Abstract
Diagnosis of scrub typhus is difficult because its symptoms are very similar to other acute febrile illnesses, such as leptospirosis, murine typhus, and other viral hemorrhagic fevers. To differentiate scrub typhus from other acute febrile diseases, a rapid and reliable serological diagnosis is important. We have developed a chimeric recombinant antigen cr56 and two other recombinant antigens, r21 and kr56, from various serotypes of Orientia tsutsugamushi. They were tested for the detection of antibodies against O. tsutsugamushi in the patient's serum samples using enzyme-linked immunosorbent assay (ELISA) and dot-blot analyses. As of conventional immunofluorescence assay (IFA), when the mixture of these three recombinant antigens was used, both sensitivity and specificity of the recombinant antigens were increased up to 98% in IgM and IgG at ELISA and dot blotting. Additionally, both sensitivity and specificity by detection of IgM and IgG antibodies at rapid diagnostic test (RDT), using the mixture of three antigens and gold conjugated antibodies, were 99%. Our results suggest the use of mixture of these recombinant antigen proteins in ELISA or RDT is suitable as a diagnostic test for scrub typhus.
Keywords: Scrub Typhus, Orientia tsutsugamushi, Recombinant Antigen, IFA, ELISA, RDT
INTRODUCTION
Scrub typhus (tsutsugamushi disease) is an acute febrile illness caused by Orientia tsutsugamushi, which belongs to the scrub typhus group of the family Rickettsiaceae (1). It occurs worldwide but is particularly frequent in Asia including Korea, Japan, China, Malaysia, Thailand, and Vietnam (2, 3). It accounts for up to 23% of all febrile episodes in endemicity in the Asia-Pacific region (2). Approximately 1 million cases of scrub typhus appear every year and more than a billion people are at risk. The pathogen is transmitted by the bite of larval-stage trombiculid mites. A patient with scrub typhus can show various symptoms ranging from mild fever to fatal status with multiple organ failure. Antibiotics, such as tetracycline or doxycycline, cure the infection completely if treated early.
It has been generally considered that the clinical diagnosis of scrub typhus is easy based on the typical symptoms and signs. However, atypical symptoms and signs have been reported in many cases. Since specific symptoms of tsutsugamushi disease, including eschar formation and typical rash, are not observed frequently, it is difficult to discriminate the scrub typhus from other acute febrile illnesses such as leptospirosis, murine typhus, dengue fever, and other viral hemorrhagic fevers (4).
The diagnostic method for scrub typhus, indirect immunofluorescent assay (IFA), is sensitive and specific in detecting the antibody to O. tsutsugamushi (5). However, it requires cell culture facility to prepare O. tsutsugamushi antigen for immediate use, since O. tsutsugamushi antigen cannot be stored for a long period of time. It also requires a fluorescent microscope for diagnosis, which can only be performed by an expert in a laboratory (6, 7). Thus, it is difficult for hospitals to perform an immunofluorescence test in rural areas, where frequency of tsutsugamushi disease is usually high. Therefore, another simple, convenient and accurate diagnostic method is necessary to be developed (7-9).
O. tsutsugamushi is classified into one species, with many serotypes having different antigenicity, of which prototypes are Gilliam, Karp, and Kato strains according to their antigenicity. Among Korean epidemic strains, Boryong and Kangwon 87-61 strain, which have different serologic reactivity from the known epidemic serotype have been reported (2, 10-12). Among the antigens of various surface proteins in O. tsutsugamushi, a 56-kDa protein antigen, constituting up to 15% of the total protein contents produced by the bacterium, is most frequently detected by the serum antibody of the patient with scrub typhus (13, 14).
The purpose of this study was to develop a reliable diagnostic recombinant protein that can detect antibody to the Korean epidemic serotype as well as the worldwide epidemic prototype, such as Gilliam, Karp and Kato. In the present work, each gene coding important epitopes of 56-kDa antigen of Gilliam, Karp or Kato strain was amplified by polymerase chain reaction (PCR) (8, 15). In addition to this, the individual antigen genes of O. tsutsugamushi coding for 21-kDa and 56-kDa antigenic proteins of Boryong and Kangwon 87-61 strain respectively were cloned and expressed separately to improve diagnostic sensitivity for scrub typhus. The sensitivity and specificity of the mixed antigen of O. tsutsugamushi were evaluated using ELISA, dot-blot immunoassay, and RDT in this study, comparing to IFA to diagnose scrub typhus.
