Abstract
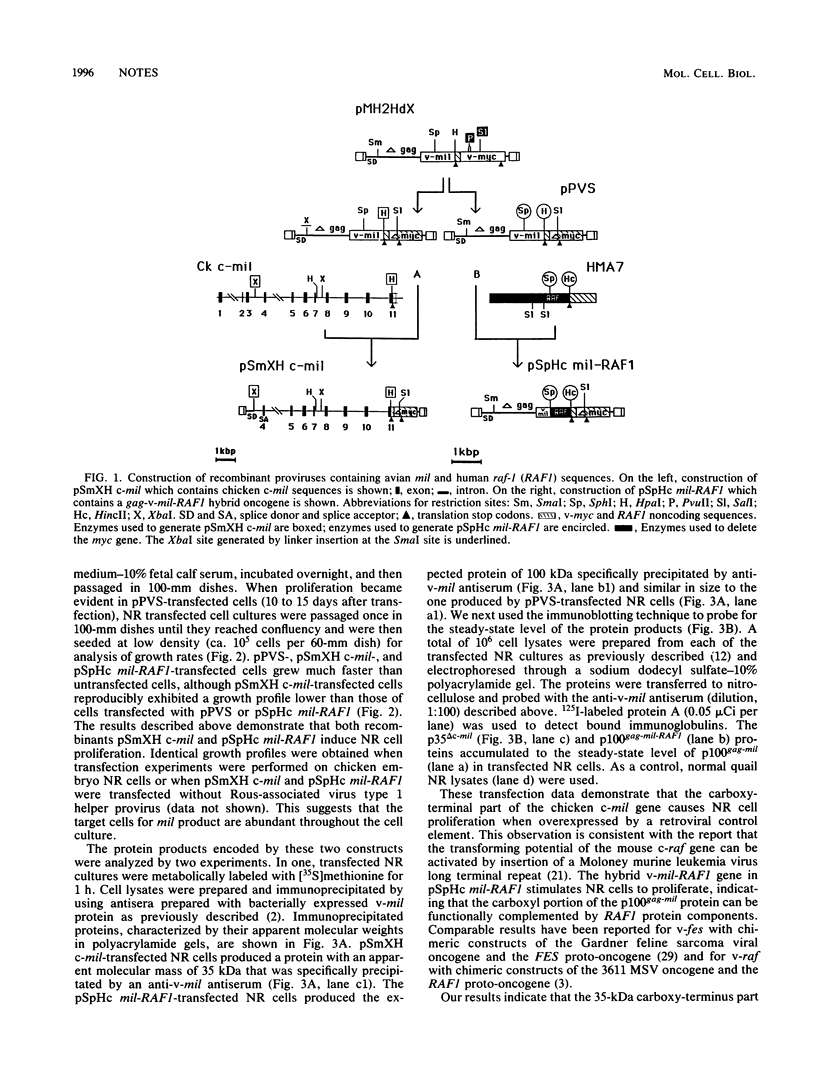
Expression of the P100gag-mil protein of avian retrovirus MH2 in cultured chicken embryo neuroretina cells was previously shown to result in the proliferation of normally quiescent cell populations. We show here that long terminal repeat activation of the carboxy terminus of the c-mil gene is sufficient to induce neuroretina cell proliferation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Bechade C., Calothy G., Pessac B., Martin P., Coll J., Denhez F., Saule S., Ghysdael J., Stéhelin D. Induction of proliferation or transformation of neuroretina cells by the mil and myc viral oncogenes. Nature. 1985 Aug 8;316(6028):559–562. doi: 10.1038/316559a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Kerby S. B., Sutrave P., Gunnell M. A., Mark G., Rapp U. R. Structure and biological activity of human homologs of the raf/mil oncogene. Mol Cell Biol. 1985 Jun;5(6):1400–1407. doi: 10.1128/mcb.5.6.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunte T., Greiser-Wilke I., Moelling K. The transforming protein of the MC29-related virus CMII is a nuclear DNA-binding protein whereas MH2 codes for a cytoplasmic RNA-DNA binding polyprotein. EMBO J. 1983;2(7):1087–1092. doi: 10.1002/j.1460-2075.1983.tb01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calothy G., Poirier F., Dambrine G., Mignatti P., Combes P., Pessac B. Expression of viral oncogenes in differentiating chick embryo neuroretinal cells infected with avian tumor viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):983–990. doi: 10.1101/sqb.1980.044.01.106. [DOI] [PubMed] [Google Scholar]
- Coll J., Righi M., Taisne C., Dissous C., Gegonne A., Stehelin D. Molecular cloning of the avian acute transforming retrovirus MH2 reveals a novel cell-derived sequence (v-mil) in addition to the myc oncogene. EMBO J. 1983;2(12):2189–2194. doi: 10.1002/j.1460-2075.1983.tb01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
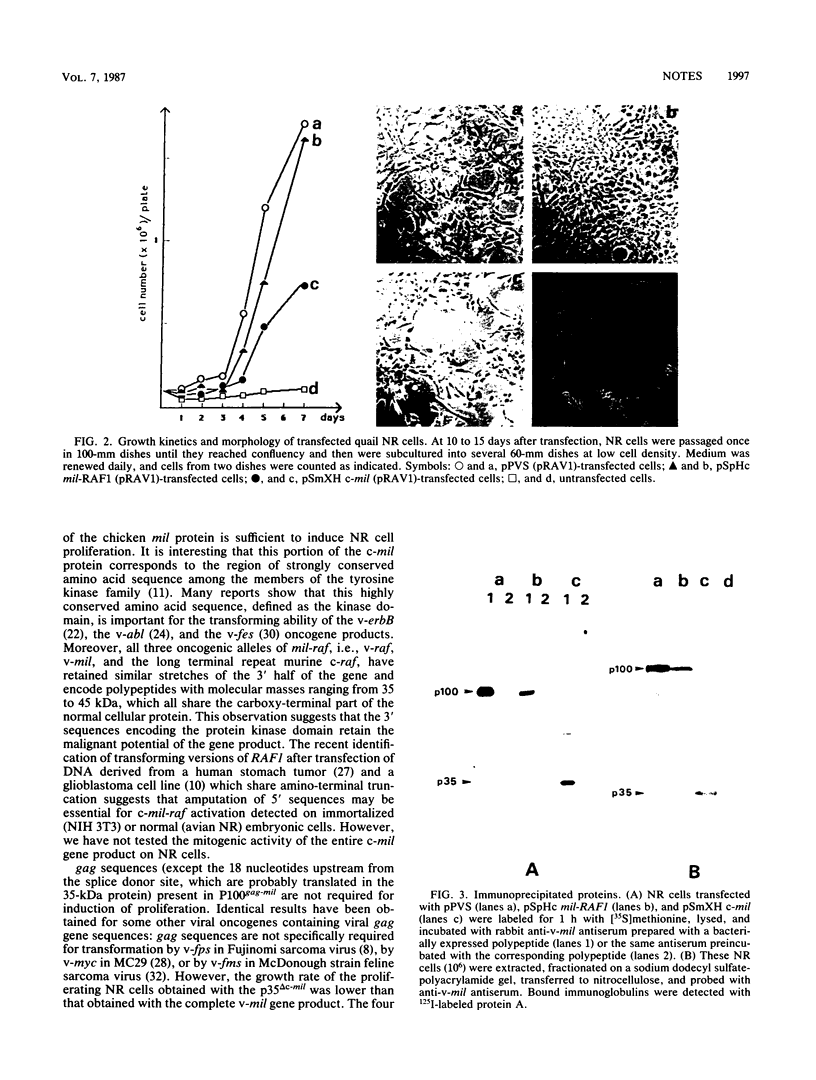
- Flordellis C. S., Kan N. C., Lautenberger J. A., Samuel K. P., Garon C. F., Papas T. S. Analysis of the cellular proto-oncogene mht/raf: relationship to the 5' sequences of v-mht in avian carcinoma virus MH2 and v-raf in murine sarcoma virus 3611. Virology. 1985 Mar;141(2):267–274. doi: 10.1016/0042-6822(85)90257-0. [DOI] [PubMed] [Google Scholar]
- Foster D. A., Hanafusa H. A fps gene without gag gene sequences transforms cells in culture and induces tumors in chickens. J Virol. 1983 Dec;48(3):744–751. doi: 10.1128/jvi.48.3.744-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. A., Shibuya M., Hanafusa H. Activation of the transformation potential of the cellular fps gene. Cell. 1985 Aug;42(1):105–115. doi: 10.1016/s0092-8674(85)80106-9. [DOI] [PubMed] [Google Scholar]
