Abstract
The use of quinolone for treatment of rickettsial diseases remains controversial. Recent clinical studies suggest that quinolone is not as effective as others in patients with rickettsial diseases including scrub typhus, although the mechanism is not well understood. In this study, we evaluated the mutation in gyrA associated with quinolone resistance. We prospectively enrolled scrub typhus patients, collected blood samples and clinical data from October, 2010 to November, 2011. Among the 21 patients enrolled, one initially received ciprofloxacin for 3 days but was switched to doxycycline due to clinical deterioration. We obtained the gyrA gene of Orientia tsutsugamushi from 21 samples (20 Boryong strain, 1 Kato strain) and sequenced the quinolone resistance-determining region. All of 21 samples had the Ser83Leu mutation in the gyrA gene, which is known to be associated with quinolone resistance. This suggests that quinolones may be avoided for the treatment of serious scrub typhus.
Keywords: Scrub Typhus, Quinolone Resistance, Genotype, Rickettsia
INTRODUCTION
Scrub typhus is a mite-borne rickettsial disease caused by Orientia tsutsugamushi endemic or reemerging in eastern and southern Asia, northern Australia, and islands of the western Pacific and Indian Ocean (1). It is estimated that there are one million new scrub typhus cases each year with one billion people at risk of infection (2). Mortality rates can be as high as 30% if left untreated. However, administration of appropriate antibiotics, including chloramphenicol, tetracyclines, macrolides and rifampicin, reduces the mortality rate to less than 5% (3-7).
Quinolone has been used for the treatment of various infectious diseases. It is a popular choice in outpatient settings due to excellent oral bioavailability, the low cost of generic drugs in developing countries, and good efficacy against intracellular pathogens. This has led some investigators to evaluate the efficacy of quinolone treatment in rickettsial diseases. However, clinical data has suggested that patients diagnosed with rickettsial disorders, such as scrub typhus (8, 9) and Mediterranean spotted fever (10), respond less favorably when treated with quinolone. Quinolone resistance has been associated with increased prevalence of the Ser83Leu mutation in the quinolone resistance-determining region (QRDR) of gyrA in the Kato strain of O. tsutsugamushi from Laos (11). It remains to be determined whether this mutation is prevalent in the Boryong strain of O. tsutsugamushi, which is the most common strain in Korea. Thus in this study, we investigated the prevalence of gyrA mutations associated with quinolone resistance in Korean patients with scrub typhus.
MATERIALS AND METHODS
Subjects
We prospectively enrolled patients > 18 yr of age who were admitted to the 1,000-bed Chonnam National University Hospital (Gwangju, Korea) from October, 2010 to November, 2011 and diagnosed with scrub typhus. Scrub typhus was diagnosed based on typical clinical manifestations and serological testing with an antibody titer of ≥ 1:80 in a single serum sample or by a fourfold or greater increase in the follow-up titration. Serological testing was performed using a passive hemagglutination assay (PHA) using the Genedia Tsutsu PHA II kit (GreenCross SangA, Yongin, Korea). Clinical data for each patient were obtained prospectively which included gender, age, comorbidity, symptoms, findings on physical examination, complications, modified acute physiology and chronic health evaluation score (APACHE II; measured within 24 hr of initial admission), treatment agents and outcomes.