MATERIALS AND METHODS
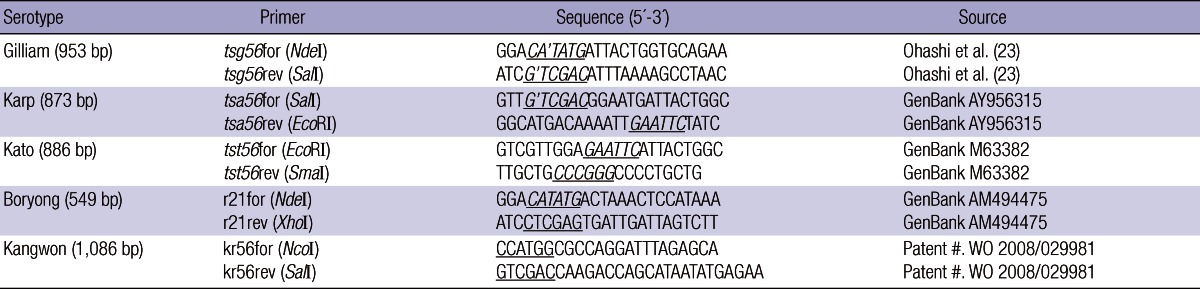
Gene cloning and expression of recombinant proteins, cr56, r21, or kr56
Each strain of O. tsutsugamushi was grown in mouse fibroblasts cells (L929), incubated at 34℃ in a humidified atmosphere of 5% CO2, and purified by percoll density gradient centrifugation as described by Ohashi et al. (1). Standard techniques for DNA manipulation, such as plasmid DNA preparation, ligation, competent cell preparation and transformation were followed as described by Sambrook and Russell (16). The DNA to make the chimeric recombinant 56-kDa (cr56) protein was isolated from O. tsutsugamushi strains grown into the L929 cells as described previously (13). Primers were designed based on the DNA sequence coding for the 56-kDa outer membrane protein gene. Homological region which showed 30% or more amino-acid sequence homology with each other at 56-kDa outer membrane proteins of O. tsutsugamushi prototype Gilliam, Karp and Kato, were selected to prepare the chimeric antigen of which oligonucleotide primers were designed in order to include each selected sequence to make the chimeric 56-kDa protein (Table 1). A 21-kDa species-specific antigen in O. tsutsugamushi serotype Boryong strain for recombinant 21-kDa protein (r21) and a 56-kDa major antigen of O. tsutsugamushi Kangwon 87-61 strain for simple recombinant 56-kDa protein (kr56) were cloned in E. coli (Patent #, WO 2008/029981 A1) (15). Each plasmid carrying the inserts coding for cr56, r21, and kr56 were induced by 0.5 mM Isoproyl-b-D-thiogalactopyranoside (IPTG) (Sigma, St. Louis, MO, USA). The recombinant His tagged proteins were purified, using His binding resins (Novagen, Darmstadt, Germany) according to the manufacturer's instruction.
Table 1.
List of O. tsutsugamushi serotype and primers used in the present study
Collection of serum samples
This study was approved by Institutional Review Board (IRB) of Hallym University. The control serum samples were obtained from healthy volunteers at Hallym University. Patient serum samples were referred from various hospitals of Hallym University Medical Center located in Chuncheon, Kangnam, Kangdong, Hangang and Sacred Heart Hospitals in Korea to diagnose scrub typhus-like symptoms from 1999 to 2000. Among them, 12 patients were confirmed with scrub typhus by the IFA test as described previously by Bozeman and Elisberg (20). All of these patients had at least two of the three symptoms of scrub typhus (fever, rash, and eschars), and all exhibited seroconversion or four-fold increment in antibody titers to O. tsutsugamushi by the IFA test. Samples were determined as scrub typhus positive by having IgM or IgG IFA titers of ≥ 1:10 or ≥ 1:40, respectively. An additional 32 serum samples were collected from scrub typhus patients who were diagnosed from only one serum sample having IgM or IgG IFA titers of ≥ 1:10 or ≥ 1:40, respectively (10, 11). For negative control, a total of 38 serum specimens were obtained from healthy blood donors, none of which reacted with O. tsutsugamushi by the IFA-IgM or -IgG test. In addition, 31 serum specimens from patients with other febrile diseases (12 specimens from hemorrhagic fever with renal syndrome [HFRS], 9 from murine typhus, and 10 from leptospirosis) were also obtained. HFRS and murine typhus were identified by the IFA test, and leptospirosis was identified by the microscopic agglutination tests as previously described (10, 17).
For RDT test, serum samples were prepared by two different hospitals separately. IFA-positive specimens were provided from Hallym University Hospital (n = 51) and Korea University Hospital (n = 58) as scrub typhus positive samples. Other serum specimens including murine typhus (n = 39) or HFRS (n = 55) infected patient serum specimens were provided by Hallym University Hospital (n = 79) and Korea University (n = 15).
Western-blot analysis and IFA
SDS-PAGE was performed as described previously (5). The expression level of each antigen was confirmed by anti-his-horse-radish peroxidase (Abcam, Cambridge, MA, USA). The antigenicity to this antigen was determined by patient serum at 1;3,000 dilution and goat anti-human IgG (H&L)-HRP (Bio-Rad Laboratories, Richmond, CA) at a 1;10,000 dilution.
Serological diagnosis of scrub typhus was performed by the IFA as described by Kim et al. (10). Briefly, O. tsutsugamushi strains Gilliam, Karp, and Kangwon 87-61 were cultured on monolayer mouse L929 cells in a humidified 5% CO2 atmosphere at 37℃ containing 2% fetal bovine serum (Gibco BRL, Grand Island, NY). When the cytopathic effect approached up to 70% of the cells, the cells were harvested and then dotted on teflon-coated spot slides, following fixation with acetone for 10 min. The slides were stored in a freezer at -70℃ without exposure to the air from outside until used. After applying two-fold serially diluted (1:10 to 1:1,280 in phosphate buffered saline [PBS]) patient serum to the antigen-coated spot on the slide for 30 min, fluorescein isothiocyanate-conjugated antibody, anti-human IgM or IgG (Cappel Laboratories, Cochranville, PA, USA), was applied to determine the positive signal. Endpoint titer against individual serum was represented as the reciprocal of the highest serum dilution at which rickettsiae exhibited clear positive fluorescence at any one of the three strains. From diagnostic study in Korea, cutoff values of titer at IgM and IgG IFA were ≥ 1:10 and ≥ 1:40 (10).
