supplementary table S6 also summarises the outcome in relation to birthweight measured continuously. There was a large span of results in relation to shift work, from an average loss of 438 gms at one extreme to a gain of 195 gms at the other, with a median estimated gain of 19 gms. Negative findings were particularly evident in one very small study (25 to 67 births) of lower quality;14 and in the three largest studies (1685 to >35,000 births)16,64,73 shift work was associated on average with a modest gain in birthweight.
Standing
Standing and SGA were analysed in 12 studies19,21-2,31,43,45,56,60,66,72,75,88 (five classed as higher quality) and including six of cohort design. The median RR from 17 estimates of effect was 1.00 (IQR 0.93 to 1.26) and only one moderately sized study, from Thailand, with higher than average potential for confounding, reported a RR as high as 2.0.60 The overall meta-estimate, assuming a cut-point of 4 hours/day, was 1.07 (95%CI 0.94-1.22), or 1.16 in sensitivity analysis, and 0.95 for exposures at this level continuing beyond the first trimester. Four estimates came from higher quality studies analysing >1,000 births,21,22,66,88 with a median of 1.06 (IQR 0.98-1.24).
Thirteen estimates of RR for LBW were available, from 9 studies,24,37,39,51,59,60,62,66,85 the median being 1.13, with no RR ≥2.0; and there were 11 estimates of birthweight analysed continuously in women who stood at work vs. not (eight studies17,24,29,59,62,65,88,94), ranging from an average weight loss of 49 gms to a weight gain of 20 gms).
Lifting
Lifting was considered in seven studies of SGA,13,21,33,45,60,72,75 with a median RR overall of 1.03 (IQR 0.73-1.15) and a similar value for the four studies of higher quality. All 11 estimates of effect were <1.2. Seven studies13,24,37,51,60,62,89 provided evidence on LBW (supplementary Table S5), but only one of nine estimated RRs was ≥2.0 (a cross-sectional study in which exposures were self-reported after delivery62). Only three studies20,24,62 looked at birthweight assessed continuously, with mixed results (Table S6), ranging from a mean reduction in birthweight of 44 gms to a mean gain of 18.9 gms in women with lifting duties.
Physical workload
SGA and physical workload were considered in 13 investigations,19,21-2,31,34,43,56,60,71,77,84,88,91 including eight of cohort design. Exposures were defined diversely. The median RR was 1.00 (IQR 0.82-1.38) (based on 14 estimates) and 0.88 in higher quality studies. Two studies43,56 reported RRs ≥2.0; both were small (about 500 births) and of lower quality.
A similar median estimate of effect was found for LBW (1.13), with RRs >2.0 in two studies, both with <800 births.26,84 Eight studies 20,24,26,29,34,48,58,88 provided 14 estimates of continuously assessed birthweight, with mixed results – a median weight loss on average of 59 gms, but ranging from an average loss of 216 gms to an average weight gain of 183 gms.
Gestational hypertension and pre-eclampsia
Case definition
Studies sub-classified pregnancy-induced hypertension (PIH) in the standard way, as: (1) gestational hypertension (raised blood pressure in a previously normotensive woman after the 20th week of gestation, which resolves after delivery); or (2) pre-eclampsia (gestational hypertension with proteinuria and oedema). However, variation existed in the level of blood pressure and degree of proteinuria underlying case definitions.
Potential confounders
Among many reported risk factors for pre-eclampsia, we considered only obesity and primiparity to be both common and to carry substantial RRs. Risk estimates were classified as having higher potential for confounding if they failed to take account of both of these variables.
Scope for meta-analysis
Because of potentially important differences in outcome definition from one study to another and a small pool of studies, we did not attempt meta-analysis for occupational associations with gestational hypertension or pre-eclampsia.
Associations with occupational activities
Eleven investigations27,30,36,42,49,50,56,61,74,78-9 (including three cohort studies) were identified concerning gestational hypertension, pre-eclampsia and occupational activity, providing 31 estimates of effect across the five categories of work exposure. However, data were sparse when individual exposure-outcome combinations were analysed separately. For example, only two estimates of effect were found respectively for standing, shift work, and lifting in relation to gestational hypertension, and only two respectively for working hours, and shift work in relation to pre-eclampsia. It may be seen, however, that median RRs, where feasible to estimate, were low (RR <1.15) and that only three studies50,56,78 reported RRs ≥2.0. In the study by Haelterman et al78 the exposure associated with a RR of 2.9 was standing on the spot for ≥1 hour at a time, but no other study assessed standing in this way. The other two studies focussed on self-reported physical activity. All three, however, were retrospective in design and rated as of lower methodological quality, two of them were also small (<600 births)50,56 and one was incompletelyreported.50
DISCUSSION
This study updates an earlier review by providing an extra six years of observation. The number of available risk estimates increased over this time by 30-50%, depending on outcome, allowing additional meta-analyses (on SGA and separately for late pregnancy) that could not be justified a relatively short while ago. Twelve of 30 new studies involved >4000 deliveries, one with >350,000 births,78 there were nine new cohort studies (in 12 reports), and eight new reports66-68,72,73,75,76,91 furnished risk estimates separately for different pregnancy trimesters, adding to the six20,24,45,46,48,54 previously identified; 40% of risk estimates were linked with a specified trimester, a much improved situation. We summarise the current evidence now as substantial for pre-term delivery, reasonably large for SGA (especially when other measures of birthweight are also considered), but still small for gestational hypertension/pre-eclampsia.