PCR and DNA sequencing
Genomic DNA for nested PCR was extracted from blood buffy coats using a QIAamp DNA Mini Kit (Qiagen Korea, Seoul, Korea), according to the manufacturer's instructions. Nucleotide primers were selected based on the nucleotide sequence of the gene encoding the 56-kDa antigen in Gilliam strains of O. tsutsugamushi. Primers 34 (5'-TCAAGCTTATTGCTAGTG CAATGTCTGC-3') and 55 (5'-AGGGATCCCTGCTGCTGTGCTTGCTGCG-3') were used in the first PCR amplification and nested PCR primers 10 (5'-GATCAAGCTTCCTCAGCCTACTATAATGCC-3') and 11 (5'-CTAGGGATCCCGACAGAT GCACTATTAGGC-3') were used in the second PCR amplification, generating a 483-bp fragment. Nested PCR was performed as described previously by Kim et al. (12). The PCR products were separated on a 1.5% agarose gel. Positive samples were eluted using a quick spin purification kit (GeneAll, Seoul, Korea). DNA sequencing was performed at Macrogen (Daejeon, Korea) using an ABI 3730 DNA sequencer (Applied Biosystems, Foster, CA, USA). The strains from which the amplified samples were derived were identified using the BLAST algorithm available at the National Center for Biotechnology Information. Regions encoding the O. tsutsugamushi 56-kDa protein were aligned using the ClustalX program. A phylogenetic tree was generated with Tree Explorer with bootstraps performed 1,000 times to increase its reliability. Homology between strains was compared using Laser Gene (DNAStar Inc., Madison, WI, USA).
Identification of mutations in the quinolone resistance-determining region of gyrA
To identify gyrA in samples, the following primers were used: TsgyAAF, 5'-TATGCTATGAGCGTAATAGT-3' and TsgyrAR, 5'-TGCCATTCCTACTGCAATTC-3'. PCR was performed with 2 µL of DNA extracted from the buffy coat. The PCR reaction (20 µL) contained 5 pM/µL primers, 10 µL AccuPower PCR PreMix (1.5 mM MgCl2, 0.25 mM dNTP, and 1U Taq DNA polymerase, 10 mM Tris-Cl, 30 mM KCl; Bioneer, Seoul, Korea) and sterile triple-distilled water. PCR products were amplified using a Bio-Rad thermo cycler (Bio-Rad, Hercules, CA, USA) under the following conditions: an initial 4 min denaturation step at 95℃; followed by 30 cycles of 30 sec of denaturation at 95℃, 30 sec of annealing at 57℃, and 1 min of extension at 72℃; and a final extension step at 72℃ for 10 min. Partial gyrA sequences containing the QRDR of O. tsutsugamushi were aligned using ClustalX to examine possible mutations known to be associated with quinolone resistance using sequences from Escherichia coli K12 for comparison.
Ethical statement
This study was approved by the institutional review board of Chonnam National University Hospital (IRB No. CNUH 2010-04-067). Written, informed consent was obtained from all patients involved in this study.
RESULTS
Clinical characteristics of the patients
Clinical data and template DNA for gyrA sequencing were obtained from blood samples of 21 patients during the study period. Clinical characteristics of the patients are shown in Table 1. Eleven (52%) of the 21 patients had serious complications and three patients were admitted to the intensive care unit. Twelve patients were initially treated with doxycycline, while eight patients were initially treated with intravenous azithromycin. All were cured without any change in antibiotic treatment or cardiac complications related to azithromycin. One patient initially received ciprofloxacin for 3 days at a primary care hospital, but the patient was transferred to our center due to clinical deterioration and the development of septic shock. The patient was subsequently treated with doxycycline and improved. All of the patients were treated successfully and recovered.
Table 1.