ELISA
To develop the optimal dilutions of the recombinant protein antigens, the recombinant protein antigens were diluted with a 0.05 M carbonate buffer (pH 9.6) and added to wells at various concentrations (18). A 96 well plate (Corning, Corning, NY, USA) was coated with the diluted recombinant protein antigens and incubated at 4℃ for 18 hr. The plate was washed twice with PBS containing 0.05% Tween 20. The antigens were blocked with 100 µL of 3% bovine serum albumin (BSA) per well, and the plate was incubated at 37℃ for 2 hr. Patient serum was diluted 1:200 in PBS, and 100 µL of the diluted serum was used as a primary antibody. A peroxidase conjugated goat anti-human IgG (H&L) (Bio-Rad, Hercules, CA, USA) was diluted to 1:6,000 and used as a secondary antibody. A 100 µL of the prepared secondary antibody was added to the wells. After washing three times, 100 µL of 0.1 M citrate phosphate buffer (pH 4.9) containing 1 mg O-phenylene diamine dihydrochloride (OPD) (Sigma) per mL and 0.03% hydrogen peroxide was added to the wells. The plate was shielded from light at room temperature for 30 min, and then 50 µL of 1 M sulfuric acid was added to terminate the reaction. The plate was read at 490 nm using a Micro-ELISA reader (SPECTRA MAX 250, Molecular Devices, Sunnyvale, CA, USA).
Dot-blot immunoassay
One microliter of each purified recombinant protein antigen (0.1 µg/µL) was dropped onto a nitrocellulose membrane (GE healthcare Biosciences, Pittsburg, PA, Germany) and then dried at 37℃ for 1 hr. The dried membrane was blocked with a TBST (Tris-buffered saline containing 0.5% Tween 20) solution containing 5% skim milk for 1 hr. Twelve patients' serum samples were diluted to 1:3,000 as a primary antibody and then added to the membrane. The reaction was allowed for 1 hr and HRP conjugated goat anti-human IgG (Bio-Rad) was used to determine a positive reaction which appeared as a distinct dark dot on the X-ray film. If no dot was seen, the reaction was interpreted as negative. The results obtained were exhibited as - for non reactivity and 1+, 2+, 3+, 4+ indicating positive based on the degree of intensity.
Rapid diagnostic Test (RDT)
A mixture of cr56, r21 and kr56 of O. tsutsugamushi as antigen was applied to RDT and this RDT was manufactured by Immunemed (15). The test procedure was briefly as follow: 300 µL diluent buffer including 3 µL serum was applied to the sample well of the test kit and then complex of antigen-serum antibody-gold conjugated anti-IgM or IgG was running on each IgM or IgG test strip. The result was read in 10 min. The red color appearing on control line (C) and test line (T) concurrently was regarded as positive. The test was considered as negative when only the control line appeared and invalid if no control line appeared.
Data analysis
KT clearinghouse (Center for Evidence-Based Medicine, Toronto, Canada) version was used to calculate all test performances. Variables indicate the number of true positives (TP), true negative (TN), false positive (FP), and false negatives (FN). Calculation of accuracy and reliability were conducted by (TP+TN)/number of all tests and ([TPXTN]-[FPXFN]/9[TP+FN][TN+FP]). Positive predictive value (PPV) indicates the value of TP/(TP+FP) and negative predictive value (NPV) is TN/(TN+FN).
Ethics statement
The study protocol was approved by the institutional review board of Hallym University (IRB, HIRB-2008-002). Informed consent was waived by the board.