Our search was restricted to publications with abstracts in English, did not extend to the ‘grey’ literature, and may therefore have not been perfectly comprehensive. However, it seems unlikely that many important papers will have been missed. On the other hand, the consistent finding that risk estimates were lower in the largest and better studies, with outliers confined to small studies, suggests that publication bias may be inflating estimates of risk.
Strengths of the evidence base, across most studies, include high response rates and ascertainment of outcomes independent of exposures (from objective sources such as birth records). Thus, response bias and non-differential misclassification of health endpoints is unlikely to have much affected findings. On the other hand, non-differential misclassification could still arise for exposures that are hard to characterise, with bias to the null.
Another continuing limitation in available evidence relates to the heterogeneity of exposure definitions, especially for lifting and physical workload. The challenge is not inconsiderable: lifting tasks, for example, may be classified according to their average daily frequency, duration, load and posture, and the optimum choice of metrics is not obvious; but there has been little move towards standardisation over time. This limitation impedes causal inference and risk communication, by precluding meta-regression and full assessment of exposure-response relationships.
One aspect of exposure that may be important is its timing during pregnancy. However, studies that presented risk estimates separately for different trimesters did not point to major differences, and in meta-analysis risks of pre-term delivery and SGA from long working hours, standing and shift work in the second and third trimesters were not noticeably higher.
As previously, we have highlighted those studies considered most susceptible to confounding and inflationary bias (which may arise particularly if workers who have suffered an adverse pregnancy event relatively over-report exposures they perceive as hazardous). Meta-estimated RRs were somewhat lower in sensitivity analyses which excluded such studies, as were summary risk estimates for larger better quality studies, and we judge these estimates to be more reliable than those overall.
Most reports emanated from Europe and North America, but findings from developing countries (16 studies, 66 effect estimates) were broadly similar to those from industrialised economies.
Current balance of evidence
Given the above strengths and limitations, we assess the balance of evidence as follows:
For pre-term delivery findings across a considerable evidence base were generally consistent and effectively rule out large effect sizes (RR≥2.0). Well powered better studies were less positive than smaller lower quality studies. Pooled estimates of risk where available pointed at most to only modest effects – e.g. excess risks of 2% to 18% with analysis restricted to higher quality reports.
For SGA the position is similar. Moreover, most meta-estimates, including those from higher quality and larger studies, were close to the null value. Studies on LBW provided somewhat higher effect estimates, but these were fewer in number and lower in quality. Findings on birthweight, similarly, were reported in relatively few studies of limited quality and were mixed in their findings, but again pointing to a limited impact on fetal growth.
For pre-eclampsia and gestational hypertension the evidence base has barely grown since 2005 and remains too limited to draw firm conclusions. Nonetheless, most estimates pointed to small or null effects.
Although there have been many narrative reviews on work and pregnancy outcomes, few have been systematic and produced meta-estimates of risk. In comparison, however, our earlier analysis11 estimated somewhat higher RRs for pre-term delivery in relation to working hours, shift work and standing, with pooled RRs of 1.31, 1.28 and 1.20 overall, and 1.20, 1.26 and 1.26 respectively in the subsets of studies of higher methodological quality. Similarly, for pre-term delivery, Mozurkewich et al (2000)99 estimated an RR of 1.26 for prolonged standing and 1.24 for shift and night work.
Implications
Findings to date seem broadly reassuring. Small levels of excess risk may exist, but it is also possible (especially given the smaller estimates from bigger and better studies, and their shrinkage over time, as more data have accumulated), that much or all of these effects are explained by a combination of chance, bias and imperfectly controlled confounding. However, a degree of residual uncertainty will always surround estimation of risks at lower levels and information on risks at extremes of exposure is very limited.