Clinical characteristics of patients diagnosed with scrub typhus
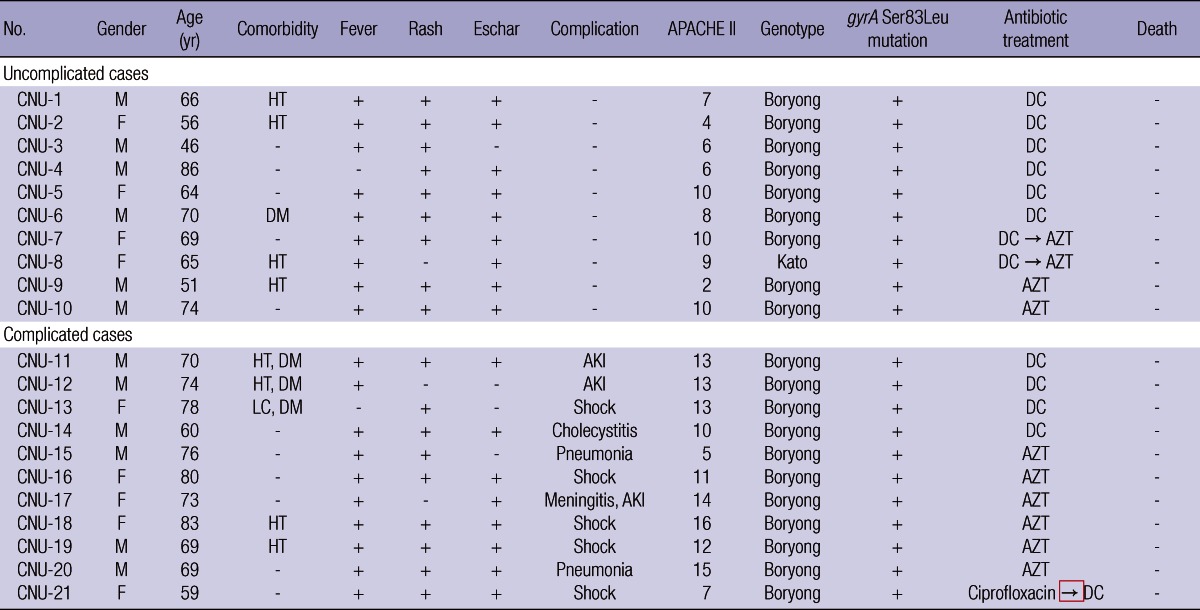
LC, liver cirrhosis; DM, diabetes mellitus; HT, hypertension; AKI, acute kidney injury; APACHE, acute physiology and chronic health evaluation; DC, doxycycline; AZT, azithromycin.
Genotype assay and sequencing of the gyrA quinolone resistance determining region
A phylogenetic tree of the PCR positive O. tsutsugamushi sequence from infected patients is shown in Fig. 1. We isolated 20 Boryong cluster isolates and one from the Kato cluster. All of the O. tsutsugamushi patient isolates, as well as the Boryong strains retrieved from the KEGG website, contained an intrinsic Ser83Leu mutation in the gyrA QRDR domain, known to be associated with quinolone resistance. A comparison of the E. coli and O. tsutsugamushi QRDR regions containing the Ser83Leu mutation is shown in Fig. 2.
Fig. 1.
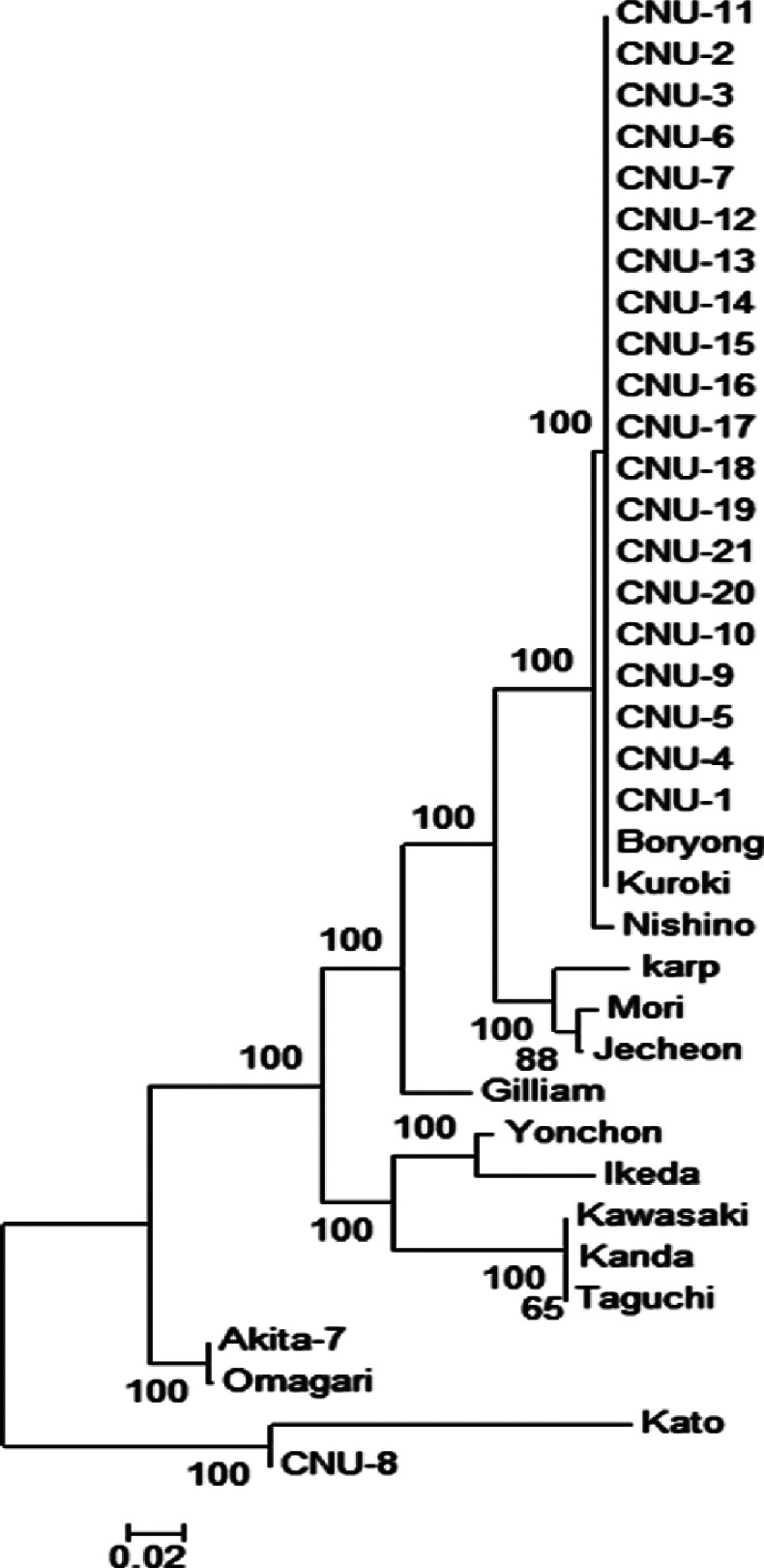
Phylogenetic tree based on the nucleotide sequences of Orientia tsutsugamushi 56-kDa genes.
Fig. 2.
DNA sequence alignment of the quinolone resistance-determining region (QRDR) domain of the gyrA genes of Orientia tsutsugamushi strains isolated from 21 patients. A comparison of the E. coli and O. tsutsugamushi QRDR regions containing the Ser83Leu mutation is shown. ECOLI, E. coli strain from the KEGG website; Boryong, Boryong strain of O. tsutsugamushi from the KEGG website.
DISCUSSION
In this study, we determined that all DNA samples of the Boryong strain of O. tsutsugamushi contained the Ser83Leu mutation in the QRDR domain, which is known to be associated with quinolone resistance. O. tsutsugamushi can be divided into several strains based on antigenic types or nucleotide sequences of the gyrA 56-kDa protein. In Korea, the Boryong strain has been reported throughout the country but predominantly in the southern areas (Chonbuk, Chonnam, Kyoungbuk, and Kyoungnam). In contrast, Karp, Kawasaki and Gilliam strains are more prevalent in the central part of Korea (Kyounggi, Kangwon and Chungcheong) (13, 14). We isolated primarily the Boryong strain from our patients, which is in agreement with previous reports and is in accordance with the location of our institute in the southern part of Korea.