RESULTS
Expression and Purification of the recombinant proteins
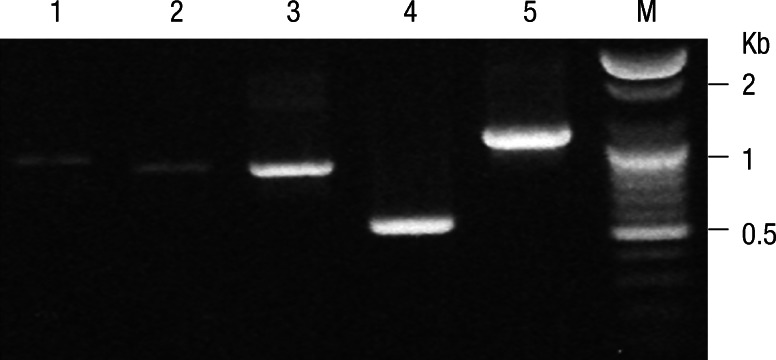
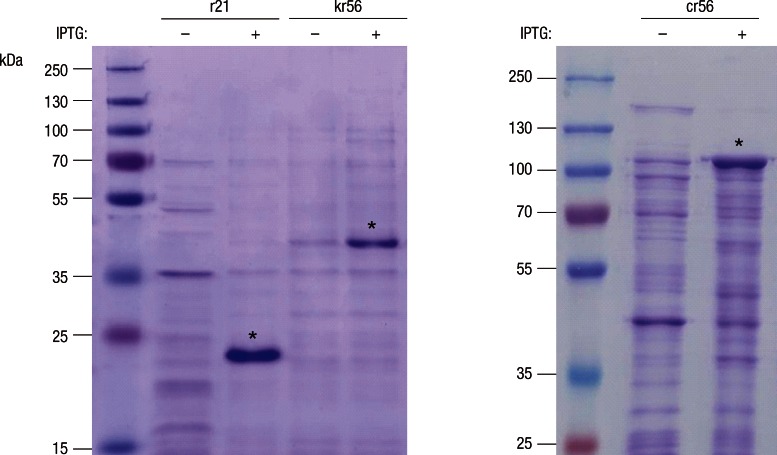
The amplified PCR products were electrophoresed and visualized by ethidium bromide staining as shown in Fig. 1 and 952-bp fragment in the Gilliam strain, 873-bp fragments in the Karp, and 886-bp fragment in the Kato strains, respectively, were fused to produce a 2,711-bp fragment. The PCR products of Boryong (549 bp) and Kangwon 87-61 (1,086 bp) were also observed. Protein expression of cr56 fusion protein, r21 protein and kr56 protein were confirmed by SDS-PAGE analysis, showing 101 kDa, 21 kDa, and 41 kDa, which are identical to the molecular mass predicted for the recombinant proteins, including His-tag (Fig. 2).
Fig. 1.
PCR amplification of antigenic genes from O. tsutsugamushi strains, Gilliam (lane 1: 952 bp), Karp (lane 2: 873 bp), Kato (lane 3: 886 bp), Boryoung (lane 4: 549 bp) and Kangwon 87-61 (lane 5: 1,086 bp) strains. The molecular size marker, 1kb (lane M).
Fig. 2.
SDS-PAGE of recombinant antigen protein r21(21 kDa), kr56 (41 kDa) and cr56 (101 kDa). Asterisks indicate the induced antigens. IPTG, Isoproyl-b-D-thiogalactopyranoside
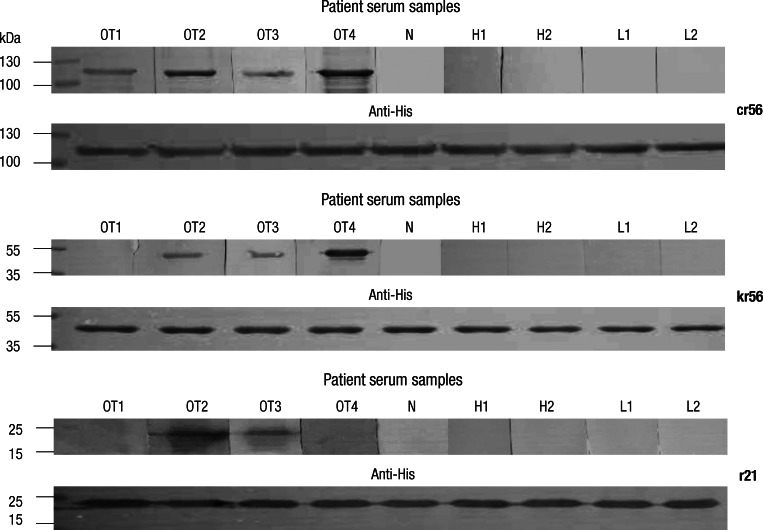
The His fusion proteins were purified with His resin and the reactivity of purified recombinant proteins with each antigen were analyzed on Western blotting, using the patient serum samples of scrub typhus (Fig. 3). Four serum samples from scrub typhus patients showed the strong and weak reactivity on the cr56 antigenic protein which includes each repetitive region of 56 kDa protein of prototype Gilliam, Karp, and Kato. On the other hand, three out of four serum samples showed the positive reaction with kr56 from Kangwon 87-61 and two out of four serum samples reacted with r21 protein from Boryong. Furthermore, some serum samples reacted to r21 or kr56, even though they reacted weakly or rarely to cr56 (data not shown).
Fig. 3.
Western blot analysis of the scrub typhus patients' serum samples using recombinant antigens, cr56, kr56 and r2. Lane 1-4: serum samples from scrub typhus patients (OT1, OT2, OT3, OT4), Lane 5: serum of healthy control (N), Lane 6 and 7: serum samples of HFRS patients (H1 and H2), Lane 8 and 9: serum samples of leptospirosis patients (L1 and L2). Loading amount of each antigen onto membrane was confirmed by Western blot, using anti-His (Anti-His).