- Fukui M., Yamamoto T., Kawai S., Maruo K., Toyoshima K. Detection of a raf-related and two other transforming DNA sequences in human tumors maintained in nude mice. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5954–5958. doi: 10.1073/pnas.82.17.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Dupont de Dinechin S., Righi M., Stehelin D. The second oncogene mil of avian retrovirus MH2 is related to the src gene family. EMBO J. 1984 Jun;3(6):1333–1338. doi: 10.1002/j.1460-2075.1984.tb01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazin C., Rigolet M., Briand J. P., Van Regenmortel M. H., Galibert F. Immunochemical detection of proteins related to the human c-myc exon 1. EMBO J. 1986 Sep;5(9):2241–2250. doi: 10.1002/j.1460-2075.1986.tb04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Moscovici C., Vogt P. K. The defectiveness of Mill Hill 2, a carcinoma-inducing avian oncovirus. Virology. 1978 Aug;89(1):162–178. doi: 10.1016/0042-6822(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Iba H., Jove R., Hanafusa H. Lack of induction of neuroretinal cell proliferation by Rous sarcoma virus variants that carry the c-src gene. Mol Cell Biol. 1985 Oct;5(10):2856–2859. doi: 10.1128/mcb.5.10.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen H. W., Bister K. Nucleotide sequence analysis of the chicken gene c-mil, the progenitor of the retroviral oncogene v-mil. Virology. 1985 Jun;143(2):359–367. doi: 10.1016/0042-6822(85)90376-9. [DOI] [PubMed] [Google Scholar]
- Jansen H. W., Trachmann C., Bister K. Structural relationship between the chicken protooncogene c-mil and the retroviral oncogene v-mil. Virology. 1984 Aug;137(1):217–224. doi: 10.1016/0042-6822(84)90028-x. [DOI] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Mark G. E., Duesberg P. H., Papas T. S. A common onc gene sequence transduced by avian carcinoma virus MH2 and by murine sarcoma virus 3611. Science. 1984 Feb 24;223(4638):813–816. doi: 10.1126/science.6320371. [DOI] [PubMed] [Google Scholar]
- Levy J. B., Iba H., Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4228–4232. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Heimann B., Beimling P., Rapp U. R., Sander T. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature. 1984 Dec 6;312(5994):558–561. doi: 10.1038/312558a0. [DOI] [PubMed] [Google Scholar]