Quinolone was used to treat one patient in our study. This patient was treated in a primary care clinic and showed clinical deterioration leading to septic shock despite 3 days of ciprofloxacin administration. The use of quinolone for the effective treatment of rickettsial diseases remains controversial since limited clinical data are available (9, 10, 15-17). However, recently, the efficacy of levofloxacin for the treatment of scrub typhus was reported in a retrospective study of 132 patients. The data suggested that levofloxacin prolongs defervescence compared with tetracycline antibiotics and results in higher mortality rates in severe cases (8). In contrast, another retrospective study of 161 cases with Mediterranean spotted fever showed that fluoroquinolone treatment was associated with increased disease severity (10). A small randomized trial performed in Korea also showed prolonged defervescence in patients with scrub typhus treated with quinolone, compared to those treated with doxycycline (9). These data suggest that quinolone has limited efficacy against rickettsial disorders and should not be used when tetracyclines and macrolides are available.
Previous in vitro and in vivo studies have supported the use of quinolone as an alternative to tetracycline (18-20). However, recent studies suggest that quinolone can cause deleterious effects on Rickettsia conorii-infected cells via a mechanism linked to toxin-antitoxin module up-regulation (21). Moreover, it has been reported that the Kato strain of O. tsutsugamushi has a higher minimum inhibitory concentration (8 µg/mL) to ciprofloxacin and ofloxacin in vitro, and all 18 Kato strain sequences obtained from patients contained the Ser83Leu mutation in the gyrA QRDR domain (11). However, there are limited data on the prevalence of this mutation in gyrA of the Boryong strain of O. tsutsugamushi, which is prevalent in Korea. For this reason, we sequenced the gyrA gene of 20 O. tsutsugamushi Boryong samples and determined that all had the Ser83Leu mutation in their QRDR domain, suggesting intrinsic resistance to quinolone.
This study has several limitations. First, ciprofloxacin was administered to only one patient in this study and thus we could not evaluate the outcome of patients treated with quinolone. Second, we performed genotypic assay of the gyrA QRDR domain in O. tsutsugamushi Boryong clinical isolates and did not verify these results using in vitro phenotypic assays, as performed by other investigators studying the Kato strain. Further study is needed to evaluate the prevalence of the QRDR domain mutation in other strains of O. tsutsugamushi such as Karp and Gilliam, which are common in other areas of Korea, and to identify this mutation in other rickettsia species.
In conclusion, we report the presence of the Ser83Leu mutation in gyrA samples of the Boryong strain of O. tsutsugamushi from 20 patients, suggesting that quinolone may be avoided for the treatment of scrub typhus, particularly in severe cases.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719. doi: 10.1128/cmr.10.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429–436. doi: 10.1097/00001432-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Sheehy TW, Hazlett D, Turk RE. Scrub typhus: a comparison of chloramphenicol and tetracycline in its treatment. Arch Intern Med. 1973;132:77–80. doi: 10.1001/archinte.132.1.77. [DOI] [PubMed] [Google Scholar]
- 4.Rajapakse S, Rodrigo C, Fernando SD. Drug treatment of scrub typhus. Trop Doct. 2011;41:1–4. doi: 10.1258/td.2010.100311. [DOI] [PubMed] [Google Scholar]
- 5.Song JH, Lee C, Chang WH, Choi SW, Choi JE, Kim YS, Cho SR, Ryu J, Pai CH. Short-course doxycycline treatment versus conventional tetracycline therapy for scrub typhus: a multicenter randomized trial. Clin Infect Dis. 1995;21:506–510. doi: 10.1093/clinids/21.3.506. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Yun HJ, Shim SK, Koo SH, Kim SY, Kim S. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin Infect Dis. 2004;39:1329–1335. doi: 10.1086/425008. [DOI] [PubMed] [Google Scholar]