The balance of evidence is against a strong effect of the reviewed activities on the reviewed pregnancy outcomes. At the same time, for none of the exposures examined was there any indication of important beneficial effects. Moreover, given the clinical importance, say, of pre-term delivery, a RR of 1.18 (the meta-estimate in better quality studies for working >40 hrs/week) might equate to 1.2 additional cases (95%CI 0.3 to 2.2) per 100 deliveries to women with that exposure, assuming a background prevalence of singleton live pre-term delivery of 6.7%100 and, if truly present, would be important to avoid. Given residual uncertainties in the evidence base and the apparent absence of benefits, there may be a precautionary case for advising women against long working hours (e.g. >40 hours/week), prolonged standing (e.g. >4 hours/day), and heavy physical work, particularly late in pregnancy, at a time in any case when fatigue limits the capacity for high demand duties. This case is not strongly driven by evidence of harm, however, and care should be taken to avoid causing undue anxiety among patients and their employers.
The need for further research is most evident for pre-eclampsia and hypertension, where studies are few, but somewhat less pressing for pre-term delivery and SGA since the database has grown substantially larger over the past few years. A relatively neglected area, deserving of more attention however, concerns the impact of work activities on intra-uterine growth trajectory and birth anthropometrics,72 given the growing evidence that poorer health in adulthood is predicted not only by SGA and birthweight but also other markers like small head circumference, reduced abdominal girth, thinness at birth, shortness at birth and low birthweight relative to placental weight.101
Supplementary Material
What this paper adds.
In theory, physical activities at work could adversely affect outcomes of pregnancy. However, an earlier systematic review indicated that long working hours, shift work, prolonged standing, heavy lifting and high physical workload have limited impact on risks of pre-term delivery and low birth weight/SGA.
This review adds 33 more reports, increases the available number of effect estimates by some 30-50%, and allows additional meta-estimates of risk.
For pre-term delivery and SGA the substantially enlarged evidence base provides greater confidence that any risks from these activities are, at most, small.
For pre-eclampsia and gestational hypertension the available evidence remains limited.
Acknowledgements
We thank Sian Williams, Penny Peel, Emily Young and the members of the HWDU Pregnancy Guidelines Development Group for their support and advice and Sue Curtis for helping to prepare this manuscript.
Funding: This work was commissioned by the Health and Work Development Unit of the Royal College of Physicians, London.
Footnotes
Competing interests: None
Contributions: KTP and MB formulated the study questions, designed the review and searches, in part undertook the quality assessment of papers, analysed the data, and prepared the first draft of this review. ECH and CL conducted the searches (which ECH helped to design), shared in the critical assessment of papers, and contributed to the writing and interpretation of this review. JPB critically appraised the review’s methods and content and contributed to the writing of the final paper, including the interpretation of findings.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Occupational and Environmental Medicine editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://oem.bmjjournals.com/misc/ifora/licenceform.shtml).
References
- 1.Labour Force Survey Quarterly Household Dataset. Apr-Jun. 2010.
- 2.Duffield M. Trends in female employment 2002. Labour Market Trends; Nov, 2002. pp. 605–16. [Google Scholar]
- 3.Department of Trade and Industry [accessed 20/6/12];Work and Parents: Competitiveness and Choice. Research and Analysis. 2000 Nov; (as cited at: http://tommys.org/
- 4.Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD000180.pub2. Art. No.: CD000180. DOI: 10.1002/14651858.CD000180.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chasan-Taber L, Evenson KR, Sternfeld B, et al. Assessment of recreational physical activity during pregnancy in epidemiologic studies of birthweight and length of gestation: methodologic aspects. Women Health. 2007;45:85–107. doi: 10.1300/J013v45n04_05. [DOI] [PubMed] [Google Scholar]
- 6.Schlussel MM, de Souza EB, Reichenheim ME, et al. Physical activity during pregnancy and maternal-child health outcomes: a systematic literature review. Cad Saude Publica. 2008;24(Suppl 4):531–44. doi: 10.1590/s0102-311x2008001600006. [DOI] [PubMed] [Google Scholar]
- 7.Lagerros YT. Physical activity—the more we measure, the more we know how to measure. Eur J Epidemiol. 2009;24:119–22. doi: 10.1007/s10654-009-9316-0. [DOI] [PubMed] [Google Scholar]
- 8.Royal College of Obstetricians and Gynaecologists [accessed 20/6/12];Exercise in pregnancy. 2006 Jan; Statement No 4. http://www.rcog.org.uk/files/rcog-corp/Statement4-14022011.pdf.