- Mölders H., Defesche J., Müller D., Bonner T. I., Rapp U. R., Müller R. Integration of transfected LTR sequences into the c-raf proto-oncogene: activation by promoter insertion. EMBO J. 1985 Mar;4(3):693–698. doi: 10.1002/j.1460-2075.1985.tb03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M., Privalsky M. L. Structural domains of the avian erythroblastosis virus erbB protein required for fibroblast transformation: dissection by in-frame insertional mutagenesis. J Virol. 1986 May;58(2):542–553. doi: 10.1128/jvi.58.2.542-553.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessac B., Defendi V. Cell aggregation: role of acid mucopolysaccharides. Science. 1972 Feb 25;175(4024):898–900. doi: 10.1126/science.175.4024.898. [DOI] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Baltimore D. The minimum transforming region of v-abl is the segment encoding protein-tyrosine kinase. J Virol. 1985 Apr;54(1):114–122. doi: 10.1128/jvi.54.1.114-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Goldsborough M. D., Mark G. E., Bonner T. I., Groffen J., Reynolds F. H., Jr, Stephenson J. R. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J., Hayman M. J., Enrietto P. J. Analysis of a deleted MC29 provirus: gag sequences are not required for fibroblast transformation. J Virol. 1985 Dec;56(3):943–950. doi: 10.1128/jvi.56.3.943-950.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Nakatsu Y., Sekiguchi M., Hokamura K., Tanaka K., Terada M., Sugimura T. Molecular cloning of an activated human oncogene, homologous to v-raf, from primary stomach cancer. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5641–5645. doi: 10.1073/pnas.82.17.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J. G., Goh W. C., Haseltine W. A. Transforming potential of a human protooncogene (c-fps/fes) locus. Proc Natl Acad Sci U S A. 1984 May;81(10):3039–3043. doi: 10.1073/pnas.81.10.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. C., Atkinson T., Smith M., Pawson T. Identification of functional regions in the transforming protein of Fujinami sarcoma virus by in-phase insertion mutagenesis. Cell. 1984 Jun;37(2):549–558. doi: 10.1016/0092-8674(84)90385-4. [DOI] [PubMed] [Google Scholar]
- Sutrave P., Bonner T. I., Rapp U. R., Jansen H. W., Patschinsky T., Bister K. Nucleotide sequence of avian retroviral oncogene v-mil: homologue of murine retroviral oncogene v-raf. Nature. 1984 May 3;309(5963):85–88. doi: 10.1038/309085a0. [DOI] [PubMed] [Google Scholar]
- Wheeler E. F., Roussel M. F., Hampe A., Walker M. H., Fried V. A., Look A. T., Rettenmier C. W., Sherr C. J. The amino-terminal domain of the v-fms oncogene product includes a functional signal peptide that directs synthesis of a transforming glycoprotein in the absence of feline leukemia virus gag sequences. J Virol. 1986 Aug;59(2):224–233. doi: 10.1128/jvi.59.2.224-233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]