Comparison of ELISA, Dot blotting and RDT
The representative distributions of IFA titer, ELISA titer, and dot blot titer from twelve serum samples obtained from confirmed scrub typhus patients are shown in Table 2. Background reactivity was assessed by analyzing healthy serum specimens and the cut off values were generally set with comparing patients' and healthy person's serum samples. In our study, the cutoff value of IgM or IgG ELISA was simplified as 0.2 through adjustment of ELISA conditions. The mean of optical density (OD) from the healthy serum specimens was 0.085 ± 0.042 (to cr56/r21, 1:1), 0.078 ± 0.038 (to cr56/r21/kr56, 5:1:3), 0.081 ± 0.044 (to cr56/r21, 5:1) and 0.075 ± 0.035 (to kr56). No serum from healthy persons had OD greater than the cutoff value in the IgM or IgG ELISA.
Table 2.
Representative Antigenicity of the recombinant antigen proteins using IFA, ELISA, and dot-blot analyses with confirmed scrub typhus serum samples
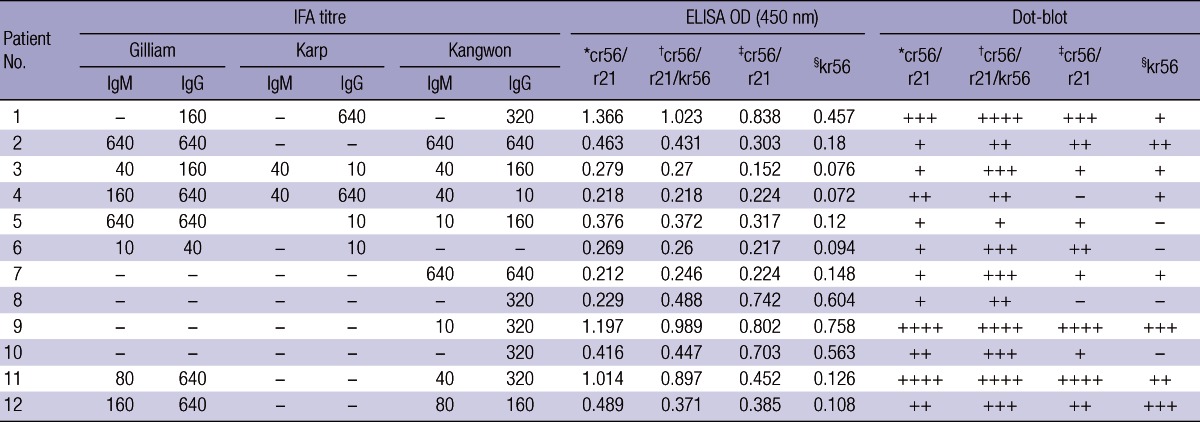
*Each antigen protein (cr56 or r21) was diluted to 1.25 µg/mL and mixed in equal proportion; healthy samples showed OD490 = 0.085 ± 0.042; †Each antigen protein, cr56, r21 or kr56 was diluted to 1.25 µg/mL and mixed in 5:1:3 ratio, respectively; healthy samples showed OD490 = 0.078 ± 0.038; ‡Each antigen protein, cr56 or r21 was diluted to 1.25 µg/mL and mixed in 5:1 ratio; healthy samples showed OD490 = 0.081 ± 0.044; §The antigen protein kr56 was used in a final concentration of 1.25 µg/mL; healthy samples showed OD490 = 0.075 ± 0.035.
In Table 3, when 44 serum samples from patients with scrub typhus, 38 serum samples from healthy person and 31 serum samples from patients with other febrile illness, were used for reactivity on recombinant antigen(s) at ELISA, mixture of cr56: r21 (1:1 or 5:1) showed 88.6-93.2% sensitivity and 91.3-92.8% specificity. In case of kr56 protein, when it was added to this mixture additionally, each positive and negative signal increased sensitivity to 95.5% and specificity to 97.1%.
Table 3.
Comparative performance of ELISA, using the recombinant proteins for detection of IgG (H+L) to O. tsutsugamushi in serum specimens
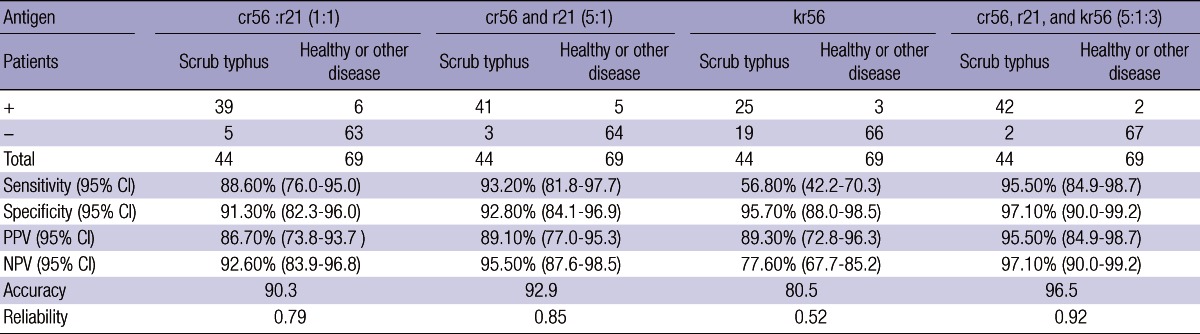
CI, confidence interval; PPV, Positive predictive value; NPV, Negative predictive value.