- 9.Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynaecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37:6–12. doi: 10.1136/bjsm.37.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonzini M, Coggon D, Palmer KT. Risk of prematurity, low birth weight, and pre-eclampsia in relation to working hours and physical activities: A systematic review. Occup Environ Med. 2007;64:228–43. doi: 10.1136/oem.2006.026872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonzini M, Palmer KT, Coggon D, et al. Shift work and pregnancy outcomes: a systematic review with meta-analysis of currently available epidemiological studies. BJOG. 2011;118:1429–37. doi: 10.1111/j.1471-0528.2011.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.RCP-NHS Plus . A national guideline. RCP; 2009. Physical and shift work in pregnancy: Occupational aspects of management. ISBN 978-1-86016-353-1. [Google Scholar]
- 13.Ahlborg GJ, Jr, Bodin L, Hogstedt C. Heavy lifting during pregnancy - a hazard to the fetus? A prospective study. Int J Epidemiol. 1990;19:90–7. doi: 10.1093/ije/19.1.90. [DOI] [PubMed] [Google Scholar]
- 14.Axelsson G, Rylander R, Molin I. Outcome of pregnancy in relation to irregular and inconvenient work schedules. Br J Ind Med. 1989;46:393–8. doi: 10.1136/oem.46.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkowitz GS, Kelsey JL, Holford TR, et al. Physical activity and the risk of spontaneous preterm delivery. J Reprod Med. 1983;28:581–8. [PubMed] [Google Scholar]
- 16.Bodin L, Axelsson G, Ahlborg G., Jr The association of shift work and nitrous oxide exposure in pregnancy with birth weight and gestational age. Epidemiology. 1999;10:429–36. doi: 10.1097/00001648-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Brink-Henriksen T, Hedegaard M, Secher NJ. Standing and walking at work and birthweight. Acta Obstet Gynecol Scand. 1995;74:509–16. doi: 10.3109/00016349509024380. [DOI] [PubMed] [Google Scholar]
- 18.Brink-Henriksen T, Hedegaard M, Secher NJ, et al. Standing at work and preterm delivery. Br J Obstet Gynaecol. 1995;102:198–206. doi: 10.1111/j.1471-0528.1995.tb09094.x. [DOI] [PubMed] [Google Scholar]
- 19.Cerón-Mireles P, Harlow SD, Sánchez-Carrillo CI. The risk of prematurity and small-for-gestational-age birth in Mexico City: the effects of working conditions and antenatal leave. Am J Public Health. 1996;86:825–31. doi: 10.2105/ajph.86.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Florack EIM, Pellegrino AEMC, Zielhuis GA, et al. Influence of occupational physical activity on pregnancy duration and birthweight. Scand J Work Environ Health. 1995;21:199–207. doi: 10.5271/sjweh.28. [DOI] [PubMed] [Google Scholar]
- 21.Fortier I, Marcoux S, Brisson J. Maternal work during pregnancy and the risks of delivering a small-for-gestational-age or preterm infant. Scand J Work Environ Health. 1995;21:412–8. doi: 10.5271/sjweh.56. [DOI] [PubMed] [Google Scholar]
- 22.Hanke W, Kalinka J, Makowiec-Dabrowska T, et al. Heavy physical work during pregnancy - a risk factor for small-for-gestational-age babies in Poland. Am J Ind Med. 1999;36:200–5. doi: 10.1002/(sici)1097-0274(199907)36:1<200::aid-ajim28>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 23.Hartikainen-Sorri AL, Sorri M. Occupational and socio-medical factors in preterm birth. Obstet Gynecol. 1989;74:13–6. [PubMed] [Google Scholar]
- 24.Hatch M, Ji BT, Shu XO, et al. Do standing, lifting, climbing, or long hours of work during pregnancy have an effect on fetal growth? Epidemiology. 1997;8:530–6. doi: 10.1097/00001648-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Hickey CA, Cliver SP, Mulvihill FX, et al. Employment-related stress and preterm delivery: a contextual examination. Public Health Rep. 1995;110:410–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Homer CJ, Beresford SAA, James SA, et al. Work-related physical exertion and risk of preterm, low birthweight delivery. Paediatr Perinat Epidemiol. 1990;4:161–74. doi: 10.1111/j.1365-3016.1990.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 27.Irwin DE, Savitz DA, St André KA, et al. Study of occupational risk factors for pregnancy-induced hypertension among active duty enlisted Navy personnel. Am J Ind Med. 1994;25:349–59. doi: 10.1002/ajim.4700250305. [DOI] [PubMed] [Google Scholar]
