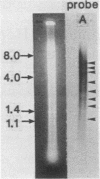
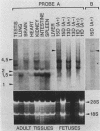
Abstract
A mouse embryonic cDNA containing two opa-like (CAX)n repeats was isolated on the basis of its cross-hybridization with a Drosophila K10 cDNA. Such repeated sequences were present in different murine mRNAs, some of which were specifically expressed during fetal life or in different adult tissues. This suggests that, as already described for Drosophila, opa-like sequences are parts of proteins involved in ontogenic or cell-type-specific functions in vertebrates. However, unlike Drosophila, such repeated sequences were not found within the murine homeo-boxes containing genes of the Hox-1 complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Beachy P. A., Helfand S. L., Hogness D. S. Segmental distribution of bithorax complex proteins during Drosophila development. Nature. 1985 Feb 14;313(6003):545–551. doi: 10.1038/313545a0. [DOI] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Voss S. D., Chowdhury K., Stewart C. L., Wagner E. F., Gruss P. Clustered homeo boxes are differentially expressed during murine development. Cell. 1985 Nov;43(1):39–45. doi: 10.1016/0092-8674(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Posakony J. W. Repetitive sequence transcripts in development. Nature. 1982 Jun 24;297(5868):633–635. doi: 10.1038/297633a0. [DOI] [PubMed] [Google Scholar]
- Duboule D., Baron A., Mähl P., Galliot B. A new homeo-box is present in overlapping cosmid clones which define the mouse Hox-1 locus. EMBO J. 1986 Aug;5(8):1973–1980. doi: 10.1002/j.1460-2075.1986.tb04452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner J. M., Nakanishi M., Ali Z., Drees B., Gustavson E., Theis J., Kauvar L., Kornberg T., O'Farrell P. H. Molecular cloning of engrailed: a gene involved in the development of pattern in Drosophila melanogaster. Cell. 1985 Aug;42(1):309–316. doi: 10.1016/s0092-8674(85)80126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon A., Carroll S. B., Storfer F. A., Riley P. D., Scott M. P. Common properties of proteins encoded by the Antennapedia complex genes of Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1985;50:253–262. doi: 10.1101/sqb.1985.050.01.032. [DOI] [PubMed] [Google Scholar]
- Laughon A., Scott M. P. Sequence of a Drosophila segmentation gene: protein structure homology with DNA-binding proteins. Nature. 1984 Jul 5;310(5972):25–31. doi: 10.1038/310025a0. [DOI] [PubMed] [Google Scholar]
- Le Bouc Y., Dreyer D., Jaeger F., Binoux M., Sondermeyer P. Complete characterization of the human IGF-I nucleotide sequence isolated from a newly constructed adult liver cDNA library. FEBS Lett. 1986 Feb 3;196(1):108–112. doi: 10.1016/0014-5793(86)80223-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Hart C. P., Gehring W. J., Ruddle F. H. Molecular cloning and chromosome mapping of a mouse DNA sequence homologous to homeotic genes of Drosophila. Cell. 1984 Oct;38(3):675–680. doi: 10.1016/0092-8674(84)90262-9. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Rusconi S., Godowski P. J., Maler B. A., Okret S., Wikström A. C., Gustafsson J. A., Yamamoto K. R. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986 Aug 1;46(3):389–399. doi: 10.1016/0092-8674(86)90659-8. [DOI] [PubMed] [Google Scholar]
- Regulski M., Harding K., Kostriken R., Karch F., Levine M., McGinnis W. Homeo box genes of the Antennapedia and bithorax complexes of Drosophila. Cell. 1985 Nov;43(1):71–80. doi: 10.1016/0092-8674(85)90013-3. [DOI] [PubMed] [Google Scholar]
- Schneuwly S., Kuroiwa A., Baumgartner P., Gehring W. J. Structural organization and sequence of the homeotic gene Antennapedia of Drosophila melanogaster. EMBO J. 1986 Apr;5(4):733–739. doi: 10.1002/j.1460-2075.1986.tb04275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Bloom F. E., Lerner R. A. Common 82-nucleotide sequence unique to brain RNA. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4942–4946. doi: 10.1073/pnas.79.16.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Yedvobnick B., Finnerty V. G., Artavanis-Tsakonas S. opa: a novel family of transcribed repeats shared by the Notch locus and other developmentally regulated loci in D. melanogaster. Cell. 1985 Jan;40(1):55–62. doi: 10.1016/0092-8674(85)90308-3. [DOI] [PubMed] [Google Scholar]