The results obtained from the dot-blot analysis were exhibited as - for non reactivity and 1+, 2+, 3+, 4+ indicating positive based on the degree of reactivity (Table 4). In the dot-blot analysis, 41 (93.2%) of the 44 serum samples from patients with scrub typhus gave at least one positive to the cr56/r21 (1:1) antigen dot, 40 (90.9%) to the cr56/r21 (5:1), and 27 (61.4%) to the kr56, but 43 serum samples (97.7%) gave at least one positive to the cr56/r21/kr56 antigen dot. None of the negative control serum sample produced positive result. These results were comparable to the data obtained from ELISA.
Table 4.
Validation of Dot blotting for detection of IgG (H+L) to O. tsutsugamushi serum specimens
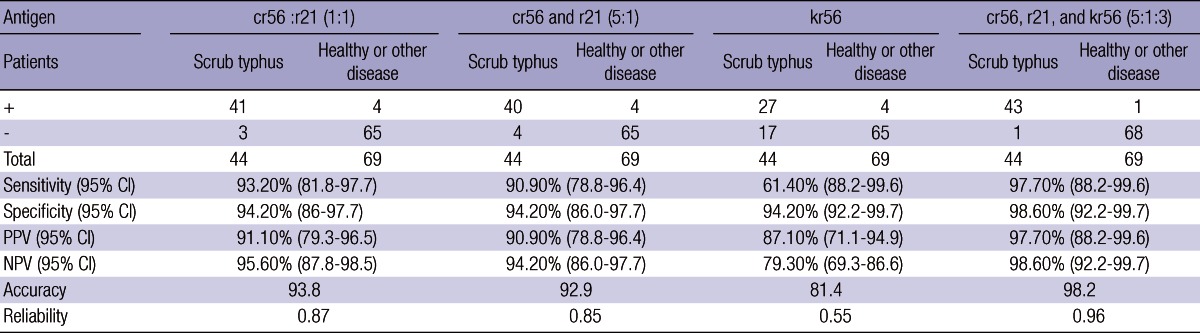
CI, confidence interval; PPV, Positive predictive value; NPV, Negative predictive value.
Finally, the efficacy of RDT against scrub typhus to use the antigen mixture cr56/r21/kr56 (5:1:3) and another patients' serum samples is presented in Table 5. Its sensitivity was 99.1% and specificity was 98.9% from reactivity to IgM and IgG, and its accuracy was 99.0%.
Table 5.
Performance of RDT tests for detection of IgM or IgG to O. tsutsugamushi in patient serum samples
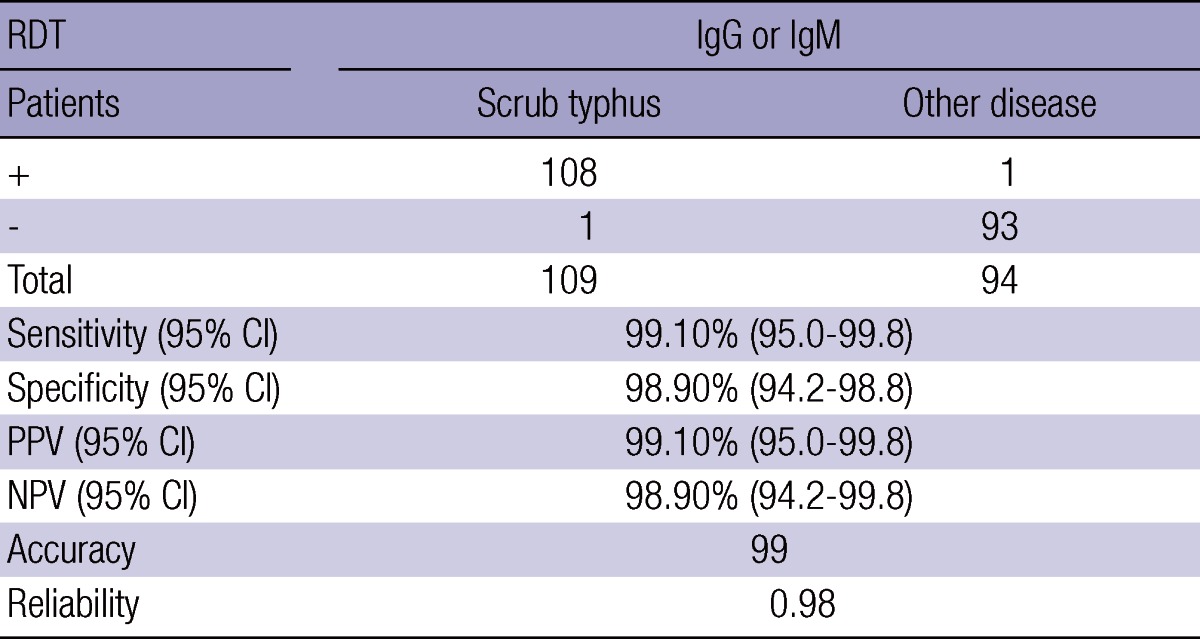
Performance of RDT was evaluated by comparing result from 3 separate tests. CI, confidence interval; PPV, Positive predictive value; NPV, Negative predictive value.