- 28.Klebanoff MA, Shiono PH, Rhoads GG. Outcomes of pregnancy in a national sample of resident physicians. N Engl J Med. 1990;323:1040–5. doi: 10.1056/NEJM199010113231506. [DOI] [PubMed] [Google Scholar]
- 29.Klebanoff MA, Shiono PH, Carey JC. The effect of physical activity during pregnancy on preterm delivery and birth weight. Am J Obstet Gynecol. 1990;163:1450–6. doi: 10.1016/0002-9378(90)90604-6. [DOI] [PubMed] [Google Scholar]
- 30.Landsbergis PA, Hatch MC. Psychosocial work stress and pregnancy-induced hypertension. Epidemiology. 1996;7:346–51. doi: 10.1097/00001648-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Launer LJ, Villar J, Kestler E, et al. The effect of maternal work on fetal growth and duration of pregnancy: a prospective study. Br J Obstet Gynaecol. 1990;97:62–70. doi: 10.1111/j.1471-0528.1990.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 32.Luke B, Mamelle N, Keith L, et al. The association between occupational factors and preterm birth: a United States nurses’ study. Am J Obstet Gynecol. 1995;173:849–62. doi: 10.1016/0002-9378(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 33.Magann EF, Evans SF, Chauhan ST, et al. The effects of standing, lifting and noise exposure on pre-term birth, growth restriction, and perinatal death in healthy low-risk working military women. J Matern Fetal Neonatal Med. 2005;18:155–62. doi: 10.1080/14767050500224810. [DOI] [PubMed] [Google Scholar]
- 34.Magann EF, Evans SF, Newnham JP. Employment, exertion, and pregnancy outcome: assessment by kilocalories expended each day. Am J Obstet Gynecol. 1996;175:182–7. doi: 10.1016/s0002-9378(96)70272-7. [DOI] [PubMed] [Google Scholar]
- 35.Mamelle N, Laumon B, Lazar P. Prematurity and occupational activity during pregnancy. Am J Epidemiol. 1984;119:309–22. doi: 10.1093/oxfordjournals.aje.a113750. [DOI] [PubMed] [Google Scholar]
- 36.Marcoux S, Bérubé S, Brisson C, et al. Job strain and pregnancy-induced hypertension. Epidemiology. 1999;10:376–82. doi: 10.1097/00001648-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 37.McDonald AD, McDonald JC, Armstrong B, et al. Prematurity and work in pregnancy. Br J Ind Med. 1988;45:56–62. doi: 10.1136/oem.45.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong BG, Nolin AD, McDonald AD. Work in pregnancy and birth weight for gestational age. Br J Ind Med. 1989;46:196–9. doi: 10.1136/oem.46.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer BA, Daling JR. Activity level of mother’s usual occupation and low infant birth weight. J Occup Med. 1985;27:841–7. doi: 10.1097/00043764-198511000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Misra DP, Strobino DM, Stashinko EE, et al. Effects of physical activity on preterm birth. Am J Epidemiol. 1998;147:628–35. doi: 10.1093/oxfordjournals.aje.a009503. [DOI] [PubMed] [Google Scholar]
- 41.Newman RB, Goldenberg MD, Moawad AH, et al. Occupational fatigue and preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;184:438–46. doi: 10.1067/mob.2001.110312. [DOI] [PubMed] [Google Scholar]
- 42.Nurminen T. Shift work, fetal development and course of pregnancy. Scand J Work Environ Health. 1989;15:395–403. doi: 10.5271/sjweh.1833. [DOI] [PubMed] [Google Scholar]
- 43.Nurminen T, Lusa S, Ilmarinen J, et al. Physical work load, fetal development and course of pregnancy. Scand J Work Environ Health. 1989;15:404–14. doi: 10.5271/sjweh.1832. [DOI] [PubMed] [Google Scholar]
- 44.Peoples-Sheps MD, Siegel E, Suchindran CM, et al. Characteristics of maternal employment during pregnancy: effects on low birthweight. Am J Public Health. 1991;81:1007–12. doi: 10.2105/ajph.81.8.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pompeii LA, Savitz DA, Evenson KR, et al. Physical exertion at work and the risk of preterm delivery and small-for-gestational age birth. Obstet Gynecol. 2005;106:1279–88. doi: 10.1097/01.AOG.0000189080.76998.f8. [DOI] [PubMed] [Google Scholar]
- 46.Rabkin CS, Anderson HR, Bland JM, et al. Maternal activity and birth weight: a prospective population-based study. Am J Epidemiol. 1990;131:522–31. doi: 10.1093/oxfordjournals.aje.a115527. [DOI] [PubMed] [Google Scholar]