- 7.Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet. 2000;356:1057–1061. doi: 10.1016/S0140-6736(00)02728-8. [DOI] [PubMed] [Google Scholar]
- 8.Tsai CC, Lay CJ, Wang CL, Ho YH, Wang LS, Chen LK. Levofloxacin versus tetracycline antibiotics for the treatment of scrub typhus. Int J Infect Dis. 2010;14:e62–e67. doi: 10.1016/j.ijid.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Oh SY, Chung MH, Oh SJ, Son MS, Ahn SW. An open clinical trial to compare the efficacy of ciprofloxacin, pefloxacin, and doxycycline in the treatment of scrub typhus. Korean J Infect Dis. 1995;27:193–198. [Google Scholar]
- 10.Botelho-Nevers E, Rovery C, Richet H, Raoult D. Analysis of risk factors for malignant Mediterranean spotted fever indicates that fluoroquinolone treatment has a deleterious effect. J Antimicrob Chemother. 2011;66:1821–1830. doi: 10.1093/jac/dkr218. [DOI] [PubMed] [Google Scholar]
- 11.Tantibhedhyangkul W, Angelakis E, Tongyoo N, Newton PN, Moore CE, Phetsouvanh R, Raoult D, Rolain JM. Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. Int J Antimicrob Agents. 2010;35:338–341. doi: 10.1016/j.ijantimicag.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DM, Kim HL, Park CY, Yang TY, Lee JH, Yang JT, Shim SK, Lee SH. Clinical usefulness of eschar polymerase chain reaction for the diagnosis of scrub typhus: a prospective study. Clin Infect Dis. 2006;43:1296–1300. doi: 10.1086/508464. [DOI] [PubMed] [Google Scholar]
- 13.Chang WH. Current status of tsutsugamushi disease in Korea. J Korean Med Sci. 1995;10:227–238. doi: 10.3346/jkms.1995.10.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong HW, Choi YK, Baek YH, Seong MH. Phylogenetic analysis of the 56-kDa type-specific protein genes of Orientia tsutsugamushi in Central Korea. J Korean Med Sci. 2012;27:1315–1319. doi: 10.3346/jkms.2012.27.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cracco C, Delafosse C, Baril L, Lefort Y, Morelot C, Derenne JP, Bricaire F, Similowski T. Multiple organ failure complicating probable scrub typhus. Clin Infect Dis. 2000;31:191–192. doi: 10.1086/313906. [DOI] [PubMed] [Google Scholar]
- 16.Jensenius M, Montelius R, Berild D, Vene S. Scrub typhus imported to Scandinavia. Scand J Infect Dis. 2006;38:200–202. doi: 10.1080/00365540500277342. [DOI] [PubMed] [Google Scholar]
- 17.Jee HG, Chung MH, Lee SG, Kim IS, Chang WH. Transmission of scrub typhus by needlestick from a patient receiving pefloxacin. Scand J Infect Dis. 1996;28:411–412. doi: 10.3109/00365549609037929. [DOI] [PubMed] [Google Scholar]
- 18.Raoult D, Roussellier P, Galicher V, Perez R, Tamalet J. In vitro susceptibility of Rickettsia conorii to ciprofloxacin as determined by suppressing lethality in chicken embryos and by plaque assay. Antimicrob Agents Chemother. 1986;29:424–425. doi: 10.1128/aac.29.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClain JB, Joshi B, Rice R. Chloramphenicol, gentamicin, and ciprofloxacin against murine scrub typhus. Antimicrob Agents Chemother. 1988;32:285–286. doi: 10.1128/aac.32.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurin M, Raoult D. Bacteriostatic and bactericidal activity of levofloxacin against Rickettsia rickettsii, Rickettsia conorii, 'Israeli spotted fever group rickettsia' and Coxiella burnetii. J Antimicrob Chemother. 1997;39:725–730. doi: 10.1093/jac/39.6.725. [DOI] [PubMed] [Google Scholar]
- 21.Botelho-Nevers E, Edouard S, Leroy Q, Raoult D. Deleterious effect of ciprofloxacin on Rickettsia conorii-infected cells is linked to toxin-antitoxin module up-regulation. J Antimicrob Chemother. 2012;67:1677–1682. doi: 10.1093/jac/dks089. [DOI] [PubMed] [Google Scholar]