DISCUSSION
Scrub typhus is a common disease in both indigenous and visiting individuals to rural areas in Asia (3, 19, 20). However, this disease is difficult to diagnose because the signs and symptoms are nonspecific and the availability of conventional IFA is not easy. The less accessibility to IFA in the field is because the epidemic or endemic serotypes of whole O. tsutsugamushi bacteria should be provided from the each specific region for diagnosis. As O. tsutsugamushi is an obligate intracellular pathogen, its culture is difficult and comparatively high in cost and requires biosafety level (BSL) 3 facilities. For these reasons, many laboratories throughout the epidemic or endemic region, in which the economic state is generally poor, do not carry out their own assays and cannot pay the high cost for commercial assay. Therefore, clinicians may not have access to laboratories that use specific assays for scrub typhus and some laboratories may use the nonspecific and insensitive Weil-Felix reaction in which cross-reactive Proteus antigens are used to diagnose rickettsial diseases (21).
Fortunately, in the last 15 yr several laboratories have developed scrub typhus assays that use recombinant proteins derived from O. tsutsugamushi. These antigens are produced by E. coli in a BSL 2 laboratory, and have been found to be very consistent from lot to lot and are quite stable. The antigen of choice has been the immunodominant outer membrane 56-kDa protein from various strains of O. tsutsugamushi (Karp, Kato, Gilliam) either used singularly or in combination (7, 9, 13, 18, 19). In nature, the antigen is quantitatively recognized by the immune systems of various hosts, including humans (9). The recombinant 56 antigen has been used successfully as a fused protein in passive hemagglutination assays and an ELISA or a truncated format in an ELISA and IFA (7, 18, 19).
The 56-kDa protein is located on the outer membrane of O. tsutsugamushi. The protein comprises 10% to 15% of the total bacterial cellular protein contents (12, 13). Many serum samples from scrub typhus patients react on 56-kDa proteins from several serotypes, although some serum samples react predominantly on a 56-kDa antigen from a certain or a few serotype strains (6, 7, 22). Several antigenic serotypes of O. tsutsugamushi were isolated and distinguishable from each other through serological cross-testing (1, 11). The serotype of the bacteria mainly depends on the antigenicity of an immunodominant 56-kDa major surface protein (6, 13, 19, 22) which contains the group-specific and serotype-specific epitopes. The 56-kDa protein is highly reactive on patient serum owing to its antigenicity and abundances, so it could be the first antigen candidate for use in the diagnosis of O. tsutsugamushi disease.
There are at least four serotypes of O. tsutsugamushi in Korea, in which serotype Boryong and Gilliam are dominant (3). Kangwon which is antigenically near Gilliam was infrequently found in Korea, and when antigen kr56 was added as near complete 56-kDa protein to the mixture antigen of cr56 and r21 with the similar molar ratio, the sensitivity and specificity was improved in the antibody detection, therefore the ratio of cr56, kr56 and r21 was 5:3:1. When other ratio was used, both sensitivity and specificity were lower than this specific ratio (data not shown).
In this report, we evaluated the ability of a new chimeric recombinant antigen protein (cr56) derived from O. tsutsugamushi Gilliam, Karp, and Kato strains, as well as antigen proteins (r21 and kr56) derived from Boryong and Kangwon 87-61 strains. Although the sensitivity and specificity of cr56 were appreciable, these were improved drastically by the use of antigen mixture (cr56, r21 and kr56). These results were supported by the data obtained from ELISA and dot-blotting. Although the parts of each antigenic motif were chosen among a few serotypes, the sequences of each recombinant antigen are showing high homology with antigen of every serotypes in the world, and therefore, it is very possible to diagnose scrub typhus globally not only in Korea.
On the basis of the results obtained in the present study, the mixture of cr56, r21 and kr56 for O. tsutsugamushi could be used as a reliable and useful antigen for diagnosis of scrub typhus with wide spectrum at least in Korea. It is suggested that this mixture of recombinant antigens will improve the diagnostic sensitivity of scrub typhus world wide.
Footnotes
This research was supported by Hallym University Research Fund, 2008 (HRF-2008-00).
The authors have no conflicts of interest to disclose.