- 47.Ramirez G, Grimes RM, Annegers JF, et al. Occupational physical activity and other risk factors for preterm birth among US army primigravidas. Am J Public Health. 1990;80:728–30. doi: 10.2105/ajph.80.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao S, Kanade A, Margetts BM, et al. Maternal activity in relation to birth size in rural India. The Pune maternal nutrition study. Eur J Clin Nutr. 2003;57:531–42. doi: 10.1038/sj.ejcn.1601582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saftlas AF, Logsden-Sackett N, Wang W, et al. Work, leisure-time physical activity, and risk of preeclampsia and gestational hypertension. Am J Epidemiol. 2004;160:758–65. doi: 10.1093/aje/kwh277. [DOI] [PubMed] [Google Scholar]
- 50.Saurel-Cubizolles MJ, Kaminski M, Llado-Arkhipoff J, et al. Pregnancy and its outcome among hospital personnel according to occupation and working conditions. J Epidemiol Community Health. 1985;39:129–34. doi: 10.1136/jech.39.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saurel-Cubizolles MJ, Kaminski M. Pregnant women’s working conditions and their changes during pregnancy: a national study in France. Br J Ind Med. 1987;44:236–43. doi: 10.1136/oem.44.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saurel-Cubizolles MJ, Subtil D, Kaminski M. Is preterm delivery still related to physical working conditions in pregnancy? J Epidemiol Community Health. 1991;45:29–34. doi: 10.1136/jech.45.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saurel-Cubizolles MJ, Zeitlin J, Lelong N, et al. Employment, working conditions, and preterm birth: results from the Europop case-control survey. J Epidemiol Community Health. 2004;58:395–401. doi: 10.1136/jech.2003.008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savitz DA, Olshan AF, Gallagher K. Maternal occupation and pregnancy outcome. Epidemiology. 1996;7:269–74. doi: 10.1097/00001648-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Schramm WF, Stockbauer JW, Hoffman HJ. Exercise, employment, other daily activities, and adverse pregnancy outcomes. Am J Epidemiol. 1996;143:211–8. doi: 10.1093/oxfordjournals.aje.a008731. [DOI] [PubMed] [Google Scholar]
- 56.Spinillo A, Capuzzo E, Colonna L, et al. The effect of work activity in pregnancy on the risk of severe preeclampsia. Aust NZ J Obstet Gynaecol. 1995;35:380–5. doi: 10.1111/j.1479-828x.1995.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 57.Stinson JC, Lee KA. Premature labor and birth: influence of rank and perception of fatigue in active duty military women. Mil Med. 2003;168:385–90. [PubMed] [Google Scholar]
- 58.Tafari N, Naeye RL, Gobezie A. Effects of maternal undernutrition and heavy physical work during pregnancy on birth weight. Br J Obstet Gynaecol. 1980;87:222–6. doi: 10.1111/j.1471-0528.1980.tb04523.x. [DOI] [PubMed] [Google Scholar]
- 59.Teitelman AM, Welch LS, Hellenbrand KG, et al. Effect of maternal work activity on preterm birth and low birth weight. Am J Epidemiol. 1990;131:104–13. doi: 10.1093/oxfordjournals.aje.a115463. [DOI] [PubMed] [Google Scholar]
- 60.Tuntiseranee P, Geater A, Chongsuvivatwong V, et al. The effect of heavy maternal workload on fetal growth retardation and preterm delivery. J Occup Environ Med. 1998;40:1013–21. doi: 10.1097/00043764-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 61.Wergeland E, Strand K. Working conditions and prevalence of pre-eclampsia, Norway 1989. Int J Gynaecol Obstet. 1997;58:189–96. doi: 10.1016/s0020-7292(97)00083-0. [DOI] [PubMed] [Google Scholar]
- 62.Wergeland E, Strand K, Børdahl PE. Strenuous working conditions and birthweight, Norway 1989. Acta Obstet Gynecol Scand. 1998;77:263–71. [PubMed] [Google Scholar]
- 63.Xu X, Ding M, Li B, et al. Association of rotating shiftwork with preterm births and low birth weight among never smoking women textile workers in China. Occup Environ Med. 1994;51:470–4. doi: 10.1136/oem.51.7.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu JL, Hjollund NH, Olsen J. Shift work, duration of pregnancy, and birth weight: The National Birth Cohort in Denmark. Am J Obstet Gynecol. 2004;191:285–91. doi: 10.1016/j.ajog.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Zuckerman BS, Frank DA, Hingson R, et al. Impact of maternal work outside the home during pregnancy on neonatal outcome. Pediatrics. 1986;77:459–64. [PubMed] [Google Scholar]
- 66.Abeysena C, Jayawardana P, Seneviratne RDA. Maternal sleep deprivation is a risk factor for small for gestational age: a cohort study. Aust NZ J Obs Gynaecol. 2009;49:382–7. doi: 10.1111/j.1479-828X.2009.01010.x. [DOI] [PubMed] [Google Scholar]
- 67.Abeysena C, Jayawardana P, Seneviratne RA. Effect of psychosocial stress and physical activity on low birthweight: a cohort study. J Obs & Gynae Research. 2010;36:296–303. doi: 10.1111/j.1447-0756.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- 68.Abeysena C, Jayawardana P, Seneviratne RA. Effect of psychosocial stress and physical activity on preterm birth: a cohort study. J Obs Gynae Research. 2010;36:260–7. doi: 10.1111/j.1447-0756.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 69.Al-Dabbagh SA, Al-Taee WY. Risk factors for pre-term birth in Iraq: A case-control study. BMC Pregnancy and Childbirth. 2006;6:13. doi: 10.1186/1471-2393-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arafa MA, Amine T, Abdel FM. Association of maternal work with adverse perinatal outcome. Canadian Journal of Public Health Revue Canadienne de Sante Publique. 2007;98:217–21. doi: 10.1007/BF03403716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bell JF, Zimmerman FJ, Diehr PK. Maternal work and birth outcome disparities. Matern Child Health J. 2008;12:415–26. doi: 10.1007/s10995-007-0264-6. [DOI] [PubMed] [Google Scholar]
- 72.Bonzini M, Coggon D, Godfrey K, et al. Occupational physical activities, working hours and outcome of pregnancy: findings from the Southampton Women’s Survey. Occup Environ Med. 2009;66:685–90. doi: 10.1136/oem.2008.043935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Both MI, Overvest MA, Wildhagen MF, et al. The association of daily physical activity and birth outcome: A population-based cohort study. Europ J Epidemiol. 2010;25:421–9. doi: 10.1007/s10654-010-9458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang PJ, Chu LC, Hsieh WS, et al. Working hours and risk of gestational hypertension and pre-eclampsia. Occup Med. 2010;60:66–71. doi: 10.1093/occmed/kqp119. [DOI] [PubMed] [Google Scholar]
- 75.Croteau A, Marcoux S, Brisson C. Work activity in pregnancy, preventive measures, and the risk of delivering a small-for-gestational-age infant. Am J Pub Health. 2006;96:846–55. doi: 10.2105/AJPH.2004.058552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Croteau A, Marcoux S, Brisson C. Work activity in pregnancy, preventive measures and the risk of preterm delivery. Am J Epidemiol. 2007;166(8):951–65. doi: 10.1093/aje/kwm171. [DOI] [PubMed] [Google Scholar]
- 77.Gisselmann MD, Hemstrom O. The contribution of maternal working conditions to socio-economic inequalities in birth outcome. Soc Sci Med. 2008;66:1297–309. doi: 10.1016/j.socscimed.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 78.Haelterman E, Marcoux S, Croteau A, et al. Population-based study on occupational risk factors for preeclampsia and gestational hypertension. Scand J Work Environ Health. 2007;33:304–17. doi: 10.5271/sjweh.1147. [DOI] [PubMed] [Google Scholar]
- 79.Jansen PW, Tiemeier H, Verhulst FC, et al. Employment status and the risk of pregnancy complications: the Generation R Study. Occup Environ Med. 2010;67:387–94. doi: 10.1136/oem.2009.046300. [DOI] [PubMed] [Google Scholar]
- 80.Jurewicz J, Hanke W, Makowiec-Dabrowska T, et al. Exposure to pesticides and heavy work in greenhouses during pregnancy: does it affect birth weight? Int Arch Occup Environ Health. 2005;78:418–26. doi: 10.1007/s00420-005-0614-x. [DOI] [PubMed] [Google Scholar]
- 81.Lawson CC, Whelan EA, Hibert EN, et al. Occupational factors and risk of preterm birth in nurses. Am J Obstet Gynecol. 2009;200:51, e1–8. doi: 10.1016/j.ajog.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meyer JD, Warren N, Reisine S. Job control, substantive complexity, and risk for low birth weight and preterm delivery: an analysis from a state birth registry. Am J Ind Med. 2007;50:664–75. doi: 10.1002/ajim.20496. [DOI] [PubMed] [Google Scholar]
- 83.Nelson K, Lohsoonthorn V, Williams MA. Preterm delivery risk in relation to maternal occupational and leisure time physical activity among Thai women. Asian Biomedicine. 2009;3:267–77. [PMC free article] [PubMed] [Google Scholar]