References
- 1.Ohashi N, Tamura A, Suto T. Immunoblotting analysis of anti-rickettsial antibodies produced in patients of Tsutsugamushi disease. Microbiol Immunol. 1988;32:1085–1092. doi: 10.1111/j.1348-0421.1988.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown GW, Robinson DM, Huxsoll DL, Ng TS, Lim KJ. Scrub typhus: a common cause of illness in indigenous populations. Trans R Soc Trop Med Hyg. 1976;70:444–448. doi: 10.1016/0035-9203(76)90127-9. [DOI] [PubMed] [Google Scholar]
- 3.Chang WH, Kang JS, Lee WK, Choi MS, Lee JH. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J Clin Microbiol. 1990;28:685–688. doi: 10.1128/jcm.28.4.685-688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuya Y, Yamamoto S, Otu M, Yoshida Y, Ohashi N, Murata M, Kawabata N, Tamura A, Kawamura A., Jr Use of monoclonal antibodies against Rickettsia tsutsugamushi Kawasaki for serodiagnosis by enzyme-linked immunosorbent assay. J Clin Microbiol. 1991;29:340–345. doi: 10.1128/jcm.29.2.340-345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozeman FM, Elisberg BL. Serological diagnosis of scrub typhus by indirect immunofluorescence. Proc Soc Exp Biol Med. 1963;112:568–573. doi: 10.3181/00379727-112-28107. [DOI] [PubMed] [Google Scholar]
- 6.Elisberg BL, Campbell JM, Bozeman FM. Antigenic diversity of rickettsia tsutsugamushi: epidemiologic and ecologic significance. J Hyg Epidemiol Microbiol Immunol. 1968;12:18–25. [PubMed] [Google Scholar]
- 7.Jiang J, Marienau KJ, May LA, Beecham HJ, 3rd, Wilkinson R, Ching WM, Richards AL. Laboratory diagnosis of two scrub typhus outbreaks at Camp Fuji, Japan in 2000 and 2001 by enzyme-linked immunosorbent assay, rapid flow assay, and Western blot assay using outer membrane 56-kD recombinant proteins. Am J Trop Med Hyg. 2003;69:60–66. [PubMed] [Google Scholar]
- 8.Choi MS, Seong SY, Kang JS, Kim YW, Huh MS, Kim IS. Homotypic and heterotypic antibody responses to a 56-kilodalton protein of Orientia tsutsugamushi. Infect Immun. 1999;67:6194–6197. doi: 10.1128/iai.67.11.6194-6197.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim IS, Seong SY, Woo SG, Choi MS, Kang JS, Chang WH. Rapid diagnosis of scrub typhus by a passive hemagglutination assay using recombinant 56-kilodalton polypeptides. J Clin Microbiol. 1993;31:2057–2060. doi: 10.1128/jcm.31.8.2057-2060.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YW, Min CH, Cho MK, Yoon CS, Cho SI, Choi MK, Kang JS, Chang WH. Murine typhus and scrub typhus in Kangwon-do Korea. Korean J Infect Dis. 1988;20:105–116. [Google Scholar]
- 11.Chang WH. Current status of tsutsugamushi disease in Korea. J Korean Med Sci. 1995;10:227–238. doi: 10.3346/jkms.1995.10.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Kim YW, Cho MK, Yoon CS, Min CH. Characterization of Rickettsia tsutsugamushi isolated in Korea by immunoblotting. J Korean Soc Microbiol. 1990;25:237–245. [Google Scholar]
- 13.Enatsu T, Urakami H, Tamura A. Phylogenetic analysis of Orientia tsutsugamushi strains based on the sequence homologies of 56-kDa type-specific antigen genes. FEMS Microbiol Lett. 1999;180:163–169. doi: 10.1111/j.1574-6968.1999.tb08791.x. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Urakami H, Tamura A. Purification of cell envelopes of Rickettsia tsutsugamushi. Microbiol Immunol. 1985;29:475–478. doi: 10.1111/j.1348-0421.1985.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim YW, Kim IS, Chang IA, Woo SD, Kim YJ, Chun JM, Kim WC, Byun YH, Cho MK. Diagnostic formulation for tsutsugamushi disease. Patent #, WO 2008/029981 A1
- 16.Sambrook J, Russell DW. Molecular Cloning: a laboratory manual. 3rd ed. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 17.Cho MK, Kim YW, Min CH, Oh HB. Serovar determination of Leptospira interrogans isolated in Korea by cross-agglutinin absorption method (1984-1987) J Korean Soc Microbiol. 1988;23:169–177. [Google Scholar]
- 18.Kim IS, Seong SY, Woo SG, Choi MS, Chang WH. High-level expression of a 56-kilodalton protein gene (bor56) of Rickettsia tsutsugamushi Boryong and its application to enzyme-linked immunosorbent assays. J Clin Microbiol. 1993;31:598–605. doi: 10.1128/jcm.31.3.598-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ching WM, Wang H, Eamsila C, Kelly DJ, Dasch GA. Expression and refolding of truncated recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi and its use in enzyme-linked immunosorbent assays. Clin Diagn Lab Immunol. 1998;5:519–526. doi: 10.1128/cdli.5.4.519-526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapmund G. Rickettsial diseases of the Far East: new perspectives. J Infect Dis. 1984;149:330–338. doi: 10.1093/infdis/149.3.330. [DOI] [PubMed] [Google Scholar]
- 21.Brown GW, Shirai A, Rogers C, Groves MG. Diagnostic criteria for scrub typhus: probability values for immunofluorescent antibody and Proteus OXK agglutinin titers. Am J Trop Med Hyg. 1983;32:1101–1107. doi: 10.4269/ajtmh.1983.32.1101. [DOI] [PubMed] [Google Scholar]
- 22.Jang WJ, Huh MS, Park KH, Choi MS, Kim IS. Evaluation of an immunoglobulin M capture enzyme-linked immunosorbent assay for diagnosis of Orientia tsutsugamushi infection. Clin Diagn Lab Immunol. 2003;10:394–398. doi: 10.1128/CDLI.10.3.394-398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohashi N, Nashimoto H, Ikeda H, Tamura A. Cloning and sequencing of the gene (tsg56) encoding a type-specific antigen from Rickettsia tsutsugamushi. Gene. 1990;91:119–122. doi: 10.1016/0378-1119(90)90171-m. [DOI] [PubMed] [Google Scholar]