- 84.Niedhammer I, O’Mahony D, Daly S, et al. Occupational predictors of pregnancy outcomes in Irish working women in the Lifeways cohort. BJOG. 2009;116:943–52. doi: 10.1111/j.1471-0528.2009.02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Omokhodion FO, Onadeko MO, Roberts OA, et al. Paid work, domestic work, and other determinants of pregnancy outcome in Ibadan, southwest Nigeria. Int J Gynaecol Obstet. 2010;111:165–70. doi: 10.1016/j.ijgo.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 86.Rodrigues T, Barros H. Maternal unemployment: an indicator of spontaneous preterm delivery risk. European J Epidemiol. 2008;23:689–93. doi: 10.1007/s10654-008-9283-x. [DOI] [PubMed] [Google Scholar]
- 87.Shirangi A, Fritschi L, Holman CD. Associations of unscavenged anesthetic gases and long working hours with preterm delivery in female veterinarians. Obstet Gynecol. 2009;113:1008–17. doi: 10.1097/AOG.0b013e31819fe996. [DOI] [PubMed] [Google Scholar]
- 88.Vrijkotte TG, van der Wal MF, van Eijsden M, et al. First-trimester working conditions and birthweight: a prospective cohort study. Am J Pub Health. 2009;99:1409–16. doi: 10.2105/AJPH.2008.138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burdorf A, Brand T, Jaddoe VW, et al. The effects of work-related maternal risk factors on time to pregnancy, preterm birth and birth weight: the Generation R Study. Occup Environ Med. 2011;68:197–204. doi: 10.1136/oem.2009.046516. [DOI] [PubMed] [Google Scholar]
- 90.Di Renzo GC, Giardina I, Rosati A, et al. Maternal risk factors for preterm birth: a country-based population analysis. Eur J Obstet Gynecol Reprod Biol. 2011;159:342–6. doi: 10.1016/j.ejogrb.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 91.Gollenberg AL, Pekow P, Bertone-Johnson ER, et al. Physical activity and risk of small-for-gestational-age birth among predominantly Puerto Rican women. Matern Child Health J. 2011;15:49–59. doi: 10.1007/s10995-009-0563-1. [DOI] [PubMed] [Google Scholar]
- 92.Herdt-Losavio ML, Lin S, Druschel CM, et al. A nested case-control study of low birthweight among cosmetologists. Int Arch Occup Environ Health. 2011;84:601–8. doi: 10.1007/s00420-010-0585-4. [DOI] [PubMed] [Google Scholar]
- 93.Lin YC, Chen MH, Hsieh CJ, et al. Effect of rotating shift work on childbearing and birth weight: a study of women working in a semiconductor manufacturing factory. World J Ped. 2011;7:129–35. doi: 10.1007/s12519-011-0265-9. [DOI] [PubMed] [Google Scholar]
- 94.Ha E, Cho SI, Park H, et al. Does standing at work during pregnancy result in reduced infant birth weight? J Occup Environ Med. 2002;44:81521. doi: 10.1097/00043764-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 95.Lima M, Ismail S, Ashworth A, et al. Influence of heavy agricultural work during pregnancy on birthweight in Northeast Brazil. Int J Epidemiol. 1999;28:469–74. doi: 10.1093/ije/28.3.469. [DOI] [PubMed] [Google Scholar]
- 96.Mamelle N, Munoz F. Occupational working conditions and preterm birth: a reliable scoring system. Am J Epidemiol. 1987;126:150–2. doi: 10.1093/oxfordjournals.aje.a114649. [DOI] [PubMed] [Google Scholar]
- 97.Nguyen N, Savitz DA, Thorp JM. Risk factors for preterm birth in Vietnam. Int J Gynaecol Obstet. 2004;86:70–8. doi: 10.1016/j.ijgo.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Ritsmitchai S, Geater AF, Chonsuviwatvong V. Prolonged standing and physical exertion at work during pregnancy increases the risk of preterm birth for Thai mothers. J Occupational Health. 1997;39:217–22. [Google Scholar]
- 99.Mozurkewich EL, Luke B, Avni M, et al. Working conditions and adverse pregnancy outcome: A meta-analysis. Obstet Gynecol. 2000;95:623–34. doi: 10.1016/s0029-7844(99)00598-0. [DOI] [PubMed] [Google Scholar]
- 100.Hospital Episode Statistics 2010. Table 22: Complications recorded in the pregnancy episode, 2009-10, NHS Hospitals, England; Table 28: Singleton and multiple deliveries by birth weight and birth status, 2009-10, NHS Hospitals, England.
- 101.Barker DJ, Osmond C, Simmonds SJ, et al. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993;306:422–6. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.